Abstract
The present study is an extension of our previous work carried out on Cynodon dactylon. This study deals with the critical evaluation of glycemic potential of ethanolic extract of defatted C. dactylon. The doses of 250, 500 and 750 mg kg−1 bw of the extract were administered orally to normal as well as Streptozotocin-induced diabetic rats to study its glycemic potential. The effect of repeated oral administration of the same doses of ethanolic extract was also studied on serum lipid profile of severely diabetic (SD) rats. The dose of 500 mg kg−1 bw was identified as the most effective dose as it lowered the blood glucose levels of normal by 42.12% and of diabetic by 43.42% during fasting blood glucose (FBG) and glucose tolerance test respectively. The SD rats were also treated daily with this identified dose of 500 mg kg−1 bw for 2 weeks and a significant reduction of 56.34% was observed in FBG level. Total cholesterol, low density lipoprotein and triglyceride levels were also decreased by 32.94, 64.06 and 48.46% respectively in SD rats whereas, cardioprotective high density lipoprotein increased by 16.45%. The reduced urine sugar level and increased body weight are additional advantages. These evidences clearly indicate that the ethanolic extract of defatted C. dactylon has high antidiabetic potential along with good hypolipidemic profile.
Keywords: antidiabetic, Cynodon dactylon, hypolipidemic, poaceae, streptozotocin
Introduction
Diabetes, a global burden, is characterized by fast elevation of blood sugar level. The incidence of diabetes mellitus is rising all over the world, especially in Asia. Many oral hypoglycemic agents, such as biguanides and sulfonylurea are available along with insulin for the treatment of diabetes mellitus but they have significant side effects (1,2) and sometimes they are found to be ineffective in chronic diabetic patients (3). Thus, there is an increasing demand of natural and synthetic products with high antidiabetic potential and lesser side effects (4). The researches conducted over the last several decades have shown that plant and plant-based therapies have high potential to treat and control diabetes (5–7) and its complications (8). Therefore, search for safe and more effective agents has continued to be an important area of active research. Since ancient times, diabetes has been treated orally with several medicinal plants or their extracts, based on folklore medicine. These herbal remedies are apparently effective, produce minimal or no side effects and are of relative low costs as compared to oral synthetic hypoglycemic agents. Furthermore, after the recommendation made by WHO on diabetes mellitus, investigation of hypoglycemic agents from medicinal plants have become more important (9–11).
Cynodon dactylon (Fam: Poaceae) is commonly known as ‘Doob’ in India. It is a weed and has been regarded to possess varied medicinal properties. It has been used to treat urinary tract infection, calculi and prostatitis. The plant possesses antimicrobial and antiviral activity (12). The aqueous fluid extract of the rhizome is used as anti-inflammatory, diuretic, anti-emetic, purifying agent and also in dysentery (13,14). The plant extract also has significant application in dropsy and secondary syphilis (15). The plant is traditionally used as an agent to control diabetes in India (16,17). The present investigation was therefore carried out to evaluate the traditional use of C. dactylon scientifically. Since the aqueous extract of C. dactylon was found to have high antidiabetic potential (18), the study of its ethanolic extract was therefore undertaken to exploit further its antidiabetic potential in Streptozotocin (STZ)-induced diabetic rats.
Methods
Preparation of Ethanolic Extract
Cynodon dactylon was collected from the campus of University of Allahabad, Allahabad, India. It was identified and authenticated by Botanical Survey of India, Allahabad branch and Prof. B.D. Singh, taxonomist, Allahabad Agriculture Institute, Naini, Allahabad. A voucher specimen (AA518) has been submitted. The whole green plant was washed with water and shade-dried. About 500 g of shade-dried plant was treated with hexane (2 × 2.5 l) to defat it and then extracted with ethanol (3 l) for 48 h. The resulting extract was filtered and concentrated in rotavapor under reduced pressure to give a residue (yield about 10.8% w/w) for further exploration.
