Abstract
Epidemiological studies suggest a correlation between severity of acquired immunodeficiency syndrome (AIDS) and selenium deficiency, indicating a protective role for this anti-oxidant during HIV infection. Here we demonstrate that thioredoxin reductase-1 (TR1), a selenium-containing pyridine nucleotide-disulfide oxidoreductase that reduces protein disulfides to free thiols, negatively regulates the activity of the HIV-1 encoded transcriptional activator, Tat, in human macrophages. We used a small interfering RNA approach as well as a high affinity substrate of TR1, ebselen, to demonstrate that Tat-dependent transcription and HIV-1 replication were significantly increased in human macrophages when TR1 activity was reduced. The increase in HIV-1 replication in TR1 small interfering RNA-treated cells was independent of the redox-sensitive transcription factor, NF-κB. These studies indicate that TR-1 acts as a negative regulator of Tat-dependent transcription. Furthermore, in vitro biochemical assays with recombinant Tat protein confirmed that TR1 targets two disulfide bonds within the Cys-rich motif required for efficient HIV-1 transactivation. Increasing TR1 expression along with other selenoproteins by supplementing with selenium suggests a potential inexpensive adjuvant therapy for HIV/AIDS patients.
The physiological functions of the micronutrient selenium are mediated through a co-translational insertion of selenocysteine (Sec),3 into specific proteins (1, 2). Commonly studied selenoproteins such as the glutathione peroxidases, thioredoxin reductases (TR), and deiodinases share a redox mechanism that is driven by Sec present in the active site making selenium a required vital nutrient. As a specific example, selenium deficiency has been strongly and independently associated with disease progression and mortality in HIV-infected individuals, increased genital shedding of HIV DNA, and increased risks of fetal and child mortality as well as intrapartum mother-to-child transmission (3–6). Recent studies have demonstrated that daily selenium supplementation of HIV-1 positive individuals suppresses the progression of HIV-1 viral burden, provides improvement of CD4 counts (7), increases vigor (8), decreases anxiety, and reduces the need for hospitalization when compared with those receiving a placebo (9–11).
HIV-1 infection alters the expression of selenoproteins, including TR (12). TRs are enzymes belonging to the flavoprotein family of pyridine nucleotide-disulfide oxidoreductases that includes lipoamide dehydrogenase, glutathione reductase, and mercuric ion reductase (1, 13, 14). Each monomer of TR includes a FAD prosthetic group, an NADPH binding site, and a selenneylsulfide active site. This active site is formed from the conserved Cys-Sec sequence, which is reduced to a selenolthiol by electrons from the redox-active disulfide of the other subunit (15, 16). Electrons are transferred from NADPH to the active site of the disulfide of TR via FAD, which then reduces the substrate. TRs exhibit catalytic redox activity toward thioredoxin (Trx), a group of small redox active proteins in the mass range of 10–12 kDa, which in turn catalyze thiol-disulfide exchange to reduce key Cys residues in transcription factors (14). Extracellular Trx inhibits the production of HIV-1, whereas its degradation product, eosinophil cytotoxicity-enhancing factor, promotes HIV-1 production (17). Apart from Trx, cytosolic TR (TR1) exhibits broad substrate specificity reducing cytotoxic protein NK-lysin, tumor suppressor protein p53, and non-protein substrates such as lipoic acid, lipid hydroperoxides, vitamin K3, dehydroascorbic acid, and the ascorbyl free radical (1, 18).
Tat is an HIV-encoded transactivating protein that is required for virus replication. Tat binds to a RNA stem loop structure (TAR) and recruits the positive transcription elongation factor, P-TEFb, to the HIV long terminal repeat (LTR), increasing RNA polymerase II processivity (19, 20). In the absence of Tat, transcription is impaired, leading to the accumulation of prematurely terminated short transcripts (21, 22). The primary structure of Tat is made up of 101 amino acids. Based on mutagenesis studies, the Cys-rich region (amino acids 20–39) has been demonstrated to be necessary for Tat activity (23, 24). Furthermore, Koken et al. (25) have shown that the transcriptionally active form of Tat is a monomer, and reducing agents strongly inhibit Tat activity, suggesting that Cys residues in Tat form intramolecular disulfide bonds. Similarly, increased transactivation activity was seen in bacterially expressed Tat that was subjected to slow-protein refolding, which permits the formation of disulfide bonds (26). These data suggest that the activity of Tat is governed in part by Cys groups via the formation of disulfide bonds and that Tat is sensitive to the redox state of the cell. Here, we report that TR1 targets the disulfide bonds in Tat and functions as a negative regulator of Tat-dependent proviral transcription.
MATERIALS AND METHODS
Cell Culture—The human U937 promonocytic cell line (ATCC, Manassas, VA) was cultured in RPMI 1640 medium supplemented with 5% defined fetal calf serum (Hyclone), 100 units/ml penicillin, 100 μg/ml streptomycin, and 0.2 m l-glutamine. Peripheral blood macrophages were isolated from whole blood obtained from healthy HIV-1-seronegative donors. Mononuclear cells were obtained by differential centrifugation using a Ficol/Hystopaque (Sigma-Aldrich) gradient and adherence to plastic culture flasks as previously described (27). Macrophages were separated from other cells by an initial adherence to plastic culture flasks overnight. After removing nonadherent cells, monocytes were cultured for 5–10 days to mature into monocyte-derived macrophages (MDM). All protocols were preapproved by the IRB at Penn State University. Fetal calf serum lots containing low selenium were selected for the study. Selenium was measured using atomic absorption spectrometry. The basal media (containing 7 nm selenium) was supplemented with selenium in the form of sodium selenite. Jurkat CD4+ T-cells stably expressing HIV-1 Tat were obtained from Drs. Antonella Caputo, William Haseltine, and Joseph Sodorski through the NIH AIDS Research and Reagent Program. The cells were cultured in RPMI 1640 medium containing 10% defined fetal calf serum and 800 μg/ml G418.
