Abstract
The study reported here was a secondary analysis of data on 157 males from a larger study of predictors of memory performance in community-dwelling elders. The males' average age was 76 years, with 13 years of education and a Mini-Mental State Exam score of 26. Measures included depression, memory performance, metamemory, and memory self-efficacy. An unusual finding was the multimodal distribution of memory self-efficacy strength scores. Using a median split, the sample was divided into low and high memory self-efficacy groups. The high efficacy group were significantly younger, had larger scores on capacity (+ = high capacity) and change (+ = greater stability). These findings provide new evidence that the memory self-efficacy of aging males influences their perceptions of cognitive performance related to memory.
Keywords: memory performance, gender difference, aging males, self-efficacy
Data from the Health and Retirement study published in Older Americans 2000: Key Indicators of Well Being suggest that in every age cohort older than 65 years, a higher percentage of males than females have moderate or severe memory impairment, defined as four or fewer words recalled out of 20 (Federal Interagency Forum of Aging-Related Statistics, 2000). In both cross-sectional and longitudinal studies of memory performance, males performed worse than females and had larger performance decrements (Barrett-Connor & Kritz-Silverstein, 1999; Zelinski, Gilewski, & Schaie, 1993). In one study healthy older males had lower scores on self-reported memory function than females (McDougall, 1998a). In another study of nursing home residents, McDougall (1998b) found scores on the capacity and change metamemory scales were significantly lower in the group with mixed depression and cognitive impairment when compared to a group with cognitive impairment and no depression. This phenomenon of compromised thinking, anxiety, and decreased confidence in memory has been described as mental frailty by McDougall and Balyer (1998) and sense of control by Lachman, Steinberg, and Trotter (1987) and Lachman and Leff (1989). Palmer, Wolkenstein, LaRue, Swan, and Smalley (1994) found that in 6% of a sample of community males (N = 1149), memory performance scores were low enough to be classified as risk for dementia. Larrabee and Crook (1993), who examined 417 male-female pairs, found that males had greater age-associated atrophy of the left hemisphere than women; however, this difference was not manifested in everyday verbal memory. Magnetic resonance imaging studies have also found greater atrophy and shrinkage in the brain structures of men than in the brains of women (Coffey et al., 1998; Larkin, 1998; Palmer et al., 1994).
Risk Factors Thought to Contribute to a Decrease in Memory Performance
Memory Complaints
Memory complaints are the subjective aspect of cognitive function and may be related to memory performance in older adults, but findings about this relationship are inconclusive (Comijs, Deeg, Dik, Twisk, & Jonker, 2002; Commissaris, Ponds, & Jolles, 1998; Hertzog, Dixon, & Hultsch, 1990a; Zelinski, Gilewski, & Anthony-Bergstone, 1990). Subjective memory complaints provide useful health information regarding cognitive aging phenomena and also may predict future cognitive decline (Blazer, Hays, Fillenbaum, & Gold, 1997; Christensen, 1991; Rowe & Kahn, 1987; Jonker, Launer, Hooijer, & Lindeboom, 1996; Verhaeghen, Geraerts, & Marcoen, 2000). Complaints are often evaluated with metamemory questionnaires; however, many studies ask only one or two questions about memory problems, such as “Do you have problems with your memory?” (Cutler & Grams, 1988; Schofield, Marder, Dooneief, Jacobs, Sano, & Stern, 1997). The individuals who have participated in memory studies tended to be individuals having memory complaints and problems, perhaps obscuring the relationship between metamemory and memory performance (Gilewski, Zelinski, & Schaie, 1990; Perlmutter, 2001; Ponds & Jolles, 1996; Ponds, Commissaris, & Jolles, 1997). Nevertheless, metamemory may influence memory performance, and therefore it has particular relevance for cognitive aging research.
Memory Self-Efficacy
Memory self-efficacy was first defined by Hertzog, Hultsch, and Dixon (1989. p. 687) as beliefs about one's capability to use memory effectively in various situations. However, Bandura (1989) determined measurement issues and noted that knowledge about memory functioning (or metamemory) is distinct from beliefs about memory efficacy. The level of distress experienced with memory problems and the actions taken in response to these problems vary depending on a number of factors. These factors include beliefs about aging, perception of symptom severity, attribution of the symptom to aging, and neurotic personality traits (Bandura, 1988, 1997; Hill & Vandervoort, 1992; Rebok & Balcerak, 1989). The differentiation of knowledge about memory from memory self-efficacy allows for the possibility that an older individual may have extensive and accurate knowledge about how his/her memory functions (metamemory) but may also believe that his or her ability to remember in a given context is poor (Jorm, Christensen, Henderson, Korten, Mackinnon, & Scott, 1994; Jorm, Christensen, Korten, Henderson, Jacomb, & Mackinnon, 1997). Therefore, to imply a causal relationship between memory self-efficacy and memory performance would exclude the influence of other health variables.
Bandura (1989) and Maibach and Murphy (1995) have explicated the methodological aspects of measuring self-efficacy. Memory self-efficacy has been defined and operationalized by a number of authors including Bandura (1989) and Berry, West, and Dennehey (1989). To meet the criteria for a domain-specific measure of self-efficacy, the measure/scale requires the individual to make two judgments regarding accomplishment of the task, in this case memory performance. First, the individual judges his/her ability to accomplish the task (yes/no). Second, a performance prediction is estimated from 10-100, indicating the strength of certainty.
