Abstract
Rationale
There is compelling support for the contribution of dopamine and the D1R-like (D1R, D5R) receptor subfamily to the behavioral and neural effects of psychostimulant drugs of abuse. The relative roles of D1R and D5R subtypes in mediating these effects are not clear.
Objectives
To directly compare (C57BL/6J congenic) D1R knockout (KO) and D5R KO mice for baseline locomotor exploration, acute locomotor responses to cocaine, and locomotor sensitization to repeated cocaine administration. To examine cocaine conditioned place preference (CPP) in D5R KO.
Methods
D1R KO, D5R KO and wild-type (WT) were assessed for baseline open field exploration, locomotor-stimulating effects of 15 mg/kg acute cocaine, and sensitized locomotor responses to cocaine following repeated home cage treatment with 20 or 30 mg/kg cocaine. D5R KO and WT were tested for CPP to 15 mg/kg cocaine.
Results
D1R KO showed modest basal hyperactivity and increased center exploration relative to WT. Acute locomotor responses to cocaine were consistently absent in D1R KO, but intact in D5R KO. D5R KO showed normal locomotor sensitization to cocaine, and normal cocaine CPP. D1R KO failed to show a sensitized locomotor response to 30 mg/kg cocaine. Failure to sensitize in D1R KO was not due to excessive stereotypies. Surprisingly, D1R KO showed evidence of modest sensitization to 20 mg/kg cocaine.
Conclusions
D5R KO does not alter acute or sensitized locomotor responses to cocaine, or cocaine CPP. D5R KO abolishes acute locomotor response to cocaine, but does not fully prevent locomotor sensitization to cocaine at all doses.
Keywords: dopamine, cocaine, knockout, mouse, psychostimulant, addiction, locomotor, reward, open field, conditioned place preference, receptor
Introduction
The neural and behavioral properties of dopamine are mediated via a diverse class of receptors, grouped into the D1-like (D1R and D5R subunits) and D2-like (D2R, D3R and D4R subunits) subfamilies (for review, see (Lachowicz and Sibley 1997)). D1R and D5R have high sequence homology and activate common intracellular signaling pathways (both positively regulate cAMP) (Grandy et al. 1991). Although there is higher expression of D1R than D5R in most brain regions in rodent and human (e.g., cortex, striatum, amygdala, but not hippocampus) (Ciliax et al. 2000; Khan et al. 2000), D5R has higher affinity for dopamine (Tiberi et al. 1991). The distinct molecular and anatomical profiles of D1R and D5R suggest differential functional roles for the subtypes.
There is strong evidence for a major role of dopamine and the D1R-like subfamily in mediating the behavioral and neural effects of most classes of drugs of abuse, including psychostimulants such as cocaine and amphetamine (Kalivas and Stewart 1991; Pierce and Kumaresan 2006). However, empirically parsing specific roles for D1R and D5R has been difficult due to the absence of subtype-selective pharmacological probes. An alternative approach has been to employ molecular genetic techniques including gene ‘knockout’ (KO). Studies of D1R KO and D5R KO mice have provided some novel insights into the functions of these subunits (reviewed in (Holmes et al. 2004; Sibley 1999; Waddington et al. 2005)). D1R KO (but not HET) mice do not show the normal increase in locomotor activity in response to acute treatment with cocaine (Corvol et al. 2007; Karasinska et al. 2005; Xu et al. 1994), mimicking the effects of D1R antagonists such as SCH23390 (Cabib et al. 1991). D1R KO mice also fail to activate key downstream signaling pathways subserving psychostimulant responses (Karasinska et al. 2005; Svenningsson et al. 2003). By comparison, one study has reported that D5R KO show an attenuated locomotor response to acute cocaine treatment (Elliot et al. 2003), and a slightly diminished locomotor and grooming stimulated responses to direct D1R/D5R agonists such as SKF81297 and SKF83959 but not A68930 (Holmes et al. 2001; O'Sullivan et al. 2005).