Expression and Activity of TR—U937 cells were cultured in basal media with or without exogenously added selenium for 1 week with three media changes in between. The washed cell pellets were lysed with mammalian protein extraction reagent (M-PER; Pierce) and clarified by centrifugation at 16,000 × g at 4 °C for 15 min. Cell lysates were used in immunoblot and enzymatic assays for TR. Assays for cytosolic TR activity in these lysates were performed as described by Holmgren and Bjornstedt (28), where recombinant Tat (rTat) was substituted for Trx in the assay. 0.1 μm purified rat liver TR1 from Sigma was used. Change in the absorbance at 314 nm was used to calculate the activity.
Transient Transfections, siRNA, and Luciferase Assay—Replication competent HXB.2, bacterial and mammalian HIV-1 Tat expression vectors, pTat86R His (29), and pCI-Tat1 (30) were obtained from the AIDS Research Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health. pCI vector (Promega, Madison, WI) was used as an empty vector control. LTR reporter constructs, LTR-Luc (wild-type–205 LTR), ΔTARLTR-Luc (–205 LTR lacking the TAR region), and ΔκBLTR-Luc (–205 LTR lacking the NF-κB binding sites) were prepared as previously described (31). DNA for transfections was prepared using plasmid purification systems from Marligen Biosciences (Ijamsville, MD) following protocols provided by the manufacturer. Transfection of U937 cells was performed using TransIT-Jurkat transfection reagent from Mirus (Madison, WI). Transfection efficiency was assessed by co-transfecting pEGFP-N3 (Clontech, Palo Alto, CA) and monitoring enhanced green fluorescence protein expression by fluorescence microscopy. Cell viability was confirmed by trypan blue staining. siRNA for TR1 as well as siRNA control were reconstituted based on the manufacturer's guidelines (Dharmacon). Luciferase assays were performed using a commercial luciferase assay kit (Promega) and a TD-20/20 luminometer (Turner BioSystems, Sunnyvale, CA). U937 cells were infected with HIV-Luc virus (1 × 106 infectious particles/1 × 106 cells). The next day cells were transfected with TR1 or control siRNA using the Amaxa nucleofector system, and luciferase activity was measured 72 h post-infection.
Generation of HIV Infectious Titers, Infections, and Transduction of Macrophages—Infectious HXB.2 virus was generated by cotransfecting 293T cells with 15 μg of HXB.2 cDNA, 3 μg of vesicular stomatitis virus-glycoprotein, and 3 μg of RSV-Rev by calcium phosphate transfection (32). Transfection efficiency was assessed by measuring p24 levels (p24 ELISA, PerkinElmer Life Sciences). We consistently generated titers of 1.0 ×106 infectious particles/ml. Supernatants were collected and filtered through 0.45-μm syringe filter (Whatman, Clifton, NJ). One ml of undiluted virus stock was added to 1.0 ×106 U937 cells or human MDMs (differentiated 10 days) for 24 h and then replaced with fresh media. Cells were then transfected with TR1 siRNA using the Amaxa nucleofector system and were subjected to luciferase assays 3–5 days post-infection. For some experiments transfected cells were cultured in the presence or absence of ebselen (5 μm; Sigma) or DMSO. Supernatants were collected at 4 days post-infection and assayed for viral replication by p24 ELISA. To assess the effect of selenium supplementation on p24 levels, 1.0 ×106 U937 cells were cultured in a selenium-deficient media and infected with HXB.2 for 24 h followed by media changes containing selenium from 0.05 μm to 1 μm. After 3 days, the culture media supernatants were used to measure p24 levels.
Immunoblots—MDMs and U937 cells were washed twice with phosphate-buffered saline, and protein extracts were prepared by treating cells with M-PER reagent (Pierce) at 4 °C for 30 min. Lysates were mixed with 2× SDS loading buffer containing dithiothreitol and heated at 100 °C for 5 min before resolving by SDS-PAGE (%T = 4–20) followed by immunoblotting onto a nitrocellulose membrane. Rabbit polyclonal antibodies against TR1 and GPX1 (Abcam) were used to analyze expression of the two selenoproteins. Horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma-Aldrich) was used as the secondary antibody. To normalize loading, membranes were treated with Western blot stripping buffer (Pierce) and reprobed with anti-β-actin or anti-glyceraldehyde-3-phosphate dehydrogenase followed by an appropriate horseradish peroxidase-conjugated secondary antibody.
Expression, Purification, and Refolding of rTat—rTat was expressed in Escherichia coli transformed with pTat86RHis as a His-tagged fusion protein using the autoinduction system (Novagen). The purification of rTat was performed using the method of Kirsch et al. (26) with some modifications. Briefly, the E. coli pellet from a 24-h culture was lysed in 6 m guanidine-HCl for 12 h. All steps were performed at 4 °C unless otherwise mentioned. The cleared cell lysate was applied to a nickel-nitrilotriacetic acid column (Novagen) and developed as described by Kirsch et al. (26). The eluate was dialyzed against 0.1 n HCl for 2 days at 4 °C followed by an overnight lyophilization. The lyophilized powder was reconstituted in 6 m urea followed by reduction of the thiols with tris[2-carboxyethyl]phosphine (25 mol/mol of Tat) in the dark for 8 h at room temperature as per the instructions of the supplier (Pierce). The mixture was subjected to sequential dialysis in 0.1 m phosphate buffer (pH 6.3) containing 4 m urea, 2 m urea, and finally in 0.1 m phosphate buffer (pH 4.3) containing 200 mm NaCl. The dialysate was filtered through 0.45-μm filter and stored in small aliquots at –20 °C for further use. Typical yields from a liter of bacterial culture were ∼10 mg of pure Tat, based on SDS-PAGE (>95%).