Experimental Animals
Male albino wistar rats of same age group and body weight 150–200 g were selected for all the experiments. Animals obtained from National Institute of Communicable Disease (NICD), New Delhi, India, were housed in polypropylene cages at an ambient temperature of 25–30°C and 45–55% relative humidity with a 12 h each of dark and light cycle. Rats were fed pellet diet (Golden feed, New Delhi India) and water ad libitum. The study was approved by the Institutional Ethical Committee (83 a/a/04/CPCSEA).
Induction of Diabetes in Rats
Diabetes was induced by a single intraperitonial injection of freshly prepared STZ (50 mg kg−1 bw) in 0.1 M citrate buffer (pH = 4.5) to overnight fasted rats. After 3 days of STZ administration, rats with marked hyperglycemia (fasting blood glucose (FBZ) > 150 mg dl−1) were selected for the study. The rats with hyperglycemia were divided into two groups.
Mild diabetic (MD) rats with FBG 120–250 mg dl−1.
Severely diabetic (SD) rats showing FBG above 250 mg dl−1.
Estimation
Blood glucose level (BGL), total cholesterol (TC), high density lipoprotein (HDL) cholesterol and triglycerides (TG) levels were estimated using standard kits of Bayer diagnostics, India. Low density lipoprotein (LDL) and very low density lipoprotein (VLDL) cholesterol levels were calculated from the above measurement by using Friedwald formula (19). Urine sugar was detected by reagent-based Uristix from Bayer Diagnostics.
VLDL = TG/5
LDL = TC − (HDL + TG/5)
Experimental Design
Evaluation of Glycemic Management in Normal Healthy Rats
Twenty-four normal healthy male rats were fasted overnight and divided equally into four groups of six rats each. Pretreatment FBG levels of each group were estimated. Group I served as untreated control given vehicle (water only) whereas, other three groups II, III and IV were given the powder of concentrated ethanolic extract suspended in distilled water (DW) orally in doses of 250, 500 and 750 mg kg−1 bw respectively. Blood samples were collected from the tail vein after 2, 4 and 6 h and percentage of hypoglycemia variations were calculated for each group.
Glycemic Potential Assessment by Glucose Tolerance in MD Rats
The hypoglycemic effect of ethanolic extract was evaluated in MD rats by glucose tolerance test. The overnight fasted rats were divided into four groups V, VI, VII and VIII of six rats each. Pretreatment FBG levels of each group were estimated. Group V (Diabetic control) received vehicle (water only) whereas, variable doses of 250, 500 and 750 mg kg−1 bw of ethanolic extract were given orally to groups VI, VII and VIII respectively. BGL of each group was further estimated after 90 min of the treatment considered as 0 h value. About 2 g kg−1 bw glucose was given to all the groups and their BGLs were estimated hourly up to 2 h after glucose administration for evaluating glycemic potential.
Study of Antidiabetic and Antilipidemic Attributes in SD Rats
The study was carried on three groups IX, X and XI of six rats each. Group IX served as normal healthy control, group X as diabetic control and group XI was orally treated daily up to 2 weeks with a single dose of 500 mg kg−1 bw of ethanolic extract suspended in DW. Control rats (group IX and X) were given vehicle (DW) only. FBG, TC, HDL cholesterol and TG levels were estimated, before and after the treatment, weekly up to 2 weeks; LDL and VLDL cholesterol levels were also calculated.
Estimation of Urine Sugar and Body Weight in SD Rats
Urine sugar and body weight were also assessed as additional observations in groups X and XI before and after the treatment, weekly up to 2 weeks.
LD50 Experiment
Toxic effect of the water extract was also studied by LD50 experiment. Two groups of rats of both the sexes (six animals per group, three females and three males), weighing about 180–200 g were orally administered by a single dose of 10.0 g and 15.0 g of the defatted ethanolic extract of C. dactylon. Then rats were observed for gross behavioral neurologic, autonomic and toxic effects continuously. Food consumption, faeces and urine were also examined at 2 h and then at 6 h intervals for 24 h.
