Abstract
Dominance status and reproductive experience are maternal characteristics that affect offspring traits in diverse taxa, including some cercopithecine primates. Maternal effects of this sort are widespread and are sources of variability in offspring fitness. We tested the hypothesis that maternal dominance rank and reproductive experience as well as a male’s own age and dominance rank predicted chronic fecal glucocorticoid (fGC) concentrations in 17 subadult wild male baboons, Papio cynocephalus (median age 6.5 years), in the Amboseli basin, Kenya. Among these variables, maternal dominance rank at a subadult male’s conception was the sole significant predictor of the male’s fGC and accounted for 42% of fGC variance; sons of lower ranking mothers had higher fGC than did those of high ranking mothers. This result is striking because subadult male baboons are approximately 4–6 years past the period of infant dependence on their mothers, and are larger than and dominant to all adult females. In addition, many males of this age have survived their mothers’ death. Consequently, the influence of maternal dominance rank persisted well beyond the stage at which direct maternal influence on sons is likely. Persistence of these major maternal influences from the perinatal period may signal organizational effects of mothers on sons’ HPA axis. Although short-term, acute, elevations in GC are part of adaptive responses to challenges such as predators and other emergencies, chronically elevated GC are often associated with stress-related pathologies and, thereby, adverse effects on fitness components.
Keywords: baboon, glucocorticoids, maternal effects, dominance rank, Papio
Introduction
Non-genetic parental effects, usually known as maternal effects, occur in many taxa (reviewed in Bernardo, 1996; Kirkpatrick and Lande, 1989; Mousseau and Fox, 1998; Wolf et al., 1998). These effects often arise from parents’ social environment and from the parent’s exposure to psychosocial stressors or access to essential resources, which may in turn impact offspring fitness (e.g., in dung beetles, Hunt and Simmons, 1997; cleaning gobies, Whiteman and Côté, 2004; savanna baboons, Altmann and Alberts, 2005; black lemurs, Bayart and Simmen, 2005; spotted hyenas Dloniak et al., 2006; bluegill sunfish, Neff and Lister 2006; dark-bellied brent geese, Poisebleau et al., 2006; mandrills, Setchell et al., 2006; mountain gorillas, Scott and Lockard, 2006; lizards, Warner et al., 2007). Maternal effects on offspring phenotype may also arise from maternal reproductive experience or age because of age-related changes in maternal condition and reproductive investment strategies (Curio, 1983; Förslund and Pärt, 1995; Stearns, 1992; Williams, 1966).
Maternal effects have been demonstrated to impact several fitness components of offspring in a range of species, including effects on birth weight or egg size (e.g., humans and chimpanzees, Fessler et al., 2005; zebra finch, Gilbert et al., 2006; rove beetle, Kyneb and Toft, 2006; soil mites, Plaistow et al., 2006; fish, Taborsky, 2006; lizard, Warner et al., 2007), growth rate and age at maturity (e.g., mandrills, Setchell et al., 2002; savanna baboons, Altmann and Alberts, 2005; beetles, Kyneb and Toft, 2006; mites, Plaistow et al., 2006), and longevity or probability of survival (e.g., mandrills, Setchell et al., 2006; fruit flies, Priest et al., 2002; herring gulls, Bogdanova et al., 2006; turtles, Paitz et al., 2007). These effects also impact the development of competitive traits such as aggression and mounting behavior (Dloniak et al., 2006; Forstmeier et al., 2004; Royle et al., 2005), and both song rate and mate choice (Forstmeier et al., 2004). In the baboon population that is the focus of the present study, both maternal dominance status and parity appear to have maternal effects, and they predict offspring growth, dominance status of daughters, and age at maturity of both sons and daughters (Alberts and Altmann 1995b; Altmann and Alberts 2003, 2005).
Although maternal effects are widespread, their role in the production of physiological traits, and the mechanisms by which they are mediated, are less well understood (but see Walker et al. 2004 for a recent review of the experimental findings for rodents and implications for humans, and Sanchez, 2006 for a review of the impact on HPA axis development of experimentally manipulated adverse care in primates). One proposal is that social or nutritional stressors experienced by a mother impact her hypothalamic-pituitary-adrenal (HPA) pathway (e.g. social: Creel, 2001; Goymann and Wingfield, 2004; nutritional: Coplan et al., 2006, Lesage et al., 2001). Glucocorticoids (GC), which are one of the end-products of this HPA pathway, may then adversely impact the fetus or young infant through direct effects during gestation and/or lactation, or through indirect effects via change in maternal behaviour. Short-term increases in the secretion of GC are adaptive and enable an individual to overcome stressful stimuli and meet acute challenges (Abbott et al., 2003; Romero, 2002, 2004; Sapolsky, 2005; Sapolsky et al., 2000). In the long term, however, chronically elevated levels of GC arise from a number of mechanistic failures, compromise major functions of the body including reproductive, immunity, and growth, and are associated with many pathological conditions (Sapolsky, 1992a).
Because maternal effects associated with either social or nutritional factors may impact offspring GC either through maternal physiology or behavior during the fetal or infant stages, concentrations of GC in offspring are particularly promising candidates for investigation of maternal effects. Moreover, persistence of maternal influences from the perinatal period into maturity may signal organizational effects of mothers on sons’ HPA axis. According to the fetal programming hypotheses (e.g. Barker et al., 1993; Phillips, 2007), the extent to which such maternal effects impose fitness costs will depend on whether differences in lifetime experiences of sons match those of their perinatal period.