Analysis of rTat Activity—U937 cells were transfected with 2 μg of LTR-Luc or an irrelevant reporter plasmid (pSV2-Luc) as a control by transient transfection. Cells were then treated with 2 μg of rTat or buffer alone, and luciferase activity was measured 48 h post-transfection as an indication of proviral transcription. After transfections, trypan blue staining was performed to control for cell viability. Transfections were performed in triplicate, and the data are presented as the % of luciferase with luciferase activity in the cells lacking Tat set at 100%.
Quantifying Free Cys Thiols in rTat—Purified and refolded rTat, as described above, was used in the standard Ellman's reagent (5,5′-dithiobis(2-nitrobenzoic acid) assay as described by the supplier (Pierce). The concentration of rTat was 2 μm, whereas the concentration of rat liver TR1 (Sigma) was 100 nm in the reaction mixture containing 0.008% bovine serum albumin, 4.3 mm EDTA, 0.43 mm NADPH in a total of 1 ml of phosphate buffer (pH 7.0) at 37 °C. Additional controls included heat-denatured TR1 and TR1 without NADPH. Free thiol (SH) groups in rTat were estimated before and after reduction by TR, reaction with heat-denatured TR, and reaction on ice (2 °C) using the standard Ellman's reagent with freshly prepared l-cysteine solution as a calibration standard (0–80 μm).
Redox Western Blot Analysis—rTat protein (20 μm) was reacted with TR1 (100 nm, from Sigma) in the presence of 0.5 mm NADPH, 5 mm EDTA, 0.1 mg/ml bovine serum albumin in 100 mm phosphate buffer (pH 7.0) for 1 h at room temperature. The resultant mixture was then incubated with 25 mm AMS (4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid; 250 mm stock in DMSO) for 30 min at 4 °C followed by 10 min at 37 °C. The mixture was subjected to a non-denaturing and nonreducing PAGE (%T = 18) followed by Western blotting with anti-Tat polyclonal antibodies. rTat (50 nm) reduced with 2.5 mm DTT and subsequently treated with AMS was used a positive control, whereas rTat (50 nm) oxidized with 2.5 mm N,N,N′,N′-tetramethylazodicarboxamide (diamide; Sigma) was used as a negative control.
Mechanism-based Affinity Chromatography—A Sepharose affinity matrix was prepared with two mutant forms of His-tagged human TR1 where the resolving Cys and Sec in the C terminus were replaced with Ser in one (denoted as SU) and both Cys and SeCys to Ser in the other (denoted as SS) as described earlier (15). The immobilized enzymes were initially incubated with 2 ml of 0.5 mm NADPH in phosphate-buffered saline for 20 min. 15 μg of rTat was applied to each column. After 1 h of incubation at room temperature on an end-over shaker, the resins were washed 5 times with phosphate-buffered saline (PBS) to remove nonspecifically bound rTat and then eluted with 500 μl of PBS containing 10 mm DTT. All the flow-through (wash) and eluate fractions were concentrated and analyzed for the presence of Tat using Western blots.
RESULTS
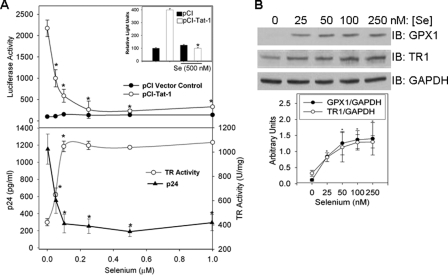
Selenium Inhibits HIV-1 Transcription—An inverse correlation between selenium levels and HIV progression has been reported (7). To gain insight into mechanisms by which selenium inhibits HIV replication, we tested whether Tat-dependent HIV transcription was sensitive to changes in cellular selenium status. The human monocytic cell line U937, cultured under selenium-deficient conditions (containing 7 nm selenium), were supplemented with selenium at 0.05–1.0 μm. Cells were co-transfected with pCI-Tat and LTR-Luc reporter that contained the TAR element. Selenium at concentrations of 100 nm significantly reduced Tat-dependent transcription compared with the cells where no exogenous selenium was added (Fig. 1). Tat was required for selenium-dependent repression of LTR-Luc activity as selenium had negligible effect on basal LTR activity in the absence of Tat. Furthermore, selenium had no effect on the ΔTARLTR-Luc (data not shown). Similar repression of Tat-dependent LTR activity was observed in human MDMs, although the amount of selenium added was 500 nm (Fig. 1A, inset). Selenium also inhibited HIV-1 replication as determined by p24 ELISA (Fig. 1A). HIV-infected U937 cells cultured in the absence of selenium produced ∼6-fold more virus than infected cells cultured in the presence of selenium. Maximum suppression of HIV-1 replication was observed at ∼0.1 μm selenium, which was the concentration that repressed Tat-dependent LTR activity. To ensure that we were effectively depleting selenium, the expression of two selenoproteins, TR1 and GPX1, was monitored. We observed a decrease in TR1 and GPX1 expression in U937 cells (Fig. 1B) and primary MDM cells (data not shown) that were cultured under selenium-deficient conditions. Selenium supplementation rescued TR1 activity (Fig. 1A) and expression (Fig. 1B) in U937 cells. The enzymatic activity of TR1 and expression of TR1 and GPX1 were saturated at ∼100 nm selenium, which corresponded to the concentration that fully inhibited Tat-dependent LTR activity, suggesting an inverse relationship between HIV-1 transcription and selenoprotein activity.
