Abstract
O-Mannosyl-linked glycosylation is abundant within the central nervous system, yet very few glycoproteins with this glycan modification have been identified. Congenital diseases with significant neurological defects arise from inactivating mutations found within the glycosyltransferases that act early in the O-mannosyl glycosylation pathway. The N-acetylglucosaminyltransferase known as GnT-Vb or -IX is highly expressed in brain and branches O-mannosyl-linked glycans. Our results using SH-SY5Y neuroblastoma cells indicate that GnT-Vb activity promotes the addition of the O-mannosyl-linked HNK-1 modification found on the developmentally regulated and neuron-specific receptor protein-tyrosine phosphatase β (RPTPβ). These changes in glycosylation accompany decreased cell-cell adhesion and increased rates of migration on laminin. In addition, we show that expression of GnT-Vb promotes its dimerization and inhibits RPTPβ intrinsic phosphatase activity, resulting in higher levels of phosphorylated β-catenin, suggesting a mechanism by which GnT-Vb glycosylation couples to changes in cell adhesion. GnT-Vb-mediated glycosylation of RPTPβ promotes galectin-1 binding and RPTPβ levels of retention on the cell surface. N-Acetyllactosamine, but not sucrose, treatment of cells results in decreased RPTP retention, showing that galectin-1 binding contributes to the increased retention after GnT-Vb expression. These results place GnT-Vb as a regulator of RPTPβ signaling that influences cell-cell and cell-matrix interactions in the developing nervous system.
Glycosylation is regulated spatially and temporally during the development of the nervous system (1). In particular, sulfated glycoconjugates, such as the human natural killer-1 epitope, are important for proper migration and adhesion during neural development (2, 3). The human natural killer-1 (HNK-1)3 epitope was originally discovered using a monoclonal antibody raised against a specific T-lymphoblastoid cell type (4). Since its discovery in lymphocytes, the HNK-1 (also known as CD57) antibody has been shown to react with many neural cell types, including glial, neuroectoderm, and neuroendocrine cells (5–8). The HNK-1 epitope consists of a glucuronic acid, transferred by glucuronyltransferases (GlcATs), that is 3-sulfated by HNK-1 sulfotransferase (HNK1st), linked to a precursor N-acetyllactosamine structure (Fig. 1). Numerous glycoproteins bearing the HNK-1 epitope have been identified in the nervous system, such as neural cell adhesion molecule (9), L1 (10), neural-glia cell adhesion molecule (11), myelin-associated glycoprotein (12), tenascin R (13), and RPTP (receptor protein-tyrosine phosphatase) (14, 15). In certain cell types, such as neural crest cells, the expression of the HNK-1 antigen is highly regulated during development (2) and is often found on migratory cells (3). The HNK-1 antigen is linked to critical functions during the formation of the nervous system, such as cell-matrix interactions (16), cell-cell adhesion (17), memory, and synaptic plasticity (15, 18, 19).2
FIGURE 1.
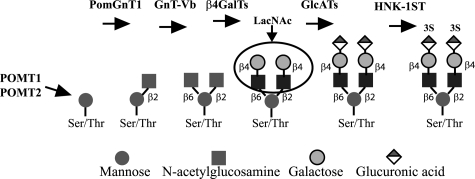
Proposed pathway for the production of O-mannosyl-linked HNK-1 epitope. POMT1/POMT2 catalyzes the transfer of mannose to a serine or threonine, followed by PomGnT1 adding GlcNAc in β1,2 linkage. GnT-Vb requires the PomGnT1 addition before it can add GlcNAc inβ1,6 linkage. After the addition of galactose residues by β4-galactosyltransferases (β4GalTs), the GlcATs (GlcATsS or GlcATsP) and the HNK-1 sulfotransferase (HNK1ST) form the HNK-1 epitope. LacNac the preferred substrate for a family of lectins known as galectins is circled.
The HNK-1 epitope is found on glycolipids (20, 21), N-linked glycans (13, 22), and O-linked glycans (23); however, recent evidence suggests that a significant portion of the HNK-1 epitope is linked to proteins via an N-glycanase-insensitive linkage, likely on O-mannose glycans (15), which can be detected by the monoclonal antibody Cat-315. Interestingly, the Cat-315 antibody has been shown to react with different perisynaptic extracellular matrix components during synapse formation, suggesting a role for O-mannosyl-linked HNK-1 in synaptogenesis (15).
O-Mannosyl-linked glycans were originally thought to be a fungus-specific glycosylation until Margolis and co-workers (24–26) demonstrated the presence of O-mannosyl glycans in brain tissue, which, in rabbit brain, makes up about one-third of the O-linked glycans and contains both 2-mono- and 2,6-di-substituted mannose-linked glycans (26, 27). In the biosynthesis of these O-mannosyl glycans, the glycosyltransferase that adds GlcNAc to mannose in the 2-position is PomGnT1 (28), whereas the enzyme that adds GlcNAc to the 6-position of mannose is GnT-Vb (29), also known as GnT-IX (30). As shown in Fig. 1, PomGnT1 addition of GlcNAc in β1,2-Man linkage is required before GnT-Vb can subsequently add the GlcNAcβ1,6-Man linkage (30). It has been proposed by Taniguchi and co-workers (30) that the HNK-1 epitope may be added following the addition of β1,6-GlcNAc. The addition of β1,6-GlcNAc would be expected to increase the amount of N-acetyllactosamine backbone required for the subsequent activity of HNK-1-specific glucuronyltransferases like GlcATs and HNK1st. Galectins, in particular galectin-1, that bind to N-acetyllactosamine have been shown to play an important role in regulating cell adhesion and migration, and could likely bind the products of GnT-Vb (reviewed in Ref. 31).
We were interested in determining whether GnT-Vb expression regulates the levels of HNK-1 epitope found on specific neural glycoproteins and exploring the phenotypic effects that result from expression of GnT-Vb. We first found that increased expression of GnT-Vb resulted in significantly reduced levels of cell-cell adhesion, increased migration, and increased binding of exogenous galectin-1 to cells. In addition, the most abundant substrate for GnT-Vb glycosylation in SH-SY5Y neuroblastoma cells was identified as a receptor protein-tyrosine phosphatase known as RPTPβ. This receptor binds ligands, such as pleiotrophin, which inhibit its intrinsic phosphatase activity and attenuate its intracellular signaling, leading to changes in cell adhesion and migration. The effects on cell behavior we observed following GnT-Vb expression may therefore result from effects on RPTPβ activity and subsequent signaling events. Our results show that GnT-Vb expression inhibits RPTPβ intrinsic phosphatase activity and its signaling via phosphorylated β-catenin. Furthermore, GnT-Vb expression results in increased cell-surface retention of RPTPβ, which is likely the cause of its attenuated phosphatase activity and downstream signaling. Increased cell-surface retention of RPTPβ would favor receptor clustering, leading to increased dimerization and inhibition of phosphatase activity. The increased retention of RPTPβ at the cell surface is accompanied by an increased association with endogenous galectin-1, suggesting a working model for the effects of GnT-Vb glycosylation of RPTPβ and changes in neural cell adhesion and migration.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents—ERK1/2 polyclonal antibody and the β-catenin H-102 antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The Cat-315 antibody has been described previously (32, 33). The CD57 antibody was purchased from BD Biosciences. The RPTPβ antibody used previously (34) was obtained from BD Biosciences. The mouse IgM antibody 5210 recognizing the extracellular region of RPTPβ was purchased from Chemicon International (Temecula, CA). The β-catenin antibody and pleiotrophin were ordered from Chemicon, and the 4G10 phosphotyrosine antibody was purchased from Upstate Biotechnology (Lake Placid, NY). The galectin-1 antibody was purchased from Santa Cruz Biotechnology. All horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology. The streptavidin-phycoerythrin and anti-IgM-Alexa Fluor 633 were obtained from Invitrogen. WGA-agarose was obtained from Vector Laboratories (Burlingame, CA). Peptide:N-glycosidase F enzyme was acquired from Prozyme. Neuraminidase and O-glycanase enzymes were purchased from Roche Diagnostics. Biotinylated galectin-1 was generously donated by Linda G. Baum (UCLA).