Repeated treatment with psychostimulants leads to an enhanced locomotor response (Post and Rose 1976). This ‘locomotor sensitization’ provides an experimental model to study the molecular mechanisms underlying the neural and behavioral adaptations occurring with repeated psychostimulant use, with potential relevance to the development of addiction (Everitt and Wolf 2002; Phillips 1997; Robinson and Berridge 2003). The development of sensitization is associated with changes in a mesoaccumbens pathway (ventral tegmental area (VTA) to nucleus accumbens (NAc)) that is rich in D1R-like receptors, particularly D1R (Henry and White 1995; Kalivas and Stewart 1991). Moreover, D1R/D5R within the NAc show increased pharmacological and physiological sensitivity following repeated cocaine treatment (Beurrier and Malenka 2002; Henry and White 1991). However, co-administration of mixed D1R/D5R antagonists (e.g., SCH23390) and cocaine does not block the development of locomotor sensitization under most conditions (Fontana et al. 1993; Kalivas and Stewart 1991; Mattingly et al. 1996; McCreary and Marsden 1993; Steketee 1998; White et al. 1998). These data leave open the question of the functional importance of D1R/D5R to cocaine-induced locomotor sensitization.
Within the mesoaccumbal pathway in rodent and non-human primate, the pattern of D1R and D5R distribution differs across regions and within certain (e.g., striatal) cell populations (Bergson et al. 1995; Surmeier et al. 1996). An important but as yet unanswered question is the relative contribution of D1R and D5R to psychostimulant sensitization. There is one report that D1R KO mice failed to develop cocaine sensitization (Xu et al. 2000), and another found that loss of Fos in D1R-expressing neurons also prevents sensitization (Zhang et al. 2006). The absence of sensitization in D1R KO was observed with the mutation placed on a mixed C57BL/6J × ‘129Sv’ genetic background. This is noteworthy because certain 129 substrains show reduced psychostimulant-induced striatal dopamine release (Chen et al. 2007) and exhibit lesser locomotor responses to repeated cocaine than C57BL/6J (Schlussman et al. 2003). Moreover, extant 129 background genes have been shown to influence KO phenotypes (Holmes et al. 2003), and there are examples of 129 genes causing false positive dopamine receptor KO motor abnormalities (Kelly et al. 1998). Thus, there is the possibility that 129 background genes may have contributed to the negative locomotor sensitization phenotype observed in D1R KO mice. Backcrossing D1R KO into a congenic C57BL/6J background reduces this potential confound.
Regarding D5R, recent work has shown that the D1R/D5R antagonist SCH23390 blocks sensitization-related changes in synaptic plasticity in VTA (Schilstrom et al. 2006). This led to the suggestion that because VTA dopamine neurons preferentially express D5R over D1R, the effects of D1R/D5R drugs on VTA plasticity may be specifically D5R-mediated (Schilstrom et al. 2006). Furthermore, an earlier study suggested that D5R may also mediate specific forms of corticostriatal plasticity (long-term depression) based on the ability of KO of D5R but not D1R to abolish this plasticity (Centonze et al. 2003). While these physiological data suggest a role for D5R in mediating neuroadaptations underlying the development of reward-related behaviors including locomotor sensitization, this remains to be tested at the behavioral level.
The objectives of the present study were to directly compare of D1R KO and D5R KO mice on a C57BL/6J congenic background for basal exploratory locomotion, acute locomotor responses to cocaine and locomotor sensitization to repeated cocaine using two different sensitizing doses. To further extend the profile of D5R KO mice by examining another measure of the reinforcing effects of repeated cocaine following D5R loss (Tzschentke 2007), D5R KO mice were also assessed for conditioned place preference to cocaine.
Methods
Subjects
D5R KO (Hollon et al. 2002) and D1R KO (Drago et al. 1994) were generated as previously described. For the current study, each KO line was separately backcrossed into C57BL/6J for >10 generations to produce a congenic genetic background (i.e., expected to be 99.9% genetically identical to the host inbred strain at all loci except the mutated gene and flanking region (Wolfer et al. 2002)). To avoid potential phenotypic abnormalities resulting from genotypic differences in maternal behavior and early life environment (Millstein and Holmes 2007), KO and WT mice of each mutant line were generated from HET × HET matings. Because D1R KO fail to thrive after weaning without palatable food supplementation, D1R KO (but not WT or D5R KO unless littermates co-housed with D1R KO) were provided with peanut butter for the first month post-weaning and then ‘bacon softies’ (Bio-Serv, Frenchtown, NJ). Mice were bred and maintained at the NIH in same-sex groups of 1−4 mice/cage in a temperature and humidity controlled vivarium under a 12 h light/dark cycle (lights on 0600 h). Mice were aged 2−8 months at the time of testing. Within any given experiment, genotypes were age-matched littermates derived from the same breeding pool. Both males and females were used. The number of mice used in each experiment is given in the figure legends. The experimenter remained blind to genotype (mice were identified by subcutaneous microchips). All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and followed the National Institute of Health guidelines outlined in ‘Using Animals in Intramural Research.’