Statistical Analysis
Data were statistically evaluated using one-way ANOVA, followed by a post hoc Scheffe's test using the SPSS computer software, version 7.5. The values were considered significant when P < 0.05.
Results
Impact of Ethanolic Extract on FBG in Normoglycemic Rats
Table 1 represents the hypoglycemic impact of graded doses of ethanolic extract of defatted C. dactylon on BGLs of normal healthy rats. All the three doses of 250, 500 and 750 mg kg−1 bw of the extract produced a significant fall of 40.4, 47.1 and 39.4% respectively, BGL after 4 h of oral administration indicating thereby a clear hypoglycemic impact. The highest impact was marked in the animals treated with a dose of 500 mg kg−1 bw. Hence this dose was identified as the most effective dose for critical evaluation of glycemic potential in case of SD models.
Table 1.
Hypoglycemic impact of graded doses of ethanolic extract of C. dactylon in normal rats (mean ± S.D.)
| BGLs (mg dl−1) |
|||||
|---|---|---|---|---|---|
| Experimental animals | Treatment | Pretreatment levels | Post-treatment levels |
||
| FBG | 2 h | 4 h | 6 h | ||
| Control | DW | 84.6 ± 3.5 | 83.2 ± 2.7 | 82.7 ± 3.5 | 80.6 ± 3.6 |
| Treated | 250 mg kg−1 | 81.5 ± 6.5 | 62.5 ± 4.4 | 48.5 ± 4.2* | 68.3 ± 4.5* |
| Treated | 500 mg kg−1 | 78.3 ± 4.5 | 57.7 ± 3.5 | 41.4 ± 4.5* | 57.2 ± 6.5 |
| Treated | 750 mg kg−1 | 78.8 ± 7.5 | 61.0 ± 6.4 | 47.7 ± 4.5* | 63.4 ± 4.3* |
*P < 0.01 as compared to pretreatment level.
Glycemic Potential of Ethanolic Extract on GTT in MD rats
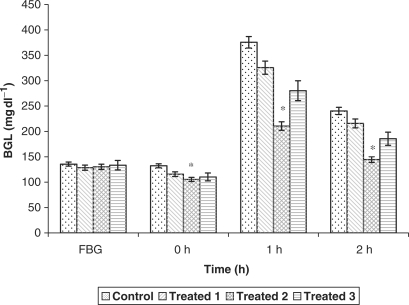
Fig. 1 depicts the effect of graded doses of 250, 500 and 750 mg kg−1 bw of defatted C. dactylon ethanolic extract on glucose tolerance up to 2 h in MD rats. This helps in deciding the dose of 500 mg kg−1 bw as the most effective dose for the study of severely diabetic models. The fall observed in BGL after 2 h of glucose administration was 10.2, 39.8 and 27.7% with the doses of 250, 500 and 750 mg kg−1 bw respectively. Since, the initial fall found at 1 h was 13.2, 43.9 and 25.3% from doses 250, 500 and 750 mg kg−1 bw respectively, suggesting thereby that 500 mg kg−1 bw is the most effective dose for assessing the antidiabetic potential of ethanolic extract in SD animals.
Figure 1.
Hypoglycemic impact of graded doses of ethanolic extract of C. dactylon on glucose tolerance in mild diabetic rats. *P < 0.01 as compared to control.
Antidiabetic and Antilipidemic Impact of the Most Effective Dose in SD Rats
The impact of repeated oral administration of defatted C. dactylon ethanolic extract on FBG and lipid profile of SD rats is shown in Table 2. FBG levels remain practically the same before and after the treatment with vehicle (water only) in case of normal control rats. Whereas, in diabetic control rats the FBG rises gradually in 2 weeks after treatment with vehicle (water only). Moreover, the 2-week treatment with the most effective dose (500 mg kg−1 bw) of the extract decreases FBG significantly from 310.6 mg dl−1 to 135.6 mg dl−1 indicating thereby a fall of 56.3% in BGL. This sharp fall of 56.3% in BGL is a clear evidence of significant antidiabetic effect of defatted C. dactylon. A distinct lowering of TC level of 32.9% was also observed with this most effective dose after two weeks. TG level also showed a significant reduction of 48.4% in the extract-treated group. The interesting observation was an increase of 16.4% in HDL level with a decrease of 64% in LDL after treatment.