Plasmids—The lentiviral GnT-Vb expression vector was produced by cloning the EcoRI/NotI fragment from 3.1-HA-Vb (35) into the EcoRI/NotI ends of pCDH1-MCS1-EF1-copGFP (Systems Biosciences, Mountain View, CA). The GnT-Vb siRNA lentiviral vector was produced by cloning the GnT-Vb-coding siRNA targeting sequences described previously (35) into the pSIH-H1-Puro vector (Systems Biosciences, Mountain View, CA) The pMD.G plasmid (encodes the envelope protein G of the vesicular stomatitis virus) and the PCMVdelta R9 packaging plasmids were gifts from the Trono laboratory (36).
Production of Lentivirus and Cell Culture—The 293T and SH-SY5Y cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. Lentivirus was produced by transfection of 293T cells using Lipofectamine 2000 with the following amounts of plasmids: 8 μg of envelope (pMD.G), 5 μg of lentiviral vector, and 8 μg of packaging plasmid. Approximately 5.5 ml of 293T cell suspension (1.2 × 106) in growth media were seeded onto Lipofectamine 2000 complexes (formed in Opti-MEM I media) in 10-cm tissue culture plates. The next day the cells were replenished with fresh media, and infectious lentivirus supernatant was collected at 48 and 72 h post-transfection. Polybrene was added to lentiviral supernatants at a final concentration of 8 μg/ml, and the virus was placed on cells overnight. Cells were refed with growth media, and the percentage of transduced cells was determined by fluorescent microscopy at 48 h post-infection. Scratch wound experiments were performed as described previously (35).
Cell-Cell Adhesion Assays—For cell-cell aggregation assays, subconfluent cells were washed with PBS and detached in the following buffer: 150 mm NaCl, 0.6 mm Na2HPO4, 10 mm glucose, 10 mm HEPES, pH 7.4, 2 mm CaCl2. Single-cell suspensions at 2.0 × 105/ml were incubated in 24-well plates coated with 1% BSA for 30 min at 37 °C with agitation at 80 rpm. Cell aggregation was determined by counting the number of cell-aggregates with three or more cells in each field at the 10-min time point. A total of five random fields per well were chosen, and the results are expressed as a percentage of the mean relative to the mean total number of cells per field.
WGA Precipitation, β-Catenin Immunoprecipitation, and Western Blotting—Cells were lysed in RIPA buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS). Total cell lysate (500 μg) was precipitated overnight at 4 °C using 50 μl of WGA-agarose. WGA precipitates were washed three times in ice-cold 1× PBS before proteins were boiled into SDS sample buffer. Proteins were separated on 4–12% BisTris gels (Invitrogen) in 1× MES buffer or 3–8% Tris acetate gels in 1× Tris acetate buffer before transfer to polyvinylidene difluoride membrane. Membranes were blocked overnight at 4 °C in 1× TBS, 0.05% Tween 20, 5% nonfat milk (TBST BLOTTO). Cat-315 antibody was diluted 1:5 in TBST BLOTTO and incubated for 1 h at room temperature. After washing, horseradish peroxidase-conjugated secondary anti-IgM antibody was diluted 1:5,000 in TBST BLOTTO and incubated for 45 min at room temperature. The CD57 antibody was diluted 1:5,000 and incubated for 1 h at room temperature. After washing the anti-IgM secondary antibody was diluted 1:5,000 in TBS BLOTTO as described. Washed blots were detected using Western Lightning® Plus (PerkinElmer Life Sciences) and exposure to Kodak Biomax chemiluminescent film. For the analysis of β-catenin phosphorylation, cells were incubated in serum-free media for 24 h before addition of pleiotrophin at 50 ng/ml for 10 min. Cells were lysed in the following buffer: 50 mm HEPES, pH 7.5, 150 mm NaCl, 1 mm EDTA, 10% glycerol, 20 mm pyrophosphate, 1% Triton X-100, 100 mm NaF, 2 mm sodium orthovanadate, 0.2 mm ammonium molybdate, protease inhibitor tablets complete (Roche Diagnostics). Lysates were centrifuged at 12,000 × g for 10 min, and 500 μg of protein was used for each β-catenin immunoprecipitation (AB19022, Chemicon). Two micrograms of β-catenin antibody was incubated with the lysate overnight at 4 °C. Immunocomplexes were captured using 50 μl of protein A/G plus agarose (Santa Cruz Biotechnology). After washing the proteins were boiled from the agarose and separated on 4–12% BisTris gels, transferred to polyvinylidene difluoride membrane, and probed with the phosphotyrosine antibody 4G10 overnight at 4 °C. The blots were detected with anti-mouse conjugated horseradish peroxidase followed by chemiluminescent detection. Blots were then detected with anti-β-catenin antibody (Santa Cruz Biotechnology, H-102) to normalize for total β-catenin levels.
Cell-surface Half-life and Protein Cross-linking—For cell-surface half-life analysis SH-SY5Y cells were washed twice in cold 1× PBS and biotinylated using 1 mg/ml sulfo-NHS-LC-biotin (Pierce) at 4 °C for 30 min. Cells were returned to culture for an additional 3 or 12 h before being lysed in 1× TBS, 1% Triton X-100/protease inhibitor mixture. Biotin-labeled proteins were precipitated with 50 μl of streptavidin magnetic beads (Promega). Proteins were separated on 4–12% BisTris gels in 1× MES buffer before transfer and Western blot detection using the indicated antibodies. For cross-linking experiments the cells were washed twice in ice-cold 1× PBS before being incubated with 3 mm BS3 (Pierce) in 1× PBS on ice for 1 h. Cross-linking reagent was quenched by the addition of 1 m Tris-Cl, pH 7.5, to a final concentration of 10 mm for 10 min on ice. Cells were lysed as described above before being used in precipitation experiments using the monoclonal 5210 RPTP antibody and galectin-1.
Flow Cytometry—Intact nonpermeabilized SH-SY5Y cells were washed with 1× PBS and released from the culture dishes with 2 mm EDTA in 1× PBS. Cells (5 × 105) per reaction were incubated with 0.2 mg/ml biotinylated galectin-1 in 1× PBS, 1% BSA for 30 min on ice in the presence of 10 mm sucrose or 10 mm N-acetyllactosamine (Sigma) in a 100-μl volume. Cells were washed in 1× PBS and incubated in phycoerythrin-conjugated streptavidin (Zymed Laboratories Inc.) at a 1:100 dilution for 15 min on ice. After washing the cells, data were acquired on a FACSCalibur flow cytometer using software by CellQuest. For the analysis of RPTPβ surface levels, SH-SY5Y cells were incubated in 10 mm sucrose or N-acetyllactosamine for 2 h in 1× PBS, 2% fetal bovine serum. Cells were detached using 2 mm EDTA before labeling the cells with antibody 5210 (1:1,000) in a 100-μl volume of 1× PBS, 1% BSA on ice for 15 min. The IgM antibody was detected using an anti-IgM Alexa Fluor 633 secondary (1:500) diluted in 1× PBS, 1% BSA on ice for 15 min.
