Abstract
Skin impedance at acupuncture points (APs) has been used as a diagnostic/therapeutic aid for more than 50 years. Currently, researchers are evaluating the electrophysiologic properties of APs as a possible means of understanding acupuncture's mechanism. To comprehensively assess the diagnostic, therapeutic and mechanistic implications of acupuncture point skin impedance, a device capable of reliably recording impedances from 100 kΩ to 50 MΩ at multiple APs over extended time periods is needed. This article describes design considerations, development and testing of a single channel skin impedance system (hardware, control software and customized electrodes). The system was tested for accuracy against known resistors and capacitors. Two electrodes (the AMI and the ORI) were compared for reliability of recording over 30 min. Two APs (LU 9 and PC 6) and a nearby non-AP site were measured simultaneously in four individuals for 60 min. Our measurement system performed accurately (within 5%) against known resistors (580 kΩ–10 MΩ) and capacitors (10 nF–150 nF). Both the AMI electrode and the modified ORI electrode recorded skin impedance reliably on the volar surface of the forearm (r = 0.87 and r = 0.79, respectively). In four of four volunteers tested, skin impedance at LU 9 was less than at the nearby non-AP site. In three of four volunteers skin impedance was less at PC 6 than at the nearby non-AP site. We conclude that our system is a suitable device upon which we can develop a fully automated multi-channel device capable of recording skin impedance at multiple APs simultaneously over 24 h.
Keywords: AMI electrode, capacitance, electrodermal screening, ORI dry electrode, resistance
Introduction
Devices that measure skin impedance at acupuncture points (APs) as an aid to diagnosing and monitoring therapeutic effects have been in use for more than 50 years (1,2). However, these devices and electrodermal screening, in general, are not yet accepted as valid medical tests. We need to acknowledge that clinical and translational Complementary and Alternative (CAM) research in the 21st century will rely upon an evidence-based model (3,4). The more objective and quantifiable the outcome measures used in acupuncture research, the more credible that research becomes. To establish the validity of electrodermal screening we must first determine whether tests of skin impedance at APs are valid and clinically useful. To date, few electrodermal instruments have been proven to be accurate and reliable (5–8) and clinical usefulness of recording skin impedance at APs is far from proven.
Three lines of scientific evidence suggest that impedance at APs is electrically distinct from non-AP sites and that changes in skin impedance at APs may be of substantial diagnostic, therapeutic and research significance. First, APs and meridians have been shown to have lower electrical skin resistance and higher capacitance than surrounding cutaneous areas (9–12). Second, in certain disease processes, higher or lower resistance has been reported at clinically relevant APs, but not at clinically unrelated APs or non-AP sites in the same patients (13–16). And third, human and animal studies demonstrate that experimentally induced physiologic dysfunction and subsequent recovery correlates with an increased or decreased electrical impedance at clinically relevant APs (17–22). The literature for this evidence base, however, is small and sometimes contradictory (23–25).
Results of a recent study by Ahn et al. (9) strengthened by the morphologic findings of Langevin et al. (26–27) have led to a renewed scientific interest in skin impedance measurements at APs as a means of understanding acupuncture's mechanism in terms of a connective tissue network.
Skin impedance measurements at APs and other skin sites, however, are prone to multiple sources of error primarily related to the skin/electrode interface. Potential confounders include probe material, probe size and shape, pressure exerted by the probe, duration of probe application, inclination of the probe tip on the skin, variations in skin condition (dry/moist, thickness and integrity of the stratum corneum) and fluctuating physiology (28–31).
Although Galvanic Skin Response is known to change from moment to moment little attention has been paid specifically to skin impedance changes at APs over time. In a recently conducted pilot study, intending to evaluate the circadian rhythm of skin impedance at APs, we recorded skin impedance at the 24 Ting APs every 20 min for 24 h (32). Our results showed significant impedance rises during sleep (up to 50–60 MΩ) and falls during wakefulness (as low as 100 KΩ). We also observed significant right/left differences in paired APs that were sometimes apparent only during sleep. These findings highlighted the need for an automated system capable of recording continuous impedance measurements at multiple AP and non-AP sites over extended time periods.
