Abstract
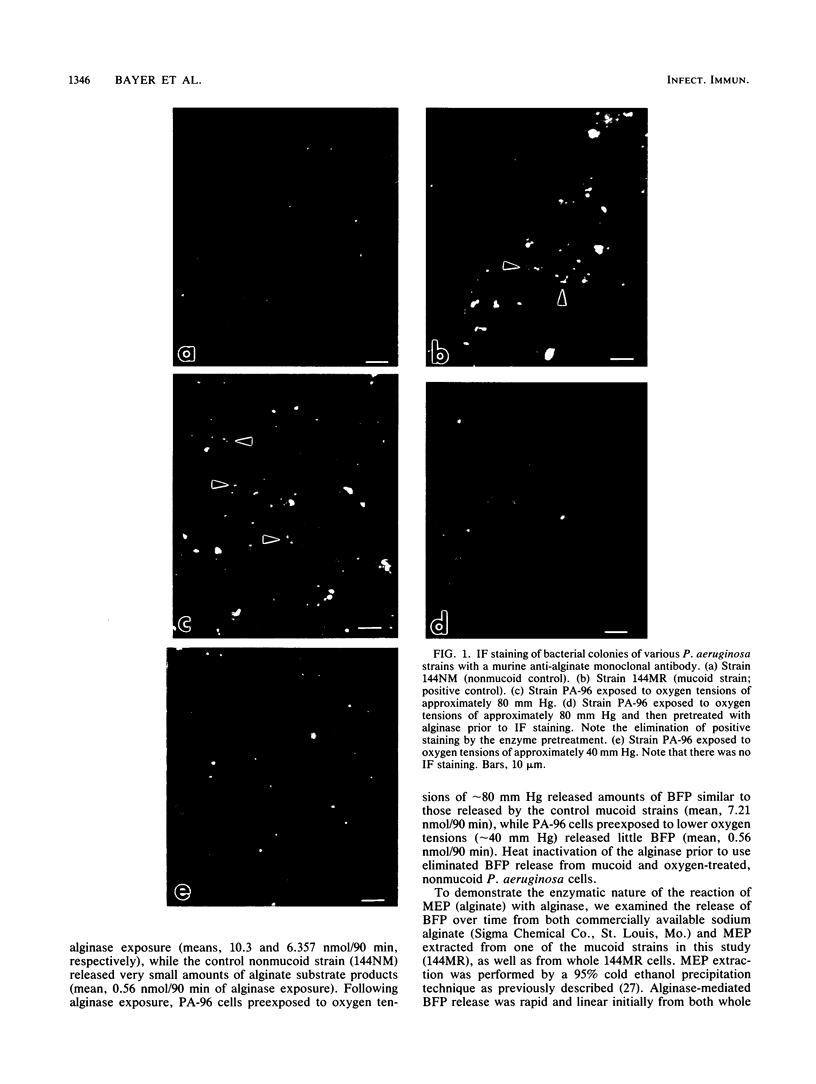
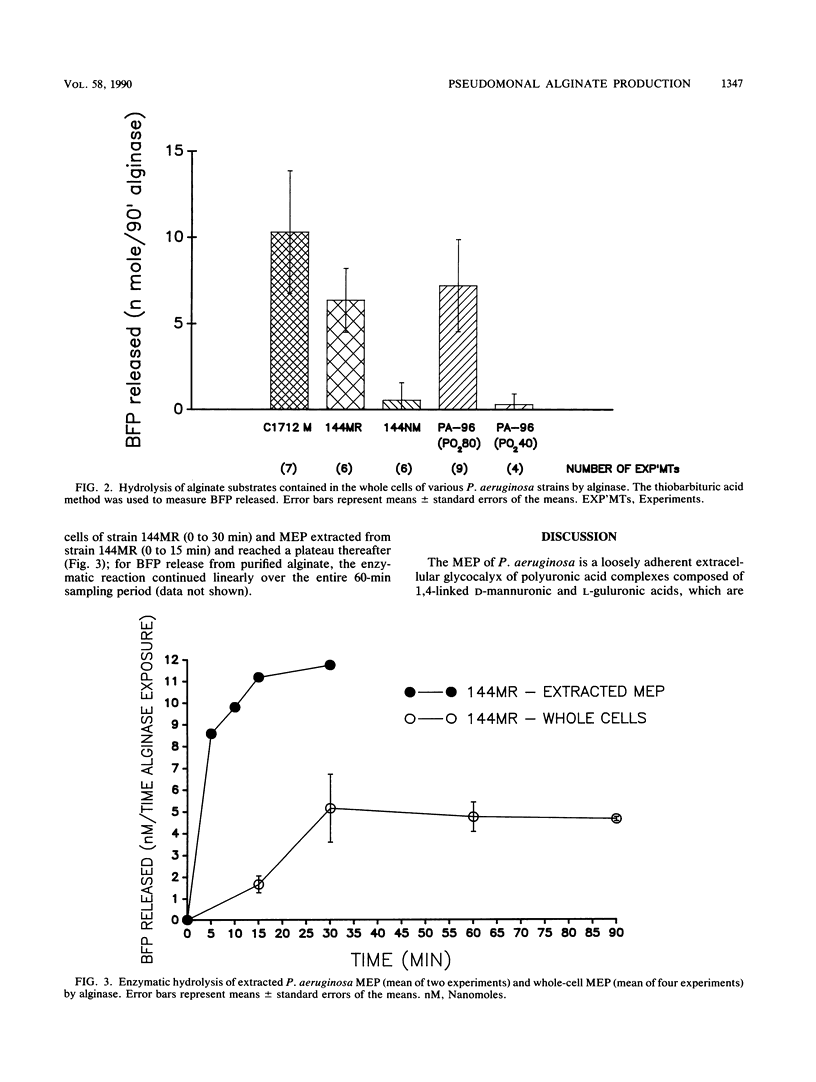
We previously showed substantial differences in Pseudomonas aeruginosa exopolysaccharide production in vitro at oxygen tensions reflective of the right versus left cardiac circuits in vivo (40 versus 80 mm Hg, respectively; A. S. Bayer, T. O'Brien, D. C. Norman, and C. C. Nast, J. Antimicrob. Chemother. 23:21-35, 1989). However, those studies did not specifically confirm this exopolysaccharide to be the characteristic P. aeruginosa mucoid alginate seen in patients with cystic fibrosis. With a murine monoclonal antibody prepared against P. aeruginosa alginate, strongly positive immunofluorescence (IF) staining of a nonmucoid P. aeruginosa strain (PA-96) was seen after its exposure in vitro to oxygen tensions (pO2) of approximately 80 mm Hg; the intensity of the IF staining under these conditions was similar to that observed with a phenotypically mucoid P. aeruginosa strain (C1712M) from a cystic fibrosis patient. In contrast, the same nonmucoid strain (PA-96), after exposure to pO2 of approximately 40 mm Hg, showed little IF staining for alginate. Following enzyme treatment with alginase, PA-96 cells previously exposed to the higher pO2 and exhibiting enhanced alginate production, as determined by IF staining, now showed no IF staining. Moreover, treatment of the oxygen-up-regulated PA-96 cells with alginase released amounts of unsaturated alginate breakdown products (uronic acids) quantitatively similar to those released by typically mucoid strains treated with the same enzyme. These data indicated that the P. aeruginosa exopolysaccharide in our studies was, indeed, mucoid alginate and that variations in oxygen tensions represent one of the trigger mechanisms for the up-regulation of mucoid exopolysaccharide production.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams B., Sklaver A., Hoffman T., Greenman R. Single or combination therapy of staphylococcal endocarditis in intravenous drug abusers. Ann Intern Med. 1979 May;90(5):789–791. doi: 10.7326/0003-4819-90-5-789. [DOI] [PubMed] [Google Scholar]
