Abstract
PPARγ is a member of the ligand-activated nuclear receptor superfamily: its ligands act as insulin sensitizers and some are approved for the treatment of metabolic disorders in humans. PPARγ has pleiotropic effects on survival and proliferation of multiple cell types, including cancer cells, and is now subject of intensive preclinical cancer research. Studies of the recent decade highlighted PPARγ role as a potential modulator of angiogenesis in vitro and in vivo. These observations provide an additional facet to the PPARγ image as potential anticancer drug. Currently PPARγ is regarded as an important target for the therapies against angiogenesis-dependent pathological states including cancer and vascular complications of diabetes. Some of the studies, however, identify pro-angiogenic and tumor-promoting effects of PPARγ and its ligands pointing out the need for further studies. Below, we summarize current knowledge of PPARγ regulatory mechanisms and molecular targets, and discuss ways to maximize the beneficial activity of the PPARγ agonists.
1. INTRODUCTION
PPARs are nuclear hormone receptors and targets for the compounds inducing peroxisome proliferation. The family encompasses three species, PPARα, PPARβ/δ, and PPARγ. PPARγ, the best researched of the three, is presented by the two isoforms, γ1 and γ2 whereas PPARγ2 contains 30 extra amino acids at the N-terminus due to initiation from the alternative transcription start (see Figure 1(a)). PPARγ, a key player in adipocyte differentiation and glucose metabolism, is abundantly expressed in adipose tissues [1]. On the other hand, it is expressed in all the cells of the normal and pathological vascular beds, including endothelial cells (EC), macrophages (MΦ), and vascular smooth muscle cells (VSMCs), in a variety of tumor cells, and, at lower levels, in lymphatic tissue, intestinal epithelium, retina, and skeletal muscle [2]. PPARγ is a potent modulator of the EC and VSMC function and inflammation: its effects on the tumor cells, tumor-associated MΦs (TAM), and tumor vasculature (EC and VSMCs) significantly attenuate tumor progression [3, 4], suggesting that PPARγ ligands may become new convenient therapeutic modifiers targeting simultaneously tumors and their microenvironment [5]. Unfortunately, recent studies reveal the tumor-promoting and pro-angiogenic PPARγ activities; while in most cases PPARγ agonists attenuate tumor growth and angiogenesis, troglitazone (TGZ, a now rejected PPARγ agonist) promotes hepatic carcinogenesis and liposarcomas. Moreover, some PPARγ agonists promote the differentiation of the circulating endothelial progenitor cells (EPC) [6] and elicit angiogenesis in vivo [7]. In some instances, PPARγ ligands increase the production of angiogenic stimuli, including VEGF or NO, by the EC or tumor cells [8]. Thus, the use of PPARγ modulators to manage tumor progression is more complex than it appears at a glance and requires precise knowledge of the molecular events involved in their pro- and antitumorigenic actions. Below we summarize the current knowledge of PPARγ effects and molecular mechanisms and delineate ways to augment PPARγ anti-angiogenic and antitumor effects while minimizing its pro-angiogenic and tumor-promoting capacities.
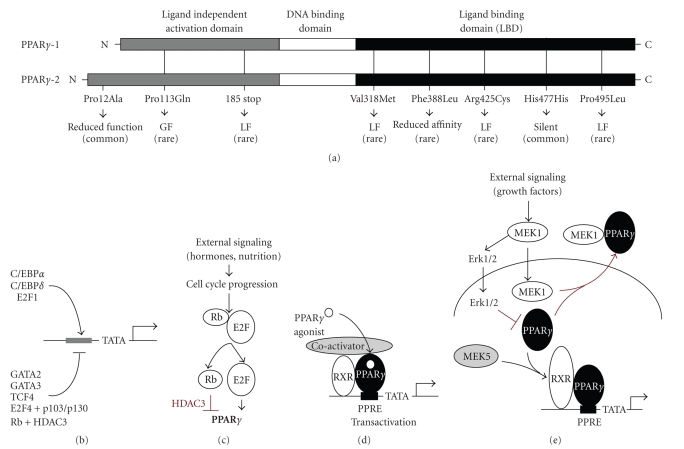
Figure 1.
PPARγ structure and regulation. (a) Schematic representation of the domain structure of the PPARγ-1 and PPARγ-2. The mutations associated with metabolic syndrome are indicated. LF: loss of function; GF: gain of function. (b) Positive and negative regulators of the PPARγ gene transcription. (c) The regulation of PPARγ levels by Rb and E2F. (d) The mechanism of ligand-dependent PPARγ activation. (e) The regulation of PPARγ activity by MEK and Erk kinases: MEK1 activates Erk-1/2, which phosphorylates PPARγ and targets it to proteasomes; in addition, MEK1 binds PPARγ in the nucleus and exports it to the cytoplasm. MEK5 can serve as coactivator for the PPARγ.
2. PPARγ AND ANGIOGENESIS
Angiogenesis is a complex process involving diverse cell types and controled by the pro- and anti-angiogenic factors produced by the ECs, VSMCs, and in vascular microenvironment by the stromal, tumor, and inflammatory cells. The balance between positive and negative angiogenesis regulators determines if the existing capillaries would expand, regress, or remain quiescent [9]. Active angiogenesis involves invasion, migration, and proliferation of the EC followed by the morphogenesis (assembly) of the neovessels. It is aided by the recruitment of the EPCs, which may constitute up to 50% of the cells in a neovessel [10]. The newly formed capillaries recruit vascular smooth muscle cells (VSMCs), which stabilize and render quiescent the newly formed capillaries: in thus stabilized mature vessels, the interactions between angiopoietin-1 (Ang-1) on the EC and Tie-2 receptor on the VSMCs generate signals that dampen EC sensitivity to the pro- and anti-angiogenic molecules [11]. Brown adipose tissue, a thermogenic organ in mammals responds to cold by increasing VEGF, thus creating permissive conditions for the fat expansion. Treatment of brown adipocytes with PPARγ ligands reduces VEGF-C mRNA pointing to their anti-angiogenic potential [12]. Moreover, chimeric mice null for PPARγ show gross defects in placental vascularization [13]. Natural and synthetic PPARγ ligands block VEGF-driven angiogenesis in vivo, in matrigel implants, in rodent cornea, and choroid [14–16]. RGZ suppresses the growth and angiogenesis of the glioblastoma, Lewis lung carcinoma, liposarcoma, and rhabdomyosarcoma in mouse models [17], which is partly due to the PPARγ-mediated apoptosis of the tumor EC and the repression of VEGF production by the tumor cells. Below, we elucidate the PPARγ pleiotropic effects on angiogenesis and suggest optimization strategies.
3. PPARγ REGULATORY MECHANISMS
PPARγ can be regulated at expression level: PPARγ gene is repressed by the GATA-2 and 3, TCF4 [18] (see Figure 1(b)), and transactivated by CAAT enhancer binding proteins (C/EBPs), predominantly C/EBPα, ADD1/SREBP1, and E2F1 (see Figure 1(b)) [19]. E2F proteins have dual effect on PPARγ expression: during cell cycle progression, phospho-Rb releases E2F1 to activate PPARγ promoter (see Figure 1(c)), however, E2F4, if bound to the p103 or p130 Rb, represses PPARγ transcription [2, 18]. Moreover, hypo-phosphorylated Rb binds PPARγ and recruits histone deacetylase (HDAC) 3 to the complexes, causing transcriptional repression (see Figure 1(c)) [19]. Multiple growth factors including platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), angiotensin II, tumor necrosis factor (TNF) α, interleukin (IL) 1β, and tumor-derived growth factor β(TGF-β) increase PPARγ expression by the vascular smooth muscle cells (VSMCs), via Egr-1. In contrast, AP-1 aided by Smad3/4 represses PPARγ promoter activity [20]. Mitotic, stress, and inflammatory signals cause PPARγ degradation via phosphorylation on Ser84 of the mouse PPARγ (Ser112 of the human molecule) in a consensus MAPK target motif PXSPP [21] by ERKs, JNKs, and p38, which leads to ubiquitination and proteasomal clearance [22]. Ser to Ala PPARγ mutant shows increased transcriptional activity, similar effect is caused by coexpression of a phosphoprotein phosphatase [21]. In human PPARγ, substitution of proline to glutamine at position 115 results in constitutive activation by blocking MAPK phosphorylation at position 114: patients with such mutation display extreme obesity [23]. Likewise, increased phosphorylation on Ser112 in Dok-1 null mice caused lean phenotype, which is lost in mice expressing phosphorylation-defective PPARγ [24]. The effect of PPARγ on angiogenesis remains to be determined.