FIGURE 1.
Selenium inhibits Tat-dependent LTR activity in human macrophages. A, U937 cells were transfected with 2 μg of LTR-Luc and then incubated with different concentrations of selenium (0.05–1.0 μm) for 72 h. Cells were lysed, and luciferase activity was measured as an indication of HIV-LTR activity. Transfections were performed in triplicate, and data are presented as the percentage of luciferase. Inset, the same experiment performed with primary human MDMs. These cells were transfected with LTR-Luc, pCI-Tat, or empty vector using the Amaxa nucleofector system. TR activity was measured in the U937 cell lysates. For replication experiments, 1.0 ×106 U937 cells were cultured in a selenium-deficient media and infected with HXB.2 for 24 h followed by media changes containing selenium from 0.05 μm to 1 μm. After 3 days the culture media supernatants were used to measure p24 levels. B, modulation of expression of selenoproteins (TR1 and GPX1) by exogenous selenium in macrophages. U937 cells cultured in low-selenium serum-containing media (7 nm selenium) were passaged for 3–5 days in media containing 0.025–0.25 μm selenium (as sodium selenite). The cell lysates were analyzed for GPX1 and TR1 expression. Membranes were stripped and reprobed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to normalize for protein loading. Densitometric evaluation of the data is shown. Representative of n = 3. *, p < 0.005. IB, immunoblot.
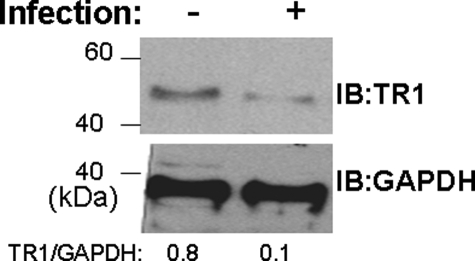
HIV-1 Infection of Macrophages Down-regulates TR1 Expression—Selenium is incorporated as Sec into enzymes such as the glutathione peroxidases and TRs that control cellular redox homeostasis. Based on cellular localization and function, we focused on the cytosolic TR1 form (also called TXNRD1 or TrxR1) rather than the thioredoxin/glutathione reductase (TR2 or TrxR3) or the mitochondrial TR3 (TXNRD2 or TrxR2) (15). To determine whether HIV-1 infection led to changes in TR expression, as reported earlier in T-cells (12), primary MDM were infected with HIV-1 and TR1 levels were monitored by immunoblotting. There was >85% decrease in TR1 expression within 48 h of HIV-1 infection (Fig. 2), suggesting that HIV-1 could potentially target selenoproteins, including TR1, to ensure generation of oxidative stress for efficient replication.
FIGURE 2.
Infection of MDMs by HIV-1 decreases the expression of TR1. Human monocyte-derived macrophages were infected with HXB.2 for 48 h. Cells were lysed, and TR1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were detected by immunoblotting (IB). Results are representative of n = 3 shown.
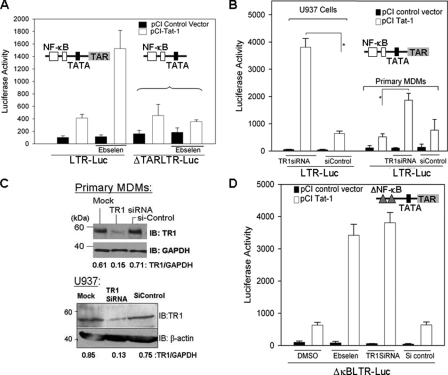
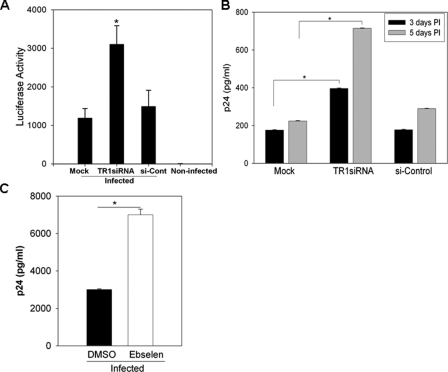
TR1 Negatively Regulates HIV-1 Transcription and Replication—The inverse correlation between TR1 activity and HIV-1 transcription and replication suggested that TR1 is a negative regulator of HIV-1 transcription. To investigate the role of TR1 in regulating Tat-mediated HIV-1 transcription, ebselen, a highly active substrate of TR (33, 34), was used to “hijack” TR activity. As shown in Fig. 3A, ebselen increased Tat-dependent transactivation of the HIV-LTR by ∼4-fold. The ability of ebselen to enhance transcription required Tat and the TAR element (Fig. 3A). In addition, we used siRNA to specifically diminish TR1 expression in U937 cells and MDM (Fig. 3B). TR1 siRNA depleted ∼80% of TR1 in MDMs and U937 cells as determined by immunoblots (Fig. 3C). In U937 cells and MDMs treated with TR1 siRNA, Tat-dependent LTR-Luc activity was increased by 8- and 4-fold, respectively (Fig. 3B). Furthermore, the decrease in TR1 did not ectopically activate NF-κB or enhance NF-κB-dependent transcription, as reducing TR1 expression and activity with siRNA or ebselen did not significantly alter activity of LTRs lacking NF-κB sites (Fig. 3D) or TAR (data not shown). To determine whether decreasing TR1 had an impact on HIV-1 provirus transcription and replication, we infected cells with HIV-Luc, a replication incompetent HIV-1 clone in which Env was replaced with a luciferase reporter gene (35). Provirus transcription was monitored by measuring luciferase activity. Consistent with the transient transfection data, we observed a 3.5-fold increase in HIV-1 provirus transcription in U937 cells when TR1 was diminished by siRNA compared with the si-control (Fig. 4A). In addition, MDMs were infected with replication competent HIV-1, and virus replication was monitored by p24 ELISA (Fig. 4B). A 3.5-fold increase in HIV replication was observed in MDMs that were transfected with TR1 siRNA. A similar increase was also observed in HIV-1 replication in U937 cells that were treated with 5 μm ebselen (Fig. 4C). Taken together, these results indicate that TR1 impacts HIV-1 transcription and replication by repressing the activity of Tat.