Basal locomotion, acute locomotor response to cocaine and locomotor sensitization following repeated 30 mg/kg cocaine
Mice were acclimated to the testing room for 60 min prior to testing (for all open field tests). The open field apparatus was a 40 × 40 × 35 cm square arena (50 lux) constructed of white Plexiglas as previously described (Hefner and Holmes 2007). The mouse was placed in the perimeter and allowed to explore the apparatus for 120 min. Total distance traveled in the whole arena, and duration and entries into the center (20 × 20 cm), was measured by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA). The apparatus was cleaned with 70% ethanol solution and dried between subjects.
Twenty-four hr after baseline testing, acute locomotor responses to cocaine were measured. Mice were habituated to the open field for 60 min then injected with 15 mg/kg cocaine and immediately returned to the perimeter of the apparatus for a further 60 min. Beginning 24 hr later, mice were intraperitoneally injected with 30 mg/kg cocaine in the home cage once daily for 5 consecutive days. Twenty-four hr after the final cocaine injection, locomotor sensitization was tested. Mice were habituated to the open field for 60 min then injected with 15 mg/kg cocaine and immediately returned to the perimeter of the apparatus for a further 60 min.
A control group of WT mice receiving repeated injections of saline rather than cocaine showed no change in locomotor activity across phases (acute response=4.48 ±0.5 meters, response following repeated injections=3.90 ±0.4 meters, paired t-test=ns). Therefore a saline-alone group was not included in subsequent experiments.
High doses of cocaine have the potential to sensitize locomotor behavior to such an extent that cocaine challenge produces locomotor stereotypies that confound measurement of locomotor stimulation and produce a false positive loss of sensitization. We addressed this issue in two ways: first, by replicating the same 30 mg/kg sensitizing procedure and quantifying stereotypies, and second by repeating the sensitizing procedure with a lower, 20 mg/kg, sensitizing dose.
Locomotor stereotypies following repeated 30 mg/kg cocaine
Naïve mice were tested using the same 30 mg/kg sensitizing procedure as above. Stereotypies were quantified concurrently with video-tracked locomotor activity during open field testing. Quantification was conducted manually every 20 sec using a time sampling method and rating scales employed previously (Creese and Iversen 1974; Daunais and McGinty 1994; Schlussman et al. 2003): 1) inactive, sleeping, 2) alert, actively grooming, 3) sniffing and exploration, 4) intermittent rearing and sniffing while rearing, 5) increased locomotion, 6) intense sniffing fixated at 1 location, 7) continuous pivoting and sniffing, 8) continuous rearing and sniffing, 9) sustained rearing and sniffing while on hind legs, 10) splayed hind limbs. Measurements were conducted during min 5−15 post-cocaine injection (=30 observations per mouse), which is the time interval corresponding to the peak locomotor activity response to cocaine.
Locomotor sensitization following repeated 20 mg/kg cocaine
Naïve mice were tested using the same procedure as above, using a 20 mg/kg rather than 30 mg/kg sensitizing dose.