RPTPβ Immunoprecipitation—Mock, GnT-Vb+, or GnT-Vb siRNA SH-SY5Y cells were lysed in 1× TBS, 1% Triton X-100 supplemented with protease inhibitors. Endogenous phosphate was removed from cell lysates using Sephadex G-25 resin (Amersham Biosciences). Lysates were pre-cleared, and 500 μg was used for each precipitation. Two micrograms of anti-RPTPβ antibody was used for each precipitation at 4 °C for 3 h. Protein A/G Plus agarose (Santa Cruz Biotechnology), 50 μl, was added to each precipitation at 4 °C for 2 h.
Tyrosine Phosphatase Activity Assay—Precipitates were washed in lysis buffer 3 times and equilibrated to 1× phosphatase buffer (20 mm imidazole, pH 7.2, 0.1 mg/ml BSA). Immunoprecipitated RPTPβ (10 μl) from a total of 50 μl was then subjected to an in vitro activity assay performed with the tyrosine phosphatase assay kit (Promega) using Tyr phosphopeptide (END(pY)INASL), where pY represents phosphotyrosine as substrate. A serially diluted standard of 1 mm KH2PO4 was used to generate a standard curve. Equivalent levels of precipitated RPTPβ were verified by Western blotting. Phosphatase activity is shown as the level of phosphate released (pmol/min/μl). Data shown are the mean of triplicate determinations from two separate experiments.
Glycanase Digestions—O-Glycanase and neuraminidase digestions were performed before N-glycanase digestion. WGA-precipitated proteins on agarose were resuspended in 20 mm Tris-Cl, pH 7.4, 20 mm sodium acetate, and 25 mm NaCl. O-Glycosidase, 20 milliunits/ml (Roche Diagnostics), and/or neuraminidase, 100 milliunits/ml (Roche Diagnostics), were added, and reactions were incubated overnight at 37 °C. The resin was equilibrated in the following buffer: 20 mm sodium phosphate, pH 7.5, 0.05% SDS, 50 mm β-mercaptoethanol and boiled for 5 min. Proteins in solution were separated from the resin, and KCl was added to a final of 10 mm. Reactions were incubated on ice for 10 min followed by centrifugation at 14,000 × g at 4 °C to remove the potassium salts of SDS. Nonidet P-40 detergent was added to a final of 0.15%, and 2 μl of peptide:N-glycosidase F (5 milliunits/50 μl) was added, and the reaction tubes were placed at 37 °C for 16–18 h. Digested proteins were recovered by ethanol precipitation prior to gel electrophoresis.
RESULTS
GnT-Vb Expression Increases the Levels of O-Mannosyl-linked HNK-1 Epitope, Contributing to Increased Cell Migration and Decreased Cell-Cell Aggregation—Our previous work demonstrated that suppression of either GnT-Vb or PomGnT1 expression decreased integrin-dependent SH-SY5Y cell migration on laminin (35). The fact that GnT-Vb and PomGnT1 suppression resulted in a similar phenotype suggested that O-mannosyl glycans played a role in adhesion and migration of these cells on laminin. In this study, we aimed to define potential acceptor substrates for GnT-Vb that could be regulating cell adhesion and migration. The neuroblastoma cell line SH-SY5Y has a neural crest lineage; the HNK-1 epitope is highly expressed in neural crest cells (37–39) and plays a role in modulating adhesion and migration to laminin (16, 40). Therefore, we tested the possibility that GnT-Vb expression regulates O-mannosyl-linked HNK-1 glycans in SH-SY5Y cells.
To determine whether increased GnT-Vb levels affected the expression of the HNK-1 epitope in SH-SY5Y cells, the cells were transduced with lentivirus from a GnT-Vb expression construct or lentivirus from empty vector, followed by SDS-PAGE and immunoblotting with antibodies specific for HNK-1 epitopes, anti-CD57 and Cat-315. The lectin WGA was employed to precipitate glycoproteins with terminal GlcNAc, concentrating any glycoproteins that may have been glycosylated by GnT-Vb. A recent report characterized the specificity of Cat-315 antibody for O-mannosyl-linked HNK-1 (15). Our results showed that the Cat-315 antibody exhibited a very low but detectable level of reactivity in mock-transfected cells, and a large increase in binding in the GnT-Vb-expressing cells (Fig. 2A). The largest increase for both Cat-315 and CD57 antibody binding occurred in a diffuse band migrating above 250 kDa (Fig. 2A, asterisk). To determine whether the Cat-315 reactivity of this large glycoprotein was due primarily to N-linked or O-linked glycans, we performed serial digestions using sialidase and O-glycanase, followed by N-glycanase digestion. Using a 3–8% gradient Tris acetate gel to allow better separation of high molecular weight proteins, immunoblotting using the Cat-315 antibody revealed that the glycoprotein(s) present in this band (migrating at ∼350–400 kDa before digestion) retained Cat-315 reactivity after all digestions (Fig. 2B). Although a size shift of the band was apparent after each digestion, demonstrating that some glycans were removed by the enzymatic treatments, the reactivity with Cat-315 remained. Additional digestions with β-hexosaminidase and β-galactosidase were also performed, but the Cat-315 epitope was resistant to these enzymes as well (data not shown). The marked resistance of the epitope to enzymatic deglycosylation using these enzymes suggests that this band represents a glycoprotein(s) that expresses the O-mannosyl-linked HNK-1 epitope.
FIGURE 2.
GnT-Vb expression increases the production of O-mannosyl-linked HNK-1 epitope. A, WGA-precipitated proteins (Ppt) were analyzed by Western blot (WB) using the Cat-315 and CD57 antibodies. B, WGA immunoprecipitation reactions from GnT-Vb-expressing cell lysates were treated with the indicated enzymes before being analyzed by Western blot using the Cat-315 antibody.
The HNK-1 epitope has been reported to play a role in cell migration during the development of the nervous system. Therefore, we examined the effect of GnT-Vb expression on cell migration induced in response to a scratch wound. Because suppression of GnT-Vb expression inhibited migration most strongly on laminin (35), we examined the effect of increased GnT-Vb expression on laminin-coated chamber slides. Confluent cells plated on laminin in serum-free medium were scraped with a yellow pipette tip, and cells were allowed to migrate for 4 and 6 h. Inspection of the cells at the 4-h time point revealed a greater number of GnT-Vb-expressing cells had migrated into the scratch wound compared with mock cells (Fig. 3A). At the 6-h time point the mock cells have begun to migrate and display a ruffled edge. However, at 6 h the GnT-Vb-expressing cells have spanned the wound, demonstrating a significant effect of GnT-Vb expression on the rate of migration (Fig. 3A).
FIGURE 3.
GnT-Vb expression increases cell migration and reduces calcium-dependent cell-cell adhesion. A, confluent mock and GnT-Vb-expressing cells on laminin-coated chamber slides were wounded with a scratch, and subsequent migration was monitored for 4 and 6 h; bar 100 μm; arrows denotes wound boundary and direction of migration. Experiment shown is representative of three separate experiments. B, mock and GnT-Vb-expressing cells were removed from culture plates and separated into single-cell suspensions in calcium containing-media with constant agitation at 37 °C. Phase contrast microscopy after 30 min of a representative field; bar 50 μm. C, cumulative results from three separate experiments counted after 10 min of constant agitation at 37 °C. Error bars represent the mean percentage (±S.D.) of cell-cell adhesion from five randomly selected fields per experiment.