FIGURE 3.
Down-regulation of TR1 activity and expression increases Tat-dependent transcription. A, ebselen treatment of macrophages increases Tat-dependent transcription. U937 cells were transfected with LTR-Luc or ΔTARLTR-Luc and pCI-Tat or empty pCI vectors. The transfected cells were cultured in the presence or absence of ebselen (5 μm) or DMSO (0.1% v/v) for 24 h. Luciferase activities in the lysates were quantitated after 48 h post-transfection and normalized to total protein. B, U937 cells were transfected with LTR-Luc and pCI-Tat or empty pCI vector and TR1 siRNA or si-control. Luciferase activities were measured as mentioned earlier. MDMs were transfected with LTR-Luc and pCI-Tat or empty pCI vector and TR1 siRNA using the Amaxa nucleofector system and processed as described earlier. C, TR1 expression in TR1 siRNA or si-control-treated U937 and MDM cells as determined by immunoblotting (IB). The blots were stripped and reprobed with either β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Densitometric values are shown below each panel. D, TR1 regulates Tat-dependent transcription independent of NF-κB. U937 cells were transfected with ΔκBLTR-Luc and pCI-Tat or empty pCI vector. Cells were then either treated with ebselen (5 μm) or TR1 siRNA or si-control. Cell lysates were used in luciferase activities 48 h post-transfection. The luciferase activity was normalized to total cell protein. *, p < 0.05.
FIGURE 4.
TR1 negatively regulates HIV-1 replication in primary monocyte-derived-macrophages. A, TR1 negatively regulates HIV-1 provirus transcription. U937 cells were infected with HIV-Luc virus. Cells (12–24 post-infection) were transfected with TR1 siRNA. Luciferase activity was measured 72 h post-infection. B, MDMs were infected with HXB.2. 12–24 h post-infection cells were transfected with TR1 siRNA or si-control using the Amaxa nucleofector system and subjected to luciferase assay 3–5 days post-infection. C, ebselen treatment increases HIV-1 replication. U937 cells were infected with HXB.2. The transfected cells were cultured in the presence or absence of ebselen (5 μm) or DMSO (0.1% v/v). Supernatants were collected at 4 days post-infection and assayed for viral replication by p24 ELISA. Each data point represents three independent infections, and error bars show the S.D. of these replicates. *, p < 0.05.
In Vitro Reduction of rTat by TR1—Tat has a Cys-rich region with three Cys-Xaa-Xaa-Cys motifs in the transactivation domain (23). Previously, increased transactivation of a LTR-Luc reporter was demonstrated by bacterially expressed rTat that was allowed to slowly refold in the presence of a reducing agent (tris[2-carboxyethyl]phosphine), indicating that the formation of intramolecular disulfide bonds was necessary for Tat function (36). Using an identical protocol, our studies also demonstrated that refolded rTat, which is efficiently taken up by cells (37), was able to activate TAR-dependent transcription in U937 cells, whereas rTat treatment did not activate the pSV2-Luc reporter, which lacks the LTR (Fig. 5). Furthermore, to demonstrate that rTat is a substrate for TR1, we used an in vitro assay with rat liver TR1 to assess the formation of free thiols from disulfide bonds using Ellman reagent (5,5′-dithiobis(2-nitrobenzoic acid)). Coincubation of rTat with rat liver TR1 and NADPH increased the number of free thiols from three to seven, indicating that four out of the seven Cys in Tat form two disulfide bonds (Fig. 6A). Redox Western blot analysis was used to examine the electrophoretic mobility of rTat before and after reduction by TR1. AMS-modified proteins increased protein mass by 500 Da, which can be detected as a modest shift on a non-reducing and non-denaturing PAGE. The results indicate an upward shift in the mobility of rTat as a result of reduction by TR1, similar to the DTT-reduced rTat control (Fig. 6B). To further confirm this observation, selenium supplementation of Jurkat T-cells that stably expressed Tat showed a dose-dependent change in the redox status of Tat with increase in selenium (data not shown). We also used a spectrophotometric assay to examine the oxidation of NADPH and observed that rat liver TR1 catalyzed the NADPH-dependent reduction of rTat in vitro. The reduction followed typical Michaelis-Menten kinetics (data not shown). Finally, we used a mechanism-based affinity chromatographic approach to demonstrate that human TR1, where the resolving Cys was mutated to Ser (denoted as SU), was able to bind to rTat. Although there was some protein seen in the flow-through fraction, the eluate fraction had a relatively higher amount of rTat (Fig. 6C), which could be due to the saturation of TR1. Using human TR1 where both Cys and SeCys were mutated to Ser (denoted as SS), we noticed relatively low levels of rTat in the eluate fractions, whereas the flow-through fraction contained most of rTat (Fig. 6D). Taken together, these studies suggest that TR1 reduces disulfides in Tat by a direct interaction.
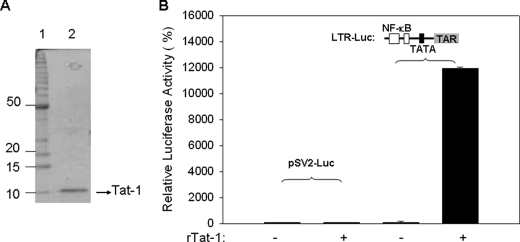
FIGURE 5.