Table 2.
Impact of oral administration of the ethanolic extract of C. dactylon on FBG and lipid profile in SD rats (mean ± S.D.)
| Experimental animals | Treatment | Pretreatment levels | Post-treatment levels |
|---|---|---|---|
| FBG (mg dl−1) | |||
| Normal (control) | DW | 90.2 ± 4.5 | 94.5 ± 5.6* |
| SD (control) | DW | 280.6 ± 5.7 | 301.4 ± 5.4* |
| SD (treated) | 500 mg kg−1 | 310.6 ± 7.5 | 135.6 ± 2.5* |
| TC (mg dl−1) | |||
| Normal (control) | DW | 57.8 ± 5.4 | 64.6 ± 8.4* |
| SD (control) | DW | 117.6 ± 6.4 | 130.8 ± 3.2* |
| SD (treated) | 500 mg kg−1 | 120.5 ± 3.8 | 80.8 ± 6.5* |
| HDL cholesterol (mg dl−1) | |||
| Normal (control) | DW | 30.7 ± 3.2 | 32.8 ± 4.6** |
| SD (control) | DW | 44.8 ± 2.8 | 27.2 ± 5.6** |
| SD (treated) | 500 mg kg−1 | 38.6 ± 3.5 | 46.2 ± 7.5** |
| TG (mg dl−1) | |||
| Normal (control) | DW | 85.3 ± 3.5 | 88.4 ± 4.5* |
| SD (control) | DW | 142.5 ± 3.2 | 156.7 ± 4.1* |
| SD (treated) | 500 mg kg−1 | 146.3 ± 2.7 | 75.4 ± 4.2* |
| LDL cholesterol (mg dl−1) | |||
| Normal (control) | DW | 10.0 ± 5.8 | 14.1 ± 7.4* |
| SD (control) | DW | 44.3 ± 6.2 | 72.2 ± 8.7* |
| SD (treated) | 500 mg kg−1 | 52.6 ± 4.2 | 18.9 ± 5.2* |
*P < 0.001 as compared to pretreatment levels.
**P < 0.05 as compared to pretreatment levels.
Effect of Ethanolic Extract on Urine Sugar and Body Weight in SD Rats
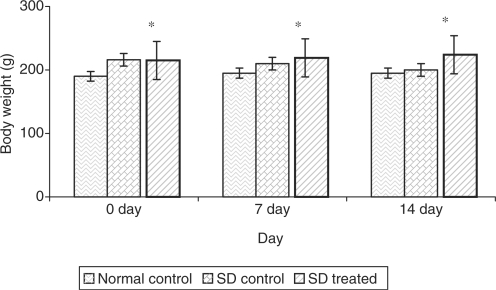
Table 3 and Fig. 2 reveal the impact of the most effective dose of 500 mg kg−1 bw of defatted C. dactylon ethanolic extract on urine sugar levels and body weights respectively of SD rats along with normal control rats. It has been observed that the urine sugar level decrease about 75% and body weight increases about 13% after the treatment of 2 weeks.
Table 3.
Impact of the most effective dose of C. dactylon ethanolic extract on urine sugar in SD rats (mean ± S.D.)
| Experimental animals | Treatment | Pretreatment levels | Post-treatment levels |
|---|---|---|---|
| Normal (control) | DW | Nil | Nil |
| SD (control) | DW | ++++ | ++++ |
| SD (treated) | 500 mg kg−1 | ++++ | + |
*P < 0.001 as compared with pretreatment.
Figure 2.