The next regulatory step involves cofactor recruitment: upon ligand binding, PPARγ forms heterodimers with the retinoic acid X receptor (RXR), and occupies twin PPAR response elements AAGGTCAnAAGGTCA (PPRE); binding of the RXR ligands further increases transcriptional activity of the PPARγ/RXR dimers (see Figure 1(d)). Coactivators including SRC1, CBP/p300, pCAF/GCN, and PGC bind PPARγ/RXR complexes in a ligand-dependent manner [19]; PGC-1α has recently been linked to HIF-independent induction of vascular endothelial growth factor (VEGF) and angiogenesis [25]. PPARγ activity can be suppressed due to phosphorylation, which results in nuclear export, both executed by MEK-1 (see Figure 1(e)) [26]. In contrast, MEK-5 acts as PPARγ coactivator (see Figure 1(e)) [27].
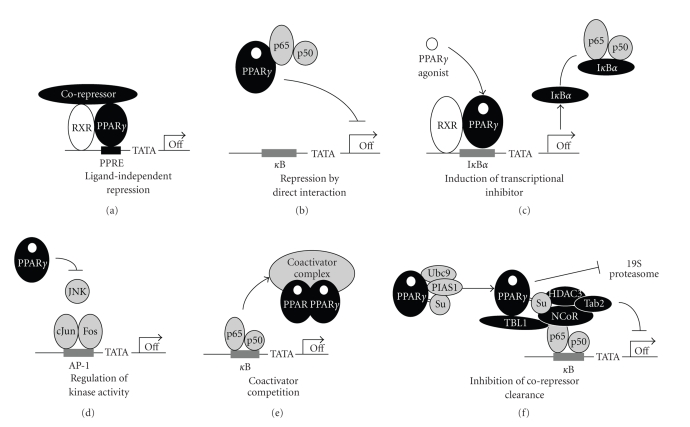
In addition to its activator function (see Figure 1(d)), PPARγ represses transcription of select genes. PPARγ transrepression of AP-1, nuclear factor of the activated T-cells (NFAT), NFκB, and STAT-1 is well documented [19, 28]. Typical PPARγ corepressors SMRT and NCoR corecruit HDAC3, transducin beta-like protein-1 (TBL-1) and TBL-1-related protein 1 (TBLR1) [29]. The repression can be ligand-independent, with PPAR/RXR dimers forming repressor complexes in the absence of the ligands (see Figure 2(a)). Ligand-dependent repression may occur by direct interaction with target transcription factors (see Figure 2(b)), modulation of the transcriptional regulators (see Figures 2(c) and 2(d)), by coactivator sequestration (see Figure 2(e)), or the blockade of corepressor clearance (see Figure 2(f)). The latter requires PPARγ sumoylation, which keeps HDAC3 associated with repressor complexes and prevents proteasomal clearance of their components [19]. NCoR complexes interact with a limited subset of promoters, which explains gene-specific repression by PPARγ.
Figure 2.
Mechanisms of transrepression by PPARγ. (a) Ligand-independent repression: preferential recruitment of corepressors in the absence of agonists. (b) Direct binding and sequestration of transcription factors on example of NFκB. (c) Activation of genes encoding inhibitors of transcription factor (e.g., NFκB inhibitor, IκBα). (d) Direct binding and inactivation of kinases, which activate transcription factors (e.g., the blockade of JNK activation of cJun). (e) Competitive binding of the coactivator complex. (f) The blockade of corepressor clearance: sumoylated PPARγ stabilizes corepressor complexes (NCoR, Tab2, and TBL1) on the promoter and facilitates the recruitment of HDAC3. In the absence of sumoylation, NCoR, Tab2, and TBL1 are subject to ubiquitination and proteasomal clearance.
4. LIGANDS
PPARγ ligands encompass wide range of structurally diverse compounds, natural and synthetic. Natural ones include long chain polyunsaturated fatty acids and derivatives (eicosanoids, prostaglandins, like 15-deoxy-Δ12,14-prostaglandin J2 (15D-PGJ2)) and nitrolinoleic acids. Synthetic ones include thiazolinediones (TZDs, or glitazones), of which rosiglitazone (RGZ) and pioglitazone (PGZ) are marketed for the treatment of type 2 diabetes and tyrosine-based derivatives (glitazars) including tesaglitazar and farglitazar, the dual agonists of PPARα and PPARγ [30]. Although their ability to alleviate insulin resistance, vascular complications, and angiogenesis is well documented, the adverse effects include hepatotoxicity, renal toxicity, weight gain, and fluid retention [30], all of which complicate the long-term use. Thus further work is required to develop PPARγ ligands into safe and efficacious treatment for diabetes, cancer, and angiogenesis-related disease. Selective PPARγ modulators (SPPARMs) represent one way to overcome this problem: they are designed to retain the desired PPARγ properties, while minimizing adverse side effects. SPPARMs can be categorized as tightly binding partial agonists (GW0072) or weakly binding full agonists of PPARγ (MCC-555/netoglitazone, NC-2100) [31].
5. ANTI-ANGIOGENIC EFFECTS OF PPARγ IN DIVERSE CELL TYPES: ENDOTHELIAL-SPECIFIC EVENTS
Human micro- and macrovascular endothelial cells (EC) express PPARγ [32]. PPARγ activation by the natural (15D-PGJ2) or synthetic ligands (TGZ, RGZ, ciglitazone, and pioglitazone) potently inhibits in vitro proliferation and morphogenesis by EC of diverse tissue origin [33]. 15D-PGJ2 and ciglitazone (CGZ) also induce EC apoptosis through PPARγ-dependent pathway. The PPARγ involvement is supported by (1) nuclear translocation, (2) increased transcriptional activity, (3) attenuation of the EC apoptosis by the decoy PPRE oligonucleotide, and (4) increased background apoptosis in PPARγ overexpressing EC, further enhanced by the ligand exposure [15]. PPARγ activation interferes with EC migration: TZDs block EC chemotaxis up the VEGF or leptin gradients, by blocking PI3K/Akt and Erk1/2 signaling [34–37]. In both cases, PPARγ/SREBP1 complex drives the transcription of PTEN tumor suppressor, which opposes the induction of Akt [38], see Figure 4(a).
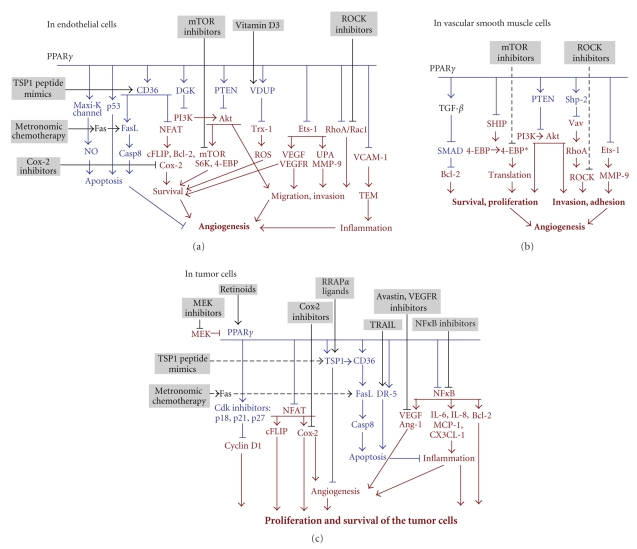
Figure 4.
PPARγ effects on the endothelial, pericytic, tumor and immune cells in the tumor microenvironment: the consequences of angiogenesis and possible ways to augment antitumor actions. Pro-angiogenic and tumor-promoting events are shown in red. The opposing effects are in blue. The proposed drugs are shown in black. (a) Summary of the PPARγ molecular effects in the endothelial cells. TEM, transendothelial migration. (b) PPARγ molecular effects on the VSMCs. (c) The effects on macrophages and tumor cells.
PPARγ ligands hamper the response of the vascular EC to VEGF by lowering VEGFR1 (Flt-1) and VEGFR2 (KDR). The regulation of VEGFR2 is biphasic: in the absence of the ligands, PPARγ enhances Sp1/Sp3 binding to the promoter and opposes it if ligands are present [39]. VEGFR2 decrease also reduces EC survival under stress or in the presence of anti-angiogenic factors, see Figure 4(a).
PPARγ induction decreases UPA and increases PAI-1 expression by the EC, thus lowering their ability to invade surrounding tissues [14, 16]. In the brain microvasculature, PPARγ stimulation dampens the activation of RhoA and Rac1 GTPases critical for the cell adhesion and migration [40], see Figure 4(a).