Next, we explored the possibility that GnT-Vb expression may influence cell-cell adhesion, considering that several proteins reported to express the HNK-1 epitope are cell adhesion molecules. Single-cell suspensions of mock and GnT-Vb cells were made, and aggregation assays were performed as described (41). After 30 min, phase contrast microscopy revealed a significant reduction of cell aggregation in the GnT-Vb-expressing cells compared with mock cells (Fig. 3B). Almost 100% of the mock cells formed large cell-cell aggregates, whereas GnT-Vb-expressing cells formed smaller cell aggregates (three or fewer cells) at a markedly reduced rate. Results quantitated at the 10-min time point from three separate experiments demonstrated a 50% reduction in the number of 3+ cell aggregates for GnT-Vb-expressing cells compared with mock cells (Fig. 3C). These results suggest that GnT-Vb glycosylation regulates homotypic cell-cell adhesion.
GnT-Vb-mediated Glycosylation of RPTPβ Inhibited Its Phosphatase Activity in Vitro and in Vivo—To understand how GnT-Vb glycosylation regulates cell-cell adhesion and cell migration, we turned our attention to identify the major HNK-1-expressing glycoprotein band. The large molecular weight of the most intensely staining band (Fig. 2A) suggested it might be the chondroitin sulfate proteoglycan RPTPβ. RPTPβ was known to express the HNK-1 epitope (14) and in particular the O-mannosyl-linked HNK-1 epitope (15). When RPTPβ was immunoprecipitated using an anti-RPTPβ antibody and analyzed by Western blot using the Cat-315 antibody, we observed an increase in reactivity for RPTPβ isolated from GnT-Vb-expressing cells compared with mock cells (Fig. 4A). Equivalent protein levels of RPTPβ were present in the mock and GnT-Vb immunoprecipitation reactions and confirmed by blotting with the anti-RPTPβ antibody. We conclude from these experiments that GnT-Vb expression leads to HNK-1 structures on RPTPβ that are likely O-mannosyl-linked. To determine whether the small, secreted form of RPTPβ, termed phosphacan, expressed Cat-315 reactivity after GnT-Vb expression, similar experiments were conducted using conditioned media collected from mock and GnT-Vb-expressing cells. No significant level of secreted Cat-315-reactive phosphacan could be detected from either cell type (data not shown).
FIGURE 4.
GnT-Vb glycosylates RPTPβ and inhibits intrinsic phosphatase activity in vitro and in vivo. A, upper box, total cell lysate (500 μg) from mock and GnT-Vb-expressing SH-SY5Y was immunoprecipitated (IP) using the anti-RPTPβ antibody, followed by Western blot (WB) analysis using the Cat-315 antibody. Lower box, anti-RPTPβ antibody Western blot analysis of the immunoprecipitations. B, tyrosine phosphatase activity assays using mock, GnT-Vb expressing, and GnT-Vb siRNA SH-SY5Y cells. RPTPβ was immunoprecipitated, and phosphatase activity was measured after 5 min using a tyrosine-phosphorylated peptide substrate (Promega). C, mock, GnT-Vb-expressing, and Gnt-Vb siRNA SH-SY5Y cells were serum-starved for 24 h before the addition of pleiotrophin at 50 ng/ml for 10 min. Lysates (500 μg) were immunoprecipitated with anti-β-catenin antibody, separated on 4–12% BisTris gels, and probed by Western blot for phosphotyrosine using the G410 antibody. Blots were re-probed for total β-catenin levels using anti-β-catenin antibody for loading normalization. Results shown are the average percentage increase in phospho-β-catenin levels following PTN treatment of GnT-Vb-expressing cells and mock-transfected cells from three separate experiments.
Because GnT-V glycosylation was observed on the larger, transmembrane form of RPTPβ, we next examined the effects of this glycosylation on its intrinsic phosphatase activity. Phosphatase activity assays utilizing a tyrosine-phosphorylated peptide substrate (Promega) were performed on RPTPβ immunoprecipitated from lysates of mock, GnT-Vb-expressing cells, and cells expressing a previously characterized siRNA target for the coding region of GnT-Vb (35). Results from two experiments indicated that GnT-Vb-mediated glycosylation inhibited RPTPβ phosphatase activity by over 55% after correcting for nonspecific background from IgG-immunoprecipitated controls (Fig. 4B). GnT-Vb expression can be reduced by >80% by the expression of the siRNA targeting sequence described previously (35). RPTPβ immunoprecipitated from GnT-Vb siRNA-expressing cells release double the phosphate compared with mock cells. After performing phosphatase activity assays, aliquots of the precipitation reactions were analyzed by Western blot to confirm equivalent RPTPβ inputs in the comparative assays (data not shown). These results indicate that GnT-Vb-mediated glycosylation either directly or indirectly inhibits RPTPβ catalytic activity.
To confirm this inhibitory effect on RPTPβ phosphatase activity, we sought an in vivo assay of an endogenous substrate of this enzyme. β-Catenin is a substrate for RPTPβ, and the cytokine pleiotrophin (PTN) is an endogenous ligand of RPTPβ that increases β-catenin phosphorylation levels by inhibiting RPTPβ phosphatase activity (34). We reasoned that if the addition of O-mannosyl-linked HNK-1 to RPTPβ indeed inhibited its phosphatase activity, after treatment with PTN an additive increase in β-catenin tyrosine phosphorylation levels would be observed for GnT-Vb-expressing cells compared with mock cells, and suppression of GnT-Vb should reduce β-catenin phosphorylation levels. To test this hypothesis, mock, GnT-Vb-expressing, and GnT-Vb siRNA SH-SY5Y cells were treated with 50 ng/ml PTN for 10 min, and then β-catenin was immunoprecipitated from cell lysates to examine the levels of β-catenin phosphorylation using the phosphotyrosine-specific antibody 4G10 (Upstate Biotechnology, Inc.). Results were analyzed from three separate experiments that were digitally quantified and normalized for differences in total β-catenin immunoprecipitation. Mock cells, GnT-Vb-expressing cells, and GnT-Vb siRNA cells showed an expected increase in β-catenin phosphorylation levels after PTN treatment compared with untreated cells. However, phospho-β-catenin levels were changing in response to GnT-Vb expression levels. For example, there was 40% more phosphorylated β-catenin observed after PTN treatment of GnT-Vb-expressing cells compared with mock cells, whereas GnT-Vb siRNA-expressing cells showed 20% less phosphorylated β-catenin compared with mock cells after PTN treatment (Fig. 4C). These results demonstrate that the modulation of RPTPβ phosphatase activity by altering GnT-Vb expression levels results in changes in the in vivo phosphorylation of an endogenous substrate of RPTPβ, β-catenin.