Conditioned place preference to cocaine
Conditioned place preference was conducted using methods previously described in our laboratory (Boyce-Rustay and Holmes 2006; Boyce-Rustay et al. 2006). The apparatus consisted of 2 compartments (each 17×13×13 cm): 1 compartment had black walls with a grid floor (3.2-mm rods) and the other had white walls with a wire mesh floor housed in a sound-attenuated chamber (Med Associates, St. Albans, VT, USA). Both compartments were equally illuminated and separated by a removable barrier. Time spent and locomotor activity within each compartment was measured by photobeams placed 1.2 cm apart across the full length of the apparatus. During the habituation and test sessions, a barrier with a 5-cm opening at the floor was placed in between the chambers. Mice were first given a habituation session to reduce the novelty of the apparatus and the mild stress of intraperitoneal injection. The grid and mesh floors were covered with solid Plexiglas to prevent any conditioning to the floor texture. Mice were given a saline injection and allowed to freely explore the apparatus for 20 min. Twenty-four hr later, the mice began conditioning trials using an unbiased design in which half of the subjects had cocaine paired with the black walls/grid floor compartment and the other half had cocaine paired with the white walls/wire mesh floor compartment. The mouse received 15 mg/kg cocaine (CS+ trials) or saline (CS− trials) and was placed into the appropriate compartment for 20 min. CS+ trials and CS− trials were alternated daily, and the order of CS+ vs. CS− trials was counterbalanced within genotype. One complete trial comprised a CS+ trial and a CS− trial. After 4 conditioning trials, mice received a preference test in which they received a saline injection and were placed in-between compartments to freely explore the whole apparatus for 20 min. CPP was assayed by the time spent on the cocaine-paired side relative to the time spent on the saline-paired side, and the relative locomotor activity (photocell beam breaks) in the paired versus unpaired side.
Drugs
Cocaine hydrochloride (Sigma, St. Louis, MO) was dissolved in 0.9% saline and injected intraperitoneally in a volume of 10 mL/kg body weight.
Statistical analysis
WT mice from the D1R and D5R lines did not differ from one another on any measure and data from all WT were combined to increase the statistical power of genotype comparisons. The effect of genotype on percent center time and center visits during baseline testing was analyzed using analysis of variance (ANOVA) and Newman-Keuls post hoc comparisons. For the sensitization experiments, the effect of genotype on locomotor activity during each of the 3 phases of testing (i.e., baseline, acute response to cocaine, response after repeated cocaine) was analyzed using ANOVA with repeated measures for time, followed by simple main effects analysis and Newman-Keuls post hoc comparisons. For conditioned place preference data, the effect of drug-paired chamber (cocaine versus saline) and genotype on time spent and locomotor activity in each side during testing was analyzed using 2-factor ANOVA with repeated measures for drug-paired chamber, followed by Newman-Keuls post hoc tests.