Proapoptotic PPARγ effects in the EC can be mediated by p53 [41–43] or by the opening of Maxi-K channel (Ca2+ activated K+ channel) whereas the protective Bcl-2 levels plummet and apoptotic Bax increases. In addition, increased eNos production causes elevated NO, which, in contrast with its usual protective effect contributes to EC death [44]. Downmodulation of the thioredoxin (Trx-1) by PPARγ via vitamin D3 upregulated protein (VDUP-1) also contributes to the EC killing, likely via formation of inactive PTEN/Trx-1 complexes [45]. PPARγ also ameliorates EC activation by glucose via the induction of diacylglycerol kinase (DGK), the reduction of diacylglycerol, which attenuates PKC activity and decreases angiogenesis [46]. Importantly, PPARγ activation enhances surface CD36, a lipid scavenger receptor, which transmits the anti-angiogenic signal of thrombospondin-1 (TSP1) [47] a potent endogenous inhibitor of angiogenesis, see Figure 4(a).
PPARγ produces complex effect on the endothelial progenitor cells (EPC): RGZ enhances the expression of the endothelial markers CD31 and VEGFR2 on the circulating EPCs, however VE-cadherin and CD146 remain low; increased uptake of oxidized lipids suggests elevated CD36, which increases the sensitivity to TSP1. EPCs from the diabetic patients treated with RGZ display better adherence to fibronectin than those from untreated diabetics and normal donors [6]. This is consistent with reduced oxidative stress and improved re-endothelialization by the EPCs from diabetic patients in RGZ-treated mice [48]. EPCs from the RGZ-treated diabetics migrate more vigorously than those from untreated subjects, but similarly to the EPC from untreated normal donors [6] suggesting that RGZ rather normalizes than increases the EPCs migratory potential. PGZ effect on cultured EPCs is twofold: it enhances the expression of endothelial markers at a lower dose (1 μm) and reduces it at higher (10 μm) concentration. PGZ also stimulates the expression of TGFβ and TGFβ receptor [49], and thus initiates EPC conversion to the VSMC phenotype [50]: increased VSMC presence may stabilize the neovasculature and thus reduce angiogenesis. This may explain why PPARγ agonists ameliorate glomerulonephritis in mouse model without increase in EPC homing [51].
6. IN VASCULAR SMOOTH MUSCLE CELLS
Genetic variations associated with atherosclerosis point to PPARγ role in associated metabolic and vascular events [52]. In atherosclerotic lesions, PPARγ promotes vascular repair and re-endothelialization, while suppressing neointima formation. PPARγ attenuates vasoconstrictive remodeling by blocking NADPH oxidases [53] and inhibits VSMCs proliferative and migratory responses to multiple cytokines and growth factors including PDGF-BB, bFGF, thrombin, insulin, and angiotensin II (AngII). PPARγ interferes with VSMC proliferation and survival by blocking the downstream targets of ERK1/2 and PI3K/Akt, SHIP2 and two important regulators of mRNA translation, p70S6 kinase and 4-EBP translation initiation inhibitor [54]. In addition, PPARγ activation enhances the expression of Shp-2 phosphatase, which dephosphorylates/inactivates Vav, a guanidine exchange factor for RhoA, impairs the activation of Rho-associated kinase (ROCK), and suppresses VSMC proliferation and migration [55]. PPARγ inhibits VSMC migration but not the attachment and motility components of the migratory response: the inhibition of PDGF-BB driven VSMC migration is due to the transcriptional repression of Ets-1, which, in turn, drives MMP-9 and invasion [56], see Figure 4(b).
PPARγ activation causes VSMC growth arrest via multiple pathways: (1) by suppressing proteasomal degradation of the p27/Kip; (2) via transrepression of the E2F target, minichromosome maintenance protein, MCM7, which blocks replication [2]; (3) by blocking Ets-1 dependent transactivation of telomerase promoter [57]. PPARγ and its agonists potently induce VSMC apoptosis (1) through direct upregulation of GADD45 and p53 via an Oct-1 dependent mechanism (PPRE are identified in GADD45 and p53 promoters) [58, 59]; (2) by inducing the TFG-β/ALK/Smad pathway, subsequent Bcl-2 repression, and Smad-dependent induction of GADD45 [60]; (3) through transcriptional upregulation of the interferon regulatory factor-1 (IRF-1), a proapoptotic, antiproliferative transcription factor [61], see Figure 4(b).
All PPARγ-dependent changes in VSMC behavior can contribute to its anti-angiogenic function: decreased VSMC migration, and proliferation, plus increased apoptosis restrict VSMC incorporation in the vasculature and therefore the stability of neovessels. Moreover, ECs of the immature, VSMC-poor vessels are vulnerable to the apoptotic signals by angiogenesis inhibitors, see Figure 4(b).
7. ANTI-INFLAMMATORY EFFECTS
PPARγ affects inflammation directly, by driving CD36-dependent apoptosis in MΦs [62, 63], or indirectly, by reducing VCAM-1 expression by the ECs and thus blocking transendothelial migration (TEM) of monocytes and MΦs during chronic inflammation typical for diabetes and cancer. In contrast, E-selectin, a mediator of the acute immune response, is not altered by PPARγ [64]. Statins increase anti-inflammatory Cox-2 in MΦs, which, in turn, increases endogenous 15D-PGJ2, activates PPARγ, and upregulates its downstream target, CD36 [65]. In addition, PPARγ ligands cause NFκB transrepression, thus reducing the production of inflammatory cytokines (IL-8, IL-6, MCP-1, and CX3CL1-1) by MΦs, and thus disrupting paracrine loop that attracts tumor-associated MΦs (TAM) and thus stimulates angiogenesis and tumor growth [66], see Figure 4(c).
8. IN TUMOR CELLS AND STROMA
PPARγ is expressed in human carcinomas of the breast, colon, esophagus, liver, lung, pancreas prostate, stomach, and thyroid, also in neuroblastoma, astrocytoma, and glioma: in all of these PPARγ ligands repress or delay xenograft growth in mouse models [67].
PPARγ ligands affect tumor cells in several ways: they reduce proliferation, enhance apoptosis, and modulate angiogenic phenotype of the tumor cells. PPARγ targets cyclin D1 via the inhibitors of cyclin-dependent kinases (Cdk), p18, p21, and p27, causing a decline in Rb phosphorylation [1] and arresting cells in G1 phase: PPARγ acts via p21 and p27 in pancreatic cancer and via p18 in hepatoma (see Figure 3(a)). On the other hand, glitazones repress the production of Cdk2, 4 and 6 in carcinomas of the bladder, breast, lung, and pancreas via GADD45 [67] (see Figure 3(b)). PPARγ activation also restores PTEN expression in tumor cells and thus blocks PI3K/Akt axis [38], it can also initiate a negative feedback loop, which consists of calcineurin phosphatase, nuclear factor of the activated T-cells (NFAT), and down syndrome critical region 1 (DSCR1), which inhibits calcineurin and blocks NFAT activity necessary for proliferation and survival (see Figure 3(c)) [68], see Figure 4(c).
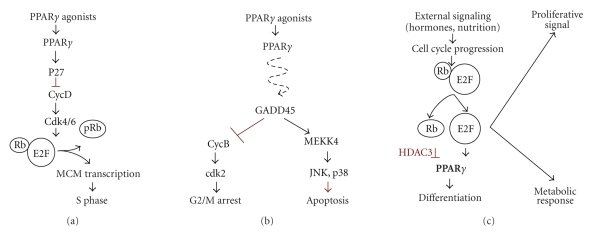
Figure 3.
PPARγ effects in cancer cells. (a) The induction of Cdk inhibitor, p27 causes growth arrest due to reduced MCM7 activity and subsequent blockade of replication. (b) The induction of GADD45 impairs Cyclin B and causes G2M growth arrest. In addition, the activation of JNK and p38 kinases via MEKK4 initiates cell death by apoptosis. (c) PPARγ activation by hormones and nutrition in normal cells and by agonists in cancer cells may activate the differentiation programs.
PPARγ induction also causes tumor cell apoptosis by downmodulating prosurvival proteins cFLIP and Bcl-2, while increasing proapoptotic Bax and BAD, as occurs in glioblastoma [69] or by the interference with the PI3K/Akt signaling [38]. Conversely, PPARγ often augments the expression of TNF-related apoptosis inducing ligand (TRAIL), which selectively eliminates cancer cells [70], see Figure 4(c).
In some cases, PPARγ activation induces tumor cell differentiation (e.g., liposarcoma, breast and pancreatic cancer, neuroblastoma, glioma, bladder carcinoma, and lung carcinoma). The differentiation is evidenced by the increase of the general markers of differentiated state, such as E-cadherin, and downregulation of the specific markers of progenitor lineages, also by morphology changes consistent with differentiated state (see Figure 3(d)) [1, 67].
Finally, treatment with the PPARγ ligands frequently downregulates the expression of pro-angiogenic factors VEGF [17], IL-8 [71], Ang-1 [72], and Cox-2 [73] and thus suspends tumor angiogenesis. Moreover, mice null for PPARγ show impaired tumorgenesis, due to the dramatic increase in TSP-1 [5], see Figure 4(c).