Current characteristics of an individual, such as age or dominance rank, regardless of genetic, maternal, or other contributions to these traits, also influence fitness components in most species (Abbott et al., 2003; Alberts et al., 2003; Alberts et al., 2006; Clutton-Brock, 1988; Côté and Festa-Bianchet, 2001; Förslund and Pärt, 1995; Holand et al., 2004; Holekamp et al., 1996; von Holst et al., 2002; Packer et al., 2000; Romero, 2004; Sapolsky, 2005). Furthermore, differences in GC levels are also sometimes associated with these current characteristics of an individual (Creel, 2001; Goymann and Wingfield, 2004; Sapolsky and Altmann, 1991). In the present research, we sought to extend our prior investigations of maternal effects in wild baboons through evaluation of both maternal effects and current traits in older offspring (specifically subadult males) and by measurement of these males’ glucocorticoids, a major component of response to challenge.
Materials and methods
Subjects
The research was conducted in a well-studied natural population of baboons in the Amboseli/Longido basin, 2° 40’S, a semi-arid short grass savannah ecosystem located at the foot of Mt. Kilimanjaro in southern Kenya (Behrensmeyer and Boaz, 1981; Western and van Praet, 1973). The subjects were 17 pre-dispersal subadult male baboons, Papio cynocephalus, that were born into five wild-feeding groups monitored by the Amboseli Baboon Research Project (ABRP). In baboon males the transition to subadult from the juvenile stage is marked by rapid testes enlargement, which is associated with production of viable sperm and occurs at a median age of 5.7 years in Amboseli (Alberts and Altmann 1995b). After about 2–3 years spent as subadults, males achieve a size and competitive ability that enables them to defeat some adult males in fights, thereby accomplishing the transition to adulthood and gaining potential access to fertile females (Alberts and Altmann 1995a; Alberts et al., 2006). Samples for GC analysis were collected across the 20 months prior to the males’ natal dispersal, another major male maturational milestone, one that usually occurs during subadulthood or early adulthood (Alberts and Altmann, 1995b). In other words, we focused on males during an important, relatively narrow life-history stage, circumscribed on the lower end by testicular enlargement and on the upper end by natal dispersal (both of which occur throughout the year, as do conception and birth in females). We included all natal males that were subadult for an appreciable period of time between September 2000 and May 2005, the period for which fecal hormone samples were available for subadults across many pre-dispersal months.
ABRP research has been ongoing for over three decades. All members of study groups are individually identifiable, and each group is the focus of detailed observations several days each week. Consequently, ages of all males born into ABRP study groups are known to within a few days. Because the study subjects and their mothers were part of this long-term research project, we had data on the focal male subjects in the months prior to their natal dispersal (their age, fGC profiles, dispersal dates, and dominance rank), as well as on their mother’s dominance rank and reproductive experience at the time of the male’s conception, approximately 6 years prior to average age at hormone sampling for the current project.
Dominance rank
Dominance ranks are assigned based on outcomes of pair-wise agonistic encounters by creating a dominance matrix from these outcomes (win/loss) with rank orderings that minimize entries below the main diagonal (Hausfater, 1975). All subadult and adult males rank above all adult females, enabling us to readily assign dominance ranks that reflect ordinal rankings of individuals within each sex. The highest ranking individual of each sex is ranked 1, the next 2, and so on. In Amboseli, rank assigned in this way predicts rates at which females are spatially supplanted (Altmann, 1980 p.98), access of adult males to estrous females (Alberts et al., 2006; Hausfater, 1975), growth rates of sexually immature offspring (Altmann and Alberts, 2005), adult rank of daughters, age at sexual maturity of sons and daughters (Alberts and Altmann, 1995b), and female reproductive rates (Altmann and Alberts, 2003, Davidson, in revision).
Female dominance rank in baboons is highly stable throughout adulthood (Pereira, 1995; Walters and Seyfarth, 1987), with the major exception that older mothers sometimes cede rank to mature daughters (Combes and Altmann, 2001); because mothers and daughters occupy adjacent ranks, however, even these rank changes are relatively small. A female’s dominance rank at her offspring’s conception has been predictive of a number of offspring characteristics; here we term this the male’s maternal dominance rank (Altmann, 1980; Altmann and Alberts, 2005). Among the subjects in this study, maternal dominance rank ranged from 1 to 13 (median 6); see Table 1. Note only are ranks of mature females highly stable in general, but on average, the rank positions of mothers in this study changed by less than one from a son’s conception through the end of his first year of life, i.e. the 18-month period of gestation and infant dependence, and no mother changed more than two rank positions during this period.
Table 1.
