Abstract
The ability to quantify ultra-low concentrations of biologically active compounds in biological matrices is essential for the study of pharmacological/toxicological effects occurring at low doses. Selective solid-phase extraction (SPE) was combined with highly sensitive capillary LC (μLC)-MS/MS analysis to achieve ultra-sensitive quantification of the anticancer drug paclitaxel in cancer cells. The optimized SPE selectively extracted paclitaxel and eliminated undesirable matrix compounds, thus enabling a high sample loading volume on the μLC column without compromising chromatographic performance and operational robustness. The validated lower limit of quantification (LOQ) was 5 pg/mL, approx. 20-fold more sensitive than published LC-MS/MS methods. The calibration curve was linear over the range of 5–6250 pg/mL. Accuracy was 98–109% and the variation (CV%) was 2.3–7.4%. This method was applied successfully to quantify temporal drug accumulation by A121a ovarian cancer cells treated with sub-ng/mL concentrations of paclitaxel.
Keywords: selective SPE, capillary LC, LC-MS/MS, sensitive quantification, paclitaxel, docetaxel
1. Introduction
The capability for ultra-sensitive quantification of small-molecule analytes in complex biological matrices such as cells, plasma, or tissues is essential in a wide range of environmental, toxicological, and pharmacological applications. For example, development of new pharmaceutical agents or therapies may require quantification of drugs or their metabolites in the low pg/mL range. Drug delivery strategies such as inhalation or intraocular injection [1] may result in long-term sustained systemic drug concentrations that are too low to be detected by current methods [1–5], but which must be quantified for therapeutic, regulatory, or safety reasons. Alternatively, administration of prodrugs such as corticosteroid acetates or propionates, can result in sustained production of low concentrations of the active agent in plasma [6,7] which may require measurement. Finally, recent studies show that sustained, ultra-low (sub-nM) concentrations of certain anti-cancer drugs may exert pharmacological actions that may be beneficial or antagonistic to the desired therapeutic effects , and therefore the capability of quantifying these drugs at low, biologically active concentrations is essential to understand pharmacological mechanisms of action and develop effective therapies.
Among anti-cancer agents that show pharmacological actions at low concentrations, paclitaxel is one of the most important clinically. It has been used widely in the treatment of human solid tumors such as ovarian, breast, and non-small cell lung cancers. Conventional high-dose therapeutic regimens of paclitaxel (e.g. 135–175 mg/m2/day) exert anticancer activity through anti-mitotic or cytotoxic effects, but can also result in severe side effects and acquired drug resistance. Numerous in vitro or preclinical experiments suggest that paclitaxel and other drugs can exert anti-vascular or anti-angiogenic effects, and some of these effects are observed at ultra-low concentrations. In vivo, low-dose paclitaxel regimens employing more frequent administration have shown activity in cancer patients without significant side effects such as myelosuppression or acquired drug resistance. Interestingly, recent studies have shown that paclitaxel can exert anti-angiogenic effects in the range of 0.1–100 pM, which is approx. 5000-fold lower than the concentrations required for anti-mitotic effects. In order to study the anti-angiogenic mechanisms or pharmacokinetics (PK) of low-dose paclitaxel, highly sensitive methods are necessary that can quantify low pg/mL concentrations of drug in complex biological matrices.
The lower limits of quantification (LOQ) of paclitaxel using HPLC-UV are reported as 5–20 ng/mL {Alexander, 2003 #52}, and for conventional LC-MS/MS are in the range of 0.1–0.25 ng/ml for biological matrices. Recent preliminary work suggests the feasibility of quantification of paclitaxel in cell lysates at concentrations as low as 20 pg/mL. However, no published method provides sufficient sensitivity and operational robustness to quantify paclitaxel routinely in the low pg/mL range, which preliminary experiments suggest will be necessary to achieve important experimental objectives.
The purpose of this work was to develop an enabling analytical approach for ultra-sensitive and selective quantification of paclitaxel in cancer cells following exposure to ultra-low but pharmacologically relevant drug concentrations. This task is challenging because the drug is expected to be present in cell lysates at low-to-medium pg/mL levels. Cell extracts represent a highly complex biological matrix, which necessitates exceptional analytical sensitivity and selectivity. Recently we developed a novel analytical strategy for ultra-sensitive quantification of corticosteroids in animal plasma samples [1]. Because electrospray ionization (ESI)-MS/MS represents a concentration-dependent detector, we employed micro-flow capillary LC (μLC), which provides much lower sample dilution than does conventional LC, and achieved high mass sensitivity for corticosteroids. One shortcoming of μLC, however, is that the loading capacity is small, which tends to counteract the sensitivity gain. Also, the increased concentration of samples is especially problematic when the biological matrices are complex. To enable loading of a relatively large injection volume on μLC columns, we devised a solid-phase extraction (SPE) procedure that selectively concentrated the corticosteroid, while removing most matrix components. This method has achieved sub-pg/mL detection limits for several corticosteroids in plasma samples [1], and in a preliminary study, we were able to improve the limit of quantification of paclitaxel to the sub-nanomolar range [24]. Here we employ a similar rationale to develop a robust approach for the ultra-sensitive quantification of paclitaxel in tumor cells. The strategy was to optimize each component of the analytical flow path: (i) the SPE extraction and elution procedure for selectivity and efficiency, (ii) the μLC loading and separation method, and (iii) the ionization and selected reaction monitoring (SRM) method for MS/MS. We also evaluated the performance of this analytical approach when employing high-resolution selected reaction monitoring (HR-SRM), a comparatively new technique that is intended to improve analytical sensitivity.
2. Experimental
2.1. Chemicals and cell lines
Paclitaxel was obtained as a gift from Phytogen Life Sciences (Vancouver, Canada). Docetaxel, the internal standard (I.S.), was obtained as a gift from Rhone Poulenc (Paris, France). HPLC-grade methanol, acetonitrile, and water were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Phosphoric acid (85%), and formic acid were from Sigma-Aldrich (St. Louis, MO, USA). Oasis HLB solid-phase extraction cartridges (30 μm particle size and 60 mg sorbent) were obtained from Waters (Milford, MA, USA). A121a human ovarian cancer cells were from Roswell Park Cancer Institute (Buffalo, NY, USA) [25], and were cultured in RPMI 1640 cell culture medium supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, USA).