Memory self-efficacy has been addressed in a few studies, but primarily as a control for memory training or as a training component (Dittmann-Kohli, Lachman, Kliegel, & Blates, 1991; Hill, Sheikh, & Yesavage, 1988; Lachman, Weaver, Bandura, Elliott, & Lewkowicz, 1992). McDougall (1994) found that healthy elders (average age 68 years) attending lifelong learning classes showed a decrease in memory self-efficacy with age. In another study, McDougall, Holston, and Wilke (2001) found that a mixed sample of black and white community-based older adults (average age 77 years, with an average of 12 years of education) had low memory self-efficacy scores. McDougall (2002) also found lower memory self-efficacy scores in a group of older adults living in a retirement village (average age 82). In an assisted living sample, McDougall (2000) found memory self-efficacy scores to be even lower than in the previous samples.
Depending on the congruence between predicted memory tasks and the memory tasks actually performed, memory self-efficacy may or may not predict performance. Memory self-efficacy predicted memory performance in a biracial sample of black and white elders without cognitive impairment (McDougall, under review). Memory self-efficacy has predicted memory performance in numerous studies with Caucasian elders (Berry, West, & Dennehey, 1989; Best, Hamlett, & Davis, 1992; Lachman et al., 1992; Rebok & Balcerak, 1989). In one study memory self-efficacy for everyday tasks (map, location, phone, and grocery) predicted memory performance for everyday tasks, but not laboratory tasks such as word, picture, digit, and maze (Berry et al., 1989). In a study designed to change memory self-efficacy beliefs, self-efficacy predicted memory performance, r(91) = .44, p < .0001, when self-efficacy was measured in terms of subjects' judgments of their highest memory capability r(91) =.30, p < .01, and when they judged their memory efficacy for the most taxing recall (Rebok & Balcerak, 1989). In the MacArthur study of successful aging, Seeman, Rodin, and Albert (1993) found that efficacy beliefs were associated with cognitive performance for men, but not for women. During longitudinal follow-up, however, Seeman, McAvay, Merrill, Albert, and Rodin (1996) found that self-efficacy beliefs did not predict performance in any cognitive domains, for either men or women. Perrig-Chiello, Perrig, and Stahelin (2000) found that memory self-efficacy predicted memory performance in men greater than 75 years of age; however, in men younger than 75, neuroticism predicted memory performance.
Depression
In older adults, depression and memory complaints are related (Derouesne et al., 1989; Grut, Jorm, & Fratiglioni, 1993; Perrig-Chiello et al., 2000; Schofield et al., 1997). Depression has also been found to be a mediating factor in the relationship between memory complaints and memory performance of older adults (Zelinski et al., 1990; Zelinski et al., 1993). In a memory training study, Weaver and Lachman (1989) found that older individuals who were less depressed became more accurate in their performance predictions over time than those who were more depressed. Dellefield and McDougall (1996) found that individuals with depression had significantly lower memory self-efficacy scores than those without depression; however, there was no difference in memory performance between the depressed and non-depressed subjects. The purpose of the study reported here was to describe the demographics, memory self-efficacy, metamemory, memory performance, and depression in older males.
Method
Procedures
Participants were 157 community-based older males recruited from high-rise apartment complexes and retirement communities in Ohio and Texas. The memory assessment consisted of several tests and scales. The Mini-Mental State Exam (MMSE) and the Rivermead Behavioural Memory Test were performance measures. The Metamemory in Adulthood Questionnaire, Memory Self-Efficacy Questionnaire, and Center for Epidemiological Studies Depression Scale were self-report questionnaires. Before the interview, a letter of consent was read and questions were answered; then the study instruments were administered. First, individuals were given the MMSE. Next, demographic information was collected, and the metamemory, memory self-efficacy, and depression instruments were administered. Then, the memory performance timed-test and a health questionnaire were administered. Finally, questions from the last part (“delayed” recall) of the memory performance test were administered.
Cognitive function was determined with a reliable and valid screening instrument, the Mini-Mental State Exam (MMSE). This instrument contains 11 questions with values that can range from 0-30, with a value of 23 or less indicating cognitive impairment (Pearson, Cherrier, & Teri, 1989; Tombaugh & McIntyre, 1992). Even though 23 is the cutoff score, individuals who scored between 17 and 30 participated in this study. This decision was made to include lower MMSE scores based on converging evidence from recent studies that suggest a dimensional relationship between cognitive status and insight and that older adults are capable of identifying their thoughts and feelings even in the presence of significant cognitive deficits (Derouesne, Thibault, Lagha-Peirucci, Baudouin-Madec, Ancri, & Lacomblez, 1999; Duke, Seltzer, Seltzer, & Vasterling, 2002; Mozley et al., 1999; Zanetti et al., 1999).