Characteristics of 17 the subadult males in the present study (see text for details).
| Maternal characteristics | Offspring characteristics | |||||
|---|---|---|---|---|---|---|
| Male | Parity | Rank | Mean age | Mean rank | # months sampled | Mean fGC |
| Ced | 4 | 6 | 7.17 | 10.40 | 17 | 42.33 |
| Dyn | 5 | 3 | 6.75 | 14.64 | 11 | 43.23 |
| Elv | 1 | 10 | 6.12 | 4.89 | 18 | 61.71 |
| Fuz | 4 | 13 | 7.11 | 11.43 | 14 | 60.05 |
| Lat | 3 | 3 | 7.03 | 7.64 | 11 | 33.23 |
| Leb | 1 | 7 | 6.97 | 12.13 | 8 | 51.41 |
| Lui | 1 | 5 | 6.48 | 12.86 | 15 | 52.20 |
| Naw | 2 | 6 | 5.83 | 14.89 | 9 | 49.67 |
| Net | 7 | 4 | 6.39 | 9.43 | 14 | 46.16 |
| Nyl | 4 | 7 | 6.95 | 11.67 | 15 | 39.61 |
| Oce | 6 | 9 | 6.81 | 2.73 | 15 | 77.89 |
| Vap | 2 | 7 | 6.29 | 7.40 | 15 | 43.20 |
| Vaz | 4 | 5 | 6.66 | 7.00 | 11 | 48.16 |
| Vei | 2 | 4 | 6.44 | 6.37 | 19 | 34.15 |
| Voy | 10 | 8 | 5.81 | 6.23 | 13 | 49.08 |
| Wes | 1 | 3 | 6.15 | 10.50 | 16 | 45.08 |
| Weu | 7 | 1 | 6.20 | 7.13 | 16 | 44.62 |
In contrast to the stability of female dominance rank, dominance rank in males is highly variable and age-dependent throughout their lives (Alberts et al., 2003; Hamilton and Bulger, 1990; Hausfater, 1975; Packer et al., 2000). The typical dominance rank trajectory for male baboons involves a rapid rank rise to high rank as males leave subadulthood and enter adulthood, soon followed by steady decline in rank as they age (Alberts et al., 2003; Hamilton and Bulger, 1990; Packer et al., 2000). Because male rank is highly labile, in this study each fGC sample was associated with the male’s dominance rank in that month, and the single dominance rank used for a male in the study was the mean rank across months during which he was sampled (see details below). Subadult males in this study ranged in average rank from 3 to 15 (median 9); see Table 1.
Maternal reproductive experience
We used parity as our measure of maternal reproductive experience; for each subject we counted the number of his mother’s pregnancies up to and including the pregnancy that produced the male subject (Altmann et al., 1977). Pregnancy assignment was made based on near-daily records of each female’s reproductive cycles, including sex-skin size and condition and observed menstruation, a long-standing observational method that has recently been validated through hormonal analysis (see Beehner et al., 2006). In this dataset, parity ranged from 1 to 10 (median 4; see Table 1), and none of the subadults had the same mother.
Fecal glucocorticoid concentration (fGC)
We collected freshly deposited fecal samples opportunistically from each individually identified subject. Although we attempted to collect fecal samples from an individual on as many observation days as possible (no more than one sample was ever collected on a single day), constraints resulting from other types of data collection meant that samples were not available for every male during each of the 20 months prior to dispersal from the natal group. The number of sampling months varied across males from 8 to 19 (median 15 months); see Table 1. Each fecal sample was associated with the male’s age on the date of collection and the male’s dominance rank during that month. In cases where males had samples from multiple days in a given month (maximum obtained=7) a single value was used for that month by taking the average fGC concentration across the male's samples that month, his average age on collection dates that month, and his dominance rank for the month. In this way, each male had a single fGC value, age, and dominance rank for each month during which any samples were obtained. We then took the mean of mean across the sampling months for each male, obtaining a single value for each that was then used in the statistical analysis. Glucocorticoid concentrations were expressed as ng/g dried fecal powder.
Fecal sample collection, storage and extraction were done as described previously (Beehner et al., 2006; Gesquiere et al., 2005; Khan et al. 2002). Fecal GC was quantified using the ICN Corticosterone RIA kit for Rats and Mice, ICN Biomedicals, Inc.Costa Mesa. Intra-assay and inter-assay coefficient of variation were 4.4±1.0% (mean ± SE) and 10.8% for the fecal extract pool, 2.5±0.5% and 8.7% for low concentration control, and 2.5±0.5% and 9.8% for high concentration control.
Statistical Analysis
We used SPSS (Version 14) linear regression analysis for modelling fGC variability in subadult males; predictor variables in the model were maternal dominance rank, parity, male age and male dominance rank, one (average) fGC value and associated set of predictor values for each male, for an N of 17 in the analysis (see Table 1). The level of significance for all statistical results was set at P<0.05.