Impact of the most effective dose of C. dactylon ethanolic extract on body weight in SD rats. *P < 0.001 as compared with pretreatment.
LD50
Experiments were carried out on normal healthy rats. The behavior of the treated rats appeared normal. No toxic effect was reported at doses up to 10 and 15 times of the effective dose of the ethanolic extract as no mortality was observed in any of these groups.
Discussion and Conclusion
The findings of this study indicate that the graded doses of 250, 500 and 750 mg kg−1 bw of the ethanolic extract of defatted C. dactylon had a significant hypoglycemic effect of 40.4, 47.1 and 39.4% after 4 h of treatment in normal rats. The effect was dose-dependent up to 500 mg kg−1 bw. However, the response decreased at higher dose of 750 mg kg−1 bw. Such a phenomenon of less hypoglycemic response at higher doses is common in indigenous plants and has already been observed in Momordica cymbalaua (20), Aegle marmelose (21) and Vinca rosea (22).
The glucose tolerance test (GTT) studies of the MD animals reveal a maximum fall of 43.9% within 1 h by the dose of 500 mg kg−1 bw whereas, the doses of 250 mg kg−1 bw and 750 mg kg−1 bw produced a fall of about 13.2 and 25.3% only in 1 h in BGL. Since this maximum fall was sustained by the dose of 500 mg kg−1 even after 2 h of glucose administration in comparison to other doses, therefore, the suggested effective dose for the study of SD animals was found to be 500 mg kg−1 bw. The results of GTT of normoglycemic animals observed were on similar pattern verifying thereby the dose of 500 mg kg−1 as the most effective dose and therefore a dose of choice for the study of severe cases. This suggested and verified effective dose showed a marked decrease in FBG level of 56.3% in SD animal after 2 weeks treatment, among all other doses used.
It has been observed mostly that hyperlipidemia has been reported to accompany hyperglycemia states (23) and the most common lipid abnormality observed is hypertriglyceridemia. The marked hyperlipidemia that characterizes the STZ-diabetic rats seems to be a consequence of uninhibited action of lipolytic hormones on adipose tissue. Since insulin inhibits adipose tissue hormone sensitive lipase and reduces lipolysis, C. dactylon may mimic insulin action. The dose of 500 mg kg−1 bw of the ethanolic extract not only lowered the TC, TG and LDL levels by 32.9, 48.4 and 64% but also enhanced the level of cardio protective HDL cholesterol by 16.4% after 2 weeks of treatment. Several studies showed that an increase in HDL cholesterol is associated with a decrease in coronary risk and most of the drugs that decrease TC also decrease HDL cholesterol (24). It is important to note that in the present study the ethanolic extract not only decreased the TC but also increased the HDL cholesterol significantly after 2 weeks treatment as an additional advantage over the existing drugs. Since diabetes is associated with coronary complications (25), which is the major cause of morbidity and deaths in diabetic subjects (26,27) due to high levels of TC and more importantly LDL cholesterol, intake of ethanolic extract of defatted C. dactylon will therefore help in reducing the incidence of coronary events.
It has been reported recently that TG level is independently related to coronary heart disease (28,29) and most of the antihypercholesterolemic drugs do not decrease TG levels (30,31). This study clearly shows that the most effective dose of 500 mg kg−1 bw of the ethanolic extract decreases TG level also by 48.8% after 2 weeks treatment.
From this study we can conclusively state that defatted C. dactylon ethanolic extract has significant hypoglycemic as well as antidiabetic effects on BGLs of normal, mild and severely diabetic models. A substantial improvement on hyperlipidemia due to diabetes was also observed. Its unique effect of increasing HDL level about 16.4% has additional advantage of reducing coronary risk. Further characterizations of active components in C. dactylon are warranted and studies are in progress to isolate, identify and characterize active components.