Expression, purification, and biological activity rTat. A, electrophoretic purity of rTat expressed in E. coli using SDS-PAGE. B, U937 cells were transfected with 2μg of LTR-Luc or pSV2-Luc reporter constructs. Transfected cells were cultured in the presence and absence of 2 μg rTat protein. Luciferase activity was measured 48 h post-transfection as an indication of proviral transcription. After transfections, trypan blue staining was performed to control for cell viability. *, p < 0.05.
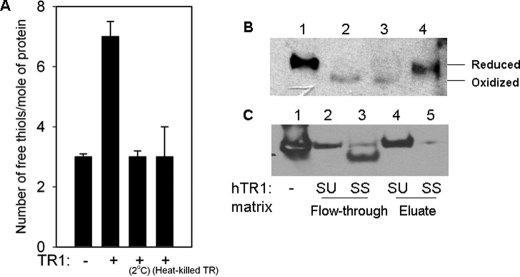
FIGURE 6.
Direct interaction of rTat with TR1 in vitro. A, 2 μm rTat was mixed with 100 nm rat liver TR1 (Sigma) and 0.4 mm NADPH as described under Materials and Methods.” Free thiols were determined using Ellman's reagent (5,5′-dithiobis(2-nitrobenzoic acid). Data shown are the mean of triplicate experiments ±S.D. B, redox Western blot analysis of the reduction of rTat by TR1. The above reaction mixture was reacted with AMS followed by Western blot analysis with anti-Tat monoclonal antibodies. Lanes 1–4 represent DTT-reduced rTat, diamide (N,N,N′,N′-tetramethylazodicarboxamide)-oxidized rTat, native rTat, and TR1-reduced rTat, respectively. Although the protein load was identical in all the lanes, we consistently observed variations in the immunoreactivity of oxidized rTat. C, mechanism-based affinity chromatography showing the binding of rTat to hTR1SU and hTR1SS mutants. Protein in the flow-through and DTT eluate fractions were concentrated and analyzed by Western blot for the presence of Tat. Lanes 1–5 represent rTat positive control, flow-through from SU column, flow-through from SS column, eluate from SU column, and eluate from SS column, respectively.
DISCUSSION
Epidemiological studies have shown a correlation between decreased selenium status and increased mortality and morbidity of HIV patients (4, 38, 39). However, there is sparse information regarding mechanisms by which selenium influences HIV-1 replication. Selenium decreases oxidative stress in HIV-infected people by neutralizing highly reactive pro-oxidant species via selenoenzymes, which is thought to positively impact patient immune function and health (11) and, thus, indirectly influence HIV-1 replication in infected cells. Based on our data, selenium appears to have a direct effect on HIV-1 transcription leading to decreased virus replication. Alternatively, selenium may directly regulate HIV-1 transcription by activating NF-κB, which is required for efficient provirus transcription. In this report, we present data indicating that selenium, through the selenoprotein TR1, negatively regulates HIV-1 transcription by targeting Tat-dependent transcription independent of NF-κB.
Tat is essential for HIV-1 replication and is required for efficient transcription elongation (40). HIV-1 Tat binds the RNA stem loop structure formed by TAR and recruits the positive transcription elongation factor, P-TEFb, to the LTR, which enhances processive transcription. Because Tat is necessary for HIV-1 replication, host mechanisms that specifically target its activity would provide attractive therapeutic options that would be complementary to current anti-retroviral treatments targeting reverse transcriptase, integrase, and protease enzymes. The conclusion that the intramolecular disulfide bonds are critical for Tat function is consistent with previous observations that reducing agents that disrupt intramolecular disulfide bonds inhibit Tat activity (25). Our results not only support earlier data but also demonstrate that TR1 negatively regulates Tat activity by reducing two disulfide bonds in Tat. Although the Cys-rich domain is not structurally well defined, recent NMR studies using recombinant Tat protein confirm that the disulfide bonds are essential for stabilizing its structure to possibly allow the cyclin T1 subunit of P-TEFb to bind Tat (41). Therefore, TR-dependent reduction of the disulfides may affect the interaction of Tat with P-TEFb, leading to a decrease in the transcription processivity. The role of individual Cys residues in the formation of disulfide bonds and the reactivity of TR1 toward various ΔCys mutant Tat proteins is being currently explored. Our results also indicate that Tat is a redox-sensing molecule, as it is subjected to negative regulation by enzymes such as TR1. Although the mechanism-based affinity chromatography experiments suggest a direct interaction between Tat and TR1, it is plausible that the redox modulation could also occur through TR modulation of Trx, as is the case for NF-κB p65 subunit (42) and glucocorticoid receptor (43). However, this latter mechanism is indirect, differing from the direct TR1-Tat interactions that our data suggest.
Specifically inhibiting TR1 activity in macrophages with either a high affinity substrate or expression using siRNA resulted in more efficient HIV-1 replication. If oxidative stress is required for efficient HIV-1 replication, then the virus would be expected to circumvent the reducing activities of TR. Selenoproteins, including TR1, are down-regulated upon HIV-1 infection and during the progression of AIDS and associated diseases. Whether HIV-1 directly alters the redox status of the cell or indirectly promotes inflammation and oxidative stress is not clear; however, we show that infection of macrophages decreases TR1 protein, which is consistent with earlier reports (12). Moreover, our data demonstrate that the expression of selenoproteins TR1 and GPX1 in U937 cells and MDMs can be modulated with selenium (Fig. 1D), providing a strategy to rescue selenoprotein expression as well as decreasing HIV-1 transcription and replication in HIV-1 infected cells. Although these studies have focused on HIV-1, we would speculate that viruses in general would have mechanisms for sensing the oxidative status of its host cell. For example, we have preliminary data indicating that replication of the parainfluenza virus type 5, a non-segmented negative strand RNA virus, is repressed by selenium supplementation (data not shown). We have not fully characterized how selenium inhibits parainfluenza virus type 5 replication, but it is interesting to note that this virus does encode a Cys-rich protein that could be potentially targeted by TR1.