Results
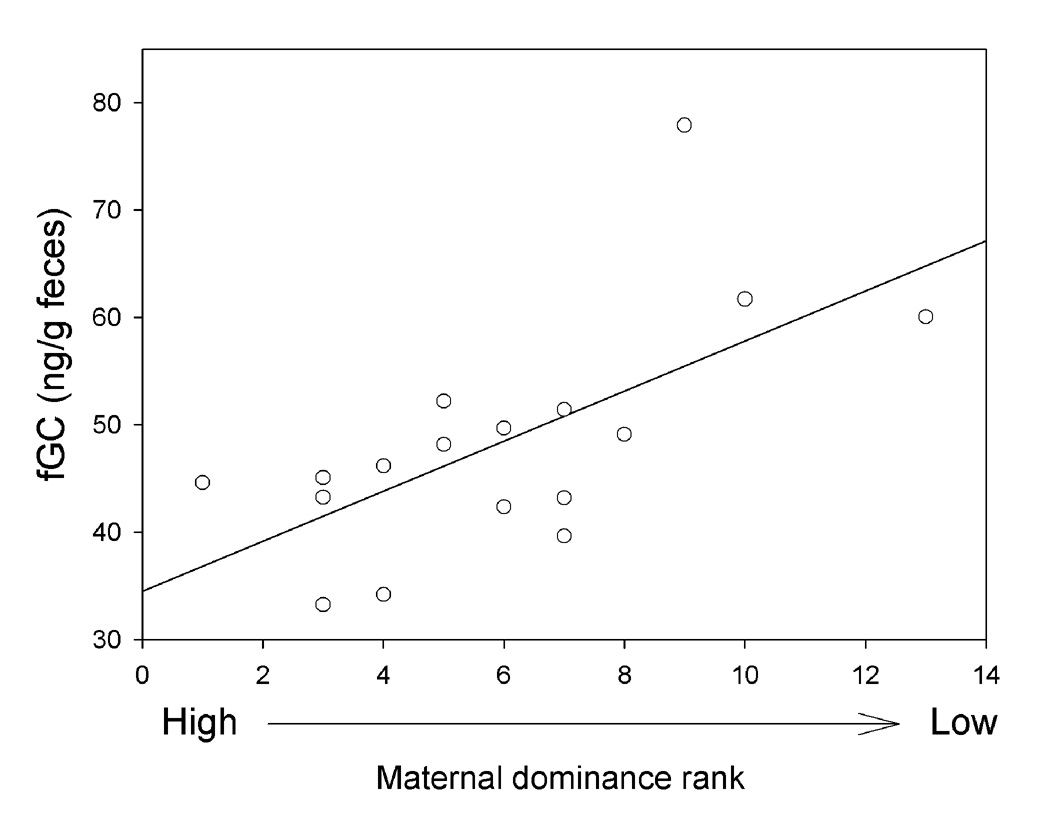
The whole model accounted for 30% of the variance (adjusted r2) in fGC concentrations of the subadult males (P = 0.082; Table 2). However, all predictive ability arose from the effect of maternal dominance rank (P = 0.012; Table 2). Specifically, subadult sons of high-ranking mothers had relatively low fGC, and sons of low-ranking mothers had relatively high fGC concentrations. In contrast, none of the other variables--maternal reproductive experience, the male’s age, or his own dominance rank--contributed to the total model (each P value > 0.46; Table 2). Not surprisingly, therefore, a simple linear regression that ignored these other variables, and predicted fGC just from maternal rank, accounted for 42% of the variance in fGC (P=0.005; Fig. 1)
Table 2.
Results of linear regression model predicting sources of variance in fecal glucocorticoids concentrations for 17 subadult male baboons. Only maternal dominance rank predicted son’s fGC concentrations.
| Predictor | Adj R2 | std. beta | F statistic | T statistic | Sig. |
|---|---|---|---|---|---|
| Whole model | 0.298 | 2.701 | 0.082 | ||
| Maternal rank | 0.642 | 2.968 | 0.012 | ||
| Parity | 0.052 | 0.236 | 0.817 | ||
| Male age | −0.100 | −0.461 | 0.653 | ||
| Male rank | −0.170 | −0.756 | 0.464 |
Figure 1.
Maternal dominance rank sign predicted fGC concentration in subadult males in a simple linear regression, y= 34.504 + 2.329x; r2=0.421, p=0.005; see text for details.
Discussion
Differences in fGC concentrations in subadult male baboons were strongly predicted by maternal dominance rank at the time of the male’s conception. This result is striking for several reasons. First, subadult male baboons are approximately 4–6 years past the period of infant dependence on their mothers. Second, subadult males are larger than and dominant to all adult females. Finally, by this age many subadults (8 of the 17 in our sample) have survived their mothers’ death. Consequently, the influence of maternal dominance rank persisted well beyond the stage at which direct maternal influence on sons is likely. Several rank-based maternal effects have been previously identified in male baboons; sons of high ranking mothers grow faster (Altmann and Alberts, 2005; Johnson, 2003) and reach reproductive maturity earlier than sons of low ranking mothers Altmann and Alberts, 2005). However, this is the first study to indicate that maternal dominance rank at the time of conception and during infancy influences the quality of life of male offspring many years later. A variety of experimental manipulations have produced either one or both of prenatal and postnatal maternal effects for laboratory primates and rodents (e.g. Sanchez, 2006; Walker et al. 2004). These experimental findings plus the natural stability of female dominance rank in baboons, including throughout the offsprings’ perinatal period, suggest the opportunity for dominance-related maternal effects such as we have demonstrated to occur at various stages durng the perinatal period and infancy in this system.
A female's dominance rank predicts her exposure to both psychosocial and nutritional stressors. Low-ranking females receive more direct aggression than high-ranking ones do. They are also subject to higher rates of spatial displacement (Altmann, 1980), more frequent invasions of personal space, and higher levels of interference and rough handling of infants that occur as a part of the attraction of others to mothers and their new infants (Kleindorfer and Wasser, 2004). Low-ranking baboon females, like low-ranking animals of most species also have less access to nutritional resources, drinking water, and perhaps to shade and the best sleeping sites (e.g. Dewsbury, 1982; Post et al., 1980; Smuts and Nicolson, 1989; Wittemyer et al., 2007), and low nutrition has been related to both neonatal and adult pathologies in offspring (e.g. as in the fetal origins hypothesis: Barker et al., 1993; Gluckman, 2001; Lesage et al., 2001; Liu et al., 1997; Meaney et al., 2007; Phillips, 2007), including disruption of the offspring’s HPA axis. During infancy as during gestation, primate infants are intimately exposed to their mother’s experiences, both ecological and social. Like infants of all anthropoid primates, during the first months of life baboon infants cling to their mother’s ventrum, positioned there for transportation, suckling, and resting throughout most of the day. Even as they spend less time in contact later in their first year, much of their time is spent within arm’s reach or a few meters of their mother (Altmann, 1980). This extended intimacy provides ideal conditions for significant and potentially diverse maternal effects.