- Anastassiou E. D., Mintzas A. C., Kounavis C., Dimitracopoulos G. Alginate production by clinical nonmucoid Pseudomonas aeruginosa strains. J Clin Microbiol. 1987 Apr;25(4):656–659. doi: 10.1128/jcm.25.4.656-659.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar H., van Biesen T., Dasgupta M., Lam K., Costerton J. W. Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother. 1989 Oct;33(10):1824–1826. doi: 10.1128/aac.33.10.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer A. S., O'Brien T., Norman D. C., Nast C. C. Oxygen-dependent differences in exopolysaccharide production and aminoglycoside inhibitory-bactericidal interactions with Pseudomonas aeruginosa--implications for endocarditis. J Antimicrob Chemother. 1989 Jan;23(1):21–35. doi: 10.1093/jac/23.1.21. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Yih J., Chiu C. Y., Nast C. C. Pathogenic effects of monocytopenia, granulocytopenia and dexamethasone on the course of experimental Pseudomonas aeruginosa endocarditis in rabbits. Chemotherapy. 1989;35(4):278–288. doi: 10.1159/000238683. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L. Influence of nutrient media on the characteristics of the exopolysaccharide produced by three mucoid Pseudomonas aeruginosa strains. Microbios. 1984;41(163):49–63. [PubMed] [Google Scholar]
- Cabral D. A., Loh B. A., Speert D. P. Mucoid Pseudomonas aeruginosa resists nonopsonic phagocytosis by human neutrophils and macrophages. Pediatr Res. 1987 Oct;22(4):429–431. doi: 10.1203/00006450-198710000-00013. [DOI] [PubMed] [Google Scholar]
- Dall L., Barnes W. G., Lane J. W., Mills J. Enzymatic modification of glycocalyx in the treatment of experimental endocarditis due to viridans streptococci. J Infect Dis. 1987 Nov;156(5):736–740. doi: 10.1093/infdis/156.5.736. [DOI] [PubMed] [Google Scholar]
- Eftekhar F., Speert D. P. Alginase treatment of mucoid Pseudomonas aeruginosa enhances phagocytosis by human monocyte-derived macrophages. Infect Immun. 1988 Nov;56(11):2788–2793. doi: 10.1128/iai.56.11.2788-2793.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. R., Linker A. Production and characterization of the slime polysaccharide of Pseudomonas aeruginosa. J Bacteriol. 1973 Nov;116(2):915–924. doi: 10.1128/jb.116.2.915-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Ohman D. E. Cloning of genes from mucoid Pseudomonas aeruginosa which control spontaneous conversion to the alginate production phenotype. J Bacteriol. 1988 Apr;170(4):1452–1460. doi: 10.1128/jb.170.4.1452-1460.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Construction and characterization of Pseudomonas aeruginosa algB mutants: role of algB in high-level production of alginate. J Bacteriol. 1987 Apr;169(4):1593–1602. doi: 10.1128/jb.169.4.1593-1602.