9. PPARγ PRO-ANGIOGENIC/TUMORIGENIC EFFECTS
In contrast to the majority of findings, a recent study suggests that PPARγ ligands may have pro-angiogenic properties both in vitro [74], in an endothelial/interstitial cell coculture assay, and in a murine corneal angiogenesis model in vivo [74]. The magnitude of the angiogenic response caused by PPARγ ligands has not been compared to the angiogenesis elicited by typical stimuli (VEGF, bFGF); also, the contradiction between these results and previous studies has not yet been addressed.
PPARγ pro-angiogenic effects are associated with the induction of VEGF and increased phosphorylation of eNOS and AKT [7, 75], which cause elevated VEGF production in human and rodent VSMCs, MΦs and tumor cells [76–79], VEGF and VEGFR levels in the ECs and myofibroblasts [80]. Although PPARγ ligands inhibit xenografted human tumors [1, 33], in one study using mouse model of colon cancer (APC/Min) PPARγ ligands increased the number of precancerous polyps, tumor frequency and size [81]. However, in two other models, APC-deficient HT-29 xenografts and azoxymetane-induced tumors PPARγ ligands suppress tumor growth and angiogenesis [82, 83]. Of the multiple small-scale clinical trials using PPARγ ligands for cancer treatment, only two showed promising results: in an early study TGZ caused prolonged PSA stabilization in prostate cancer patients [84], while PGZ combined with low-dose chemotherapy and rofexoxib produced moderate improvement in the patients with high-grade glioma [85]. In contrast, patients with breast, colon, and thyroid cancers showed no significant response [86–88]. Thus, the use of PPARγ ligands in clinical practice obviously requires optimization, and the answers may come from the use of combination or complementation treatments.
10. PPARγ LIGANDS IN COMBINATION TREATMENTS: CAN WE AUGMENT THE BENEFICIAL EFFECTS?
The information above narrows down the list of PPARγ targets critical for its anti-angiogenic and antitumor effects (see Figure 4(a)). PPARγ reverses angiogenic functions in the ECs by blocking the expression of VEGF-A and its receptor, VEGFR2 by blocking Ets-1 transcription factor, and by dampening the prosurvival PI3K/Akt cascade, likely via PTEN induction. It also deactivates RhoA/Rac1 small GTPases which enable EC migration. NFAT deactivation lowers the levels of the apoptosis inhibitors, cFLIP and Bcl-2, and critical invasion molecules UPA and MMP 9. In addition, PPARγ promotes the following proapoptotic events: it elevates expression of the proapoptotic CD36 and TSP1 receptor-ligand duo; increases p53 stability; opens of the Maxi-K channel to upregulate nitric oxide (NO), which, paradoxically, causes apoptosis. In addition, PPARγ suppresses Trx-1 and ROS levels by upregulating VDUP-1, a vitamin D3 target. Finally, PPARγ ligands block protein synthesis via 4-eBP and p70S6 kinase, both the targets of mTOR pathway.
In the VSMC, PPARγ represses the activation of prosurvival Erk-1 and PI3K/Akt and SHIP thus sustaining the unphosphorylated, active state of 4-EPB, a negative regulator of translation. It also enhances the activity of Shp-2 phosphatase, which blocks Vav, the trigger of RhoA/ROCK pathway necessary for survival and migration; PPARγ also interferes with VSMC Bcl-2 expression by enhancing TGFβ/Smad2 and disrupts MMP-9 production by blocking Ets-1 (see Figure 4(b)).
In MΦs and tumor cells, PPARγ through transrepression of NFκB and NFAT lowers the production of multiple growth factors and inflammatory cytokines including VEGF, Ang-1, cyclo-oxygenase (Cox) 2, IL-6, IL-8, MCP-1, and CX3CL-1. PPARg also enhances the production of thrombospondin (TSP) 1: therefore angiogenic balance tips in favor of vascular quiescence. In addition, PPARγ lowers the resistance of tumor cells and tumor-associated MΦs (TAM) to stress and apoptotic stimuli by blocking cyclin D1 via cdk inhibitors p18, p21, p27, by repressing antiapoptotic Bcl-2 and FLIP, by upregulating proapoptotic CD36 in MΦs, and Bax and BAD in tumor cells (see Figure 4).
This comprehensive list of PPARγ targets and interacting proteins can be used for intelligent design of the optimal combination therapies based on PPARγ ligands to achieve the best anti-angiogenic and anticancer activity. For example, it stands to reason to expect that EC apoptosis caused by PPARγ can be augmented by supplying CD36 ligand, TSP1 or its peptide mimics, such as ABT-510 [89]. Indeed, PPARγ ligands 15PG-E2, TGZ and RGZ, and TSP1 anti-angiogenic peptide ABT-510 synergistically block angiogenesis and curtail the growth of lung and bladder carcinoma xenografts, by initiating CD36-dependent apoptotic events in remodeling tumor endothelium [47]. Furthermore, TSP1 expression is enhanced by the low-dose metronomic chemotherapy, including cytoxan, docetaxel, and 5-fluorouracil [90–92]. Thus cytoxan, docetaxel, and 5-fluorouracil are likely to potentiate the PPARγ anti-angiogenic effects in EC and to reduce tumor-associated inflammation responses by killing TAMs. This is supported by the fact that 15D-PGE2 enhances antitumor activity of docetaxel against lung carcinoma cell lines [93]. In addition, metronomic chemotherapy enhances the expression of Fas, a critical apoptosis mediator induced by the TSP1/CD36 interaction and thus potentiates the activity of TSP1 derivatives, such as ABT-510 [94, 95]. Hence, combined use of PPARγ ligands and metronomic regimens of chemotherapy agents is likely to be more effective than individual treatments.
PPARγ blockade of the EC and VSMC migration involves the inhibition of RhoA/ROCK signaling [40, 65], which makes ROCK inhibitors likely candidates for the use in combination with PPARγ ligands. This is doubly important, since ROCK activates Myc pathway and thus abolishes TSP1 expression by the tumor cells [96]. ROCK inhibitors show strong toxic effects at therapeutic doses, thus their clinical use is problematic. However, combined use with PPARγ ligands may allow to lower their effective concentration and therefore limit drug-induced toxicity.
Since PPARα strongly increases TSP1 production, combined use of PPARα and PPARγ agonists or the use of dual PPARα/γ ligands may present an advantage. Interestingly, TZD18, a novel PPARα/γ dual agonist induces apoptosis of glioma cells with high efficiency [97]. Unfortunately, glitazars have carcinogenic activity of their own [98].
PPARγ ligands sensitize leukemic, lung and endothelial cells to the TRAIL-induced apoptosis by enhancing DR5 expression [99, 100] pointing to possible synergy between PPARγ agonists and TRAIL therapies.
The inhibition of VEGFR2 expression by vascular endothelium, which contributes to the antiangiogenesis by the PPARγ, could be assisted by VEGF sequestering agents, such as Avastin, or by the inhibitors VEGF RTK activity, such as sunitinib, sorafenib or VEGF decoy receptor. This hypothesis is yet to be tested.
The downstream target of the PI3K/Akt pathway, which is blocked by PPARγ via PTEN activation, is tuberous sclerosis tumor suppressor complex, which, when phosphorylated by Akt, allows the activation of mammalian target of rapamycin (mTOR) kinase, protein synthesis, and cell survival [101]. On the other hand, PPARγ ligands interfere with translation by augmenting the activity of 4-EBP and blocking S6 kinase [102]. Thus PPARγ disrupts mTOR regulation of protein synthesis at two distinct steps. Moreover, the blockade of mTOR pathway is likely to suppress VEGF in all cell types in the tumor microenvironment [103]. Hence, mTOR inhibitors such as tacrolimus are likely to complement the anti-angiogenic and antitumor activity of PPARγ agonists. Cyclic AMP analogs, which block mTOR activity via AMPK1 pathway [101], may also contribute to the PPARγ beneficial effects: this is particularly important, since cAMP analogs are capable of increasing PPARγ activity (Schulze-Hoepfner and Volpert, unpublished observations). The fact that amino acid deprivation, the main off switch for the mTOR, enhances PPARγ proapoptotic effects in tumor cells [104] lends further support to this hypothesis.
PPARγ transrepression of NFκB and NFAT signaling leads to the inhibition of multiple angiogenic stimuli, including interleukins 6 and 8, MCP-1 and CX3CL-1, as well as protective Ang-1 and proinflammatory Cox-2. This PPARγ function suggests a wide range of possible treatment combinations with NFκB inhibitors, including synthetic inhibitors of IKK kinases [105] or naturally occurring plant substances, like curcumin [106]. On the other hand, the inhibition of Cox-2 with highly selective agents, like celexoxib, has direct anti-angiogenic tumor-preventing effects [107] and is quite likely to contribute to the PPARγ antitumor and anti-angiogenic activities, especially in the light of potentiating effect of celexoxib on docetaxel treatment [108] and beneficial effects of PGZ combined with rofexoxib and low-dose chemotherapy [85].