2.2. Optimization of SPE wash and elution conditions
In order to extract paclitaxel and docetaxel (the I.S.) selectively from cell lysates, while simultaneously reducing the quantity of matrix components that were also derived from the samples, the SPE wash and elution conditions were optimized by the following procedure. Oasis HLB cartridges (Waters) were conditioned with 5 mL of methanol and then equilibrated with 5 mL of water. Paclitaxel was spiked into 1.8-mL aliquots of A121a cell lysates (cell density 0.9–1.3 ×106 cells/mL) to a final concentration of 2 ng/mL. Phosphoric acid was added to a final concentration of 3% in order to dissociate protein-bound drug [1,26], and then samples were loaded batch-wise onto 17 SPE cartridges at approx. 2–3 mL/min. After loading, all columns were washed with 5 mL 3% phosphoric acid.
After sample loading and the initial washing, the cartridges were divided into two groups. The first group was devoted to the optimization of washing conditions that would remove more polar sample matrix components without eluting the analyte (“Strong-wash” step). The second group was devoted to optimization of conditions that would elute the target analyte while minimizing the elution of less polar sample matrix components (“Elution” step).
For group 1, each analyte-loaded cartridge was washed with 5 mL of one of the following concentrations of methanol: 55%, 60%, 65%, 70%, 75%, 80%, 85%, or 90% (v/v in water). After washing, the absorbed analyte was then eluted from all cartridges with 5 mL of methanol containing 0.1% formic acid. Docetaxel (the I.S.) was then added to each eluate to a final concentration of 300 pg/mL.
For group 2, all analyte-loaded cartridges were washed with 5 mL of 5% methanol, and the absorbed analytes were eluted with 5 mL of 0.1% formic acid in one of the following concentrations of methanol: 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or 100% (v/v in water). The I.S. was then added to each eluate to a final concentration of 300 pg/mL.
The eluates from both groups of samples were evaporated and reconstituted as described below (Section 2.3). This optimization procedure was performed in triplicate, and the recovery of paclitaxel in the samples was determined by μLC-MS/MS using a Vinj of 0.5 μL.
2.3. Cell sample preparation
A121a ovarian cancer cells [25] were grown in 25 cm2 (T-25) flasks and were used for method development or drug uptake studies when 90% confluence was reached. To harvest cells for analysis, the cell culture medium was removed and the cell monolayer was washed rapidly with 3 mL of phosphate buffered saline (PBS) containing 5% FBS, then with 3 mL PBS, and then incubated in 0.5 mL of 0.05% trypsin containing 1 mM ethylenediamine tetraacetate (EDTA). After 3–4 minutes of incubation at 37°C, the dissociated cells were collected into a tube. The tissue culture flask was washed with an additional 1.5 mL PBS, which was combined with the cell suspension. From the 2 mL of cell suspension that was recovered, a volume of 0.2 mL was removed and the cells were counted using a Coulter Z2 Counter (Hialeah, FL, USA). Typical cell densities of the suspension were in the range of 0.9–1.3 ×106/ml. Fifty % methanol (v/v in water) was added to the remaining 1.8 mL of cell suspension to achieve a final volume of 5 mL. The samples were then snap frozen in liquid nitrogen and thawed in a 37°C water bath. After a second rapid freeze-thaw cycle, the samples were stored at −80°C until analysis.
For analysis, the frozen samples were thawed and phosphoric acid was added to a final concentration of 3%. Samples were spiked with the I.S. to a final concentration of 300 pg/mL and vortexed for 30 seconds. SPE cartridges, washed and conditioned as described above, were loaded with cell lysates, and then washed first with 5 mL of 35% methanol containing 3% phosphoric acid (“Weak-wash” step), and next with 5 mL of 70% methanol, which was determined to be the optimal Strong-Wash solvent composition in experiments described above. Following this second wash, the analyte was eluted with 5 mL of 97.5% methanol containing 0.1% formic acid, which was identified (Section 2.2, above) as the optimized elution solvent. The SPE eluates were evaporated to dryness under a gentle nitrogen stream at 45°C, washed with 1 mL of methanol, and dried again under the same conditions. The samples were finally reconstituted with 100 μL of 35% methanol, transferred into fresh 1.5 ml polypropylene centrifuge tubes, and centrifuged at 10,000g for 20 minutes to eliminate particulates. The supernatant was then analyzed by μLC-MS/MS.
2.4 μLC-MS/MS
The samples were injected via a Spark Holland Endurance micro-scale autosampler (Emmen, The Netherlands). Analysis by μLC-MS/MS was performed on an Eksigent direct-flow capillary/nano LC system (Dublin, CA, USA) coupled to a Thermo Scientific Quantum Ultra EMR triple-quadrupole mass spectrometer via an ESI interface (San Jose, CA, USA). The Eksigent LC system included two micro-flow pumps powered by pressurized nitrogen (100 p.s.i.= 689,476 Pa) with a fast-response active flow-rate control system. A double transport liquid wash with mobile phases A and B (sequentially) was employed to eliminate carryover by the autosampler. The analytical column was a Zorbax C18 Stablebond capillary column (150 mm × 0.5 mm I.D.) having a particle size of 3.5 μm and a pore size of 100 Å (Agilent, Santa Clara, CA, USA). The injection volume was 8 μL unless specified otherwise. The μLC flow rate was 10 μL/min, and the mobile phases consisted of (A) 10:90 acetonitrile:water containing 2 mM ammonium acetate adjusted to pH 3.2 with formic acid, and (B) 90:10 acetonitrile:water containing 2 mM ammonium acetate. The percentage of mobile phase B was held at 25% for the first three minutes, increased to 85% over 12 minutes, and then increased to 98% over two minutes. To obtain optimal microspray performance, a 34-gauge microbore ESI stainless steel spray needle having a recommended flow range of 0.5–20 μL/min was used. The ionization voltage, skimmer offset, and capillary transduction tube temperature were set at 3.2 kv, 12 v, and 200°C, respectively. The pressure of the N2 sheath gas was set at 15 (arbitrary units) and the argon collision gas pressure was set at 1.5 mTorr (0.2 Pa). SRM conditions for paclitaxel and the I.S., including m/z of SRM pairs, collision energy, spray voltage, and tube lens voltage, were optimized by direct infusion of the compounds into the MS instrument at a concentration of 5 μg/mL. The resulting optimized conditions are shown in Table 1. The dwell time of each SRM transition was 300 ms. The Q1 and Q3 resolutions were 0.7 mass units FWHM (full width at half maximum). For HR-SRM, the conditions were the same as for SRM, except that the resolution for Q1 was set at 0.2 mass units FWHM.
Table 1.