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Bass J. A., Mattingly S. J. Aeration selects for mucoid phenotype of Pseudomonas aeruginosa. J Clin Microbiol. 1986 Dec;24(6):986–990. doi: 10.1128/jcm.24.6.986-990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Learn D. B., Brestel E. P., Seetharama S. Hypochlorite scavenging by Pseudomonas aeruginosa alginate. Infect Immun. 1987 Aug;55(8):1813–1818. doi: 10.1128/iai.55.8.1813-1818.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Wolff S. M., Puziss M. Summary of a workshop on infections in patients with cystic fibrosis. J Infect Dis. 1979 Aug;140(2):252–256. doi: 10.1093/infdis/140.2.252. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Desjardins D., Aguilar T., Barnard M., Speert D. P. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J Clin Microbiol. 1986 Aug;24(2):189–196. doi: 10.1128/jcm.24.2.189-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Saunders J. M., Ames P., Edwards M. S., Auerbach H., Goldfarb J., Speert D. P., Hurwitch S. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. N Engl J Med. 1987 Sep 24;317(13):793–798. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Pier G. B. Role of Pseudomonas aeruginosa mucoid exopolysaccharide in adherence to tracheal cells. Infect Immun. 1985 Jan;47(1):1–4. doi: 10.1128/iai.47.1.1-4.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Current problems in the treatment of infective endocarditis due to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):314–321. doi: 10.1093/clinids/5.2.314. [DOI] [PubMed] [Google Scholar]
- Sherbrock-Cox V., Russell N. J., Gacesa P. The purification and chemical characterisation of the alginate present in extracellular material produced by mucoid strains of Pseudomonas aeruginosa. Carbohydr Res. 1984 Dec 15;135(1):147–154. doi: 10.1016/0008-6215(84)85012-0. [DOI] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. The penetration of antibiotics through sodium alginate and through the exopolysaccharide of a mucoid strain of Pseudomonas aeruginosa. Lancet. 1981 Sep 5;2(8245):502–503. doi: 10.1016/s0140-6736(81)90885-0. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Farmer S. W., Campbell M. E., Musser J. M., Selander R. K., Kuo S. Conversion of Pseudomonas aeruginosa to the phenotype characteristic of strains from patients with cystic fibrosis. J Clin Microbiol. 1990 Feb;28(2):188–194. doi: 10.1128/jcm.28.2.188-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]





