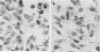
Abstract
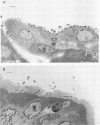
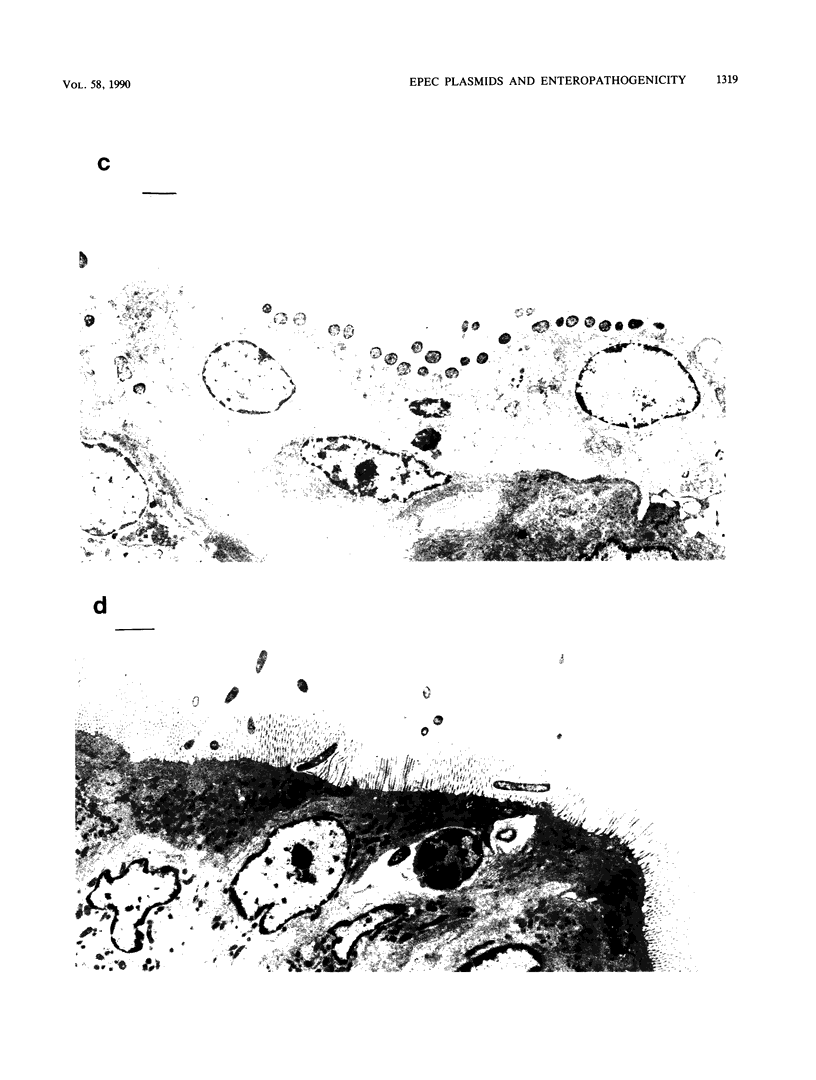
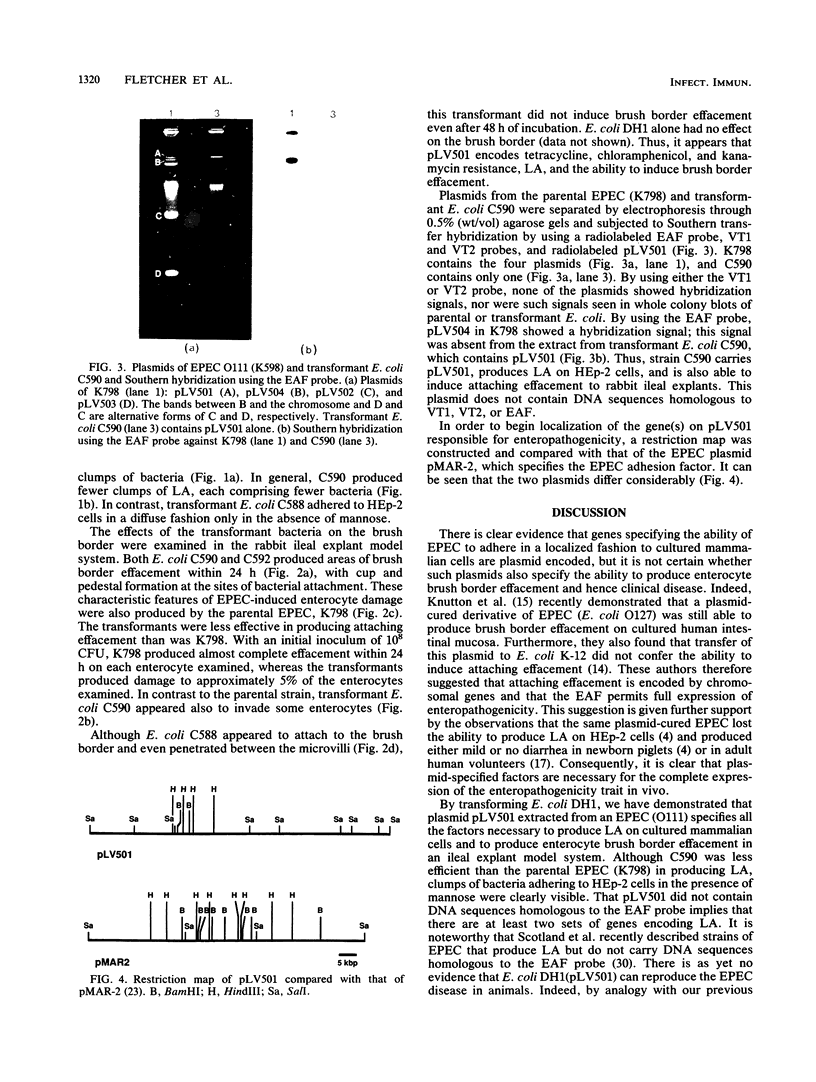
An enteropathogenic Escherichia coli (EPE) O111 serotype a,b,H- strain carried the following four plasmids: pLV501 (96.5 kilobase pairs [kbp]) specifying resistance to chloramphenicol, tetracycline, and kanamycin; pLV502 (8 kbp) specifying ampicillin resistance; pLV503 (1.9 kbp) specifying streptomycin resistance; and pLV504 (80 kbp) with no resistance markers. This EPEC attached to HEp-2 cells to produce localized clumps of bacteria (localized adhesion) and attached intimately to the enterocyte surface, leading to loss of the brush border (attaching effacement). Plasmid pLV501 was also found to specify the ability to produce localized adhesion on HEp-2 cells and attaching effacement in a rabbit ileal explant model system. Restriction maps showed considerable dissimilarities between pLV501 and pMAR-2, an EPEC plasmid carrying the EPEC adherence factor (EAF) genes. Furthermore, pLV501 did not hybridize with the EAF probe, whereas pLV504 did. There was sequence homology between pLV501 and large plasmids in all seven other well-characterized EPEC, only five of which hybridized with the EAF probe. These findings indicate that pLV501 carries at least one of the genes responsible for production of the brush border damage characteristic of EPEC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Moore B. F., Hart C. A., Saunders J. R. Plasmid-mediated conjugative transfer of Klebsiella sp. rcs genesable to induce colanic acid capsular polysaccharide biosynthesis in Escherichia coli. FEMS Microbiol Immunol. 1988 Jan;1(1):19–25. doi: 10.1111/j.1574-6968.1988.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Allen P. M., Roberts I., Boulnois G. J., Saunders J. R., Hart C. A. Contribution of capsular polysaccharide and surface properties to virulence of Escherichia coli K1. Infect Immun. 1987 Nov;55(11):2662–2668. doi: 10.1128/iai.55.11.2662-2668.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade J. R., Da Veiga V. F., De Santa Rosa M. R., Suassuna I. An endocytic process in HEp-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989 Jan;28(1):49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Batt R. M., Hart C. A., McLean L., Saunders J. R. Organ culture of rabbit ileum as a model for the investigation of the mechanism of intestinal damage by enteropathogenic Escherichia coli. Gut. 1987 Oct;28(10):1283–1290. doi: 10.1136/gut.28.