The major fragments, MRM transitions, and optimal collision energies of paclitaxel and docetaxel
| Compound | Major fragments /relative abundance (%) | MRM transition (precursor/product) | Optimal collision energy (eV) |
|---|---|---|---|
| Paclitaxel | 509/100, 569/80, 776/35, 239/10 | 854/569 | 9 |
| Docetaxel | 526/100, 509/58, 708/10, 689/8 | 808/526 | 10 |
2.5 Calibration and method validation
Calibration standards were prepared by spiking the appropriate quantity of paclitaxel into 1.8 mL of pooled blank A121a cell lysate to final concentrations of 5, 10, 50, 250, 1250, and 6250 pg/mL. Docetaxel (the I.S.) was spiked to a final concentration of 300 pg/mL. For method validation, quality control (QC) samples were prepared in cell lysates of the same volume spiked to final concentrations of 30, 250 and 3000 pg/ml paclitaxel and the final concentration of the I.S. was 300 pg/mL.
Data processing was carried out using the Quan Brower module of the Xcalibur program (Thermo Scientific, San Jose, CA, USA). The peak area ratios of analyte: I.S. extracting ion current (XIC) were used for the construction of calibration curves. Weighted least-squares linear regression analysis was used for both construction of the calibration curve and sample quantification.
For method validation, accuracies were obtained by comparing concentrations calculated from the standard curve against the nominal concentrations at the three QC levels. Precision of the assay was determined by repeated analysis of spiked cell samples, and the relative standard deviations (RSD%) of the replicate measurements was calculated to determine intra-day and inter-day variability. For intra-day validation, each QC sample was evaluated three times in a day; for inter-day validation, each QC sample was measured on 3 days (Monday, Tuesday and Wednesday) in a week, once per day.
2.6 Determination of paclitaxel uptake by A121a cells at ultra-low drug concentrations
As proof-of-concept for applying this approach for ultra-sensitive quantification of paclitaxel in complex biological matrices, uptake of paclitaxel by cancer cells exposed to ultra-low drug concentrations was evaluated. A121a human ovarian cancer cells were grown to 90% confluence in T-25 flasks. At the time of treatment, the cell culture medium was removed and replaced with fresh medium containing paclitaxel at one of the following concentrations: 0.2, 0.8, 2 and 5 ng/mL. The incubation was continued at 37°C for various time intervals (10, 30, 60, 180, and 360 min), and then the cells were harvested as described above (Section 2.3), extracted using the selective SPE procedure (Section 2.2), and analyzed by μLC-MS/MS. Each time point consisted of triplicate samples.
3. Results and Discussion
Although conventional LC-MS/MS analysis has achieved LOQs of 100–250 pg/mL for paclitaxel [16–23], preliminary experiments suggested that a LOQ far below this range was necessary to investigate pharmacological responses to ultra-low (sub-nM) drug concentrations. To achieve higher-sensitivity quantification, low-flow μLC was selected as a means to increase peak concentrations for ESI/MS. However, the loading capacity of μLC columns is correspondingly lower because of the reduced mass of stationary phase [27], and this factor limits the overall analytical sensitivity that can be achieved. The most practical approach for improving sensitivity is therefore to increase the injection volume (Vinj). However, for highly complex biological samples, numerous matrix compounds may be retrieved by standard sample preparation procedures such as protein precipitation or generic SPE. These co-extracted compounds can readily exceed the μLC column capacity unless a very low Vinj is used, as was observed in preliminary studies with the analyte and the types of samples of interest here.
Previously we demonstrated that application of a selective SPE procedure, which extracts the target analytes selectively from complex biological samples while simultaneously removing undesirable compounds to the extent possible, enabled ultra-sensitive quantification of corticosteroids in whole blood [1]. Based on this strategy, we set out to develop a selective SPE approach to concentrate paclitaxel from cellular samples, thereby enabling a relatively large Vinj on the capillary column and increasing sensitivity.
3.1. Optimization of selective solid-phase extraction
Optimization of the SPE procedure was carried out to meet three objectives. First, the overall conditions selected would provide the highest and most consistent absolute recovery of the target analyte. Second, the conditions for washing the SPE cartridges containing bound analyte would remove unwanted matrix components to the greatest extent possible without eluting the target analyte or I.S.. Third, elution conditions would subsequently recover the analyte efficiently while minimizing the elution of less polar matrix components.
Oasis HLB SPE cartridges were selected after evaluation of the analyte recovery and reproducibility of cartridges produced by several manufacturers. To enable calculation of the absolute recovery of paclitaxel through the SPE procedure, cell lysates were spiked with known concentrations of paclitaxel, and the I.S. was added to the final eluate. Because of the relatively high protein binding of paclitaxel and the structurally-similar I.S., phosphoric acid was added to the cell lysates to a final concentration of 3% (v/v) to disrupt drug/protein binding before loading onto SPE cartridges [28,29].
After the SPE cartridges were loaded with analyte-spiked cell lysates, they were washed in two steps. First, 3% phosphoric acid in 35% methanol (“Weak-wash” step) was used to remove residual cellular proteins and thus avoid precipitation and column blocking that could occur with subsequent washes that would employ higher-organic solvent conditions. Second, a higher concentration of methanol, identified through detailed optimization, was used to elute relatively polar matrix components without loss of the analyte (“Strong-wash” step). Finally, the target analyte was eluted quantitatively using a solvent composition optimized to recover it efficiently while leaving more hydrophobic matrix components on the cartridge (Experimental Section 2.2).