GnT-Vb-mediated Glycosylation of RPTPβ Increased Cell-surface Retention through a Galectin-1-binding Mechanism—To understand how GnT-Vb-mediated changes in glycosylation of the extracellular domain of RPTPβ could inhibit phosphatase activity, we examined the rate of RPTPβ synthesis and cell-surface half-life. The rationale for these experiments is based on previous studies for receptor protein-tyrosine phosphatases showing that clustering of the extracellular domains and subsequent dimerization can inhibit phosphatase activity (42, 43). Glycosylation has been shown to enhance the stability of some receptors at the cell surface (44, 45); therefore, we were interested in determining whether GnT-Vb-mediated glycosylation of RPTPβ could influence the rate of endocytosis. Increased RPTPβ receptor on the cell surface could promote clustering, leading to dimerization of the receptor, therefore reducing phosphatase activity. We measured the synthesis and transport of RPTPβ to the cell surface using a pulse-chase experiment and found the rates to be similar for mock and GnT-Vb-expressing cells (data not shown). To measure changes in the cell-surface half-life of RPTPβ, we surface-labeled with a cell-impermeable biotin reagent and re-cultured the cells for various times before precipitating the surface proteins with streptavidin bound to magnetic beads. After dissociating the streptavidin-bound proteins by boiling, RPTPβ was detected by Western blot using an anti-RPTPβ antibody. We found that the RPTPβ cell-surface half-life was dramatically increased in GnT-Vb-expressing cells compared with mock cells (Fig. 5A). The large increase of RPTPβ at the surface could be explained by increased association of nonlabeled receptor with labeled receptor during the time course. To investigate the possibility that GnT-Vb glycosylation was promoting dimerization, we treated cells with a cross-linker and analyzed total cell lysates on 3–8% Tris acetate gels before Western blot detection of RPTPβ. We were able to observe consistently an increase in dimer formation of RPTPβ for GnT-Vb-expressing cells compared with mock cells (Fig. 5B). Cumulative results from three separate experiments normalized to monomer RPTPβ levels reveal on average a 20% increase in dimerization following GnT-Vb expression (Fig. 5C). These data suggest that increased dimerization may be responsible, in part, for the increased RPTPβ levels on the cell surface.
FIGURE 5.
GnT-Vb glycosylation promotes RPTPβ cell-surface retention through a galectin-1 binding mechanism. A, RPTPβ cell-surface half-life was measured following cell surface biotinylation. Cells were re-cultured for 3 and 12 h before making cell lysates and pulling down biotinylated proteins by streptavidin magnetic bead precipitation, followed by detection of RPTPβ by Western blot using anti-RPTPβ antibody. B, RPTPβ dimerization was analyzed by chemical cross-linking using BS3 as described under “Experimental Procedures.” Total cell lysates (100 μg) were separated on a 3–8% Tris acetate gel before Western blot detection of RPTPβ. The results shown are representative of three separate experiments. C, densitometry analysis of dimerization experiments. Error bars represent the means (±S.D.) of the density of the dimer band normalized to monomer band density. D, galectin-1 cell-surface half-life was measured as described for A, except that anti-galectin-1 antibody was used following streptavidin magnetic bead precipitation. E, RPTPβ association with galectin-1 was assayed by protein cross-linking using BS3 as described under “Experimental Procedures” followed by immunoprecipitation (IP) and Western blotting using the indicated antibodies.
GnT-Vb expression would be expected to increase on glycoprotein glycans the levels of LacNAc (Galβ1–4GlcNAc, circled in Fig. 1), the preferred carbohydrate-binding structure for the family of lectins known as galectins (46). To test if the increased surface half-life of RPTPβ observed in GnT-Vb-expressing cells was lectin-mediated, we analyzed the surface retention of galectin-1, the most abundant galectin in SH-SY5Y cells. We found that galectin-1 showed an increase in surface retention similar to that observed for RPTPβ in the GnT-Vb-expressing cells (Fig. 5D). To determine whether galectin-1 and RPTPβ were associated at the cell surface, we performed cross-linking and then immunoprecipitation using antibodies to either component, followed by immunoblotting. Immunoprecipitation using an RPTPβ antibody followed by Western blot analysis for galectin-1 indicated an increased level of galectin-1 associating with RPTPβ in GnT-Vb-expressing cells compared with mock cells (Fig. 5E). We also found that higher levels of RPTPβ associated with galectin-1 in GnT-Vb-expressing cells compared with mock cells (Fig. 5E) using a galectin-1 antibody immunoprecipitation followed by Western blot analysis with RPTPβ antibody. The similar increases observed in cell-surface retention for both components in the GnT-Vb-expressing cells, coupled with the increased co-immunoprecipitation of galectin-1 and RPTPβ, provide evidence that galectin-1 and RPTPβ likely associate at the cell surface. These results indicate that galectin-1 binding is responsible at least in part for the increased surface retention of RPTPβ after GnT-Vb expression.
If galectin-1 binding to RPTPβ in GnT-Vb-expressing cells was responsible for the elevated surface levels of RPTPβ, then the addition of Galβ1–4GlcNAc (LacNAc) should compete for lectin binding and reduce RPTPβ surface levels back to mock cell levels. To determine whether cell surface binding sites for galectin-1 increased after GnT-Vb expression and whether LacNAc could inhibit galectin-1 binding to SH-SY5Y cells, biotinylated recombinant galectin-1 in the presence of 10 mm sucrose or 10 mm LacNAc was added to mock and GnT-Vb-expressing SH-SY5Y cells in vitro. Galectin-1 binding was measured by flow cytometry of intact cells using streptavidin phycoerythrin detection. Exogenous galectin-1 binding was elevated in GnT-Vb-expressing cells compared with mock cells after incubation in sucrose (Fig. 6A, left panel), whereas Lac-NAc incubation completely inhibited galectin-1 binding to both mock and GnT-Vb-expressing cells (Fig. 6A, right panel). This experiment demonstrates that GnT-Vb glycosylation increases galectin-1 cell surface ligands and that 10 mm LacNAc is sufficient to completely inhibit galectin-1 binding.
FIGURE 6.
Inhibition of galectin-1 binding reduces cell-surface retention of RPTPβ. A, galectin-1 binding to the cell surface was measured by flow cytometry after the addition of biotinylated galectin-1 to suspended mock (shaded) or GnT-Vb-expressing (white) cells at 4 °C for 30 min in the presence of 10 mm sucrose (left) or 10 mm LacNAc (right). Bound biotinylated galectin-1 was detected using streptavidin-phycoerythrin. B, RPTPβ cell surface expression was monitored by flow cytometry after incubating mock (shaded) or GnT-Vb-expressing (white) cells for 2 h in the presence of 10 mm sucrose (left) or 10 mm LacNAc (right). Surface RPTPβ levels were detected on intact cells using an antibody to the extracellular region of RPTPβ, followed by detection using anti-IgM Alexa Fluor 633 secondary. Data presented have been subtracted for secondary-only fluorescence and are representative of two separate experiments.
Next, we wanted to determine whether endogenous galectin-1 binding is contributing to the increased cell-surface retention of RPTPβ. For this experiment, we incubated mock and GnT-Vb-expressing cells in 10 mm sucrose or 10 mm LacNAc for 2 h in culture before examining the levels of cell surface RPTPβ by flow cytometry. We observed a 39% increase in cell surface RPTPβ for GnT-VB-expressing cells, compared with mock cells, after sucrose incubation (Fig. 6B, left panel). These results agree with those of the cell-surface half-life experiments described above. After LacNAc incubation, we found that the increased cell surface levels of RPTPβ on GnT-Vb-expressing cells were reduced to only a 9% elevation over mock cells (Fig. 6B, right panel). These results indicate that competition of galectin-1 binding by prolonged LacNAc exposure reduced RPTPβ cell surface levels almost to the level of mock cells. Taken together, these results support the hypothesis that GnT-Vb glycosylation of RPTPβ increases galectin-1-binding sites, promoting galectin-1 binding, resulting in dimerized receptor with increased cell-surface retention and attenuated phosphatase activity.