In conclusion, we have identified TR1 as a critical host selenoprotein that plays a pivotal role in repressing HIV-1 transcription by modulating the redox-status of the key viral protein, Tat (Fig. 7). It remains to be seen if the redox status of Tat correlates with the plasma selenium in HIV-1 seropositive individuals. Such studies are necessary to understand the therapeutic benefits of selenium as an adjuvant in the management of HIV/AIDS. These studies partially elucidate one mechanism by which selenium inhibits HIV-1 replication. Furthermore, these findings suggest that administration of selenium to HIV-1 seropositive individuals at a daily recommended intake of 55 μg/day, as per the National Institute of Medicine, may provide an effective method to slow the progression of AIDS, reduce morbidity, and enhance survival partly through the redox-dependent regulation of Tat-dependent transcription. Such supplementation studies need to be evaluated with caution given the toxicity associated with selenium, seen in the form of nausea, blotchy nails, vomiting, and diarrhea at doses higher than the tolerable upper intake levels of 400 μg/day. However, understanding the mechanism of action of selenium will allow more direct targets to be examined and set optimal doses to mitigate toxicity and achieve the desired effect.
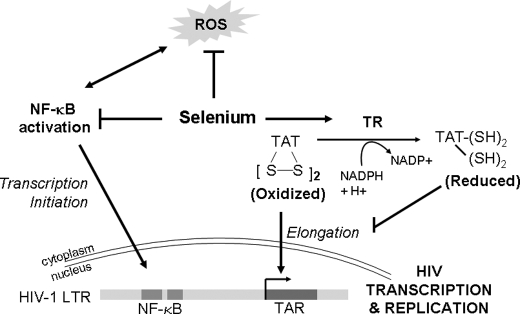
FIGURE 7.
Proposed model of regulation of HIV-1 transcription by TR1. Selenium supplementation of macrophages leads to the decrease in reactive oxygen species (ROS) and NF-κB-dependent pathways of pro-inflammatory gene expression possibly by the increased cellular peroxide scavenging ability of selenoenzymes. Although such effects may have an impact on the HIV-1 transcription, a more direct mechanism of action of the selenoprotein, TR1, is possible. Increased expression and activity of TR1 in selenium-supplemented cells reduces proviral transcription by targeting the disulfide bonds in Tat. Thus, TR1 acts as a negative regulatory molecule capable of suppressing proviral transcription of HIV-1 in in vitro culture models.
Acknowledgments
We are grateful to Dr. Vadim N. Gladyshev, University of Nebraska, Lincoln, NE for help with the mechanism-based affinity chromatography experiments and to the National Institutes of Health AIDS Research and Reference Reagent Program for providing the HXB.2 viral cDNA constructs, LTR-luciferase reporters, and Tat monoclonal antibody. We thank Dr. C. Channa Reddy for invaluable suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 AI062467 (to A. J. H.) and R01 DK077152 (to K. S. P.) from the Public Health Service. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Sec, selenocysteine; TR, thioredoxin reductase; siRNA, small interfering RNA; HIV, human immunodeficiency virus; Trx, thioredoxin; LTR, long terminal repeat; MDM, monocyte-derived macrophages; AMS, 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid; ELISA, enzyme-linked immunosorbent assay; DTT, dithiothreitol; rTat, recombinant Tat.
References
- 1.Mustacich, D., and Powis, G. (2000) Biochem. J. 346 1–8 [PMC free article] [PubMed] [Google Scholar]
- 2.Stadtman, T. C. (1990) Annu. Rev. Biochem. 59 111–127 [DOI] [PubMed] [Google Scholar]
- 3.Baum, M. K., Shor-Posner, G., Lai, S., Zhang, G., Lai, H., Fletcher, M. A., Sauberlich, H., and Page, J. B. (1997) J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 15 370–374 [DOI] [PubMed] [Google Scholar]
- 4.Kupka, R., Msamanga, G. I., Spiegelman, D., Morris, S., Mugusi, F., Hunter, D. J., and Fawzi, W. W. (2004) J. Nutr. 134 2556–2560 [DOI] [PubMed] [Google Scholar]
- 5.Kupka, R., Msamanga, G. I., Spiegelman, D., Rifai, N., Hunter, D. J., and Fawzi, W. W. (2005) Eur. J. Clin. Nutr. 59 1250–1258 [DOI] [PubMed] [Google Scholar]
- 6.Kaiser, J. D., Campa, A. M., Ondercin, J. P., Leoung, G. S., Pless, R. F., and Baum, M. K. (2006) J. Acquired Immune Defic. Syndr. 42 523–528 [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz, B. E., Klaus, J. R., Llabre, M. M., Gonzalez, A., Lawrence, P. J., Maher, K. J., Greeson, J. M., Baum, M. K., Shor-Posner, G., Skyler, J. S., and Schneiderman, N. (2007) Arch. Intern. Med. 167 148–154 [DOI] [PubMed] [Google Scholar]
- 8.Shor-Posner, G., Lecusay, R., Miguez, M. J., Moreno-Black, G., Zhang, G., Rodriguez, N., Burbano, X., Baum, M., and Wilkie, F. (2003) Int. J. Psychiatry Med. 33 55–69 [DOI] [PubMed] [Google Scholar]
- 9.Burbano, X., Miguez-Burbano, M. J., McCollister, K., Zhang, G., Rodriguez, A., Ruiz, P., Lecusay, R., and Shor-Posner, G. (2002) HIV Clin. Trials 3 483–491 [DOI] [PubMed] [Google Scholar]