Taken together, our results and these various characteristics of female dominance and of primate life histories with long gestation and intensive maternal care suggest the possibility that maternal GC might be rank related in mothers and/or that maternal GC is the mechanism by which maternal rank affects sons’ GC. We cannot yet directly evaluate either of these possibilities; our hormone data do not yet span enough years for us to have both maternal fGC and fGC of subadult sons. However, we do have some indications that the relationships are not simple in this system; in a recent one-year study of hormones and behavior during the perinatal period, Nguyen et al. (in press) found that maternal fGC levels predicted maternal responsiveness to infant distress but maternal dominance rank did not predict maternal fGC levels. This is not surprising: even considering only nutritional limitation, which has received the most attention in the literature and in a highly manipulative series of laboratory studies, experimental studies indicate a complex and contingent set of effects on maternal and fetus HPA axis reviewed in Lesage et al., 2001; see also commentary by Gluckman, 2001 and see Phillips, 2007 for a recent review of the fetal origins hypothsis).
Unlike maternal dominance rank, maternal parity did not predict fGC levels in the subadult males. Moreover, in an alternative analysis we examined whether the effect of parity might be limited to a difference between first-time mothers (parity 1) and experienced, multiparous, ones (see, e.g., Altmann and Alberts, 2005); we found no effect of parity 1 versus later parities (unpublished data). The lack of an effect of maternal reproductive experience in the present study may be due to the transient nature of parity: firstborn infants are at early risk but those that survive may be especially strong and consequently may experience no permanent effects of birth status (see discussion in Altmann and Alberts, 2005). Alternatively, the disadvantages of being a firstborn offspring may be partially ameliorated through extended parental care (Altmann and Alberts, 2005; Wasser and Wasser, 1995).
That neither a male's own dominance rank nor his age predicted fGC concentrations might initially seem surprising. Glucocorticoid levels change with age across the lifespan in baboons and other species (Goncharova and Lapin, 2002, 2004; Sapolsky and Altmann, 1991). These age changes are often quite small or gradual across most of the lifespan, however, and the age range of our subjects was narrow relative to other studies of baboons, which often included both subadult and adult males or even spanrf all age classes. Glucocorticoid levels are also sometimes related to dominance, albeit positively or negatively depending on species and perhaps sex (Abbott, 2003; Creel, 2001). Other factors also sometimes affect the relationship between rank and GC levels, even among male baboons, such as Sapolsky’s reports for olive baboons of a relationship during times of rank stability among post-dispersal males but not during times of rank instability, when ‘personality’ predicted hypercortisolism (Ray and Sapolsky, 1992; Sapolsky, 1992b, 1993; also Sapolsky et al., 1997, in whivh either low dominance status or social isolation predicted hypercortisolism among adult males in the Amboseli baboon population). The lack of importance of male rank in the present study may arise because although males can be linearly ranked during the subadult period as during other life stages, dominance rank among pre-dispersal subadult males does not function in the same way that it does for adults; subadult males are subordinate to all adult males and dominant to juvenile males and all females. They also exhibit relatively low rates of social interaction, are often peripheral to the social group, and cannot access reproductive females. That is, subadult males are in a phase of life that is relatively quiescent with respect to social interactions and reproductive activity. Thus the fact that a male's own dominance rank did not predict fGC in our study may reflect the narrow age range and particular life history phase of our subjects and also the low immediate functional social consequences of dominance within this life history phase. We are gradually developing a dataset for adult males of known maternal and early life experience because some males born into study groups disperse into other study groups. This dataset will provide the first test of the changing relative impact of early experience and current status during adulthood on glucocorticoids. We predict that across adulthood physiological traits such as GC levels will be affected not just by maternal traits but also by individuals’ current status, and cumulative lifetime stressors, i.e. cumulative ‘allostatic load’ (Goymann and Wingfield, 2004; McEwen, 2000; McEwen and Wingfield, 2003).
In conclusion, our results indicate that rank-based maternal effects are strongly predictive of GC in subadult sons such that sons of low ranking mothers have higher fGC concentrations than sons of high-ranking mothers many years after independence and even after the mothers die. These findings suggest potential organizational effects of maternal traits on offspring HPA axis as shown for various aspects of biology in experimental studies of captive primates (e.g. manipulation of sex differences through prenatal hormone injections reviewed in Wallen (2005); see also nutritional effects discussed, above). To the extent that chronically elevated levels of GC are associated with many stress-related pathologies, sons of low ranking mothers may experience fitness costs of their mother's low rank past juvenile stages, into subadulthood and adulthood. Additional research will be needed to elucidate lifetime fitness consequences of maternal dominance rank effects in this as well as in other group-living species.