Performance Data
Memory performance was evaluated with the Rivermead Behavioural Memory Test (RBMT) (Wilson, Cockburn, & Baddeley, 1985; Wilson, Cockburn, Baddeley, & Hiorns, 1989). The RBMT is designed to reflect everyday memory and serves as a bridge between laboratory-based measures of memory and assessments obtained by questionnaires and observations. The components are remembering a name (first and surname), hidden belonging, appointment, picture recognition, brief news article, face recognition, new route (immediate), new route (delayed), message, orientation, and date. Questions are designed so that normal subjects would pass but individuals with everyday memory problems would fail. For each subtest, two scores are produced, a pass/fail screening score and a standardized profile score from 0-2 (0 points = abnormal; 1 point = borderline; 2 points = normal). Thus each subject's evaluation results in two scores, a Screening Score (SS) ranging from 0-12 and a Standardized Profile Score (SPS) ranging from 0-24. To help control for practice effect, the test is available in four alternate (parallel) forms. Test-retest reliabilities have been reported as .78 for the screening score and .85 for the profile score. Alpha reliabilities for this sample were .86 for the profile score and .80 for the screening score.
Self-Report Data
Memory self-efficacy was operationalized with the Memory Self-Efficacy Questionnaire (MSEQ) derived from Bandura's self-efficacy theory (Bandura, 1993; Berry et al., 1989). The MSEQ is a Guttman scale consisting of 50 questions, five for each of ten daily tasks: groceries, telephone, picture, location, word, digit, map, errands, photographs, and a maze. Subjects rate their self-efficacy level (SEL) from 1 to 5 for each of the five levels of the task (least difficult to most difficult) and the strength of their confidence self-efficacy strength score (SEST) in performing each task from 10% to 100%. Alpha reliability for this sample was .93.
Depression was operationalized with the Center for Epidemiological Studies Depression Scale CES-D on which individuals respond using a four-point-Likert scale from rarely or none of the time to most or all of the time. Scores range from 0 to 60, with higher scores indicating more depressive symptomatology; composite score is acceptable (Radloff & Teri, 1986). The CES-D has been tested with older African, Caucasian, and Mexican-American adults and found stable when subscale and total scores were reported (Himmelfarb & Murrell, 1983). Alpha reliability with this sample was .84.
Metamemory was measured with the Metamemory in Adulthood Questionnaire (MIA), which captures the subjective memory components of knowledge, beliefs, and affect (Dixon, Hultsch, & Hertzog, 1988). The MIA consists of 108 statements, with responses rated on a five-point Likert scale, from “all of the time” or “always” to “none of the time” or “none.” The seven subscales measure achievement, anxiety, capacity, change, locus, task, and strategy. Achievement is the perceived importance of having a good memory and of performing well on memory tasks (+ = high motivation). Anxiety is the rating of the influence of anxiety and stress on performance (+ = greater anxiety). Capacity is the perception of memory capabilities as measured by predictive report of performance on given tasks (+ = high capacity). Change is the perception of memory abilities as generally stable or subject to long-term decline (+ = stability). Locus is the individual's perceived personal control over remembering abilities (+ = internal locus). Task is knowledge of basic memory processes, especially knowledge of how most people perform (+ = greater knowledge). Cronbach's alphas for the seven subscales have been reported as .79 to .84. Cronbach's alphas for the subscales in this sample were: Achievement, .69, Anxiety, .85, Capacity, .87, Change, Locus, .69, Strategy, .82, and Task, .68.
Results
Data Analyses
First, using the maximum number of cases available for each variable, means, standard deviations, and ranges were calculated for study variables. Second, Pearson product moment correlation coefficients were computed between study variables. Third, independent t tests were calculated between the low and high memory self-efficacy groups.
Of the 157 males in the sample, memory self-efficacy data was available on 111 individuals; for the remaining variables, available data varied (Table 1). The majority of the sample were Caucasian males; however, there were 24 African-American males. The sample had a mean age of 75.49 years (Range = 60-97 years), had on average 13 years of education (Range = 4-25 years), and had an average MMSE score of 26. The participants had high perceived health scores M = 9.06, SD = 2.50, R = 4-13. Although their CES-D scores (N = 79) were in the nondepressed range M = 9.93, SD = 8.81, 24% (N = 19) of the males scored in the depressed range with scores > 16. Memory self-efficacy scores were low (M = 37.60, SD = 20.38). In order to explore the associations between the demographic variables and the performance tasks, a series of statistical analyses were performed.
Table 1. Means and Standard Deviations for Study Variables.
| Mean | SD | Range | N | |
|---|---|---|---|---|
| Age | 75.49 | 8.00 | 60-97 | 151 |
| Education | 13.12 | 4.19 | 4-25 | 118 |
| MMSE | 26.49 | 3.41 | 17-30 | 112 |
| Depression | 9.93 | 8.81 | 0-37 | 79 |
| Health | 9.06 | 2.50 | 4-13 | 79 |
| Achievement | 3.73 | .39 | 2.81-4.75 | 131 |
| Anxiety | 3.12 | .63 | 1.79-4.36 | 132 |
| Capacity | 2.98 | .63 | 1.18-4.76 | 144 |
| Change | 2.58 | .70 | 1.06-4.44 | 144 |
| Locus | 3.42 | .53 | 2.11-4.67 | 151 |
| Strategy | 3.36 | .57 | 1.78-4.44 | 144 |
| Task | 3.85 | .37 | 2.00-4.94 | 133 |
| Memory Self-efficacy | 37.60 | 20.38 | .80-93.60 | 111 |
| Memory Profile | 15.97 | 5.75 | .00-24.00 | 92 |
| Memory Screen | 6.76 | 2.93 | .00-12.00 | 92 |
Significant (p ≤ .05) correlation coefficients are reported between study variables (Table 2). The memory profile scores, with a range of 0-24, were used in the correlations. Memory self-efficacy was associated with age (r = -.39), cognition (r = .28), memory performance (r = .30), anxiety (r = -.25), capacity (r = .51), change (r = .46), and locus (r = .22). Age was related to memory performance (r = -.21), capacity (r = -.17), and change (r = -.29). Depression was associated with education (r = -.37) and anxiety (r = .34). Anxiety was inversely associated with education (r = -.23). As an individual grew older, memory self-efficacy and memory performance decreased. In addition, with increased age, memory capacity decreased and worsened toward instability. An older male with less education was more likely to be anxious about his memory and was more likely to have signs of depression.