PPARγ activity is opposed by MEK kinases: thus MEK inhibitors are likely to improve the efficacy of PPARγ ligands: indeed, MEK-1 inhibitor, PD98059, improves CGZ antitumor effect in colon cancer xenografts [109]. PPARγ activity is also augmented by RXR ligands: 9-cis retinoic acid (RA) enhances PPARγ-induced differentiation and gene expression. In colon cancer, PPARγ and RXR ligands induce differentiation and apoptosis more potently than each individual compound [110, 111]. Nine-cis retinoic acid partially overcomes RXR phosphorylation, which reduces PPARγ/RXR dimerization and opposes PPARγ activity: MEK-1 inhibitors improve the combined effect of CGZ and 9-cis RA [109]. Finally, HDAC inhibitor, trichostatin A, potentiates the effects of phenolfibrate on the differentiation and attenuation of stemness of the lung adenocarcinoma cells [112]. While combining PPARγ agonists with other drugs, particular attention should be paid to the agonist dosage: studies of PPARγ effects metabolic syndrome demonstrate that overactive and hypoactive mutants cause similar metabolic consequences and suggest the use of SPPARMs versus full agonists [113].
The list of agents with the potential to enhance the antitumor and anti-angiogenic effects of PPARγ ligands is not limited by the examples above, however we hope that it provides a convincing example of rational design of the complementation therapies, based on the knowledge of molecular mediators of a given agent. The examples, which demonstrate the improved efficacy of predicted combinations, provide an impetus for the evaluation of the combinations, which have not yet been tested.
ABBREVIATIONS
- ADD1:
Adducin 1
- AMPK1:
Adenosine Monophosphate Protein Kinase
- Ang-1:
Angiopoietin-1
- APC:
Adenomatous Polyposis Coli
- Bcl:
B-cell Leukemia
- bFGF:
Basic Fibroblast Growth factor
- C/EBPs:
CAAT enhancer binding proteins
- CBP:
CREB binding Protein
- Cdk:
Cyclin-Dependent Kinases
- cFLIP:
FLICE Inhibitory Protein, a caspase-8 inhibitor
- CGZ:
Ciglitazone
- Cox:
Cyclooxygenase
- DGK:
Diacylglycerol Kinase
- 15D-PGJ2:
15-deoxy-Δ12,14-prostaglandin J2
- DSCR1:
Down Syndrome Critical Region 1
- EC:
Endothelial Cells
- Egr:
Early Growth Response
- EPC:
Endothelial Progenitor Cells
- ERKs:
Extracellular Signal-Regulated Kinase
- GATA:
GATA-binding transcription factor
- HDAC:
Histone deacetylase
- HIF:
Hypoxia Inducible factor
- IKK:
Inhibitor of Kappa Beta Kinase
- IL:
Interleukin
- MAPK:
Mitogen-Activated Protein Kinase
- MCM7:
Minichromosome Maintenance Protein
- MCP:
Monocyte Chemotactic Protein-1
- MMP:
Matrix Metalloproteinase
- mTOR:
Mammalian Target of Rapamycin
- NCoR:
Nuclear Co-Repressor
- NADPH:
Nicotinamide Dinucleotide Phosphate
- NFAT:
Nuclear Factor of the Activated T-cells
- NFκB:
Nuclear Factor Kappa Beta
- JNKs:
Jun N-terminal Kinase
- MΦ:
Macrophages
- PPAR:
Peroxisome Proliferator Activated Receptor
- PAI-1:
Plasminogen Activator Inhibitor
- PDGF-BB:
Plateled-Derived Growth Factor
- PI3K:
Phosphatidil Inositol-3 Kinase
- PGZ:
Pioglitazone
- PPRE:
PPARg Response Element
- PTEN:
Phosphatase and Tensin Analog
- Rb:
Retinobalsoma
- ROCK:
Rho-associated kinase
- RXR:
Retinoic Acid X receptor
- STAT:
Signal Transducers and Activators of Transcription
- SMRT:
Silencing Mediator of Retinoic Acid and Thyroid Hormone Receptor
- SPPARMs:
Selective PPARγ modulators
- SREBP:
Serum response Element Binding Protein
- TAM:
Tumor-associated Macrophages
- TBL-1:
Transducin Beta-Like Protein-1
- TCF:
T-cell factor
- TNF:
Tumor Necrosis Factor
- TGF-β:
Tumor-Derived Growth Factor β
- TGZ:
Troglitazone
- TRAIL:
TNF-related apoptosis inducing ligand
- Trx:
Thioredoxin
- TSP1:
Thrombospondin-1
- TZDs:
Thiazoliediones
- UPA:
Urokinase Plasminogen Activator
- VDUP:
Vitamin D3 Upregulated Protein
- VEGF:
Vascular Endothelial Growth Factor
- VSMC:
Vascular Smooth Muscle Cells
- VCAM:
Vascular Cell Adhesion Molecule-1.
References
- 1.Wang T, Xu J, Yu X, Yang R, Han ZC. Peroxisome proliferator-activated receptor γ in malignant diseases. Critical Reviews in Oncology/Hematology. 2006;58(1):1–14. doi: 10.1016/j.critrevonc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Bruemmer D, Blaschke F, Law RE. New targets for PPARγ in the vessel wall: implications for restenosis. International Journal of Obesity. 2005;29(supplement 1):S26–S30. doi: 10.1038/sj.ijo.0802910. [DOI] [PubMed] [Google Scholar]
- 3.Margeli A, Kouraklis G, Theocharis S. Peroxisome proliferator activated receptor-γ (PPAR-γ) ligands and angiogenesis. Angiogenesis. 2003;6(3):165–169. doi: 10.1023/B:AGEN.0000021377.13669.c0. [DOI] [PubMed] [Google Scholar]
- 4.Panigrahy D, Shen LQ, Kieran MW, Kaipainen A. Therapeutic potential of thiazolidinediones as anticancer agents. Expert Opinion on Investigational Drugs. 2003;12(12):1925–1937. doi: 10.1517/13543784.12.12.1925. [DOI] [PubMed] [Google Scholar]
- 5.Panigrahy D, Huang S, Kieran MW, Kaipainen A. PPARγ as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biology and Therapy. 2005;4(7):687–693. doi: 10.4161/cbt.4.7.2014. [DOI] [PubMed] [Google Scholar]
- 6.Pistrosch F, Herbrig K, Oelschlaegel U, et al. PPARγ-agonist rosiglitazone increases number and migratory activity of cultured endothelial progenitor cells. Atherosclerosis. 2005;183(1):163–167. doi: 10.1016/j.atherosclerosis.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 7.Chu K, Lee S-T, Koo J-S, et al. Peroxisome proliferator-activated receptor-γ-agonist, rosiglitazone, promotes angiogenesis after focal cerebral ischemia. Brain Research. 2006;1093(1):208–218. doi: 10.1016/j.brainres.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama Y, Xin B, Shigeto T, et al. Clofibric acid, a peroxisome proliferator-activated receptor α ligand, inhibits growth of human ovarian cancer. Molecular Cancer Therapeutics. 2007;6(4):1379–1386. doi: 10.1158/1535-7163.MCT-06-0722. [DOI] [PubMed] [Google Scholar]
- 9.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends in Molecular Medicine. 2002;8(7):330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Isner JM. Endothelial progenitor cells for vascular regeneration. Journal of Hematotherapy and Stem Cell Research. 2002;11(2):171–178. doi: 10.1089/152581602753658385. [DOI] [PubMed] [Google Scholar]
- 11.Shim WSN, Ho IAW, Wong PEH. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Molecular Cancer Research. 2007;5(7):655–665. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- 12.Asano A, Irie Y, Saito M. Isoform-specific regulation of vascular endothelial growth factor (VEGF) family mRNA expression in cultured mouse brown adipocytes. Molecular and Cellular Endocrinology. 2001;174(1-2):71–76. doi: 10.1016/s0303-7207(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 13.Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PPAR expression and function during vertebrate development. International Journal of Developmental Biology. 2002;46(1):105–114. [PubMed] [Google Scholar]
- 14.Xin X, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator-activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. The Journal of Biological Chemistry. 1999;274(13):9116–9121. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 15.Bishop-Bailey D, Hla T. Endothelial cell apoptosis induced by the peroxisome proliferator-activated receptor (PPAR) ligand 15-deoxy-Δ12,14-prostaglandin J2 . The Journal of Biological Chemistry. 1999;274(24):17042–17048. doi: 10.1074/jbc.274.24.17042. [DOI] [PubMed] [Google Scholar]
- 16.Murata T, He S, Hangai M, et al. Peroxisome proliferator-activated receptor-γ ligands inhibit choroidal neovascularization. Investigative Ophthalmology and Visual Science. 2000;41(8):2309–2317. [PubMed] [Google Scholar]
- 17.Panigrahy D, Singer S, Shen LQ, et al. PPARγ ligands inhibit primary tumor growth and metastasis by inhibiting angiogenesis. Journal of Clinical Investigation. 2002;110(7):923–932. doi: 10.1172/JCI15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miard S, Fajas L. Atypical transcriptional regulators and cofactors of PPARγ . International Journal of Obesity. 2005;29(supplement 1):S10–S12. doi: 10.1038/sj.ijo.0802906. [DOI] [PubMed] [Google Scholar]