The purpose of this project was therefore, to develop and pretest a single channel system (the prototype for a future multi-channel system) that would accurately and reliably record a wide range of skin impedance (resistance and capacitance) continuously for up to 1 h.
Methods
Four major tasks were completed in this project: (i) Hardware design and development, (ii) control software development, (iii) reliability testing of two electrode types and (iv) pilot testing skin impedance at two APs and a nearby non-AP site.
Considerations for Hardware Design and Development
Most traditional methods for measuring skin impedance have used a current source of 1–25 μA (micro-Ampere), although Nakatani (2), when developing his Ryodoraku method, used a moist electrode that applied a current of ∼200 μA. (It should be noted, however, that the Committee on Electrocardiology recommends currents of 10 μA or less for patient-connected leads (33)). The voltage is measured across the skin under test. Although a constant current source works well when impedances are <1 MΩ, in our previous research we found that skin impedance at APs during sleep can be of the order of 50–60 MΩ (34). Using the traditional approach would require a constant current source capable of developing over 50 V across the skin patch, an impractical solution. This led to the development of our present constant voltage measurement system.
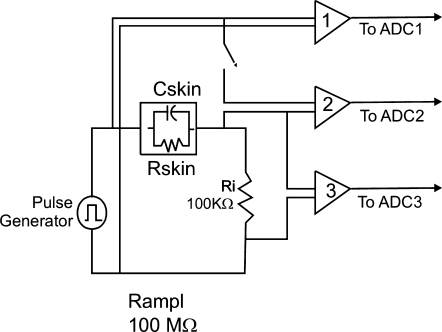
Since most of the impedance is determined by the resistance value, we used voltage amplifiers to accommodate the three orders of magnitude in resistance measurements (from 50 KΩ to 50 MΩ). We assume that the skin impedance is a parallel resistor and capacitor network as shown in Fig. 1. A block diagram of the system to measure the values of the components is also shown in Fig. 1. Amplifiers one and two have fixed gains, but amplifier three requires variable gain.
Figure 1.
Block diagram of measurement system. The ADC1 and ADC3 signals are used to evaluate the resistance value. ADC2 is used to determine the capacitance value after the resistance value has been determined.
We assembled our system with off-the-shelf components and used it to develop the basic algorithm for resistance and capacitance measurements. The generator is an Agilent 33220A Function Generator, Agilent Technologies, Palo Alto, CA, USA. The basic acquisition system, an OMB Daqboard 2000, IoTech, Stamford, CT, USA provides for 16 channels of acquisition and interfaces to a personal computer. Our device collects only a single channel of data since the goal at this prototype development stage was to determine if accurate, reliable, near-continuous skin impedance data could be collected. Our final system will be fully automated for continuous data collection form eight individual channels. Near continuous data collection was achievable by moving the connector to multiple sites on a universal breadboard sequentially. For each skin site measured, only one electrode needed to be connected since the reference electrode is fixed. Three UFI2122i bioamplifiers with programmable gains (UFI, Morro Bay, CA, USA) are incorporated in the circuit as shown above. Amplifiers one and three, connected to the ADC1 and ADC3 inputs of the Daqboard, are used to determine the resistance of the load.
A square wave of period 0.5 s with 50% duty cycle is generated for the impedance measurements, yielding, which is for all practical purposes a DC measurement. Other authors have used AC measurement approaches with square waves of higher frequency (12,35,36). An AC measurement yields a combined impedance of the resistor and capacitor at the square wave frequency; however, a disadvantage of an AC measurement system is that the signal must be continuously applied for the duration of the measurement. With a DC step measurement, the signal is applied only for the duration of the ON cycle, with no stimulation of the skin between cycles.
With our DC measurement, in the first phase only the resistance value of the skin is measured. To obtain this value, we can neglect the 50 Ω internal resistance of the pulse generator given that the amplifier input impedance of 10 MΩ and the 100 KΩ resistance Ri are much larger than the 50 Ω. After reaching an essentially steady state (about 400 ms), the skin resistance Rskin is given by Equation (1) where V1 and V3 are the steady state voltages measured in the ADC1 and ADC3 of the Daqboard. V1 can be considered fixed at 5 V. Time averaging is used to reduce the measurement noise in V3.
| (1) |
Capacitance is measured in the second phase. Amplifier two, connected to the ADC2 port of the Daqboard, is switched in. Since the skin capacitance to be measured ranges from a few to hundreds of nanoFarads (nF), we can neglect the amplifier capacitance, estimated to be on the order of 100 pF. The 10 MΩ input of the amplifier cannot, however, be neglected at high values of skin resistance and must be taken into account.