DISCUSSION
We have reported that expression levels of N-acetylglucosaminyltransferase Vb modulate integrin-dependent neuroblastoma cell-matrix adhesion and migration on laminin, using siRNA specific for this enzyme (35). These effects were shown to be due to changes in O-mannosyl glycan expression, because the effects were also observed when expression of POMGnT-1 was knocked down using siRNA. The results in this study extend these findings and show that GnT-Vb expression increases the levels of O-mannosyl-linked HNK-1 epitope in SH-SY5Y cells. The HNK-1 epitope is a terminal sulfoglucuronyl carbohydrate structure that plays important roles in neural cell adhesion and migration (3) and has been shown to be expressed on O-mannosyl-linked glycans (27). In this study, increased GnT-Vb expression led to increased levels of a major Cat-315 antibody-reactive glycoprotein with a concomitant decrease in cell-cell adhesion and increase in laminin-dependent migration. The Cat-315 antibody binds to a predominant glycoprotein that most likely expresses O-mannosyl-linked glycans because of the resistance of the Cat-315 epitope to N-glycanase and several other glycosidases. The possibility that the Cat-315 epitope may also react with the HNK-1 epitope expressed on other glycans resistant to enzymatic de-glycosylation cannot be excluded, however.
The predominate substrate of GnT-Vb in SH-SY5Y cells was identified as RPTPβ, and evidence that GnT-Vb-mediated glycosylation can inhibit RPTPβ phosphatase activity both in vitro and in vivo was presented. The functional consequences of suppressed RPTPβ activity was demonstrated by showing that β-catenin, a substrate of RPTP β, has increased tyrosine phosphorylation levels in GnT-Vb-expressing cells following PTN treatment compared with mock cells that were also PTN-treated, resulting in decreased levels of cell-cell adhesion and increased migration.
RPTPβ is constitutively active in its monomeric form, and it has been hypothesized, based on structural studies of another receptor protein-tyrosine phosphatase known as RPTPα, that dimerization triggered by ligand interaction with the extracellular domain leads to inactivation of the enzyme (43). Glycosylation, in particular, N-linked sialylation, has been shown to regulate the homodimerization of another RPTP known as CD45 in T-cells (47). Our cross-linking data suggest that there is an increase in dimerization when GnT-Vb is overexpressed, consistent with the CD45 and RPTPα results.
Pleiotrophin, a ligand of RPTPβ, interacts with this receptor and inhibits phosphatase activity (48). We examined the levels of endogenous PTN following GnT-Vb expression using quantitative reverse transcription-PCR but find no differences between mock and GnT-Vb-expressing cells (data not shown). Furthermore, we labeled recombinant PTN with biotin and examined the binding efficacy of RPTPβ isolated from mock and GnT-Vb-expressing cells. We found no difference in PTN affinity for RPTPβ after GnT-Vb expression (data not shown). Therefore, we conclude that GnT-Vb-mediated inhibition of RPTPβ phosphatase activity is not because of increased pleiotrophin abundance or binding affinity.
Our results show an increase in the number of cell surface ligands for galectin-1 binding after GnT-Vb expression; galectin-1 is the predominant lectin expressed in SH-SY5Y cells. Based on the binding specificity of galectin-1, these ligands contain LacNAc or poly-LacNAc (49). RPTPβ was identified as an acceptor for GnT-Vb based on increases in Cat-315 antibody binding, which is thought to be specific for the HNK-1 epitope expressed on glycans that are resistant to several glycosidases, including N-glycanase. We have no direct evidence, however, that a single glycan species distal to the β(1,6)-linked GlcNAc transferred by GnT-Vb contains both galectin-1-binding sites, as well as a terminal HNK-1 epitope. We do not know of a study that has measured the affinity of galectin-1 for a glycan containing LacNAc or poly-LacNAc that terminates in the HNK-1 epitope, although it is clear that galectin-1 has affinity for some sulfated glycans (46).
Several studies using T-cells have shown that galectin-1 binding to, and clustering of, CD45 results in decreased phosphatase activity (50–52). Interestingly, immune cells and neural cells are both cell types that communicate via synapse formation. These results support our observations in SH-SY5Y cells, indicating that increased galectin-1 binding, as a result of GnT-Vb glycosylation, modulates RPTPβ cell-surface retention and attenuates its phosphatase activity.
We propose a working model in which the O-mannosyl-linked HNK-1 N-acetyllactosamine structure that results from GnT-Vb activity favors galectin-1 binding, leading to increased cell-surface retention of RPTPβ that results in decreased catalytic activity through the promotion of receptor dimerization (Fig. 7), or possibly a ligand-induced conformational change. RPTPβ is highly active as a monomer, causing one of its key substrates, β-catenin, to become dephosphorylated, resulting in increased levels of cell-cell adhesion (Fig. 7A). As RPTPβ becomes increasingly glycosylated by GnT-Vb and the other members of the O-mannosyl HNK-1 biosynthetic pathway, galectin-1 binding increases and results in increased RPTPβ cell-surface retention. Greater cell-surface retention of RPTPβ likely induces receptor dimerization and possibly conformational changes that inactivate its intrinsic phosphatase activity, contributing to more phosphorylated β-catenin. Higher levels of phosphorylated β-catenin then destabilize the β-catenin-α-cadherin complex, resulting in reduced cell-cell adhesion (Fig. 7B) and increased migration.
FIGURE 7.
Working model for the inhibition of RPTPβ activity and subsequent increases in β-catenin phosphorylation by GnT-Vb-mediated glycosylation and galectin-1 binding. A, in the absence of significant levels of O-mannosylated N-acetyllactosamine structures, which may include the HNK-1 epitope, RPTPβ is predominantly an active monomer. The active form of RPTPβ promotes the dephosphorylation (Y to Y) of tyrosine residues in β-catenin, causing more cell-cell adhesion and less cell motility. B, expression of GnT-Vb leads to more N-acetyllactosamine structures, including the HNK-1 epitope, that are bound by galectin-1. Galectin-1 binding stabilizes RPTPβ on the cell surface, promoting the dimerization of the receptor. RPTPβ dimerization then inhibits the phosphatase activity, leading to increased tyrosine phosphorylation (Y) of β-catenin that promotes the dissociation of the catenin-cadherin complex, resulting in decreased cell-cell adhesion and increased cell motility.
Both galectin-1 and GnT-Vb are expressed within the adult mouse subventricular zone during neuromorphogenesis where neurons are actively dividing and migrating (53).3 The long receptor form of RPTPβ that is a substrate for GnT-Vb in SH-SY5Y cells is expressed at high levels in the developing brain, whereas the shorter form of RPTPβ increases expression postnatally (54). RPTPβ, galectin-1, and GnT-Vb have each been implicated in regulating neural outgrowth and migration (35, 48, 55). Our model links these proteins in a mechanism capable of regulating key signaling events that reduce cell-cell adhesion and promote cell migration during brain development. Aberrant expression of the embryonic long receptor form of RPTPβ in invasive glioblastoma likely contributes to the highly invasive phenotype of this tumor (56). Moreover, this form of RPTPβ has also been consistently found expressed in different subtypes of human breast cancer, suggesting that its expression, and perhaps glycosylation, may affect the adhesion of non-neural carcinomas (57).