Acknowledgments
We are grateful to the Office of the President, Republic of Kenya and to the Kenya Wildlife Service. We also thank the team of field assistants: R. S. Mututua, S. Sayialel, and J. K. Warutere. Laboratory assistance was provided in Nairobi by T. Wango and in Princeton by L. Okpala, and C. Markham. M. Wikelski made helpful comments on previous drafts. Financial support was provided by the Chicago Zoological Society, US National Science Foundation (IBN-0322613, IBN-0322781, BCS-0323553, BCS-0323596) and the US National Institutes of Health (R03 MH65294). The research was conducted in compliance with all relevant regulations in Kenya Research Permit (MOEST 13/001/C351 Vol. II) and in the US (Princeton University IACUC 1547).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne FB, Bercovitch , Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Altmann J. Balancing costs and opportunities: dispersal in male baboons. American Naturalist. 1995a;145:279–306. [Google Scholar]
- Alberts SC, Altmann J. Preparation and activation: determinants of age at reproductive maturity in male baboons. Behavioural Ecology and Sociobiology. 1995b;36:397–406. [Google Scholar]
- Alberts SC, Buchan JC, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Animal Behaviour. 2006;72:1177–1196. [Google Scholar]
- Alberts SC, Watts HE, Altmann J. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Animal Behaviour. 2003;65:821–840. [Google Scholar]
- Altmann J. Baboon mothers and infants. Chicago: University of Chicago Press; 1980. [Google Scholar]
- Altmann J, Alberts SC. Variability in reproductive success viewed from a life-history perspective in baboons. American Journal of Human Biology. 2003;15:401–409. doi: 10.1002/ajhb.10157. [DOI] [PubMed] [Google Scholar]
- Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behavioral Ecology and Sociobiology. 2005;57:490–501. [Google Scholar]
- Altmann J, Altmann SA, Hausfater G, McCuskey S. Life history of yellow baboons: physical development, reproductive parameters, and infant mortality. Primates. 1977;18:315–330. [Google Scholar]
- Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Bayart F, Simmen B. Demography, range use, and behavior in Black lemurs (Eulemur macaco macaco) at Ampasikely, northwest Madagascar. American Journal of Primatology. 2005;67:299–312. doi: 10.1002/ajp.20186. [DOI] [PubMed] [Google Scholar]
- Beehner JC, Nguyen N, Wango EO, Alberts SC, Altmann J. The endocrinology of pregnancy and fetal loss in wild baboons. Hormones and Behavior. 2006;49:688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Behrensmeyer AK, Boaz DD. Late Pleistocene geology and paleontology of Amboseli National Park, Kenya. In: Coetzee JA, v EM, Bakker ZS, editors. Paleontology of Africa and the surrounding islands. Vol. 13. Rotterdam: A. A. Balkema; 1981. pp. 175–188. [Google Scholar]
- Bernardo J. Maternal effects in animal ecology. American Zoologist. 1996;36:83–105. [Google Scholar]
- Bogdanova MI, Nager RG, Monaghan P. Does parental age affect offspring performance through differences in egg quality? Functional Ecology. 2006;20:132–141. [Google Scholar]
- Clutton-Brock TH. Reproductive success. In: Clutton-Brock TH, editor. Reproductive success. University of Chicago Press; 1988. [Google Scholar]
- Combes SL, Altmann J. Status change during adulthood: life-history byproduct or kin-selection based on reproductive value? Proceedings of the Royal Society of London. Series B, Biological Sciences. 2001;268:1367–1373. doi: 10.1098/rspb.2001.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Smith ELP, Altemus M, Mathew SJ, Perera T, Kral JG, Gorman JM, Owens MJ, Nemeroff CB, Rosenblum LA. Maternal-infant response to variable foraging demand in nonhuman primates - Effects of timing of stressor on cerebrospinal fluid corticotropin-releasing factor and circulating glucocorticoid concentrations. Annals of the New York Academy of Sciences. 2006;1071:525–533. doi: 10.1196/annals.1364.057. [DOI] [PubMed] [Google Scholar]
- Côté SD, Festa-Bianchet M. Reproductive success in female mountain goats: the influence of age and social rank. Animal Behaviour. 2001;62:173–181. [Google Scholar]
- Creel S. Social dominance and stress hormones. Trends in Ecology and Evolution. 2001;16:491–497. [Google Scholar]
- Curio E. Why do young birds reproduce less well? Ibis. 1983;125:400–404. [Google Scholar]
- Davidson R, Altmann J, Alberts SC. Best of times, worst of times: the impact of environmental variation and social factors on baboon life histories (in revision) [Google Scholar]
- Dloniak SM, French JA, Holekamp KE. Rank-related maternal effects of androgens on behaviour in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Dominance rank, corpulatory behavior, and differential reproduction. Quarterly Review of Biology. 1982;57:135–159. doi: 10.1086/412672. [DOI] [PubMed] [Google Scholar]
- Fessler DMT, Navarrete CD, Hoptins W, Izard MK. Examining the terminal investment hypothesis in humans and chimpanzees: associations among maternal age, parity and birth weight. American Journal of Physical Anthropology. 2005;127:95–104. doi: 10.1002/ajpa.20039. [DOI] [PubMed] [Google Scholar]