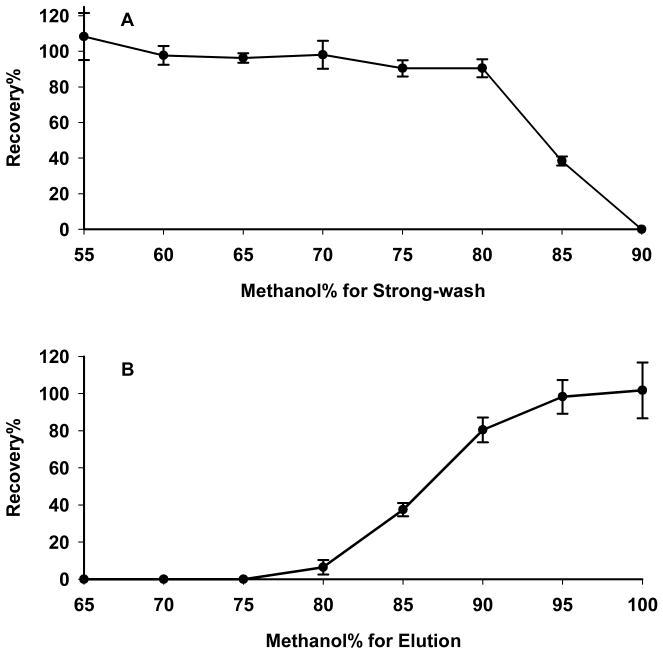
Absolute recovery of analyte through the SPE procedure was investigated using (i) Strong-wash solvent compositions ranging from 55–95% methanol and 100% methanol for elution, and (ii) Stong-wash solvent of 35% methanol and elution solvent compositions of 60%–100% methanol. The results are shown in Fig. 1. In preliminary investigations [24], it was found that complete elution of paclitaxel from the Oasis cartridge was difficult even with 100% methanol. Here, after evaluating a number of mobile phase modifiers in the present study, it was found that 0.1% formic acid in the elution solvent resulted in satisfactory elution of paclitaxel when ≥95% methanol was used (Fig. 1B), perhaps by increasing the polarity of paclitaxel in solution. Based on the results, 75% methanol was selected as optimal for Wash-2, and 0.1% formic acid in 97.5% methanol was selected as optimal for the elution step.
Figure 1. Optimization of solvent composition for maximizing absolute recovery of paclitaxel after SPE extraction.
Solvents used in the Elution step were composed of 0.1% formic acid in the indicated solvent composition. (A) Optimization of the Strong-wash solvent: triplicate SPE cartridges were washed with a range of 55–95% methanol; drug was eluted from all cartridges with 100% methanol; (B) optimization of the Elution solvent: triplicate SPE cartridges were washed with 5% methanol and eluted with methanol concentrations ranging from 60–100%.
We also investigated the effect of the composition of the wash and elution solvents upon the recovery of docetaxel, the internal standard, during the SPE procedure. Docetaxel is the second taxane approved for clinical use, and is an important anticancer agent in its own right. Using paclitaxel as the I.S. for these studies, we found that the optimal SPE conditions for docetaxel were nearly identical to those for paclitaxel (data not shown). This finding indicates not only that both the analyte (paclitaxel) and the I.S. (docetaxel) are extracted efficiently under the wash and elution conditions selected, thus ensuring quantitative accuracy, but also that a similarly-designed strategy for ultra-sensitive quantification of docetaxel is feasible with little modification of the procedure described here.
3.2 μLC-MS/MS Optimization
3.2.1. Optimization of SRM conditions
The MS and MS/MS behavior of paclitaxel and docetaxel under positive ion ESI was investigated by direct infusion of standard solutions into the mass spectrometer. In preliminary experiments, it was observed that both taxanes in methanol/water formed strong [M + Na]+ and [M + K]+ ions along with relatively weaker [M + H]+ (spectra not shown). However, [M + H]+ ions predominated when 0.1% formic acid was added into the solution. Further study showed that an acidic 2 mM ammonium acetate/water/acetonitrile buffer, adjusted to pH 3.2 with formic acid, increased the signal-to-noise-ratio (S/N) of [M + H]+ further and suppressed the Na+ and K+ adducted ions significantly (data not shown). This solvent system therefore was selected for both MS/MS optimization and LC separation.
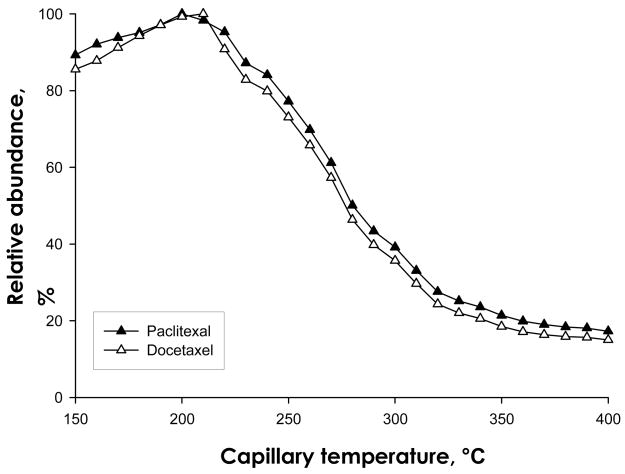
We observed that both paclitaxel and docetaxel appear to undergo thermal degradation during the desolvation and/or ion transmission process. The temperature of the ion transduction tube, which is a metal capillary located before the tube lens/skimmer, seldom is a critical parameter for analysis of most compounds. In our experience, a capillary temperature within the range of 270–320°C (in accordance with the manufacturer’s recommendation) usually provides the optimal ion intensity at LC flow rates of 50 μL/min or lower, and modification of capillary temperatures by +/−50°C does not affect ion intensity appreciably. However, we observed that as the capillary temperature was decreased from 320°C, the ion intensity for both paclitaxel and docetaxel increased significantly. Therefore, ionization intensity was examined systematically as a function of capillary temperature. We observed that the ion intensity increased more than 2.5-fold for both drugs when the capillary temperature was decreased from 300°C to 200°C (Fig. 2). Below 200°C, the ion intensity decreased, probably as a result of insufficient desolvation at lower temperatures. As a result of these investigations, a capillary temperature of 200°C was selected as optimal.
Figure 2. Intensity of protonated paclitaxel and docetaxel as a function of capillary temperature.
Various capillary temperatures were investigated for the effect upon the intensity of the protonated paclitaxel and docetaxel ion. The optimal temperature was determined to be 200 °C.
Selection of the paclitaxel ion for quantification was also investigated. The collisionally activated dissociation (CAD) spectra for paclitaxel and the I.S. were investigated individually, and the major fragments are listed in Table 1. Among the most intense product ions, the transitions of 854/569 and 808/526, which correspond to the taxane nucleus obtained after cleavage of the side chain of paclitaxel and docetaxel, respectively, were selected for MRM analysis. MS/MS conditions for each transition were then optimized in order to achieve the maximum S/N for all compounds. The optimized collision energies for the MRM transitions are shown in Table 1.
3.2.2. μLC optimization
The development of the chromatographic separation conditions was approached in terms of three inter-related variables: the column, the chromatographic separation strategy, and the injection volume. The use of smaller inner-diameter (I.D.) columns and lower flow rates can improve the sensitivity of ESI-MS/MS considerably [30]. However, loading capacities are proportionally lower, and analysis can be less robust compared with larger I.D. columns [31]. After balancing these considerations, we selected a 0.5 mm I.D. μLC column.