The RC (Resistance/capacitance) time constant of the equivalent circuit for capacitance measurement is given by (Rskin//Ramp2//Ramp3//Ri) Cskin, where the Ramp2 is the input resistance of amplifier two and Ramp3 is the input resistance of amplifier three and notation R1//R2 is used to denote two resistances in parallel. For medium values of skin resistance, 50 KΩ − 1 MΩ, the time constant is determined by the skin capacitance and the 100 KΩ current measurement resistance, Ri, in parallel with the skin resistance. For large values of skin resistance, the time constant is determined by the skin capacitance and the 100 KΩ Ri. Since the input resistance of the amplifier can be comparable with the skin resistance and thus, complicate the measurement of the skin resistance, it is necessary to switch the second amplifier off during the first phase of the resistance measurement.
Assuming that the rise time of the voltage measured at amplifier two is a simple exponential, the 10–90% rise time is related to the RC time constant through Equation (2).
| (2) |
It is interesting to note that, with our constant voltage (rather than constant current) approach, the skin resistance is not a limiting factor in the calculation of the RC time constant. The time constant is Cskin*100 KΩ for large values of Rskin, such as 50 MΩ. With a 100 nF capacitance, the time constant is only 10 ms. Had a current source approach been used, the time constant would have been 5 s, for 100 nF and 50 MΩ. With a 10 ms time constant, the corresponding rise time is on the order of 22 ms. Thus, as far as the measurements are concerned, we could have used a square wave up to 10 Hz to obtain the same result. In a final implementation, we will keep the 2 Hz rate but reduce the duty cycle to 15–20%. This will allow longer than 400 ms between skin stimulations.
It should also be noted that we chose to measure the voltage across the load for the capacitance measurement. An alternative is to use the voltage across Ri since amplifier three is already connected. Then we would measure a 90–10% fall time rather than a rise time. The problem with this approach is that our amplifier bandwidth does not allow us to measure the initial voltage surge. As a result, the 100% reference level cannot be determined accurately. With a rise time measurement, the amplifier bandwidth is not a limiting factor.
Control Software Development
The control software was developed using Labview version 7.0 under Microsoft XP Professional edition running on a Pentium 4 processor. The software program acquires the data and then calculates resistance and capacitance using the equations above. It then saves and stores the data in a text file with a unique ID code. To begin impedance measurements, the operator selects the channel, the sampling frequency and the file name in which the data will be saved. The program automatically acquires data from the DAQ board. The program calculates the average value and displays a graph. The operator visually observes the waveforms on the computer screen and saves the averaged data as soon as the waveform has stabilized (∼6–10 s after the current has been injected). The maximum and minimum voltage values are captured in resistance Ri and calculated as an average of the previous 5–10 resistance readings. In the next step the operator can exit or save the data to the file. The file includes the date, the average skin resistance value and the average skin capacitance value.
Participants
Preliminary testing in three separate experiments was performed on eight volunteers (aged 27–62 years). The research protocols for each experiment were conducted according to the ethical standards in the Declaration of Helsinki (37). In Experiment no. 1, we compared the repeatability of skin impedance measurements recorded with two electrodes in each of eight volunteers over 30 min. In Experiment no. 2 capacitance and resistance measurements were tracked simultaneously in a single volunteer. In Experiment no. 3 involving four volunteers, skin impedance at two APs and a non-AP site was measured continuously over 60 min.
Reliability Testing Of Electrodes
To circumvent the problems of probe application (pressure, duration and inclination of the probe on the skin), the electrodes were positioned flatly on the skin and securely affixed with a clear adhesive wrapped circumferentially around the forearm. Electrodes remained in place for 15 min prior to recording and were then left in place, undisturbed, during 60 min recording. The pressure exerted by the undisturbed electrodes was not measured as it was presumed to remain constant during the duration of the recordings.