These results suggest that the product of C. dactylon may provide a new therapeutic avenue against diabetes and diabetes-related complications—a global burden. Moreover, natural health products of vegetable origin were clearly indicated as a promising avenue for the prevention of chronic diseases (32), as raised notably by a panel of experts that convened in Montreal in 2004 (33). Wide-spread research is currently taking place in China, India and other countries too in order to explore new traditional Chinese, Indian and Western medicines that will prevent and treat diabetes mellitus and its chronic complications (34–36).
Acknowledgement
The authors are thankful to NMPB, Ministry of Health and Family Welfare Government of India, India for providing the financial assistance.
References
- 1.Holmann RR, Turner RC. Oral agents and insulin in the treatment of NIDDM. In: Pickup J, Williams G, editors. Textbook of Diabetes. Oxford: Backwell; 1991. pp. 407–69. [Google Scholar]
- 2.Williams G, Pickup JC. Textbook of Diabetes. II. Oxford: Blackwell; 1991. pp. 977–93. [Google Scholar]
- 3.Nagarajan S, Jain HC, Aulakh GS. Indegenous Plants used for Control of Diabetes. New Delhi: Pub. and Inf. Directorate; 1987. p. 86. [Google Scholar]
- 4.Rao BK, Kesavulu MM, Giri RAC. Fruit power in alloxan diabetic rats. J Ethnopharmacol. 1999;67:103–09. doi: 10.1016/s0378-8741(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 5.Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–75. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 6.Bailey LJ, Day C. Traditional plant medicine as treatment for diabetes. Diab Care. 1989;12:553–64. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 7.Marles RJ, Fransworth NR. Antidiabetic plants and their active constituents. Phytomed. 1995;2:137–89. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 8.Grover JK, Vats V, Rathi SS, Dawar R. Traditional Indian antidiabetic plants attenuate renal hypertrophy, urine volume and albuminuria in streptozotocin induced diabetic mice. J Ethnopharmacol. 2001;76:233–38. doi: 10.1016/s0378-8741(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 9.Alberti KGMM, Zimmet PZ. Definition diagnosis and classification of diabetes mellitus and its complications. Part I. Diagnosis and classification of diabetes mellitus provisinol report of a WHO consultation. Diab Med. 1998;13:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Gupta RK, Kesari AN, Murthy PS, Chandra R, Tandan V, Watal G. Hypoglycemic and antidiabetic effect of ethanolic extract of leaves of Annona squamosa L. in experimental animals. J Ethnopharmacol. 2005;99:75–81. doi: 10.1016/j.jep.2005.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Kesari AN, Gupta RK, Watal G. Hypoglycemic effects of Murraya koenigii on normal and alloxan diabetic rabbits. J Ethnopharmacol. 2005;97(2):247–51. doi: 10.1016/j.jep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Dhar ML, Dhawan M, Melhrotra Screening of Indian plants for biological activity Part I. Ind J Exp Biol. 1968;6:232–47. [PubMed] [Google Scholar]
- 13.Ahmed S, Reza MS, Jabbar A. Antimicrobial activity of Cynodon dactylon. Fitoterapia. 1994;65:463–64. [Google Scholar]
- 14.Kritikar KK, Basu BD. Indian Medicinal Plants. 2nd. Vol. 2. Lalit Mohan Publication, 1935: 1980. p. 2650. [Google Scholar]
- 15.Kesari AN, Gupta RK, Singh SK, Diwakar S, Watal G. Hypoglycemic and antihyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. J Ethnopharmacol. 2006;107:374–79. doi: 10.1016/j.jep.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 16.Kirtikar KR, Basu BD. Indian Medicinal Plants. IV. Allahabad: CM Basu; 1933. p. 1020. [Google Scholar]