In terms of the LC separation strategy, it would have been possible to employ a minimal μLC separation procedure to enable higher throughput. However, sufficient μLC resolution was desirable for two reasons. First, when minimal separation was employed, a severe ion suppression effect that originated with the sample matrix was observed, using an approach we established previously [32] (data not shown). This suppression effect severely compromised sensitivity, but could be eliminated by increasing μLC separation. Second, it was found that the formation of both [M+Na]+ and [M+K]+ ions was relatively high when minimal μLC separation was used, even with the addition of acidified 2 mM ammonium acetate/formate as a mobile phase modifier. However, adequate chromatographic separation reduced these undesirable adduct ions and enhanced the formation of [M+H]+. The most likely explanation of this observation is that K+ and Na+ salts do not co-elute with the target compounds when sufficient chromatographic separation is employed.
The selective SPE procedure reduced matrix components in the cell lysate samples and therefore permitted considerable concentration of the SPE eluate. Nonetheless, the matrix components that co-elute with the analyte during SPE limit the degree to which the eluate can be concentrated for μLC analysis. Large injection volumes can contribute significantly to the sensitivity of quantification, but may cause peak broadening as a result of both long sample injection times and the large volume of sample solvent. Furthermore, the smaller column volume selected here requires that Vinj be held to considerably smaller volumes in order to avoid column overcapacity and fouling [27]. To address these issues, we developed an approach that would enable a high Vinj of the selectively extracted samples without causing peak broadening and column fouling. A two-segment gradient was designed, consisting of a focusing segment and a separation segment. The following theoretical rationale suggests the use of a low-organic mobile phase to load and focus a large Vinj on the column. The maximum acceptable injection volume (Vmax) of an LC system can be expressed as follows [33]:
where θ is the fractional loss of the column plate number caused by the injection, K is a constant describing the injection profile, ε is the column porosity, dc is the column inner diameter, L is the column length, k is the retention factor and N is the theoretical plate number. For a given set of chromatographic conditions and column, θ, K, ε, L, dc and N can be considered roughly as constants. As a result, Vmax is approximately proportional to the retention factor k. Therefore, to maximize Vmax, low-organic mobile phases, which would provide a high k value for both paclitaxel and its I.S., would focus the analytes on the column face during the sample loading process.
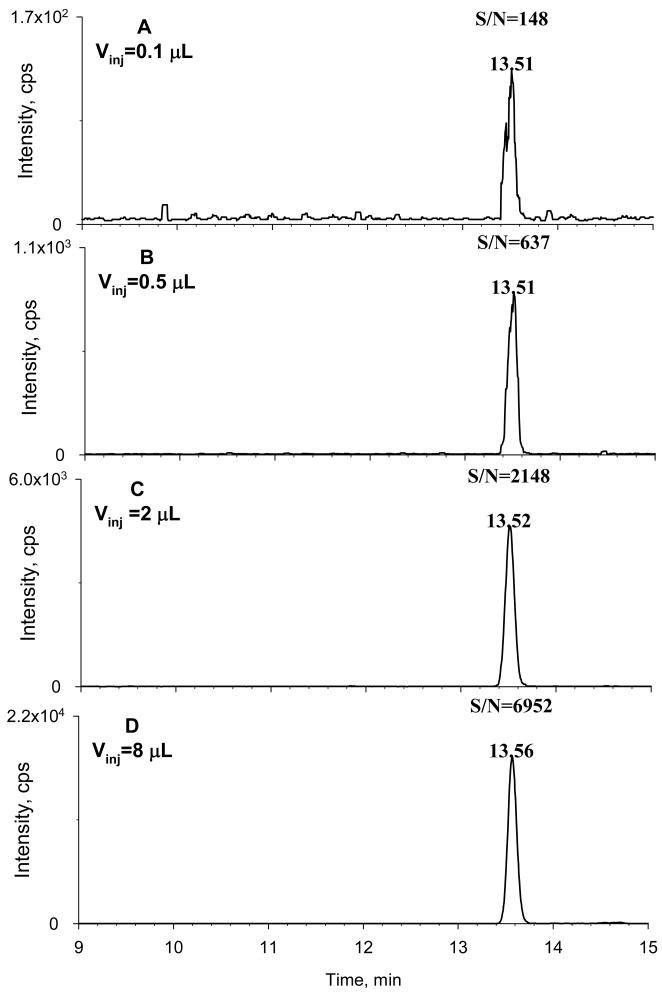
The manufacturer-recommended Vinj for the 0.5 mm I.D. column used here is 0.2–0.4 μL. However, we hypothesized that the complexity of cell lysates would be reduced considerably by the selective SPE approach, and therefore a considerably higher Vinj could be tolerated without compromising chromatographic separation, provided the sample focusing strategy was also employed. To test this hypothesis, we evaluated μLC performance as a function of Vinj using cell lysates spiked with the analyte. Typical chromatograms are shown in Fig. 3. The retention times of the target compounds increased only slightly as Vinj was increased, and peak broadening or peak shape deterioration was not observed over the range of Vinj from 0.2 to 8 μL. Furthermore, both the intensity and S/N for paclitaxel increased roughly in proportion with Vinj. This comfirmed that the selective SPE procedure, in conjunction with the sample focusing strategy, permits a high Vinj on the μLC column without causing column overcapacity. A Vinj of 8 μL was selected for quantification of paclitaxel in cell lysates, which is 20–40-fold higher than the manufacturer’s recommendation. Column durability was not compromised; after approximately 300 injections of 8 μL of cell lysate samples on the μLC column, the separation of the target analytes remained highly reproducible, with neither loss of resolution nor increase in backpressure.
Figure 3. Optimization of sample injection volume.
Various injection volumes (Vinj) of spiked cell lysate samples were investigated to optimize Vinj. Lysates were spiked with 250 pg/mL of paclitaxel and extracted by selective SPE. The Vinj was (A) 0.1 μL; (B) 0.5 μL; (C) 2.0 μL; (D) 8.0 μL. A 15 cm × 0.5 mm I.D. capillary column was used for separation; the manufacturer-recommended Vinj for the column was 0.1–0.2 μL. Selective SPE and a two-segment focusing and gradient separation procedure enabled a large Vinj on the capillary column without deterioration of chromatographic performance, which provided a significantly higher sensitivity than the manufacturer-recommended Vinj.