Changes in glycan expression and galectin binding regulate specific glycoprotein cell-surface retention and affect function (42, 44, 45, 58). These effects are suggestive of the clustering reported for galectins in vitro (59, 60). In one case, the Glut-2 transporter on mouse pancreatic islet cells displayed significantly reduced cell-surface retention, and subsequent poor uptake of glucose in animals that lack a specific glycosyltransferase, GnT-Iva, that is active in N-linked biosynthesis (45). In this case Glut-2 surface transporter retention on the cell surface is down-regulated by elimination or reduction of GnT-IVa expression, which in turns causes reduced binding by galectin-9, the galectin expressed at highest levels in this cell type. Our data using neuroblastoma cells suggest that galectin-1, the predominant lectin expressed in these cells, is involved in regulating cell-surface retention of RPTPβ and its phosphatase activity. This regulation is likely through O-mannosyl glycans that are synthesized by GnT-Vb, in contrast to the N-linked branched glycans implicated in other studies that are synthesized by GnT-IVa and GnT-Va. Moreover, in the case of the Glut-2 receptor, increased glycosylation by GnT-IVa results in increased galectin-9 binding, increased cell-surface retention, and high levels of glucose transport. Increased GnT-Va glycosylation of some growth factor receptors has been reported to result in higher levels of galectin-3 binding which, in turn, causes increased cell-surface retention, increased tyrosine phosphorylation, and increased receptor signaling via these phosphorylated residues (61). By contrast, however, inhibiting GnT-Va activity with siRNA expression in a human breast carcinoma cell line stimulated by epidermal growth factor resulted in increased cell-surface retention, lowered ERK phosphorylation, and increased SHP-2 phosphatase activity (62). Increased GnT-Va in all cases, however, does appear to result in decreased cadherin-mediated cell-cell and integrin-mediated cell-matrix adhesion, promoting increased cell migration and an invasive phenotype. Likewise, increased GnT-Vb glycosylation of RPTPβ led to decreased cell-cell adhesion and increased migration of neuroblastoma cells, because of an inhibition of RPTPβ intrinsic phosphatase activity and increased tyrosine phosphorylation of β-catenin. Intriguingly, specific acceptor glycosylation by both paralogs, GnT-Va and GnT-Vb, appear to regulate cell-cell interactions, although by distinct mechanisms.
Acknowledgments
We thank Mabel Pang and Linda Baum for providing recombinant galectin-1. We acknowledge Julie Nelson for help with the flow cytometry, Amanda Anderson for technical assistance, and Huabei Guo for help with cell-cell adhesion assays. We also thank Drs. Ron Schnaar and Linda Baum for helpful discussions and advice during the preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1 CA64462 (NCI). This work was also supported by a Canary Fund/American Cancer Society Postdoctoral Fellowship (to K. A.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement”in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HNK-1, human natural killer-1; GlcAT, glucuronyltransferase; siRNA, short interfering RNA; PBS, phosphate-buffered saline; BSA, bovine serum albumin; WGA, wheat germ agglutinin; TBS, Tris-buffered saline; PTN, pleiotrophin; MES, 4-morpholineethanesulfonic acid; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; LacNAc, N-acetyllactosamine; RPTP, receptor protein-tyrosine phosphatase; ERK, extracellular signal-regulated kinase.
R. T. Matthews, unpublished results.
References
- 1.Breen, K. C., Coughlan, C. M., and Hayes, F. D. (1998) Mol. Neurobiol. 16 163–220 [DOI] [PubMed] [Google Scholar]
- 2.Schwarting, G. A., Jungalwala, F. B., Chou, D. K., Boyer, A. M., and Yamamoto, M. (1987) Dev. Biol. 120 65–76 [DOI] [PubMed] [Google Scholar]
- 3.Bronner-Fraser, M. (1986) Dev. Biol. 115 44–55 [DOI] [PubMed] [Google Scholar]
- 4.Abo, T., and Balch, C. M. (1981) J. Immunol. 127 1024–1029 [PubMed] [Google Scholar]
- 5.Lipinski, M., Braham, K., Caillaud, J. M., Carlu, C., and Tursz, T. (1983) J. Exp. Med. 158 1775–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuller-Petrovic, S., Gebhart, W., Lassmann, H., Rumpold, H., and Kraft, D. (1983) Nature 306 179–181 [DOI] [PubMed] [Google Scholar]
- 7.Wernecke, H., Lindner, J., and Schachner, M. (1985) J. Neuroimmunol. 9 115–130 [DOI] [PubMed] [Google Scholar]
- 8.Caillaud, J. M., Benjelloun, S., Bosq, J., Braham, K., and Lipinski, M. (1984) Cancer Res. 44 4432–4439 [PubMed] [Google Scholar]
- 9.Rathjen, F. G., Wolff, J. M., Frank, R., Bonhoeffer, F., and Rutishauser, U. (1987) J. Cell Biol. 104 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathjen, F. G., and Schachner, M. (1984) EMBO J. 3 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grumet, M., Rutishauser, U., and Edelman, G. M. (1982) Nature 295 693–695 [DOI] [PubMed] [Google Scholar]
- 12.McGarry, R. C., Helfand, S. L., Quarles, R. H., and Roder, J. C. (1983) Nature 306 376–378 [DOI] [PubMed] [Google Scholar]
- 13.Woodworth, A., Pesheva, P., Fiete, D., and Baenziger, J. U. (2004) J. Biol. Chem. 279 10413–10421 [DOI] [PubMed] [Google Scholar]
- 14.Maeda, N., Hamanaka, H., Shintani, T., Nishiwaki, T., and Noda, M. (1994) FEBS Lett. 354 67–70 [DOI] [PubMed] [Google Scholar]
- 15.Dino, M. R., Harroch, S., Hockfield, S., and Matthews, R. T. (2006) Neuroscience 142 1055–1069 [DOI] [PubMed] [Google Scholar]
- 16.Hall, H., Carbonetto, S., and Schachner, M. (1997) J. Neurochem. 68 544–553 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt, J. T., and Schachner, M. (1998) J. Neurobiol. 37 659–671 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto, S., Oka, S., Inoue, M., Shimuta, M., Manabe, T., Takahashi, H., Miyamoto, M., Asano, M., Sakagami, J., Sudo, K., Iwakura, Y., Ono, K., and Kawasaki, T. (2002) J. Biol. Chem. 277 27227–27231 [DOI] [PubMed] [Google Scholar]
- 19.Senn, C., Kutsche, M., Saghatelyan, A., Bosl, M. R., Lohler, J., Bartsch, U., Morellini, F., and Schachner, M. (2002) Mol. Cell. Neurosci. 20 712–729 [DOI] [PubMed] [Google Scholar]
- 20.Ilyas, A. A., Quarles, R. H., and Brady, R. O. (1984) Biochem. Biophys. Res. Commun. 122 1206–1211 [DOI] [PubMed] [Google Scholar]