- Förslund P, Pärt T. Age and reproduction in birds: hypotheses and tests. Trends in Ecology and Evolution. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- Forstmeier W, Coltman DW, Birkhead TR. Maternal effects influence the sexual behavior of sons and daughters in the zebra finch. Evolution. 2004;58:2574–2583. doi: 10.1111/j.0014-3820.2004.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Altmann J, Khan MZ, Couret J, Yu JC, Endres CS, Lynch JW, Ogola P, Fox EA, Alberts SC, Wango EO. Coming of age: steroid hormones of wild immature baboons (Papio cynocephalus) American Journal of Primatology. 2005;67:83–100. doi: 10.1002/ajp.20171. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Williamson KA, Hazon N, Graves JA. Maternal effects due to male attractiveness affect offspring development in the zebra finch. Proceedings of the Royal Society of London Series B, Biological Sciences. 2006;273:1765–1771. doi: 10.1098/rspb.2006.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD. Editorial: nutrition, glucocorticoids, birth size, and adult disease. Endocrinology. 2001;142:1689–1691. doi: 10.1210/endo.142.5.8205. [DOI] [PubMed] [Google Scholar]
- Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mechanisms of Ageing and Development. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Goncharova ND, Lapin BA. Age-related endocrine dysfunction in nonhuman primates. Annals of the New York Academy of Sciences. 2004;1019:321–325. doi: 10.1196/annals.1297.054. [DOI] [PubMed] [Google Scholar]
- Goymann W, Wingfield JC. Allostatic load, social status and stress hormones: the costs of social status matter. Animal Behaviour. 2004;67:591–602. [Google Scholar]
- Hamilton WJ, III, Bulger JB. Natal male baboon rank rises and successful challenges to resident alpha males. Behavioral Ecology and Sociobiology. 1990;26:357–362. [Google Scholar]
- Hausfater G. Dominance and reproduction in baboons (Papio cynocephalus) S. Kager: Basel; 1975. [PubMed] [Google Scholar]
- Holand O, Weladji RB, Gjostein H, Kumpula J, Smith ME, Nieminen M, Roed KH. Reproductive effort in relation to maternal social rank in reindeer (Rangifer tarandus) Behavioral Ecology and Sociobiology. 2004;57:69–76. [Google Scholar]
- Holekamp KE, Smale L, Szykman M. Rank and reproduction in the female spotted hyaena. Journal of Reproduction and Fertility. 1996;108:229–237. doi: 10.1530/jrf.0.1080229. [DOI] [PubMed] [Google Scholar]
- von Holst D, Hutzelmeyer H, Kaetzke P, Khaschei M, Rodel HG, Schrutka H. Social rank, fecundity and lifetime reproductive success in wild European rabbits (Oryctolagus cuniculus) Behavioral Ecology and Sociobiology. 2002;51:245–254. [Google Scholar]
- Hunt J, Simmons LW. Patterns of fluctuating asymmetry in beetle horns: an experimental examination of the honest signalling hypothesis. Behavioral Ecology and Sociobiology. 1997;41:109–114. [Google Scholar]
- Johnson SE. Life history and the competitive environment: trajectories of growth, maturation, and reproductive output among chacma baboons. American Journal of Physical Anthropology. 2003;120:83–98. doi: 10.1002/ajpa.10139. [DOI] [PubMed] [Google Scholar]
- Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. General and Comparative Endocrinology. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. [DOI] [PubMed] [Google Scholar]
- Kleindorfer S, Wasser SK. Infant handling and mortality in yellow baboons (Papio cynocephalus): evidence for female reproductive competition. Behavioral Ecology and Sociobiology. 2004;56:328–337. [Google Scholar]
- Kyneb A, Toft S. Effects of maternal diet quality on offspring performance in the rove beetle Tachyporus hypnorum. Ecological Entomology. 2006;31:322–330. [Google Scholar]
- Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in newborn rat. Endocrinology. 2001;142:1692–1702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochemical Research. 2000;25:1219–1231. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Hormones and Behavior. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. TRENDS in Molecular Medicine. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends in Ecology and Evolution. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Neff BD, Lister JS. Genetic life history effects on juvenile survival in bluegill. Journal of Evolutionary Biology. 2006;20:517–525. doi: 10.1111/j.1420-9101.2006.01265.x. [DOI] [PubMed] [Google Scholar]
- Nguyen N, Gesquiere L, Wango EO, Alberts SC, Altmann J. Late pregnancy glucocorticoid levels predict responsiveness in wild baboon mothers (Papio cynocephalus) Animal Behaviour. in press. [Google Scholar]
- Packer C, Collins AD, Eberly LE. Problem with primate sex ratios. Philosophical Transactions of the Royal Society of London. B, Biological Sciences. 2000;355:1627–1635. doi: 10.1098/rstb.2000.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paitz RT, Harms HK, Bowden RM, Janzen FJ. Experience pays: offspring survival increases with female age. Biology Letters. 2007;3:44–46. doi: 10.1098/rsbl.2006.0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira ME. Development and social dominance among group-living primates. American Journal of Primatology. 1995;37:143–175. doi: 10.1002/ajp.1350370207. [DOI] [PubMed] [Google Scholar]
- Phillips DIW. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? (Symposium) Journal of Internal Medicine. 2007;261:453–460. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Plaistow SJ, Lapsley CT, Benton T. Context-dependent intergenerational effects: the interaction between past and present environments and its effect on population dynamics. American Naturalist. 2006;167:206–215. doi: 10.1086/499380. [DOI] [PubMed] [Google Scholar]
- Poisbleau M, Fritz H, Valeix M, Perroi P, Dalloyau S, Lambrechts MM. Social dominance correlates and family status in wintering dark-bellied brent geese, Branta bernicla bernicla. Animal Behaviour. 2006;71:1351–1358. [Google Scholar]