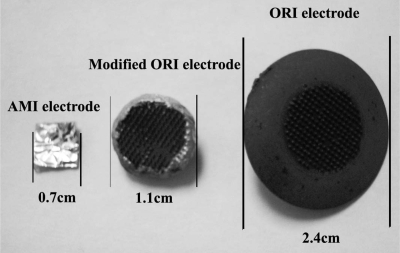
Two electrodes were compared for reliability of measurements on the volar surface of the forearm. The ORI electrode* developed by Orbital Research Inc. Cleveland, OH, USA for long term use in electrocardiography (ECG) is a dry, 2.4 cm diameter electrode with tiny micro anchors on the surface in contact with the skin (38). The ECG version of these electrodes was FDA approved in December 2006 (FDA 510k#K062760). With permission from Orbital Research, Inc. we reduced the size of this electrode by grinding down the platform to a 1.1 cm diameter and coated the exposed surface with conductive epoxy (Fig. 2). This modification does not affect the sensing element and or skin contact portion of the device. The other electrode, a 7 mm2 stick-on Ag-AgCl gel electrode covered with conductive foil is used with the AMI (Apparatus for measuring the functioning of Meridians and their corresponding Internal organs device). Our reference electrode an Ag/AgCl conductive adhesive ECG electrode (MediTrace 530) with a diameter of 2.4 cm was placed ∼8 cm proximal to the recording electrodes on the volar surface of the ipsilateral forearm. Skin resistance at two adjacent sites on the volar surface of the forearm was recorded over a 30 min period in eight volunteers. The between- and within-components of variance were computed across the eight participants using a standard method (39). The reliability of the measurements taken with each electrode was estimated as the ratio of the between person variance to the total variance.
Figure 2.
Dimensions of AMI and modified ORI electrodes.
Comparing Impedance at Two APs and a Non-AP Site
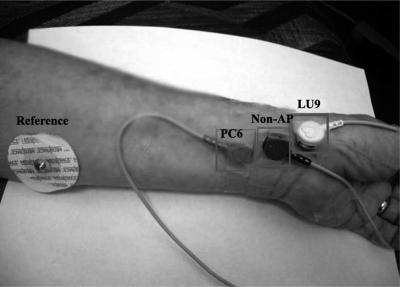
Two APs (LU 9 and PC 6) were compared with a nearby non-AP site (Fig. 3). APs were located using standard acupuncture charts, anatomical landmarks and digital palpation. The non-AP site was located at a point midway on an imaginary line from wrist to elbow that was equidistant between the two APs.
Figure 3.
Modified ORI dry electrodes on LU 9 and PC 6 acupuncture points and a nearby non-AP site.
The skin at the selected sites was cleansed with ethyl alcohol and allowed to dry prior to electrode application. Electrodes were affixed to the skin with clear tape and left in place undisturbed for the duration of the recordings. We assumed the pressure of the electrode on the skin was constant during the recordings as the electrodes were not touched during the course of the experiments.
All volunteers were tested in the Psychophysiology Laboratory at the Helfgott Research Institute which was kept at a constant temperature of 69 and 70°F. Testing occurred between 9 am and 4 pm on 3 days.
Results
Measurement Accuracy of the Prototype against Known Resistors and Capacitors
The accuracy of the resistance measurements recorded with our system was evaluated by comparison with reference resistors obtained from a Radio Shack multimeter (Model 22–1666B). Accuracy for measuring resistance is within 5% of the reference values in the range of interest (10 KΩ–10 MΩ). Accuracy of capacitance measurements was tested with six different resistors (simulating various skin resistances) in the circuit. The circuit tested had the ‘unknown’ capacitor in parallel with the indicated resistance. Accuracy is within 5% of reference values for capacitors between 10 and 150 nF at resistances of 560 KΩ – 10 MΩ.
Resistance and Capacitance Measurements
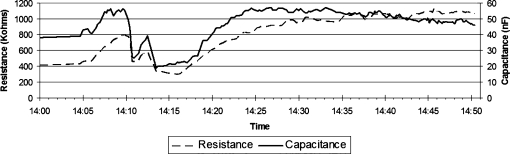
Resistance and capacitance measurements tracked well over 50 min in a single subject as shown in Fig. 4. Absolute resistance and capacitance values in this volunteer ranged from 400–1000 KΩ and 20–55 nF, respectively. These values leveled out to ∼1000 KΩ and 48–55 nF during the final 20 min of recording.