- 19.Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochimica et Biophysica Acta. 2007;1771(8):926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu M, Zhang J, Lin Y, et al. Early stimulation and late inhibition of peroxisome proliferator-activated receptor γ (PPARγ) gene expression by transformino growth factor β in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. Biochemical Journal. 2003;370(3):1019–1025. doi: 10.1042/BJ20021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diradourian C, Girard J, Pégorier J-P. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87(1):33–38. doi: 10.1016/j.biochi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Genini D, Catapano CV. Block of nuclear receptor ubiquitination: a mechanism of ligand-dependent control of peroxisome proliferator-activated receptor δ activity. The Journal of Biological Chemistry. 2007;282(16):11776–11785. doi: 10.1074/jbc.M609149200. [DOI] [PubMed] [Google Scholar]
- 23.Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. The New England Journal of Medicine. 1998;339(14):953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 24.Hosooka T, Noguchi T, Kotani K, et al. Dok1 mediates high-fat diet-induced adipocyte hypertrophy and obesity through modulation of PPAR-γ phosphorylation. Nature Medicine. 2008;14(2):188–193. doi: 10.1038/nm1706. [DOI] [PubMed] [Google Scholar]
- 25.Arany Z, Foo S-Y, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α . Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 26.Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARγ . Cell Cycle. 2007;6(13):1539–1548. doi: 10.4161/cc.6.13.4453. [DOI] [PubMed] [Google Scholar]
- 27.Winn RA, Van Scoyk M, Hammond M, et al. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor γ . The Journal of Biological Chemistry. 2006;281(37):26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- 28.Blanquart C, Barbier O, Fruchart JC, Staels B, Glineur C. Peroxisome proliferator-activated receptors: regulation of transcriptional activities and roles in inflammation. Journal of Steroid Biochemistry and Molecular Biology. 2003;85(2–5):267–273. doi: 10.1016/s0960-0760(03)00214-0. [DOI] [PubMed] [Google Scholar]
- 29.Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends in Cell Biology. 2007;17(6):292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Rubenstrunk A, Hanf R, Hum DW, Fruchart J-C, Staels B. Safety issues and prospects for future generations of PPAR modulators. Biochimica et Biophysica Acta. 2007;1771(8):1065–1081. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Balint BL, Nagy L. Selective modulators of PPAR activity as new therapeutic tools in metabolic diseases. Endocrine, Metabolic & Immune Disorders-Drug Targets. 2006;6(1):33–43. doi: 10.2174/187153006776056620. [DOI] [PubMed] [Google Scholar]
- 32.Collins AR. Pleiotropic vascular effects of PPARγ ligands. Drug News and Perspectives. 2003;16(4):197–204. doi: 10.1358/dnp.2003.16.4.829330. [DOI] [PubMed] [Google Scholar]
- 33.Giaginis C, Margeli A, Theocharis S. Peroxisome proliferator-activated receptor-γ ligands as investigational modulators of angiogenesis. Expert Opinion on Investigational Drugs. 2007;16(10):1561–1572. doi: 10.1517/13543784.16.10.1561. [DOI] [PubMed] [Google Scholar]
- 34.Goetze S, Eilers F, Bungenstock A, et al. PPAR activators inhibit endothelial cell migration by targeting Akt. Biochemical and Biophysical Research Communications. 2002;293(5):1431–1437. doi: 10.1016/S0006-291X(02)00385-6. [DOI] [PubMed] [Google Scholar]
- 35.Hsueh WA, Jackson S, Law RE. Control of vascular cell proliferation and migration by PPAR-γ: a new approach to the macrovascular complications of diabetes. Diabetes Care. 2001;24(2):392–397. doi: 10.2337/diacare.24.2.392. [DOI] [PubMed] [Google Scholar]
- 36.Cho D-H, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) γ-dependent and PPARγ-independent signaling pathways. The Journal of Biological Chemistry. 2004;279(4):2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- 37.Goetze S, Bungenstock A, Czupalla C, et al. Leptin induces endothelial cell migration through Akt, which is inhibited by PPARγ-ligands. Hypertension. 2002;40(5):748–754. doi: 10.1161/01.hyp.0000035522.63647.d3. [DOI] [PubMed] [Google Scholar]
- 38.Teresi RE, Planchon SM, Waite KA, Eng C. Regulation of the PTEN promoter by statins and SREBP. Human Molecular Genetics. 2008;17(7):919–928. doi: 10.1093/hmg/ddm364. [DOI] [PubMed] [Google Scholar]
- 39.Sassa Y, Hata Y, Aiello LP, Taniguchi Y, Kohno K, Ishibashi T. Bifunctional properties of peroxisome proliferator-activated receptor γ1 in KDR gene regulation mediated via interaction with both Sp1 and Sp3. Diabetes. 2004;53(5):1222–1229. doi: 10.2337/diabetes.53.5.1222. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez SH, Heilman D, Morsey B, Potula R, Haorah J, Persidsky Y. Activation of peroxisome proliferator-activated receptor γ (PPARγ) suppresses Rho GTPases in human brain microvascular endothelial cells and inhibits adhesion and transendothelial migration of HIV-1 infected monocytes. The Journal of Immunology. 2008;180(3):1854–1865. doi: 10.4049/jimmunol.180.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho T-C, Yang Y-C, Chen S-L, et al. Pigment epithelium-derived factor induces THP-1 macrophage apoptosis and necrosis by the induction of the peroxisome proliferator-activated receptor gamma. Molecular Immunology. 2008;45(4):898–909. doi: 10.1016/j.molimm.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Ho T-C, Chen S-L, Yang Y-C, Liao C-L, Cheng H-C, Tsao Y-P. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovascular Research. 2007;76(2):213–223. doi: 10.1016/j.cardiores.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y-C, Tsao Y-P, Ho T-C, Choung I-P. Peroxisome proliferator-activated receptor-γ agonists cause growth arrest and apoptosis in human ovarian carcinoma cell lines. International Journal of Gynecological Cancer. 2007;17(2):418–425. doi: 10.1111/j.1525-1438.2006.00866.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim KY, Cheon HG. Antiangiogenic effect of rosiglitazone is mediated via peroxisome proliferator-activated receptor γ-activated maxi-K channel opening in human umbilical vein endothelial cells. The Journal of Biological Chemistry. 2006;281(19):13503–13512. doi: 10.1074/jbc.M510357200. [DOI] [PubMed] [Google Scholar]
- 45.Billiet L, Furman C, Larigauderie G, et al. Enhanced VDUP-1 gene expression by PPARγ agonist induces apoptosis in human macrophage. Journal of Cellular Physiology. 2008;214(1):183–191. doi: 10.1002/jcp.21179. [DOI] [PubMed] [Google Scholar]
- 46.Verrier E, Wang L, Wadham C, et al. PPARγ agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circulation Research. 2004;94(11):1515–1522. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- 47.Huang H, Campbell SC, Bedford DF, et al. Peroxisome proliferator-activated receptor γ ligands improve the antitumor efficacy of thrombospondin peptide ABT510. Molecular Cancer Research. 2004;2(10):541–550. [PubMed] [Google Scholar]
- 48.Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Circulation. 2007;116(2):163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 49.Redondo S, Hristov M, Gümbel D, Tejerina T, Weber C. Biphasic effect of pioglitazone on isolated human endothelial progenitor cells: involvement of peroxisome proliferator-activated receptor-γ and transforming growth factor-β1. Thrombosis and Haemostasis. 2007;97(6):979–987. [PubMed] [Google Scholar]
- 50.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 51.Westerweel PE, den Ouden K, Nguyen TQ, Goldschmeding R, Joles JA, Verhaar MC. Amelioration of anti-Thy1-glomerulonephritis by PPAR-γ agonism without increase of endothelial progenitor cell homing. American Journal of Physiology. 2008;294(2):F379–F384. doi: 10.1152/ajprenal.00019.2007. [DOI] [PubMed] [Google Scholar]