- 21.Chou, D. K., Ilyas, A. A., Evans, J. E., Costello, C., Quarles, R. H., and Jungalwala, F. B. (1986) J. Biol. Chem. 261 11717–11725 [PubMed] [Google Scholar]
- 22.Burger, D., Simon, M., Perruisseau, G., and Steck, A. J. (1990) J. Neurochem. 54 1569–1575 [DOI] [PubMed] [Google Scholar]
- 23.Ong, E., Suzuki, M., Belot, F., Yeh, J. C., Franceschini, I., Angata, K., Hindsgaul, O., and Fukuda, M. (2002) J. Biol. Chem. 277 18182–18190 [DOI] [PubMed] [Google Scholar]
- 24.Finne, J., Krusius, T., Margolis, R. K., and Margolis, R. U. (1979) J. Biol. Chem. 254 10295–10300 [PubMed] [Google Scholar]
- 25.Kiang, W. L., Margolis, R. U., and Margolis, R. K. (1981) J. Biol. Chem. 256 10529–10537 [PubMed] [Google Scholar]
- 26.Chai, W., Yuen, C. T., Kogelberg, H., Carruthers, R. A., Margolis, R. U., Feizi, T., and Lawson, A. M. (1999) Eur. J. Biochem. 263 879–888 [DOI] [PubMed] [Google Scholar]
- 27.Yuen, C. T., Chai, W., Loveless, R. W., Lawson, A. M., Margolis, R. U., and Feizi, T. (1997) J. Biol. Chem. 272 8924–8931 [DOI] [PubMed] [Google Scholar]
- 28.Zhang, W., Betel, D., and Schachter, H. (2002) Biochem. J. 361 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaneko, M., Alvarez-Manilla, G., Kamar, M., Lee, I., Lee, J. K., Troupe, K., Zhang, W., Osawa, M., and Pierce, M. (2003) FEBS Lett. 554 515–519 [DOI] [PubMed] [Google Scholar]
- 30.Inamori, K., Endo, T., Gu, J., Matsuo, I., Ito, Y., Fujii, S., Iwasaki, H., Narimatsu, H., Miyoshi, E., Honke, K., and Taniguchi, N. (2004) J. Biol. Chem. 279 2337–2340 [DOI] [PubMed] [Google Scholar]
- 31.Elola, M. T., Wolfenstein-Todel, C., Troncoso, M. F., Vasta, G. R., and Rabinovich, G. A. (2007) Cell. Mol. Life Sci. 64 1679–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lander, C., Kind, P., Maleski, M., and Hockfield, S. (1997) J. Neurosci. 17 1928–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lander, C., Zhang, H., and Hockfield, S. (1998) J. Neurosci. 18 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng, K., Rodriguez-Pena, A., Dimitrov, T., Chen, W., Yamin, M., Noda, M., and Deuel, T. F. (2000) Proc. Natl. Acad. Sci. U. S. A. 97 2603–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abbott, K. L., Troupe, K., Lee, I., and Pierce, M. (2006) Exp. Cell Res. 312 2837–2850 [DOI] [PubMed] [Google Scholar]
- 36.Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M., and Trono, D. (1996) Science 272 263–267 [DOI] [PubMed] [Google Scholar]
- 37.Pedraza, L., Spagnol, G., Latov, N., and Salzer, J. L. (1995) J. Neurosci. Res. 40 716–727 [DOI] [PubMed] [Google Scholar]
- 38.Dharmesh, S. M., Skelton, T. P., and Baenziger, J. U. (1993) J. Biol. Chem. 268 17096–17102 [PubMed] [Google Scholar]
- 39.Johnson, G., and Moore, S. W. (2001) Int. J. Dev. Neurosci. 19 439–445 [DOI] [PubMed] [Google Scholar]
- 40.Hall, H., Deutzmann, R., Timpl, R., Vaughan, L., Schmitz, B., and Schachner, M. (1997) Eur. J. Biochem. 246 233–242 [DOI] [PubMed] [Google Scholar]
- 41.Guo, H. B., Lee, I., Kamar, M., and Pierce, M. (2003) J. Biol. Chem. 278 52412–52424 [DOI] [PubMed] [Google Scholar]
- 42.Hernandez, J. D., Nguyen, J. T., He, J., Wang, W., Ardman, B., Green, J. M., Fukuda, M., and Baum, L. G. (2006) J. Immunol. 177 5328–5336 [DOI] [PubMed] [Google Scholar]
- 43.Bilwes, A. M., den Hertog, J., Hunter, T., and Noel, J. P. (1996) Nature 382 555–559 [DOI] [PubMed] [Google Scholar]
- 44.Partridge, E. A., Le Roy, C., Di Guglielmo, G. M., Pawling, J., Cheung, P., Granovsky, M., Nabi, I. R., Wrana, J. L., and Dennis, J. W. (2004) Science 306 120–124 [DOI] [PubMed] [Google Scholar]
- 45.Ohtsubo, K., Takamatsu, S., Minowa, M. T., Yoshida, A., Takeuchi, M., and Marth, J. D. (2005) Cell 123 1307–1321 [DOI] [PubMed] [Google Scholar]
- 46.Allen, H. J., Ahmed, H., and Matta, K. L. (1998) Glycoconj. J. 15 691–695 [DOI] [PubMed] [Google Scholar]
- 47.Xu, Z., and Weiss, A. (2002) Nat. Immunol. 3 764–771 [DOI] [PubMed] [Google Scholar]
- 48.Maeda, N., and Noda, M. (1998) J. Cell Biol. 142 203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stowell, S. R., Arthur, C. M., Mehta, P., Slanina, K. A., Blixt, O., Leffler, H., Smith, D. F., and Cummings, R. D. (2008) J. Biol. Chem. 283 10109–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amano, M., Galvan, M., He, J., and Baum, L. G. (2003) J. Biol. Chem. 278 7469–7475 [DOI] [PubMed] [Google Scholar]
- 51.Fouillit, M., Joubert-Caron, R., Poirier, F., Bourin, P., Monostori, E., Levi-Strauss, M., Raphael, M., Bladier, D., and Caron, M. (2000) Glycobiology 10 413–419 [DOI] [PubMed] [Google Scholar]
- 52.Walzel, H., Schulz, U., Neels, P., and Brock, J. (1999) Immunol. Lett. 67 193–202 [DOI] [PubMed] [Google Scholar]
- 53.Sakaguchi, M., Imaizumi, Y., and Okano, H. (2007) Cell. Mol. Life Sci. 64 1254–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canoll, P. D., Petanceska, S., Schlessinger, J., and Musacchio, J. M. (1996) J. Neurosci. Res. 44 199–215 [DOI] [PubMed] [Google Scholar]
- 55.McGraw, J., Gaudet, A. D., Oschipok, L. W., Steeves, J. D., Poirier, F., Tetzlaff, W., and Ramer, M. S. (2005) Pain 114 7–18 [DOI] [PubMed] [Google Scholar]
- 56.Schreiber, E., Matthias, P., Muller, M. M., and Schaffner, W. (1989) Nucleic Acids Res. 17 6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Pinera, P., Garcia-Suarez, O., Menendez-Rodriguez, P., Mortimer, J., Chang, Y., Astudillo, A., and Deuel, T. F. (2007) Biochem. Biophys. Res. Commun. 362 5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lagana, A., Goetz, J. G., Cheung, P., Raz, A., Dennis, J. W., and Nabi, I. R. (2006) Mol. Cell. Biol. 26 3181–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris, S., Ahmad, N., Andre, S., Kaltner, H., Gabius, H. J., Brenowitz, M., and Brewer, F. (2004) Glycobiology 14 293–300 [DOI] [PubMed] [Google Scholar]
- 60.Brewer, C. F., Miceli, M. C., and Baum, L. G. (2002) Curr. Opin. Struct. Biol. 12 616–623 [DOI] [PubMed] [Google Scholar]
- 61.Lau, K. S., Partridge, E. A., Grigorian, A., Silvescu, C. I., Reinhold, V. N., Demetriou, M., and Dennis, J. W. (2007) Cell 129 123–134 [DOI] [PubMed] [Google Scholar]
- 62.Guo, H. B., Randolph, M., and Pierce, M. (2007) J. Biol. Chem. 282 22150–22162 [DOI] [PubMed] [Google Scholar]