Table 2. Correlation Matrix for Study Variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||||
| Education | .09 | |||||||||||
| Cognition | -.09 | .24* | ||||||||||
| Depression | .01 | -.37* | -.07 | |||||||||
| Memory SE | -.39 | .11 | .28* | -.11 | ||||||||
| Memory | -.21* | .10 | .64* | -.09 | .30* | |||||||
| Achievement | -.09 | -.06 | .09 | -.02 | .01 | .02 | ||||||
| Anxiety | .02 | -.23* | -.14 | .34* | -.25* | -.23 | .26* | |||||
| Capacity | -.17* | -.15 | -.04 | .03 | .51* | .21 | -.10 | -.51* | ||||
| Change | -.29* | -.06 | .14 | -.09 | .46* | .30* | -.15 | -.53* | .68* | |||
| Locus | -.19 | -.09 | .12 | -.06 | .22* | .04 | .34* | -.31* | .39* | .50* | ||
| Strategy | -.01 | .31* | .31* | -.06 | .05 | .29* | .28* | .15* | -.22* | -.10 | .16 | |
| Task | -.08 | .30* | .39* | -.15 | .14 | .07 | .18* | .06 | -.01 | -.09* | .23 | .33* |
| p ≤ .05 |
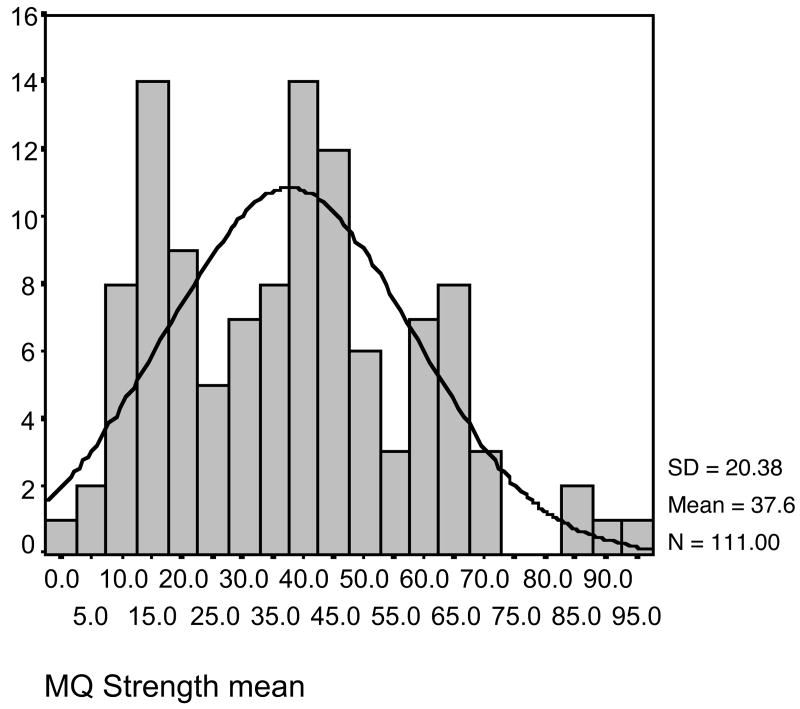
After observing a multimodal distribution (Figure 1) of memory self-efficacy, a median split of 38.40 was used to divide the sample into two memory self-efficacy groups (low, N = 56 [M = 21.21, SD = 9.92] and high, N = 55 [M = 54.31, SD = 13.52]). Next, t tests were computed on study variables using the available data for group comparisons (Tables 3-5). Because the number of cases varied, equal variances were not assumed to be present. The high memory self-efficacy group was significantly (p ≤ .05) younger (72 vs. 77) than the low efficacy group. There were no other group differences on demographic variables. On the metamemory scale, there were significant (p ≤ .05) group differences on two subscales, capacity and change. The high self-efficacy group had higher scores on capacity (3.25 vs. 2.79) and change (2.89 vs. 2.39). The low self-efficacy group's scores on change indicated an unstable or worsening memory (Table 2). There were no group differences on memory performance (screening or profile).
Figure 1.
Histogram of memory self-efficacy scores.