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp C. A., Greene K. D., Downes F. P., Sowers E. G., Wells J. G., Wachsmuth I. K. Unusual verotoxin-producing Escherichia coli associated with hemorrhagic colitis. J Clin Microbiol. 1987 Aug;25(8):1486–1489. doi: 10.1128/jcm.25.8.1486-1489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Embaye H., Batt R. M., Saunders J. R., Getty B., Hart C. A. Interaction of enteropathogenic Escherichia coli 0111 with rabbit intestinal mucosa in vitro. Gastroenterology. 1989 Apr;96(4):1079–1086. doi: 10.1016/0016-5085(89)91626-0. [DOI] [PubMed] [Google Scholar]
- Hall G. A., Reynolds D. J., Chanter N., Morgan J. H., Parsons K. R., Debney T. G., Bland A. P., Bridger J. C. Dysentery caused by Escherichia coli (S102-9) in calves: natural and experimental disease. Vet Pathol. 1985 Mar;22(2):156–163. doi: 10.1177/030098588502200210. [DOI] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987 Mar;155(3):377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Xu J. G., Kaper J. B., Lior H., Prado V., Tall B., Nataro J., Karch H., Wachsmuth K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987 Jul;156(1):175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- Mathewson J. J., Cravioto A. HEp-2 cell adherence as an assay for virulence among diarrheagenic Escherichia coli. J Infect Dis. 1989 Jun;159(6):1057–1060. doi: 10.1093/infdis/159.6.1057. [DOI] [PubMed] [Google Scholar]
- Miliotis M. D., Koornhof H. J., Phillips J. I. Invasive potential of noncytotoxic enteropathogenic Escherichia coli in an in vitro Henle 407 cell model. Infect Immun. 1989 Jul;57(7):1928–1935. doi: 10.1128/iai.57.7.1928-1935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyenuddin M., Rahman K. M., Sack D. A. The aetiology of diarrhoea in children at an urban hospital in Bangladesh. Trans R Soc Trop Med Hyg. 1987;81(2):299–302. doi: 10.1016/0035-9203(87)90247-1. [DOI] [PubMed] [Google Scholar]
- NETER E. Enteritis due to enteropathogenic Escherichia coli; present-day status and unsolved problems. J Pediatr. 1959 Aug;55(2):223–239. doi: 10.1016/s0022-3476(59)80091-3. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Baldini M. M., Kaper J. B., Black R. E., Bravo N., Levine M. M. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis. 1985 Sep;152(3):560–565. doi: 10.1093/infdis/152.3.560. [DOI] [PubMed] [Google Scholar]