- 17.Brahmvarchas K. Ayurveda ka pran: vanaushdi vigyan. 3rd edn. 2003. p. 188. [Google Scholar]
- 18.Singh SK, Kesari AN, Gupta RK, Watal G. Assessment of Antidiabetic Potential of Cynodon dactylon Extract in Streptozotocin Diabetic Rats. J Ethnopharmacol. 2006 doi: 10.1016/j.jep.2007.07.039. (Revised) [DOI] [PubMed] [Google Scholar]
- 19.Friedwald J, Levy YR, Friedrickson SD. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.Rao VV, Dwivedi SK, Swarup D, Sharma SR. Antidiabetic and hypolipidemic effects of Momordica cymbalaua Hook. Fruit power in alloxan diabetic rats. Curr Sc. 1995;69:103–209. [Google Scholar]
- 21.Sharma SR, Dwivedi SK, Varshney VP, Swarup D. Antihyperglycemic and Insulin release effects of Aegle marmelos leaves in streptozotocin-diabetic rats. Phytother Res. 1996a;10:426–28. [Google Scholar]
- 22.Chattopadhyay RR, Sarkar SK, Ganguly S, Banerjee RN, Basu TK. Hypoglycemic and Antihyperglycemic effects of leaves of Vinca rosea Linn. Ind J Physiol and Pharmacol. 1991;35:145–51. [PubMed] [Google Scholar]
- 23.Sharma SR, Dwivedi SK, Swarup D. Hypoglycaemic and hypolipidemic effects of Cinnamomum tamala Nees leaves. Ind J Exp Biol. 1996b;34:372–74. [Google Scholar]
- 24.Randle PJ, Gailand PB, Hales CN, Neiosholine EA. The glucose fatty acid cycle: its role in insulin sensitivity and metabolic disturbance of diabetes. The Lancet. 1963;1:785. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 25.Baynes JW. Role of oxidative stress in the development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PWF. High density lipoprotein, low density lipoprotein and coronary heart disease. Am J Card. 1990;66:7–10A. doi: 10.1016/0002-9149(90)90562-f. [DOI] [PubMed] [Google Scholar]
- 27.Temme EH, Van HPG, Schouten EG, Kesteloot H. Effect of a plant sterol-enriched spread on serum lipids and lipoprotein in mildly hypercholesterolaemic subjects. Acta card. 2002;57:111–15. doi: 10.2143/AC.57.2.2005382. [DOI] [PubMed] [Google Scholar]
- 28.Huse DM, Oster G, Killen AR, Lacey MJ, Golditz GA. The economic costs of non-insulin dependent diabetes mellitus. J A Med Assoc. 1988;262:2708–13. doi: 10.1001/jama.262.19.2708. [DOI] [PubMed] [Google Scholar]
- 29.Bainton D, Miller NE, Botton CH, Yarnell JWG, Suretmen PM, Baker IA, et al. Plasma triglyceride and high density lipoprotein cholesterol as predictors of ischemic heart disease in British man. Brit Heart J. 1992;68:60–6. doi: 10.1136/hrt.68.7.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taskinen MR. Lipoprotein and apoproteins in diabetes. Diab Res. 1993;12:122–34. [Google Scholar]
- 31.El-Hazmi MA, Warsy AS. Evaluation of serum cholesterol and triglyceride levels in 1-6-year-old Saudi children. J Tropical Pediatrics. 2001;47:181–85. doi: 10.1093/tropej/47.3.181. [DOI] [PubMed] [Google Scholar]
- 32.Punitha ISR, Rajendran K, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haddad PS, Beauchamp G, Co⁁té G, Boivin M. Maintenance of an efficient and equilibrated immune system through the novel use of natural health products: synopsis of a symphosium. Evid Based Complement Alternat Med. 2005;2:237–38. [Google Scholar]
- 35.Xia R, Huang P, Shao GM. Nourishing yin and promoting blood circulation of TCM to treat hemorheologic disorder induced by diabetes mellitus in rats. Evid Based Complement Alternat Med. 2006;24:1–5. doi: 10.1093/ecam/nel088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Punitha ISR, Rajendran K, Shirwaikar A, Shirwaikar A. Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid Based Complement Alternat Med. 2005;2:375–81. doi: 10.1093/ecam/neh099. [DOI] [PMC free article] [PubMed] [Google Scholar]