Table 3. Means and Standard Deviations of Demographic Variables for Memory Self-Efficacy Groups.
| Low Mean | SD | High Mean | SD | p | |
|---|---|---|---|---|---|
| Agea | 77.11 | 7.28 | 72.15 | 6.42 | .000 |
| Cognitionb | 26.63 | 3.08 | 27.50 | 2.80 | NS |
| Educationc | 12.83 | 3.54 | 13.87 | 4.36 | NS |
| Depressiond | 10.14 | 9.06 | 9.59 | 8.53 | NS |
N = 111
N = 77
N = 80
N = 79
Table 5. Means and Standard Deviations of Memory Performance Variables.
| Low Mean | SD | High Mean | SD | p | |
|---|---|---|---|---|---|
| Memory Perf.—SPSa | 15.67 | 5.34 | 17.90 | 4.30 | NS |
| Memory Perf.—SSa | 6.49 | 2.92 | 7.55 | 2.36 | NS |
| Memory Self-Efficacyb | 21.21 | 9.92 | 54.31 | 13.52 | .05 |
N = 78
N = 111
Discussion
Age and memory performance were associated; similarly in longitudinal studies of memory performance, age has been found the strongest predictor of memory decline (Federal Interagency Forum of Aging-Related Statistics, 2000; Hultsch, Hertzog, Small, McDonald-Miszczak, & Dixon, 1992; Zelinski & Stewart, 1998). The memory performance scores of the participants in this study were similar to those of Cockburn and Smith's (1989, 1991) community participants over 70 years of age, but poorer than those of van Baleen, Westland, and Mulder's (1996) older adults greater than 69 years of age. In this study, the older males read and understood English and had an average of 13 years of education; however, without intelligence test scores, it is impossible to determine if their memory performance scores were above or below average scores.
Memory self-efficacy was positively associated with everyday memory performance and inversely associated with anxiety. The inverse relationship between anxiety and memory self-efficacy supports Bandura's (1988) conception of anxiety; that is, as the strength of one's self-efficacy increases, their anxiety arousal decreases. Memory self-efficacy has predicted memory performance in numerous studies with African- and Caucasian-American elders (Berry, West, & Dennehey, 1989; Best, Hamlett, & Davis, 1992; Lachman, Weaver, Bandura, Elliott, & Lewkowicz, 1992; McDougall, under review; Rebok & Balcerak, 1989). Memory self-efficacy has predicted performance for everyday tasks (Berry, West, & Dennehey, 1989; Rebok & Balcerak, 1989). Seeman, Rodin, and Albert (1993) found that efficacy beliefs were associated with cognitive performance for men, but not for women. However, these findings were not maintained at longitudinal follow-up for either men or women (Seeman, McAvay, Merrill, Albert, & Rodin, 1996). Memory self-efficacy predicted memory performance in men greater than 75 years of age (Perrig-Chiello, Perrig, & Stahelin, 2000). The low self-efficacy group's scores were similar to those in McDougall's (2000, McDougall, 2001) studies, which included males from assisted living and nursing homes. Participants believed their memory capacity was low and worsening. The capacity and change scores were significantly different between the two self-efficacy groups. There were no differences in memory performance between the groups; however, the low efficacy group believed their everyday memory performance was worsening.
Even though there were no group differences on the Locus subscale, it was positively associated with health and negatively associated with age and anxiety. Locus refers to the individual's perceived sense of control over remembering skills (Hertzog, Dixon, & Hultsch, 1990b). Does the individual believe he or she has control, or is the control outside of self? Items include statements that memory depends largely on hard work, practice, and determination, and, conversely, that it depends largely on health, other persons' activities, age, or other factors outside the respondent's control. Higher scores indicate internal or personal control and have been associated with higher performance assessments (Lachman, Steinberg, & Trotter, 1987). The findings indicated that as anxiety increases in intensity, the sense of control over remembering abilities decreases. An individual's perception of his or her sense of control over memory performance may be moderated by experience; however, the testing was novel and participants had no previous experience with this type of memory testing.
Sense of control over cognitive abilities is also influenced by health status. In a five-year longitudinal study of perceived control and intellectual functioning, Lachman and Leff (1989) found that external-control beliefs were more likely to be adopted by those who had more medical problems, more education, and lower levels of fluid intelligence. Results of this study, that is, a positive association between the Locus subscale and health, support Lachman and Leff's findings. Self-rated health data is a necessary and unique source of information needed to further efforts in prevention and treatment related to memory aging (Lachman, Steinberg, & Trotter, 1987; Schecter & Herrmann, 1997).
Recent studies of mild cognitive impairment (MCI), a transitional state between the cognitive changes of normal aging and Alzheimer's disease (AD), have illuminated the need to treat individuals with memory loss so that their illness might not progress to a dementia (Peterson et al., 2001; Peterson, Smith, Waring, Ivnik, Tangalos, & Kokmen, 1999). Numerous investigators have articulated that subjective memory complaints provide useful health information regarding cognitive aging phenomena and also may predict future cognitive decline (Blazer et al., 1997; Christensen, 1991; Jonker et al., 1996; Rowe & Kahn, 1987; Verhaeghen et al., 2000). The subjective aspects of memory performance clearly have an impact on perceptions of health and well-being. McDougall and Balyer (1998) introduced the concept of mental frailty, which includes compromised thinking, anxiety, and decreased confidence in memory; this study suggests that if depression and memory self-efficacy were boosted, that in itself would decrease these symptoms of worsening memory function.
This study was a secondary analysis of existing data. Further, since it focused on only males, the findings can only be interpreted for males; and then, the majority of the sample was Caucasian. In addition, there was a high level of overlap of the distributions of subjects across the analyzed measures. Despite these limitations, the study provides new information on the cognitive performance of aging males living in the community.