The subsequent gradient steps constitute the second (‘separation’) segment of the chromatographic strategy, in which a gradually increasing percentage of mobile phase B elutes the compounds that were focused on the column during the initial gradient step. To determine the optimal separation conditions, we investigated chromatographic retention vs. S/N for cell lysate samples spiked with 250 pg/mL of paclitaxel and I.S.. The S/N of the target analytes improved considerably when their chromatographic retention increased, but beyond a certain point, the S/N approached a maximum (data not shown). This behavior was observed previously when a conceptually similar chromatographic strategy was used for quantification of corticosteroids in plasma [1]. As summarized in the Experimental section, the μLC separation conditions that utilized the shortest run time but achieved the maximum S/N for paclitaxel were chosen, based on these investigations.
3.3 Ultra-sensitive quantification of paclitaxel in cell lysates
The specificity of the developed method was evaluated with blank cell lysates spike with I.S.. No interfering peaks were detected at the retention time of paclitaxel. Calibration curves for paclitaxel spiked into cell lysates showed good linearity over the concentration range of 5–6250 pg/mL (r2=0.992). Method validation was carried out as described in the Experimental section, and the results are reported in Table 2. Intra-day accuracies for paclitaxel quantification were 103–109%, and the inter-day accuracies were 98–105%. The intra- and inter- day variations, expressed as relative standard deviations (RSD %), were in the range of 2.3–5.0% and 3.2–6.5%, respectively. The stability of the drug in extracted samples was good, with minimal loss upon storage of the final extract at −20°C for up to three weeks.
Table 2.
a Precision and accuracy for μLC/MS/MS quantification of paclitaxel in cell lysates
| Intra-day
|
Inter-day
|
|||||
|---|---|---|---|---|---|---|
| TCb, pg/ml | C, pg/mL | RSD % | Accuracy% | C, pg/mL | RSD % | Accuracy% |
| 30 | 31 | 4.9 | 103 | 29 | 6.5 | 98 |
| 250 | 272 | 7.4 | 109 | 261 | 3.8 | 104 |
| 3000 | 3253 | 2.3 | 108 | 3161 | 3.2 | 105 |
Intra-day assays were evaluated with triplicate samples within 1 day and inter-day assays were performed on 3 days in a week (n=3).
TC = Theoretical concentration of paclitaxel standard; C = mean of the calculated concentrations; RSD = relative standard deviation (precision).
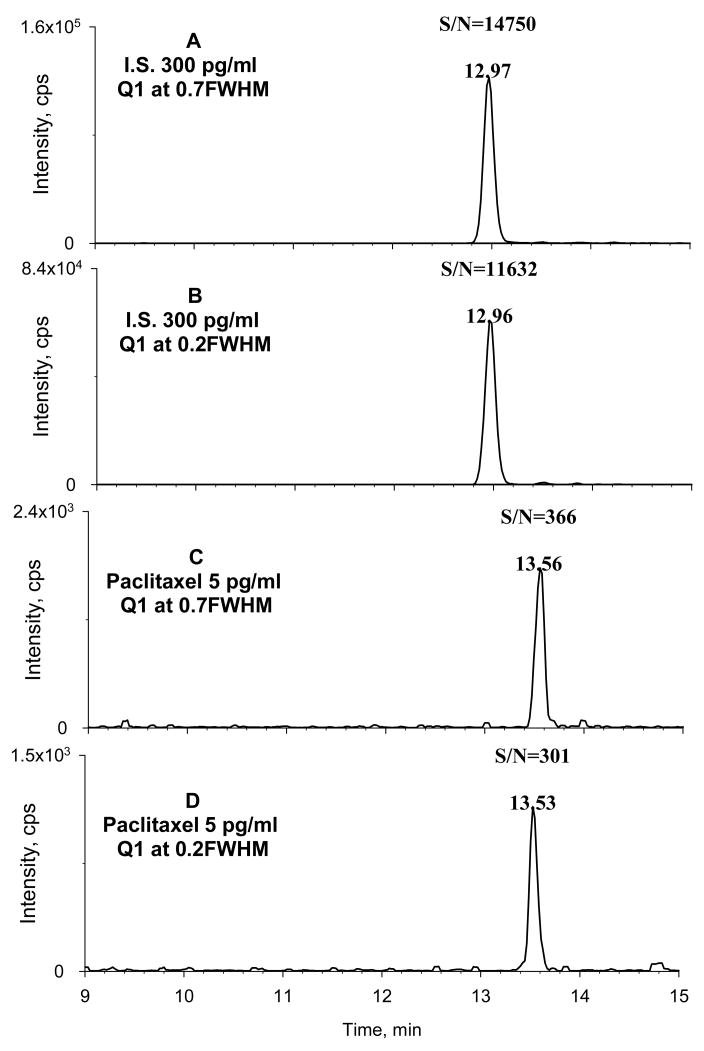
An ultra-low detection limit (defined as S/N=3) was achieved for paclitaxel in cell lysates: 0.5 pg/mL. This detection limit is 200–500 fold lower than conventional LC-MS/MS methods published previously. The LOQ was validated at 5 pg/mL; representative chromatograms of cell lysates spiked with this concentration of drug are shown in Fig 4a–4b. Accuracy and precision of the LOQ were 102% and 4.1% respectively, which are satisfactory according to prevailing criteria. Matrix effects that could compromise quantitative accuracy seriously were not observed under the optimized SPE and μLC-MS/MS conditions.
Figure 4. Typical μLC-MS/MS chromatograms of paclitaxel and docetaxel.
Cell lysates were spiked with paclitaxel to a final concentration equaling the LOQ (5 pg/mL) and with the I.S. docetaxel at a final concentration of 300 pg/mL. Both SRM and HR-SRM were evaluated. (A) SRM of docetaxel; (B) HR-SRM of docetaxel; (C) SRM of paclitaxel; (D) HR-SRM of paclitaxel.
For the mass spectrometer used in this study, the manufacturer suggests that higher MS resolution on either quadrupole can be utilized without drastic loss of sensitivity. This feature enables a practical high resolution SRM approach (HR-SRM), which employs a higher MS resolution for Q1 to decrease chemical noise for biological analysis and thus could increase the S/N [34–36]. We examined the sensitivity of the method described above using HR-SRM. When the S/N obtained for paclitaxel in cell lysates using HR-SRM was compared to the S/N obtained using conventional SRM, it appeared that HR-SRM resulted in a comparable or lower S/N for both paclitaxel and docetaxel, the I.S. (Fig 4c–4d).