Figure 4.
Example of resistance and capacitance measurements recorded from volar skin surface of left forearm in one subject. Resistance and capacitance track each other consistently over 50 min.
Reliability of Impedance Measurements with ORI and AMI Electrodes
Reliability of impedance measurements for the AMI electrode and the modified ORI electrode were both good and comparable (r = 0.87 and r = 0.79, respectively). We chose the modified ORI electrode for Experiment no. 3 because that electrode was more easily held in place (using ECG clips and wires) for extended time periods than the AMI electrode. The AMI electrode having been designed for intermittent measurements, using the light touch of a hand held probe, rather than secured in place for continuous measurements, could not be securely connected via a wire to the universal breadboard.
Comparing Continuous Skin Impedance at Two APs and a Non-AP Site
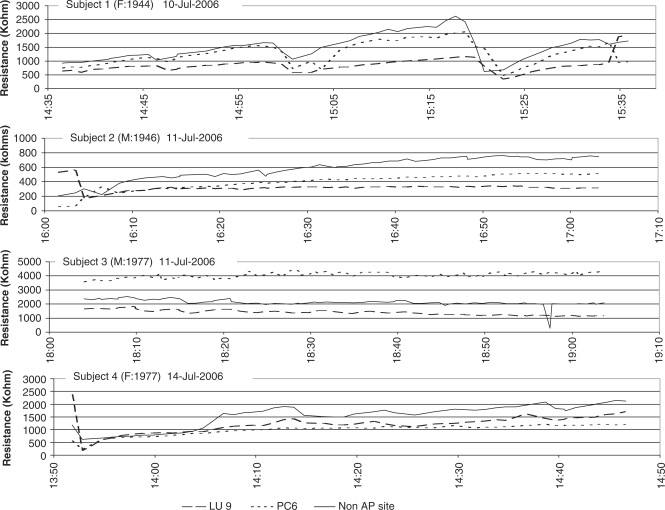
Near-continuous impedance measurements for 60 min were recorded at APs LU 9 and PC 6 and a non-AP site (located midway between these two points) in four volunteers, two males and two females, age range 29–61-years (Fig. 5). In all four individuals consistently lower impedance was recorded at LU 9 compared with the nearby non-AP site. In three of the four individuals, lower impedance was recorded at PC 6 than at the non-AP site. In Subject 3, the impedance at PC 6 was higher than at the nearby non-AP site (Fig. 5). Of interest is the fact that the relationship between impedance measurements at each of the three skin sites was maintained throughout the 60 min recording period in all four volunteers. Unexplained events or artifacts, which appear to be cyclical, are indicated in the traces from Subject 1 at time 15:04 and 15:23.
Figure 5.
Skin resistance at three sites (LU 9 and PC 6 acupuncture points and a nearby non-AP site) recorded in four volunteers over 60 min.
Discussion
With the exception of the AMI (5), the Prognos (6,7) and the recently developed device of Wiegele et al. (8), commercially available electrodermal screening devices have not generally been tested for accuracy and reliability. As electrodermal measurements are prone to many sources of error we thought it was essential that, prior to developing a marketable system, a single channel prototype be rigorously evaluated. In this project we designed, developed and pilot tested the hardware and software of our prototype.
Several challenges were overcome when designing the hardware circuitry in order to measure a wide range of skin resistances and capacitances for periods up to 60 min. To overcome the skin/electrode interface problems, including electrode size and materials, probe pressure problems and the inclination of the probe (28–31) and physiologic fluctuations, we evaluated two electrodes of different sizes, shapes and materials that could be affixed to the skin and left undisturbed during 60 min measurements. Both electrodes performed reliably. The modified ORI dry electrode was selected for further studies because of the ease with which it could be secured by an ECG clip to the skin and connected to the universal breadboard. Preliminary testing of our system (instrument plus electrodes) at two APs and a non-AP site suggests that should differences in skin impedance between APs and non-AP sites exist, our system will be capable of distinguishing them.