Table 4. Means and Standard Deviations of Metamemory Variables (Self-Report).
| Low Mean | SD | High Mean | SD | p | |
|---|---|---|---|---|---|
| Achievementa | 3.65 | .38 | 3.69 | .40 | NS |
| Anxietyb | 3.15 | .56 | 3.00 | .66 | NS |
| Capacityb | 2.79 | .56 | 3.25 | .60 | .000 |
| Changeb | 2.39 | .58 | 2.89 | .77 | .000 |
| Locusc | 3.36 | .45 | 3.53 | .59 | NS |
| Strategyb | 3.32 | .58 | 3.35 | .58 | NS |
| Taska | 3.83 | .32 | 3.89 | .28 | NS |
N =105
N =106
N =157
Acknowledgments
Support for this research was provided by NINR Grant R15 NR0420 and the American and Rehabilitation Nurses Foundations. The research was presented at the 2001 Gerontological Society of America annual scientific meeting in Chicago, IL, and the 2002 Southern Nursing Research Society in San Antonio, TX. A special thanks to the graduate nursing students who assisted with this project.
References
- Bandura A. Self-efficacy conception of anxiety. Anxiety Research. 1988;1:77–98. [Google Scholar]
- Bandura A. Regulation of cognitive processes through perceived self-efficacy. Developmental Psychology. 1989;25(5):729–735. [Google Scholar]
- Bandura A. Perceived self-efficacy in cognitive development and functioning. Educational Psychologist. 1993;28(2):117–148. [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- Barrett-Connor E, Kritz-Silverstein D. Gender differences in cognitive function with age: the Rancho Bernardo study. Journal American Geriatrics Society. 1999;47(2):159–164. doi: 10.1111/j.1532-5415.1999.tb04573.x. [DOI] [PubMed] [Google Scholar]
- Berry JM, West RL, Dennehey DM. Reliability and validity of the memory self-efficacy questionnaire. Developmental Psychology. 1989;25(5):701–713. [Google Scholar]
- Best DL, Hamlett KW, Davis SW. Memory complaint and memory performance in the elderly: The effects of memory skills training and expectancy change. Applied Cognitive Psychology. 1992;6:405–416. [Google Scholar]
- Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline. Journal of Aging and Health. 1997;9(2):171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- Christensen H. The validity of memory complaints by elderly persons. International Journal of Geriatric Psychiatry. 1991;6:307–312. [Google Scholar]
- Cockburn J, Smith PT. The Rivermead Behavioural Memory Test Supplement 3: Elderly people. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1989. [Google Scholar]
- Cockburn J, Smith PT. The relative influence of intelligence and age on everyday memory. Journal of Gerontology: Psychological Sciences. 1991;46:31–31. doi: 10.1093/geronj/46.1.p31. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, et al. Sex differences in brain aging: A quantitative magnetic resonance imaging study. Archives Neurology. 1998;55(2):169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Comijs HC, Deeg DJH, Dik MG, Twisk JWR, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics. A 6-year follow-up study. Journal of Affective Disorders. 2002;72:157–165. doi: 10.1016/s0165-0327(01)00453-0. [DOI] [PubMed] [Google Scholar]
- Commissaris CJAM, Ponds RWHM, Jolles J. Subjective forgetfulness in a normal Dutch population: Possibilities for health education and other interventions. Patient Education and Counseling. 1998;34:25–32. doi: 10.1016/s0738-3991(98)00040-8. [DOI] [PubMed] [Google Scholar]
- Cutler SJ, Grams AE. Correlates of self-reported everyday memory problems. Journal of Gerontology: Social Sciences. 1988;43(3):S82–90. doi: 10.1093/geronj/43.3.s82. [DOI] [PubMed] [Google Scholar]
- Dellefield KS, McDougall GJ. Increasing metamemory in community elderly. Nursing Research. 1996;45(5):284–290. doi: 10.1097/00006199-199609000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouesne C, Alperovitch A, Arvay N, Migeon P, Moulin F, Vollant M, et al. Memory complaints in the elderly: A study of 367 community-dwelling individuals from 50 to 80 years old. Archives Gerontology and Geriatrics Supplement. 1989;1:151–163. [PubMed] [Google Scholar]
- Derouesn MC, Thibault S, Lagha-Peirucci S, Baudouin-Madec V, Ancri D, Lacomblez L. Decreased awareness of cognitive deficits in patients with mild dementia of the Alzheimer type. International Journal of Geriatric Psychiatry. 1999;14(12):1019–1030. [PubMed] [Google Scholar]
- Dittmann-Kohli F, Lachman ME, Kliegl R, Baltes P. Effects of cognitive training and testing on intellectual efficacy beliefs in elderly adults. Journal of Gerontology. 1991;46(4):162–164. doi: 10.1093/geronj/46.4.p162. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Hultsch DF, Hertzog C. The metamemory in adulthood (MIA) questionnaire. Psychopharmacology Bulletin. 1988;24(4):671–688. [PubMed] [Google Scholar]
- Duke LM, Seltzer B, Seltzer JE, Vasterling JJ. Cognitive components of deficit awareness in Alzheimer's disease. Neuropsychology. 2002;16(3):359–369. doi: 10.1037//0894-4105.16.3.359. [DOI] [PubMed] [Google Scholar]
- Federal Interagency Forum of Aging-Related Statistics. Older Americans 2000: Key indicators of well-being. Washington, DC: U.S. Government Printing Office; 2000. [Google Scholar]