There are several possible explanations for this observation. First, the low chemical noise observed in the SRM chromatograms suggest that the biological matrix had been simplified significantly, which was the intended outcome of our strategy to combine selective SPE with sufficient μLC separation. Reduction in sample complexity may de-emphasize the advantage of HR-SRM. Second, the m/z of the precursors of both paclitaxel and docetaxel are relatively high (854 and 808, respectively) and lie in a relatively ‘quiet’ m/z region, in which chemical noise interferes much less than in lower m/z ranges. Perhaps because the chemical noise was already low for SRM, HR-SRM did not provide further gain in the S/N.
3.4 Temporal accumulation of paclitaxel in ovarian cancer cells at ultra-low drug concentrations
The quantification of cellular uptake of paclitaxel under ultra-low drug exposure conditions will provide essential insight into the pharmacological actions of this important class of drugs. However, the analytical challenges are significant, and previous methods have not provided this capability. For example, we estimated that a LOQ of 20 pg/mL in cell lysates would be needed to quantify paclitaxel uptake by cells exposed to approx. 1 ng/mL drug, but the detection limits of published LC-MS/MS based methods were in the range of 0.1–0.25 ng/mL [16–23]. Furthermore, concentrations of paclitaxel lower than 1 ng/mL are pharmacologically active, and therefore a LOQ lower than 20 pg/mL is desirable.
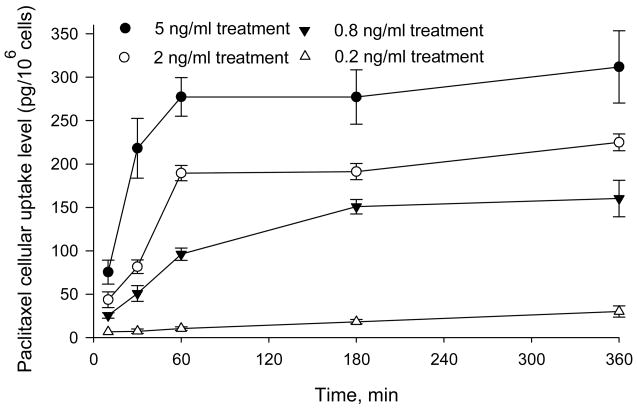
Using the optimized analytical procedure described above, we investigated the accumulation of paclitaxel in human ovarian cancer cells exposed to ultra-low paclitaxel concentrations. The cells were exposed to drug at concentrations ranging from 0.2 to 5 ng/mL, and the temporal dependence of cellular accumulation was quantified (Fig. 5). The drug was measurable at all time points and under all treatment conditions: the lowest measured intracellular accumulation was 6.5 pg/106 cells, observed 10 min after the addition of 0.2 ng/mL paclitaxel to cells. The detected concentration in this sample corresponded to approximately 9.6 pg/mL drug in the cell lysate, or nearly twice the LOQ.
Figure 5. Paclitaxel uptake by A121a ovarian cancer cells at ultra-low drug concentrations.
Cells were incubated with 0.2, 0.8, 2.0, and 5.0 ng/ml paclitaxel, and intracellular drug levels were quantified at different time points after treatment (n=3 samples per time point).
For most concentrations of paclitaxel, it appeared that the intracellular drug concentrations increased rapidly with exposure time and reached a plateau within 1–3 h. However, for cells exposed to the lowest concentration (0.2 ng/mL), the maximum intracellular drug concentration apparently was not achieved within 6 h, which was longest time interval investigated. In future studies, these data will be expanded to include additional concentrations and exposure times, and analyzed according to cellular pharmacokinetic models such as those published previously [29].
4. Conclusions
The ability to quantify low pg/mL concentrations of paclitaxel in biological samples is essential to investigate important pharmacological effects induced by ultra-low doses. To address this need, we employed a strategy we developed for ultra-sensitive quantification of corticosteroids [1], which combines selective SPE and μLC-MS/MS. SPE washing and elution conditions were optimized to extract paclitaxel from cell lysates selectively, which significantly simplified the sample matrix. Formic acid at a final concentration of 0.1% in the SPE elution solvent improved the recovery of paclitaxel from the SPE to approx. 100%. Because of the selective extraction procedure, the acceptable Vinj of cell lysate samples onto the μLC column was increased significantly, and this enhanced sensitivity without compromising chromatographic performance or operational robustness. An additional benefit of the selective SPE strategy was an improved S/N that resulted from reduced ion suppression. During sample loading onto the μLC column, a sample focusing procedure, employing a low-organic mobile phase, prevented the peak broadening that would otherwise result from a large Vinj. The conditions for μLC separation and SRM of paclitaxel were optimized also. Sufficient μLC separation maximized the S/N and minimized matrix effects. It was observed that the taxanes were heat-sensitive during the desolvation process, and that a reduced capillary temperature improved S/N. An ultra-low detection limit of 0.5 pg/mL paclitaxel in cell lysates was achieved, and the validated LOQ was 5 pg/mL. HR-SRM did not improve analytical sensitivity beyond that achieved through the approaches described above.
The analytical approach developed permitted consistent quantification of paclitaxel accumulation in human ovarian cancer cells at ultra-low, pharmacologically relevant concentrations. It is robust and can be implemented using commercially available μLC columns, SPE cartridges, and commonly available μLC-MS/MS instruments. Given the prevailing need for highly sensitive quantification of a broad range of physico-chemically similar compounds in biological, pharmaceutical, and environmental applications, this method has potentially widespread applications.