From a previous study (34) in which we assessed the normal physiologic variability of AP skin impedance over 24 h, we realized that an automated system would be essential if we intend to fully characterize skin impedance at APs at multiple sites over several hours. Multichannel impedance measuring systems have been developed for assessing skin impedance at APs at particular moments in time (8,29,35,40–43). However, to our knowledge, no researchers other than Motoyama (44) have attempted to simultaneously assess electrical skin changes at different APs over time. Motoyama reports an experiment in which he simultaneously recorded skin potentials at APs (using insulated needle electrodes) in response to stimulating acupuncture point TE 4. He observed a response specifically in the TE meridian (at TE 1, TE 10, TE 23) and in its linked meridian (at PC1, PC 3 and PC 9) (44). It should be pointed out that Motoyama evaluated skin potentials (rather than skin impedance) and found the generalized Galvanic Skin Response that occurred at all recording sites, to be different from what he termed a ‘meridian reaction’ which occurred only in related acupuncture meridians. To our knowledge, no other researchers have replicated Motoyama's experiments. Our multichannel system will allow us to attempt to verify Motoyama's work and to test several other fundamental aspects of the bioelectromagnetic mechanistic theories of acupuncture.
Conclusion
Preliminary measurements of skin impedance suggest that our single channel device when used with a modified ORI dry electrode records repeatable skin resistance and capacitance over time and can differentiate impedances at skin sites in close proximity to each other. The next step in our program of research is to develop a multi-channel, fully automated system capable of simultaneously measuring skin impedance at 24 A and eight non-acupuncture sites for periods up to 24 h.
Acknowledgments
We would like to thank Aaron Rood, of Orbitral Research, Inc. for supplying the ORI electrodes used in the study and for his ongoing advice.
Conflict of interest statement The individual authors report no conflict of interest in this Research Project.
References
- 1.Voll R. Twenty years of electroacupuncture diagnosis in Germany. A progress report. Am J Acupunct. 1975;3:7–17. [Google Scholar]
- 2.Nakatani Y. On the nature of the acupuncture points and meridias. J Japan Orient Med. 1953;3:39–49. [Google Scholar]
- 3.Chiappelli F, Prolo P, Cajulis OS. Evidence-based research in complementary and alternative medicine I: history. Evid Based Complement Alternat Med. 2005;2:453–8. doi: 10.1093/ecam/neh106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiappelli F, Prolo P, Rosenblum M, Edgerton M, Cajulis OS. Evidence-based research in complementary and alternative medicine II: the process of evidence-based research. Evid Based Complement Alternat Med. 2006;3:3–12. doi: 10.1093/ecam/nek017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessel-Kenyon J, Pfeiffer L, Brenton M. A statistical comparison of repeatability in three commonly used bioelectric devices: Kirlian photography, the segmental electrogram and the AMI of Motoyama. Acupunct Med. 1998;16:40–2. [Google Scholar]
- 6.Colbert AP, Hammerschlag R, Aickin M, McNames J. Reliability of the Prognos electrodermal device for measurements of electrical skin resistance at acupuncture points. J Altern Complement Med. 2004;10:610–6. doi: 10.1089/acm.2004.10.610. [DOI] [PubMed] [Google Scholar]
- 7.Treugut H, Gorner C, Ludtke R, Burghardt VV. Reliabilitat der energetischen Meridianmessung mit Prognos A(R) Forsch Komplementarmed. 1998;5:284–9. doi: 10.1159/000021160. [DOI] [PubMed] [Google Scholar]
- 8.Wiegele B, Schober G, Kuder J, Kolb FP, Irnich D. [A new device for measurements of electrical skin resistance at acupuncture points in humans] Forsch Komplementarmed. 2006;13:227–32. doi: 10.1159/000094704. [DOI] [PubMed] [Google Scholar]