- Gilewski MJ, Zelinski EM, Schaie KW. The memory functioning questionnaire for assessment of memory complaints in adulthood and old age. Psychology and Aging. 1990;5(4):482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- Grut M, Jorm AE, Fratiglioni L. Memory complaints of elderly people in a population survey: Variation according to dementia stage and depression. Journal American Geriatrics Society. 1993;41:1295–1300. doi: 10.1111/j.1532-5415.1993.tb06478.x. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Hultsch DF, Dixon RA. Evidence for the convergent validity of two self-report metamemory questionnaires. Developmental Psychology. 1989;25:687–700. [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF. Relationships between metamemory, memory predictions, and memory task performance in adults. Psychology and Aging. 1990a;5(2):215–227. doi: 10.1037//0882-7974.5.2.215. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Dixon RA, Hultsch DF. Metamemory in adulthood: Differentiating knowledge, belief, and behavior. In: Hess TH, editor. Aging and cognition: Knowledge organization and utilization. Amsterdam, North Holland: Elsevier Science Publishers B. V.; 1990b. pp. 161–212. [Google Scholar]
- Hill RD, Sheikh JI, Yesavage J. The effect of mnemonic training on perceived recall confidence in the elderly. Experimental Aging Research. 1988;13(4):185–188. doi: 10.1080/03610738708259323. [DOI] [PubMed] [Google Scholar]
- Hill RD, Vandervoort D. The effects of state anxiety on recall performance in older learners. Educational Gerontology. 1992;18:597–605. [Google Scholar]
- Himmelfarb S, Murrell SA. Reliability and validity of five mental health scales in older persons. Journal of Gerontology. 1983;38:333–339. doi: 10.1093/geronj/38.3.333. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, McDonald-Miszczak L, Dixon RA. Short-term longitudinal change in cognitive performance in later life. Psychology and Aging. 1992;7(4):571–584. doi: 10.1037//0882-7974.7.4.571. [DOI] [PubMed] [Google Scholar]
- Jonker C, Launer LJ, Hooijer C, Lindeboom J. Memory complaints and memory impairment in older individuals. Journal American Geriatrics Society. 1996;44:44–49. doi: 10.1111/j.1532-5415.1996.tb05636.x. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Korten AE, Mackinnon A, Scott R. Complaints of cognitive decline in the elderly: A comparison of reports by subjects and informants in a community survey. Psychological Medicine. 1994;24(2):365–374. doi: 10.1017/s0033291700027343. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Korten AE, Henderson AS, Jacomb PA, Mackinnon A. Do cognitive complaints either predict future cognitive decline or reflect past cognitive decline? A longitudinal study of an elderly community sample. Psychological Medicine. 1997;27(1):91–98. doi: 10.1017/s0033291796003923. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Leff R. Perceived control and intellectual functioning in the elderly: A 5-year longitudinal study. Developmental Psychology. 1989;25(5):722–728. [Google Scholar]
- Lachman ME, Steinberg ES, Trotter SD. Effects of control beliefs and attributions on memory self-assessments and performance. Psychology and Aging. 1987;2(3):266–271. doi: 10.1037//0882-7974.2.3.266. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Weaver SL, Bandura M, Elliott E, Lewkowicz CJ. Improving memory and control beliefs through cognitive restructuring and self-generated strategies. Journal of Gerontology: Psychological Sciences. 1992;47(5):P293–P299. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- Larkin M. Brain shrinkage more rapid in men than women. Lancet. 1998;351(9102):575. doi: 10.1016/S0140-6736(05)78568-8. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Crook TH., 3rd Do men show more rapid age-associated decline in simulated everyday verbal memory than do women? Psychology and Aging. 1993;8(1):68–71. doi: 10.1037//0882-7974.8.1.68. [DOI] [PubMed] [Google Scholar]
- Maibach E, Murphy DA. Self-efficacy in health promotion research and practice: Conceptualization and measurement. Health Education Research. 1995;10(1):37–50. [Google Scholar]
- McDougall GJ. Predictors of metamemory in older adults. Nursing Research. 1994;43(4):212–218. [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Gender differences in coping and control with memory aging. Journal of Women and Aging. 1998a;10(1):21–40. doi: 10.1300/j074v10n01_03. [DOI] [PubMed] [Google Scholar]
- McDougall GJ. Memory awareness in nursing home residents. Gerontology. 1998b;44:281–287. doi: 10.1159/000022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Memory improvement in assisted living elders. Issues in Mental Health Nursing. 2000;21(2):217–233. doi: 10.1080/016128400248202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Rehabilitation of memory and memory self-efficacy in cognitively impaired nursing home residents. Clinical Gerontologist. 2001;23(34):127–139. doi: 10.1300/J018v23n03_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ. Memory improvement in octogenarians. Applied Nursing Research. 2002;15(1):2–10. doi: 10.1053/apnr.2002.29518. [DOI] [PubMed] [Google Scholar]
- McDougall GJ. Memory self-efficacy, metamemory, and memory performance in African- and Caucasian-American elders under review. [Google Scholar]