- 52.Al-Shali KZ, House AA, Hanley AJG, et al. Genetic variation in PPARG encoding peroxisome proliferator-activated receptor γ associated with carotid atherosclerosis. Stroke. 2004;35(9):2036–2040. doi: 10.1161/01.STR.0000138784.68159.a5. [DOI] [PubMed] [Google Scholar]
- 53.Mujumdar VS, Tummalapalli CM, Aru GM, Tyagi SC. Mechanism of constrictive vascular remodeling by homocysteine: role of PPAR. American Journal of Physiology. 2002;282(5):C1009–C1015. doi: 10.1152/ajpcell.00353.2001. [DOI] [PubMed] [Google Scholar]
- 54.Benkirane K, Amiri F, Diep QN, El Mabrouk M, Schiffrin EL. PPAR-γ inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. American Journal of Physiology. 2006;290(1):H390–H397. doi: 10.1152/ajpheart.00662.2005. [DOI] [PubMed] [Google Scholar]
- 55.Wakino S, Hayashi K, Kanda T, et al. Peroxisome proliferator-activated receptor γ ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circulation Research. 2004;95(5):e45–e55. doi: 10.1161/01.RES.0000142313.68389.92. [DOI] [PubMed] [Google Scholar]
- 56.Goetze S, Kintscher U, Kim S, et al. Peroxisome proliferator-activated receptor-γ ligands inhibit nuclear but not cytosolic extracellular signal-regulated kinase/mitogen-activated protein kinase-regulated steps in vascular smooth muscle cell migration. Journal of Cardiovascular Pharmacology. 2001;38(6):909–921. doi: 10.1097/00005344-200112000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa D, Nomiyama T, Nakamachi T, et al. Activation of peroxisome proliferator-activated receptor γ suppresses telomerase activity in vascular smooth muscle cells. Circulation Research. 2006;98(7):e50–e59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 58.Bruemmer D, Yin F, Liu J, et al. Regulation of the growth arrest and DNA damage-inducible gene 45 (GADD45) by peroxisome proliferator-activated receptor γ in vascular smooth muscle cells. Circulation Research. 2003;93(4):e38–e47. doi: 10.1161/01.RES.0000088344.15288.E6. [DOI] [PubMed] [Google Scholar]
- 59.Okura T, Nakamura M, Takata Y, Watanabe S, Kitami Y, Hiwada K. Troglitazone induces apoptosis via the p53 and Gadd45 pathway in vascular smooth muscle cells. European Journal of Pharmacology. 2000;407(3):227–235. doi: 10.1016/s0014-2999(00)00758-5. [DOI] [PubMed] [Google Scholar]
- 60.Ruiz E, Redondo S, Gordillo-Moscoso A, Tejerina T. Pioglitazone induces apoptosis in human vascular smooth muscle cells from diabetic patients involving the transforming growth factor-β/activin receptor-like kinase-4/5/7/Smad2 signaling pathway. Journal of Pharmacology and Experimental Therapeutics. 2007;321(2):431–438. doi: 10.1124/jpet.106.114934. [DOI] [PubMed] [Google Scholar]
- 61.Lin Y, Zhu X, McIntee FL, et al. Interferon regulatory factor-1 mediates PPARγ-induced apoptosis in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(2):257–263. doi: 10.1161/01.ATV.0000109170.43400.2f. [DOI] [PubMed] [Google Scholar]
- 62.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr., Nicholson AC. Transforming growth factor-β1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ . The Journal of Biological Chemistry. 2000;275(2):1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 63.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. The Journal of Biological Chemistry. 1998;273(40):25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki M, Jordan P, Welbourne T, et al. Troglitazone, a PPAR-γ activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-α . BMC Physiology. 2005;5, article 3:1–12. doi: 10.1186/1472-6793-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yano M, Matsumura T, Senokuchi T, et al. Statins activate peroxisome proliferator-activated receptor γ through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circulation Research. 2007;100(10):1442–1451. doi: 10.1161/01.RES.0000268411.49545.9c. [DOI] [PubMed] [Google Scholar]
- 66.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends in Immunology. 2007;28(12):551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Koeffler HP. Peroxisome proliferator-activated receptor γ and cancers. Clinical Cancer Research. 2003;9(1):1–9. [PubMed] [Google Scholar]
- 68.Bush CR, Havens JM, Necela BM, et al. Functional genomic analysis reveals cross-talk between peroxisome proliferator-activated receptor γ and calcium signaling in human colorectal cancer cells. The Journal of Biological Chemistry. 2007;282(32):23387–23401. doi: 10.1074/jbc.M702708200. [DOI] [PubMed] [Google Scholar]
- 69.Zang C, Wächter M, Liu H, et al. Ligands for PPARγ and RAR cause induction of growth inhibition and apoptosis in human glioblastomas. Journal of Neuro-Oncology. 2003;65(2):107–118. doi: 10.1023/b:neon.0000003728.80052.a8. [DOI] [PubMed] [Google Scholar]
- 70.Kim Y, Suh N, Sporn M, Reed JC. An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. The Journal of Biological Chemistry. 2002;277(25):22320–22329. doi: 10.1074/jbc.M202458200. [DOI] [PubMed] [Google Scholar]
- 71.Ohama Y, Harada T, Iwabe T, Taniguchi F, Takenaka Y, Terakawa N. Peroxisome proliferator-activated receptor-γ ligand reduced tumor necrosis factor-α-induced interleukin-8 production and growth in endometriotic stromal cells. Fertility and Sterility. 2008;89(2):311–317. doi: 10.1016/j.fertnstert.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 72.Fu Y-G, Sung JJY, Wu K-C, et al. Inhibition of gastric cancer cells associated angiogenesis by 15d-prostaglandin J2 through the downregulation of angiopoietin-1. Cancer Letters. 2006;243(2):246–254. doi: 10.1016/j.canlet.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 73.Grau R, Iñiguez MA, Fresno M. Inhibition of activator protein 1 activation, vascular endothelial growth factor, and cyclooxygenase-2 expression by 15-deoxy-Δ12,14-prostaglandin J2 in colon carcinoma cells: evidence for a redox-sensitive peroxisome proliferator-activated receptor-γ-independent mechanism. Cancer Research. 2004;64(15):5162–5171. doi: 10.1158/0008-5472.CAN-04-0849. [DOI] [PubMed] [Google Scholar]
- 74.Biscetti F, Gaetani E, Flex A, et al. Selective activation of peroxisome proliferator-activated receptor (PPAR)α and PPARγ induces neoangiogenesis through a vascular endothelial growth factor-dependent mechanism. Diabetes. 2008;57(5):1394–1404. doi: 10.2337/db07-0765. [DOI] [PubMed] [Google Scholar]
- 75.Ptak-Belowska A, Pawlik MW, Krzysiek-Mączka G, Brzozowski T, Pawlik WW. Transcriptional upregulation of gastrin in response to peroxisome proliferator-activated receptor gamma agonist triggers cell survival pathways. Journal of Physiology and Pharmacology. 2007;58(4):793–801. [PubMed] [Google Scholar]
- 76.Jozkowicz A, Dulak J, Piatkowska E, Placha W, Dembinska-Kiec A. Ligands of peroxisome proliferator-activated receptor-γ increase the generation of vascular endothelial growth factor in vascular smooth muscle cells and in macrophages. Acta Biochimica Polonica. 2000;47(4):1147–1157. [PubMed] [Google Scholar]
- 77.Yamakawa K, Hosoi M, Koyama H, et al. Peroxisome proliferator-activated receptor-γ agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2000;271(3):571–574. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- 78.Eibl G, Takata Y, Boros LG, et al. Growth stimulation of COX-2-negative pancreatic cancer by a selective COX-2 inhibitor. Cancer Research. 2005;65(3):982–990. [PubMed] [Google Scholar]
- 79.Fauconnet S, Lascombe I, Chabannes E, et al. Differential regulation of vascular endothelial growth factor expression by peroxisome proliferator-activated receptors in bladder cancer cells. The Journal of Biological Chemistry. 2002;277(26):23534–23543. doi: 10.1074/jbc.M200172200. [DOI] [PubMed] [Google Scholar]
- 80.Chintalgattu V, Harris GS, Akula SM, Katwa LC. PPAR-γ agonists induce the expression of VEGF and its receptors in cultured cardiac myofibroblasts. Cardiovascular Research. 2007;74(1):140–150. doi: 10.1016/j.cardiores.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 81.Lefebvre A-M, Chen I, Desreumaux P, et al. Activation of the peroxisome proliferator-activated receptor γ promotes the development of colon tumors in C57BL/6J-APCMIin/+ mice. Nature Medicine. 1998;4(9):1053–1057. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 82.Sarraf P, Mueller E, Jones D, et al. Differentiation and reversal of malignant changes in colon cancer through PPARγ . Nature Medicine. 1998;4(9):1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka T, Kohno H, Yoshitani S-I, et al. Ligands for peroxisome proliferator-activated receptors α and γ inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Research. 2001;61(6):2424–2428. [PubMed] [Google Scholar]