Acknowledgments
This work was supported partially by grant R01CA107570 from the National Cancer Inst., National Institutes of Health (NIH), U.S.A. to RMS and an unrestricted gift from the Kapoor Charitable Foundation to the School of Pharmacy and Pharmaceutical Sciences, University at Buffalo (UB), State University of New York (SUNY), U.S.A.. The LC-MS/MS was obtained through a Shared Instrumentation Grant (S10RR14592) from the National Center for Research Resources, NIH, U.S.A.. We acknowledge the diverse support provided to the New York State Center of Excellence in Bioinformatics and Life Sciences (SUNY) that contributed to the extensive liquid chromatography/mass spectrometry capabilities in the instrumentation facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Qu J, Qu Y, Straubinger RM. Anal Chem. 2007;79:3786. doi: 10.1021/ac062184r. [DOI] [PubMed] [Google Scholar]
- 2.Degenring RF, Jonas JB. Am J Ophthalmol. 2004;137:1142. doi: 10.1016/j.ajo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sandstrom T, Svensson K, Tattersfield A. Am J Respir Crit Care Med. 2001;164:1392. doi: 10.1164/ajrccm.164.8.2104102. [DOI] [PubMed] [Google Scholar]
- 4.Guan FY, Uboh C, Soma L, Hess A, Luo Y, Tsang DS. J Mass Spectrom. 2003;38:823. doi: 10.1002/jms.495. [DOI] [PubMed] [Google Scholar]
- 5.Olsen TW, Feng X, Wabner K, Conston SR, Sierra DH, Folden DV, Smith ME, Cameron JD. Am J Ophthalmol. 2006;142:777. doi: 10.1016/j.ajo.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 6.Caloni F, Belloli C, Crescenzo G, Ormas P, Archimbault P. Vet Hum Toxicol. 2000;42:345. [PubMed] [Google Scholar]
- 7.Harrison TW, Tattersfield AE. Thorax. 2003;58:258. doi: 10.1136/thorax.58.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bocci G, Nicolaou KC, Kerbel RS. Cancer Res. 2002;62:6938. [PubMed] [Google Scholar]
- 9.Ling X, Bernacki RJ, Brattain MG, Li F. J Biol Chem. 2004;279:15196. doi: 10.1074/jbc.M310947200. [DOI] [PubMed] [Google Scholar]
- 10.Kim SC, Yu J, Lee JW, Park ES, Chi SC. J Pharm Biomed Anal. 2005;39:170. doi: 10.1016/j.jpba.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 11.Jordan MA, Kamath K. Curr Cancer Drug Targets. 2007;7:730. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- 12.Belotti D, Vergani V, Drudis T, Borsotti P, Pitelli MR, Viale G, Giavazzi R, Taraboletti G. Clin Cancer Res. 1996;2:1843. [PubMed] [Google Scholar]
- 13.Merchan JR, Jayaram DR, Supko JG, He XY, Bubley GJ, Sukhatme VP. Int J Cancer. 2005;113:490. doi: 10.1002/ijc.20595. [DOI] [PubMed] [Google Scholar]
- 14.Wang JY, Lou PP, Lesniewski R, Henkin J. Anti-Cancer Drugs. 2003;14:13. doi: 10.1097/00001813-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Shahi P, Pineda I. Cancer Invest. 2008;26:104. doi: 10.1080/07357900701662509. [DOI] [PubMed] [Google Scholar]
- 16.Alexander MS, Kiser MM, Culley T, Kern JR, Dolan JW, McChesney JD, Zygmunt J, Bannister SJ. J Chromatogr B. 2003;785:253. doi: 10.1016/s1570-0232(02)00913-3. [DOI] [PubMed] [Google Scholar]
- 17.Stokvis E, Ouwehand M, Nan LGAH, Kemper EM, van Tellingen O, Rosing H, Beijnen JH. J Mass Spectrom. 2004;39:1506. doi: 10.1002/jms.747. [DOI] [PubMed] [Google Scholar]
- 18.Parise RA, Ramanathan RK, Zamboni WC, Egorin MJ. J Chromatogr B. 2003;783:231. doi: 10.1016/s1570-0232(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 19.Hou WY, Watters JW, McLeod HL. J Chromatogr B. 2004;804:263. doi: 10.1016/j.jchromb.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Grozav AG, Hutson TE, Zhou X, Bukowski RM, Ganapathi R, Xu Y. J Pharm Biomed Anal. 2004;36:125. doi: 10.1016/j.jpba.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, Johnson JL, Khan S, Ahmad A, Ahmad I. Anal Biochem. 2005;336:213. doi: 10.1016/j.ab.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 22.Wang LZ, Goh BC, Grigg ME, Lee SC, Khoo YM, Lee HS. Rapid Commun Mass Spectrom. 2003;17:1548. doi: 10.1002/rcm.1091. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson DL, Long ME, Zirrolli JA, Duncan MW, Holden SN, Pierson AS, Eckhardt SG. Cancer Chemother Pharmacol. 2003;52:159. doi: 10.1007/s00280-003-0622-z. [DOI] [PubMed] [Google Scholar]
- 24.Gaspar JR, Qu J, Straubinger NL, Straubinger RM. Analyst. 2008 doi: 10.1039/b806856a. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crickard K, Niedbala M, Crickard U, Yoonessi M, Sandberg A, Okuyama K, Bernacki R, Satchidanand S. Gyne Oncol. 1989:163. doi: 10.1016/s0090-8258(89)80028-9. [DOI] [PubMed] [Google Scholar]
- 26.Samtani MN, Lohle M, Grant A, Nathanielsz PW, Jusko WJ. Drug Metab Dispos. 2005;33:1124. doi: 10.1124/dmd.105.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vissers JPC, Claessens HA, Cramers CA. J Chromatogr A. 1997;779:1. [Google Scholar]
- 28.Kumar GN, Walle UK, Bhalla KN, Walle T. Res Commun Chem Pathol Pharmacol. 1993;80:337. [PubMed] [Google Scholar]
- 29.Kuh HJ, Jang SH, Wientjes MG, Au JLS. J Pharmacol Exp Ther. 2000;293:761. [PubMed] [Google Scholar]
- 30.Emmett MR, Caprioli RM. J Am Soc Mass Spectrom. 1994;5:605. doi: 10.1016/1044-0305(94)85001-1. [DOI] [PubMed] [Google Scholar]
- 31.Saito Y, Jinno K, Greibrokk T. J Sep Sci. 2004;27:1379. doi: 10.1002/jssc.200401902. [DOI] [PubMed] [Google Scholar]
- 32.Qu J, Wang YM, Luo GA. J Chromatogr A. 2001;919:437. doi: 10.1016/s0021-9673(01)00849-4. [DOI] [PubMed] [Google Scholar]
- 33.Chervet JP, Ursem M, Salzmann JB. Anal Chem. 1996;68:1507. doi: 10.1021/ac9508964. [DOI] [PubMed] [Google Scholar]
- 34.Jemal M, Ouyang Z. Rapid Commun Mass Spectrom. 2003;17:24. doi: 10.1002/rcm.872. [DOI] [PubMed] [Google Scholar]
- 35.Xu XY, Veals J, Korfmacher WA. Rapid Commun Mass Spectrom. 2003;17:832. doi: 10.1002/rcm.985. [DOI] [PubMed] [Google Scholar]
- 36.Pucci V, Bonelli F, Monteagudo E, Laufer R. Rapid Commun Mass Spectrom. 2006;20:1240. doi: 10.1002/rcm.2439. [DOI] [PubMed] [Google Scholar]