- Nataro J. P., Maher K. O., Mackie P., Kaper J. B. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1987 Oct;55(10):2370–2377. doi: 10.1128/iai.55.10.2370-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Scaletsky I. C., Kaper J. B., Levine M. M., Trabulsi L. R. Plasmid-mediated factors conferring diffuse and localized adherence of enteropathogenic Escherichia coli. Infect Immun. 1985 May;48(2):378–383. doi: 10.1128/iai.48.2.378-383.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. E., Charlier G. J., Halen P. H. Pathogenicity of attaching effacing enteropathogenic Escherichia coli isolated from diarrheic suckling and weanling rabbits for newborn rabbits. Infect Immun. 1984 Dec;46(3):690–696. doi: 10.1128/iai.46.3.690-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A., Mainil J. G., Baljer G., Moon H. W. Attaching and effacing bacteria in the intestines of calves and cats with diarrhea. Vet Pathol. 1987 Jul;24(4):330–334. doi: 10.1177/030098588702400407. [DOI] [PubMed] [Google Scholar]
- Rothbaum R., McAdams A. J., Giannella R., Partin J. C. A clinicopathologic study of enterocyte-adherent Escherichia coli: a cause of protracted diarrhea in infants. Gastroenterology. 1982 Aug;83(2):441–454. [PubMed] [Google Scholar]
- Scotland S. M., Willshaw G. A., Smith H. R., Gross R. J., Rowe B. Adhesion to cells in culture and plasmid profiles of enteropathogenic Escherichia coli isolated from outbreaks and sporadic cases of infant diarrhoea. J Infect. 1989 Nov;19(3):237–249. doi: 10.1016/s0163-4453(89)90729-9. [DOI] [PubMed] [Google Scholar]
- Sherman P., Soni R., Karmali M. Attaching and effacing adherence of Vero cytotoxin-producing Escherichia coli to rabbit intestinal epithelium in vivo. Infect Immun. 1988 Apr;56(4):756–761. doi: 10.1128/iai.56.4.756-761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Hart A., Batt R. M., McDougall C., McLean L. Ultrastructural and biochemical changes in human jejunal mucosa associated with enteropathogenic Escherichia coli (0111) infection. J Pediatr Gastroenterol Nutr. 1986 Jan;5(1):70–73. doi: 10.1097/00005176-198601000-00013. [DOI] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Vial P. A., Robins-Browne R., Lior H., Prado V., Kaper J. B., Nataro J. P., Maneval D., Elsayed A., Levine M. M. Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis. 1988 Jul;158(1):70–79. doi: 10.1093/infdis/158.1.70. [DOI] [PubMed] [Google Scholar]