- 9.Ahn AC, Wu J, Badger GJ, Hammerschlag R, Langevin HM. Electrical impedance along connective tissue planes associated with acupuncture meridians. BMC Complement Altern Med. 2005;5:10. doi: 10.1186/1472-6882-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyvarinen J, Karlsson M. Low-resistance skin points that may coincide with acupuncture loci. Med Biol. 1977;55:88–94. [PubMed] [Google Scholar]
- 11.Reichmanis M, Marino AA, Becker RO. Electrical correlates of acupuncture points. IEEE Trans Biomed Eng. 1975;22:533–5. doi: 10.1109/tbme.1975.324477. [DOI] [PubMed] [Google Scholar]
- 12.Johng HM, Cho JH, Shin HS. Frequency dependence of impedances at the acupuncture point Quze (PC3) IEEE Eng Med Biol Mag. 2002;21:33–6. doi: 10.1109/memb.2002.1000183. [DOI] [PubMed] [Google Scholar]
- 13.Krop J, Lewith GT, Gziut W, Radulescu C. A double blind, randomized, controlled investigation of electrodermal testing in the diagnosis of allergies. J Altern Complement Med. 1997;3:241–8. doi: 10.1089/acm.1997.3.241. [DOI] [PubMed] [Google Scholar]
- 14.Margolin A, Avants SK, Birch S, Falk CX, Kleber HD. Methodological investigations for a multisite trial of auricular acupuncture for cocaine addiction: a study of active and control auricular zones. J Subst Abuse Treat. 1996;13:471–81. doi: 10.1016/s0740-5472(96)00065-7. [DOI] [PubMed] [Google Scholar]
- 15.Oleson TD, Kroening RJ, Bresler DE. An experimental evaluation of auricular diagnosis: the somatotopic mapping of musculoskeletal pain at ear acupuncture points. Pain. 1980;8:217–29. doi: 10.1016/0304-3959(88)90009-7. [DOI] [PubMed] [Google Scholar]
- 16.Saku K, Mukaino Y, Ying H, Arakawa K. Characteristics of reactive electropermeable points on the auricles of coronary heart disease patients. Clin Cardiol. 1993;16:415–9. doi: 10.1002/clc.4960160509. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Hayes MF., Jr Acupuncture, electric phenomenon of the skin and postvagotomy gastrointestinal atony. Am J Surg. 1973;125:176–80. doi: 10.1016/0002-9610(73)90023-8. [DOI] [PubMed] [Google Scholar]
- 18.Szopinski J, Lukasiewicz S, Lochner GP, Nasiek DJ, Krupa-Jezierska B, Warakomski P, et al. Influence of general anesthesia and surgical intervention of the electrical parameters of auricular organ projection areas. Med Acupunct. 2002;14:40–42. [Google Scholar]
- 19.Ogata H, Matsumoto T, Tsukahara H. Electrical skin resistance changes in meridians during ophthalmic surgery with local anesthesia. Am J Chin Med. 1983;11:130–6. doi: 10.1142/S0192415X83000215. [DOI] [PubMed] [Google Scholar]
- 20.Usichenko TI, Lysenyuk VP, Groth MH, Pavlovic D. Detection of ear acupuncture points by measuring the electrical skin resistance in patients before, during and after orthopedic surgery performed under general anesthesia. Acupunct Electrother Res. 2003;28:167–73. doi: 10.3727/036012903815901606. [DOI] [PubMed] [Google Scholar]
- 21.Kawakita K, Kawamura H, Keino H. Development of low impediance points in the auricular skin of experimental peritonitis rats. Am J Chin Med. 1991;19:199–205. doi: 10.1142/S0192415X91000272. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt S. The electrodermal properties of acupuncture points. Am J Acupunct. 1982;10:131–7. [Google Scholar]
- 23.Semizzi M, Senna G, Crivellaro M, Rapacioli G, Passalacqua G, Canonica WG, et al. A double-blind, placebo-controlled study on the diagnostic accuracy of an electrodermal test in allergic subjects. Clin Exp Allergy. 2002;32:928–32. doi: 10.1046/j.1365-2222.2002.01398.x. [DOI] [PubMed] [Google Scholar]