- Post DG, Hausfater G, McCuskey SA. Feeding behaviour of yellow baboons (Papio cynocephalus): relationship to age, gender, and dominance rank. Folia Primatologica. 1980;34:170–195. doi: 10.1159/000155954. [DOI] [PubMed] [Google Scholar]
- Priest NK, Mackowiak B, Promislow DEL. The role of parental age effects on the evolution of aging. Evolution. 2002;56:927–935. doi: 10.1111/j.0014-3820.2002.tb01405.x. [DOI] [PubMed] [Google Scholar]
- Ray JC, Sapolsky RM. Styles of male social behavior and their endocrine correlates among high-ranking wild baboons. American Journal of Primatology. 1992;28:231–250. doi: 10.1002/ajp.1350280402. [DOI] [PubMed] [Google Scholar]
- Romero LM. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. General and Comparative Endocrinology. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. [DOI] [PubMed] [Google Scholar]
- Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends in Ecology and Evolution. 2004;19:249–255. doi: 10.1016/j.tree.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Royle NJ, Lindström J, Metcalfe NB. A poor start in life negatively affects dominance status in adulthood independent of body size in green swordtails Xiphophorus helleri. Proceedings of the Royal Society of London. Series B, Biological Sciences. 2005;272:1917–1922. doi: 10.1098/rspb.2005.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Neuroendocrinology of the stress response. In: Becker JB, Breedlove SB, Crews D, editors. Behavioral Endocrinology. Cambridge: Massachusettes: MIT Press; 1992a. pp. 287–324. [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992b;17:701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology Alfresco: psychoendocrine studies of wild baboons. Recent Progress in Hormone Research. 1993;48:437–468. doi: 10.1016/b978-0-12-571148-7.50020-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Altmann J. Incidence of hypercorticolism and dexamethasone resistence increase with age among wild baboons. Biological Psychiatry. 1991;30:1008–1016. doi: 10.1016/0006-3223(91)90121-2. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Alberts SC, Altmann J. Hypercorticolism associated with social subordinance or social isolation among wild baboons. Archives of General Psychiatry. 1997;54:1137–1143. doi: 10.1001/archpsyc.1997.01830240097014. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Scott J, Lockard JS. Captive female gorilla agonistic relationships with clumped defendable food resources. Primates. 2006;47:199–209. doi: 10.1007/s10329-005-0167-3. [DOI] [PubMed] [Google Scholar]
- Setchell JM, Lee PC, Wickings EJ, Dixson AF. Reproductive parametersand maternal investment in mandrills (Mandrillus sphinx) International Journal of Primatology. 2002;23:51–68. [Google Scholar]
- Setchell JM, Wickings EJ, Knapp LA. Life history in male mandrills (Mandrillus sphinx): physical development, dominance rank, and group association. American Journal of Physical Anthropology. 2006;131:498–510. doi: 10.1002/ajpa.20478. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Nicolson N. Reproduction in wild female olive baboons. American Journal of Primatology. 1989;19:229–246. doi: 10.1002/ajp.1350190405. [DOI] [PubMed] [Google Scholar]
- SPSS. Student Version 14 Software and user’s guide 2003 SPSS Inc [Google Scholar]
- Stearns SC. The evolution of life histories. New York: Oxford University Press; 1992. [Google Scholar]
- Taborsky B. Mothers determine offspring size in response to own juvenile growth conditions. Biology Letters. 2006;2:225–228. doi: 10.1098/rsbl.2005.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C-D, Deschamps S, Proulx K, Tu M, Salzman C, Woodside B, Lupien S, Gallo-Payet N, Richard D. Mother to infant or infant to mother? Reciprocal regulation of responsiveness to stress in rodents and the implications for humans. Journal of Psychiatry & Neuroscience. 2004;29:364–382. [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Frontiers in Neuroendocrinology. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Walters JR, Seyfarth RM. Conflict and cooperation. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago: Chicago University Press; 1987. pp. 306–317. [Google Scholar]
- Warner DA, Lovern MB, Shine R. Maternal nutrition affects reproductive output and sex allocation in a lizard with environmental sex determination. Proceedings of the Royal Society of London. Series B, Biological Sciences4. 2007;274:883–890. doi: 10.1098/rspb.2006.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser LM, Wasser SK. Environmental variation and developmental rate among free ranging yellow baboons (Papio cynocephalus) American Journal of Primatology. 1995;35:15–30. doi: 10.1002/ajp.1350350103. [DOI] [PubMed] [Google Scholar]
- Western D, van Praet C. Cyclical changes in the habitat and climate of an east African ecosystem. Nature London. 1973;241:104–106. [Google Scholar]
- Whiteman E, Côté I. Dominance hierarchies in group-living cleaning gobies: causes and foraging consequences. Animal Behaviour. 2004;67:239–247. [Google Scholar]
- Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I. Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to conservation behavior. Behavioral Ecology and Sociobiology. 2007;61:1919–1931. [Google Scholar]
- Williams GC. Adaptations and natural selection: a critique of some recent evolutionary thought. Princeton, NJ: Princeton University Press; 1966. [Google Scholar]
- Wolf JB, Brodie ED, III, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends in Ecology and Evolution. 1998;13:64–69. doi: 10.1016/s0169-5347(97)01233-0. [DOI] [PubMed] [Google Scholar]