- McDougall GJ, Balyer J. Decreasing mental frailty in at-risk elders. Geriatric Nursing. 1998;19(4):220–224. doi: 10.1016/s0197-4572(98)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall GJ, Holston EC, Wilke P. Recruiting African Americans into research on cognitive aging. Ethnicity and Disease. 2001;11:124–133. [PMC free article] [PubMed] [Google Scholar]
- Mozley CG, Huxley P, Sutcliffe C, Bagley H, Burns A, Challis D, et al. “Not knowing where I am doesn't mean I don't know what I like”: Cognitive impairment and quality of life responses in elderly people. International Journal of Geriatric Psychiatry. 1999;14:776–783. [PubMed] [Google Scholar]
- Palmer CG, Wolkenstein BH, LaRue A, Swan GE, Smalley SL. Commingling analysis of memory performance in elderly men. Genetic Epidemiology. 1994;11(5):443–449. doi: 10.1002/gepi.1370110506. [DOI] [PubMed] [Google Scholar]
- Pearson JL, Cherrier M, Teri L. The mini-mental state exam and the mental status questionnaire: Depression in Alzheimer's disease. Clinical Gerontologist. 1989;8(4):31–37. [Google Scholar]
- Perlmutter M. Metamemory. In: Maddox GL, editor. Encyclopedia of aging. 3rd. New York: Springer Publishing Company; 2001. pp. 684–686. [Google Scholar]
- Perrig-Chiello P, Perrig WJ, Stahelin HB. Differential aspects of memory self-evaluation in old and very old people. Aging & Mental Health. 2000;4(92):130–135. [Google Scholar]
- Peterson RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Archives Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Peterson RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Ponds RW, Commissaris KJ, Jolles J. Prevalence and covariates of subjective forgetfulness in a normal population in the Netherlands. International Journal of Aging and Human Development. 1997;45(3):207–221. doi: 10.2190/MVQ1-WB58-875H-Y4X0. [DOI] [PubMed] [Google Scholar]
- Ponds RWHM, Jolles J. Memory complaints in elderly people: The role of memory abilities, metamemory, depression, and personality. Educational Gerontology. 1996;22:341–357. [Google Scholar]
- Radloff LS, Teri L. Use of the center for epidemiological studies-depression scale with older adults. Clinical Gerontologist. 1986;5(12):119–136. [Google Scholar]
- Rebok GW, Balcerak LJ. Memory self-efficacy and performance differences in young and old adults: The effect of mnemonic training. Developmental Psychology. 1989;25(5):714–721. [Google Scholar]
- Rowe JW, Kahn RL. Human aging: Usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Schecter S, Herrmann D. The proper use of self-report questions in effective measurement of health outcomes. Evaluation & the Health Professions. 1997;20:28–46. doi: 10.1177/016327879702000103. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. American Journal Psychiatry. 1997;154(5):609–615. doi: 10.1176/ajp.154.5.609. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Rodin J, Albert M. Self-efficacy and cognitive performance in high-functioning older individuals. Journal of Aging and Health. 1993;5(4):455–476. [Google Scholar]
- Seeman T, McAvay G, Merrill S, Albert M, Rodin J. Self-efficacy beliefs and change in cognitive performance: MacArthur studies of successful aging. Psychology and Aging. 1996;11(3):538–551. doi: 10.1037//0882-7974.11.3.538. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: A comprehensive review. Journal of the American Geriatrics Society. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- van Balen HGG, Westzaan PSH, Mulder T. Stratified norms for the Rivermead Behavioural Memory Test. Neuropsychological Rehabilitation. 1996;6(3):203–217. [Google Scholar]
- Verhaeghen P, Geraerts MA, Marcoen A. Memory complaints, coping, and well-being in old age: A systematic approach. The Gerontologist. 2000;40(50):540–548. doi: 10.1093/geront/40.5.540. [DOI] [PubMed] [Google Scholar]
- Weaver SL, Lachman ME. Enhancing memory self-conceptions and strategies in young and old adults. In: Hill RD, Berry J, editors. What is memory training the training of?; Symposium conducted at the meeting of the American Psychological Association; New Orleans, LA. 1989. Aug, Chairs. [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. Titchfield: Thames Valley Test Company; 1985. [Google Scholar]
- Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. Journal of Clinical and Experimental Neuropsychology. 1989;11(6):855–870. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- Zanetti O, Vallotti B, Frisoni GB, Geroldi C, Baincheti A, Pasqualetti P, et al. Insight in dementia: When does it occur? Evidence for a nonlinear relationship between insight and cognitive status. Journal of Gerontology: Psychological Sciences. 1999;54(2):P100–P106. doi: 10.1093/geronb/54b.2.p100. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Anthony-Bergstone CR. The memory functioning questionnaire: Concurrent validity with memory performance and self-reported memory failures. Psychology and Aging. 1990;5(3):388–399. doi: 10.1037/0882-7974.5.3.388. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Schaie KW. Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging. 1993;8(2):176–186. doi: 10.1037//0882-7974.8.2.176. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Stewart ST. Individual differences in 16-year memory changes. Psychology and Aging. 1998;13(4):622–630. doi: 10.1037//0882-7974.13.4.622. [DOI] [PubMed] [Google Scholar]