- 24.Lewith GT, Kenyon JN, Broomfield J, Prescott P, Goddard J, Holgate ST. Is electrodermal testing as effective as skin prick tests for diagnosing allergies? A double blind, randomised block design study. Brit Med J. 2001;322:131–4. doi: 10.1136/bmj.322.7279.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewith GT. Can we evaluate electrodermal testing? Complement Ther Med. 2003;11:115–7. doi: 10.1016/s0965-2299(03)00057-8. [DOI] [PubMed] [Google Scholar]
- 26.Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec. 2002;269:257–65. doi: 10.1002/ar.10185. [DOI] [PubMed] [Google Scholar]
- 27.Langevin HM, Bouffard NA, Badger GJ, Churchill DL, Howe AK. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: evidence for a mechanotransduction-based mechanism. J Cell Physiol. 2006;207:767–74. doi: 10.1002/jcp.20623. [DOI] [PubMed] [Google Scholar]
- 28.Fraden J. Active acupuncture point impedance and potential measurements. Am J Acupunct. 1979;7:137–44. [Google Scholar]
- 29.Martinsen OG, Grimnes S, Morkrid L, Hareide M. Line patterns in the mosaic electrical properties of human skin–a cross-correlation study. IEEE Trans Biomed Eng. 2001;48:731–4. doi: 10.1109/10.923791. [DOI] [PubMed] [Google Scholar]
- 30.McCarroll GD, Rowley BA. An investigation of the existence of electrically located acupuncture points. IEEE Trans Biomed Eng. 1979;26:177–81. doi: 10.1109/tbme.1979.326392. [DOI] [PubMed] [Google Scholar]
- 31.Noordergraaf A, Silage D. Electroacupuncture. IEEE Trans Biomed Eng. 1973;:364–6. doi: 10.1109/TBME.1973.324289. [DOI] [PubMed] [Google Scholar]
- 32.Colbert A, Hayes M, Aickin M, Hammerschlag R. Physiologic variability of electrical skin resistance measurements at the ting acupuncture points. Med Acupunct. 2006;17:12–9. [Google Scholar]
- 33.Laks MM, Arzbaecher R, Bailey JJ, Geselowitz DB, Berson AS. Recommendations for safe current limits for electrocardiographs. A statement for healthcare professionals from the committee on electrocardiography, American heart association. Circulation. 1996;93:837–9. doi: 10.1161/01.cir.93.4.837. [DOI] [PubMed] [Google Scholar]
- 34.Colbert APHM, Aickin M, Hammerschlag R. Physiologic variability of electrical skin resistance measurements at the ting acupuncture points. Medical Acupuncture. 2006;17:12–9. [Google Scholar]
- 35.Fukumoto T, Ohba S, Futami R, Tanaka H, Hoshimiya N. A basic study about multichannel measurement of skin impedance vector loci at the acupuncture points. 23rd Annual EMBS International Conference; Istanbul, Turkey. 2001. [Google Scholar]
- 36.Chizmadzhev YA, Indenbom AV, Kuzmin PI, Galichenko SV, Weaver JC, Potts RO. Electrical properties of skin at moderate voltages: contribution of appendageal macropores. Biophys J. 1998;74:843–56. doi: 10.1016/S0006-3495(98)74008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Organization WM. Declaration of Helsinki. Brit Med J. 1996;313:1448–9. [Google Scholar]
- 38.Lisy F. Dry micromachined physiological recording electrodes. CRISP database. 2003 [Google Scholar]
- 39.Winer B. Statistical Principles in Experimental Design. 2nd. New York: McGraw Hill; 1962. [Google Scholar]
- 40.Halek J, Opavsky J, Kolarova J. Problems of the skin resistance measuring in randomly chosen and so-called active points of the skin. Acta Univ Palacki Olomuc Fac Med. 1984;107:51–62. [PubMed] [Google Scholar]
- 41.Mayer-Gindner A, Lek-Uthai A, Abdallah O, Bolz A. Newly explored electrical properties of normal skin and special skin sites. Biomed Tech (Berl) 2004;49:117–24. doi: 10.1515/BMT.2004.024. [DOI] [PubMed] [Google Scholar]
- 42.Kwok G, Cohen M, Cosic I. Mapping acupuncture points using multi channel device. Australas Phys Eng Sci Med. 1998;21:68–72. [PubMed] [Google Scholar]
- 43.Robert O Becker, Reichmanis M, Marino AA, Spadaro JA. Electrophysiological correlates of acupuncture points and meridians. Psychoenergetic Systems. 1976;1:105–12. [Google Scholar]
- 44.Motoyama H. Measurements of Ki Energy: Diagnoses and Treatments. Tokyo: Human Science Press; 1997. [Google Scholar]