- 10.McClelland, R. S., Baeten, J. M., Overbaugh, J., Richardson, B. A., Mandaliya, K., Emery, S., Lavreys, L., Ndinya-Achola, J. O., Bankson, D. D., Bwayo, J. J., and Kreiss, J. K. (2004) J. Acquired Immune Defic. Syndr. 37 1657–1663 [DOI] [PubMed] [Google Scholar]
- 11.McDermid, J. M., Lalonde, R. G., Gray-Donald, K., Baruchel, S., and Kubow, S. (2002) J. Acquired Immune Defic. Syndr. 29 158–164 [DOI] [PubMed] [Google Scholar]
- 12.Gladyshev, V. N., Stadtman, T. C., Hatfield, D. L., and Jeang, K. T. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker, K., Gromer, S., Schirmer, R. H., and Muller, S. (2000) Eur. J. Biochem. 267 6118–6125 [DOI] [PubMed] [Google Scholar]
- 14.Arner, E. S., and Holmgren, A. (2000) Eur. J. Biochem. 267 6102–6109 [DOI] [PubMed] [Google Scholar]
- 15.Turanov, A. A., Su, D., and Gladyshev, V. N. (2006) J. Biol. Chem. 281 22953–22963 [DOI] [PubMed] [Google Scholar]
- 16.Zhong, L., Arner, E. S., and Holmgren, A. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 5854–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman, G. W., Balcewicz-Sablinska, M. K., Guarnaccia, J. R., Remold, H. G., and Silberstein, D. S. (1994) J. Exp. Med. 180 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arner, E. S., Nordberg, J., and Holmgren, A. (1996) Biochem. Biophys. Res. Commun. 225 268–274 [DOI] [PubMed] [Google Scholar]
- 19.Herrmann, C. H., and Rice, A. P. (1995) J. Virol. 69 1612–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgeois, C. F., Kim, Y. K., Churcher, M. J., West, M. J., and Karn, J. (2002) Mol. Cell. Biol. 22 1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao, S. Y., Calman, A. F., Luciw, P. A., and Peterlin, B. M. (1987) Nature 330 489–493 [DOI] [PubMed] [Google Scholar]
- 22.Marciniak, R. A., and Sharp, P. A. (1991) EMBO J. 10 4189–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuppuswamy, M., Subramanian, T., Srinivasan, A., and Chinnadurai, G. (1989) Nucleic Acids Res. 17 3551–3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frankel, A. D., and Pabo, C. O. (1988) Cell 55 1189–1193 [DOI] [PubMed] [Google Scholar]
- 25.Koken, S. E., Greijer, A. E., Verhoef, K., van Wamel, J., Bukrinskaya, A. G., and Berkhout, B. (1994) J. Biol. Chem. 269 8366–8375 [PubMed] [Google Scholar]
- 26.Kirsch, T., Boehm, M., Schuckert, O., Metzger, A. U., Willbold, D., Frank, R. W., and Rosch, P. (1996) Protein Expression Purif. 8 75–84 [DOI] [PubMed] [Google Scholar]
- 27.Henderson, A. J., and Calame, K. L. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 8714–8719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holmgren, A., and Bjornstedt, M. (1995) Methods Enzymol. 252 199–208 [DOI] [PubMed] [Google Scholar]
- 29.Patki, A. H., and Lederman, M. M. (1996) Cell. Immunol. 169 40–46 [DOI] [PubMed] [Google Scholar]
- 30.Frankel, A. D., Bredt, D. S., and Pabo, C. O. (1988) Science 240 70–73 [DOI] [PubMed] [Google Scholar]
- 31.Henderson, A. J., Zou, X., and Calame, K. L. (1995) J. Virol. 69 5337–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pear, W. S., Nolan, G. P., Scott, M. L., and Baltimore, D. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao, R., and Holmgren, A. (2002) J. Biol. Chem. 277 39456–39462 [DOI] [PubMed] [Google Scholar]
- 34.Zhao, R., Masayasu, H., and Holmgren, A. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connor, R. I., Chen, B. K., Choe, S., and Landau, N. R. (1995) Virology 206 935–944 [DOI] [PubMed] [Google Scholar]
- 36.Nordberg, J., and Arner, E. S. (2001) Free Radic. Biol. Med. 31 1287–1312 [DOI] [PubMed] [Google Scholar]
- 37.Herce, H. D., and Garcia, A. E. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 20805–20810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baum, M. K., and Shor-Posner, G. (1998) Nutr. Rev. 56 S135–S139 [DOI] [PubMed] [Google Scholar]
- 39.Ogunro, P. S., Ogungbamigbe, T. O., Elemie, P. O., Egbewale, B. E., and Adewole, T. A. (2006) Niger. Postgrad. Med. J. 13 1–5 [PubMed] [Google Scholar]
- 40.Karn, J. (1999) J. Mol. Biol. 293 235–254 [DOI] [PubMed] [Google Scholar]
- 41.Shojania, S., and O'Neil, J. D. (2006) J. Biol. Chem. 281 8347–8356 [DOI] [PubMed] [Google Scholar]
- 42.Hirota, K., Murata, M., Sachi, Y., Nakamura, H., Takeuchi, J., Mori, K., and Yodoi, J. (1999) J. Biol. Chem. 274 27891–27897 [DOI] [PubMed] [Google Scholar]
- 43.Makino, Y., Yoshikawa, N., Okamoto, K., Hirota, K., Yodoi, J., Makino, I., and Tanaka, H. (1999) J. Biol. Chem. 274 3182–3188 [DOI] [PubMed] [Google Scholar]