- 84.Mueller E, Smith M, Sarraf P, et al. Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):10990–10995. doi: 10.1073/pnas.180329197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hau P, Kunz-Schughart L, Bogdahn U, et al. Low-dose chemotherapy in combination with COX-2 inhibitors and PPAR-gamma agonists in recurrent high-grade gliomas—a phase II study. Oncology. 2007;73(1-2):21–25. doi: 10.1159/000120028. [DOI] [PubMed] [Google Scholar]
- 86.Kebebew E, Peng M, Reiff E, et al. A phase II trial of rosiglitazone in patients with thyroglobulin-positive and radioiodine-negative differentiated thyroid cancer. Surgery. 2006;140(6):960–967. doi: 10.1016/j.surg.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 87.Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-γ ligands for the treatment of breast cancer. Expert Opinion on Investigational Drugs. 2005;14(6):557–568. doi: 10.1517/13543784.14.6.557. [DOI] [PubMed] [Google Scholar]
- 88.Debrock G, Vanhentenrijk V, Sciot R, Debiec-Rychter M, Oyen R, Van Oosterom A. A phase II trial with rosiglitazone in liposarcoma patients. British Journal of Cancer. 2003;89(8):1409–1412. doi: 10.1038/sj.bjc.6601306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCarty MF, Barroso-Aranda J, Contreras F. PPARgamma agonists can be expected to potentiate the efficacy of metronomic chemotherapy through CD36 up-regulation. Medical Hypotheses. 2008;70(2):419–423. doi: 10.1016/j.mehy.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 90.Ooyama A, Oka T, Zhao H-Y, Yamamoto M, Akiyama S-I, Fukushima M. Anti-angiogenic effect of 5-Fluorouracil-based drugs against human colon cancer xenografts. Cancer Letters. 2008;267(1):26–36. doi: 10.1016/j.canlet.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 91.Damber J-E, Vallbo C, Albertsson P, Lennernäs B, Norrby K. The anti-tumour effect of low-dose continuous chemotherapy may partly be mediated by thrombospondin. Cancer Chemotherapy and Pharmacology. 2006;58(3):354–360. doi: 10.1007/s00280-005-0163-8. [DOI] [PubMed] [Google Scholar]
- 92.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Ichite N, Singh M. 15-deoxy-Δ12,14-prostaglandin J2 enhances docetaxel anti-tumor activity against A549 and H460 non-small-cell lung cancer cell lines and xenograft tumors. Anti-Cancer Drugs. 2007;18(1):65–78. doi: 10.1097/CAD.0b013e3280101006. [DOI] [PubMed] [Google Scholar]
- 94.Quesada AJ, Nelius T, Yap R, et al. In vivo upregulation of CD95 and CD95L causes synergistic inhibition of angiogenesis by TSP1 peptide and metronomic doxorubicin treatment. Cell Death and Differentiation. 2005;12(6):649–658. doi: 10.1038/sj.cdd.4401615. [DOI] [PubMed] [Google Scholar]
- 95.Yap R, Veliceasa D, Emmenegger U, et al. Metronomic low-dose chemotherapy boosts CD95-dependent antiangiogenic effect of the thrombospondin peptide ABT-510: a complementation antiangiogenic strategy. Clinical Cancer Research. 2005;11(18):6678–6685. doi: 10.1158/1078-0432.CCR-05-0621. [DOI] [PubMed] [Google Scholar]
- 96.Watnick RS, Cheng Y-N, Rangarajan A, Ince TA, Weinberg RA. Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell. 2003;3(3):219–231. doi: 10.1016/s1535-6108(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 97.Liu D-C, Zang C-B, Liu H-Y, Possinger K, Fan S-G, Elstner E. A novel PPAR alpha/gamma dual agonist inhibits cell growth and induces apoptosis in human glioblastoma T98G cells. Acta Pharmacologica Sinica. 2004;25(10):1312–1319. [PubMed] [Google Scholar]
- 98.Egerod FL, Nielsen HS, Iversen L, Thorup I, Storgaard T, Oleksiewicz MB. Biomarkers for early effects of carcinogenic dual-acting PPAR agonists in rat urinary bladder urothelium in vivo. Biomarkers. 2005;10(4):295–309. doi: 10.1080/13547500500218682. [DOI] [PubMed] [Google Scholar]
- 99.Lipkowitz S, Dennis PA. PPARγ agonists follow an unknown TRAIL in lung cancer. Cancer Biology and Therapy. 2007;6(1):107–109. doi: 10.4161/cbt.6.1.3751. [DOI] [PubMed] [Google Scholar]
- 100.Zou W, Liu X, Yue P, Khuri FR, Sun S-Y. PPARγ ligands enhance TRAIL-induced apoptosis through DR5 upregulation and c-FLIP downregulation in human lung cancer cells. Cancer Biology and Therapy. 2007;6(1):99–106. doi: 10.4161/cbt.6.1.3555. [DOI] [PubMed] [Google Scholar]
- 101.Ellisen LW. Growth control under stress: mTOR regulation through the REDD1-TSC pathway. Cell Cycle. 2005;4(11):1500–1502. doi: 10.4161/cc.4.11.2139. [DOI] [PubMed] [Google Scholar]
- 102.Cho D-H, Choi YJ, Jo SA, et al. Troglitazone acutely inhibits protein synthesis in endothelial cells via a novel mechanism involving protein phosphatase 2A-dependent p70 S6 kinase inhibition. American Journal of Physiology. 2006;291(2):C317–C326. doi: 10.1152/ajpcell.00491.2005. [DOI] [PubMed] [Google Scholar]
- 103.Stoeltzing O, Meric-Bernstam F, Ellis LM. Intracellular signaling in tumor and endothelial cells: the expected and, yet again, the unexpected. Cancer Cell. 2006;10(2):89–91. doi: 10.1016/j.ccr.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Núñez NP, Liu H, Meadows GG. PPAR-γ ligands and amino acid deprivation promote apoptosis of melanoma, prostate, and breast cancer cells. Cancer Letters. 2006;236(1):133–141. doi: 10.1016/j.canlet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 105.McIntyre KW, Shuster DJ, Gillooly KM, et al. A highly selective inhibitor of IκB kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis and Rheumatism. 2003;48(9):2652–2659. doi: 10.1002/art.11131. [DOI] [PubMed] [Google Scholar]
- 106.Brennan P, O'Neill LAJ. Inhibition of nuclear factor κB by direct modification in whole cells—mechanism of action of nordihydroguaiaritic acid, curcumin and thiol modifiers. Biochemical Pharmacology. 1998;55(7):965–973. doi: 10.1016/s0006-2952(97)00535-2. [DOI] [PubMed] [Google Scholar]
- 107.Sinicrope FA. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Molecular Carcinogenesis. 2006;45(6):447–454. doi: 10.1002/mc.20232. [DOI] [PubMed] [Google Scholar]
- 108.Fulzele SV, Shaik MS, Chatterjee A, Singh M. Anti-cancer effect of celecoxib and aerosolized docetaxel against human non-small cell lung cancer cell line, A549. Journal of Pharmacy and Pharmacology. 2006;58(3):327–336. doi: 10.1211/jpp.58.3.0006. [DOI] [PubMed] [Google Scholar]
- 109.Yamazaki K, Shimizu M, Okuno M, et al. Synergistic effects of RXRα and PPARγ ligands to inhibit growth in human colon cancer cells—phosphorylated RXRα is a critical target for colon cancer management. Gut. 2007;56(11):1557–1563. doi: 10.1136/gut.2007.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szatmari I, Vámosi G, Brazda P, et al. Peroxisome proliferator-activated receptor γ-regulated ABCG2 expression confers cytoprotection to human dendritic cells. The Journal of Biological Chemistry. 2006;281(33):23812–23823. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- 111.Cesario RM, Stone J, Yen W-C, Bissonnette RP, Lamph WW. Differentiation and growth inhibition mediated via the RXR:PPARγ heterodimer in colon cancer. Cancer Letters. 2006;240(2):225–233. doi: 10.1016/j.canlet.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Chang T-H, Szabo E. Enhanced growth inhibition by combination differentiation therapy with ligands of peroxisome proliferator-activated receptor-γ and inhibitors of histone deacetylase in adenocarcinoma of the lung. Clinical Cancer Research. 2002;8(4):1206–1212. [PubMed] [Google Scholar]
- 113.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-γ calls for activation in moderation: lessons from genetics and pharmacology. Endocrine Reviews. 2004;25(6):899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]