Results
Baseline locomotor exploration
Baseline locomotor data from cocaine sensitizing experiments were combined for analysis. There was a significant genotype × time interaction for baseline total distance traveled (F56,1288=1.63, P<.01). Post hoc analysis showed that D1R KO were significantly more active than WT controls during most time bins during the session, while D5R KO were significantly less active than WT controls during min 75−80 and 115−120 (Figure 1A). Taking the session as a whole, D1R KO mice were significantly more active than WT controls, while D5R KO mice were significantly less active than WT (post hoc comparisons: P<.01).
Figure 1.
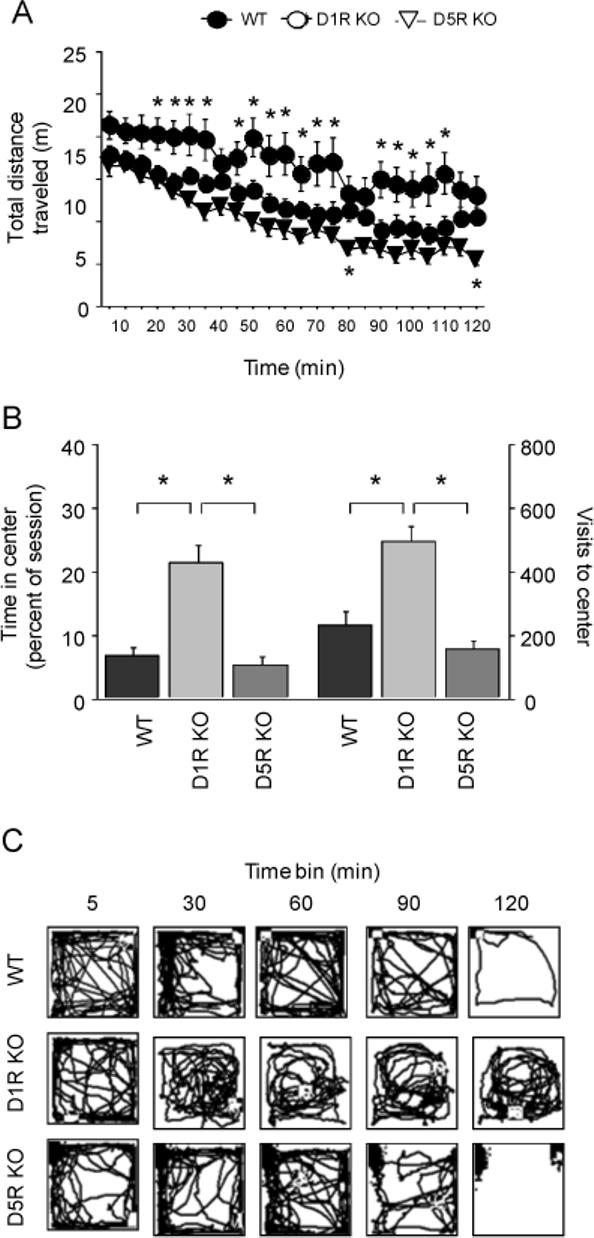
Baseline locomotor exploration in D1R KO and D5R KO mice. (A) D1R KO mice showed heightened levels of activity during the majority of the session as compared to WT, while D5R KO showed a modest decrease in activity during latter periods of the session. (B) D1R KO showed more exploration of the center (time and visits) of the open field than D5R KO or WT. (C) Representative depictions of 5-min exploration patterns across the test session in WT, D1R KO and D5R KO. n=17−24/genotype. Filled circles=WT, Open circles=D1R KO, Open triangles=D5R KO. *P<.05 vs. WT, or as indicated. Data are means ±SEM.
There was also significant effect of genotype for entries into (F2,56=19.08, P<.01) and percent time spent in (F2,56=24.90, P<.01) the center of the open field across the session. Post hoc tests showed that D1R KO mice showed significantly more exploration of the center than WT controls (Figure 1B). Genotype differences in spatial distribution and across-session habituation are depicted as track tracings in Figure 1C.
Acute locomotor response to cocaine
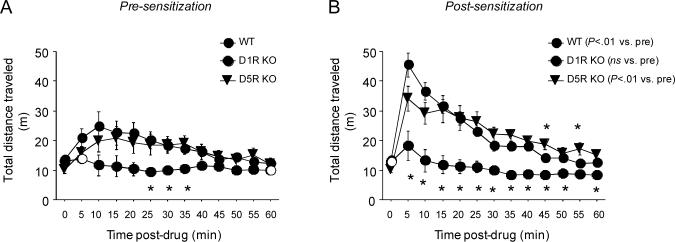
There was a significant genotype × time interaction for total distance traveled following acute injection with cocaine (F24,384=2.00, P<.01). Post hoc analysis showed that D5R KO and WT mice were more active than D1R KO mice during min 20−35 following cocaine injection (Figure 2A). Genotypes were equivalent by the end of the session (Figure 2A).
Figure 2.
Locomotor responses to cocaine pre- and post-sensitization in D1R KO and D5R KO using a 30 mg/kg sensitizing cocaine dose. (A) Pre-sensitization, D5R KO and WT, but not D1R KO, showed a locomotor-stimulant response to cocaine. (B) Post-sensitization, D5R KO and WT, but not D1R KO, showed a locomotor-stimulant response to cocaine that was greater than pre-sensitization. Filled circles=WT, Open circles=D1R KO, Open triangles=D5R KO. n=9−16/genotype. *P<.05 D1R KO vs. WT and D5R KO. Data are means ±SEM.
Locomotor response to a 30 mg/kg sensitizing dose of cocaine
There was a significant genotype × time interaction for total distance traveled following cocaine injection after mice had received repeated cocaine treatment (F24,384=6.17, P<.01). Post hoc analysis showed that D5R KO and WT mice were more active than D1R KO mice during all time points post cocaine injection except min 50−55, while D5R KO were more active than WT controls during min 40−45 and 50−55 (Figure 2B).
Analysis of the change in locomotor activity between the pre- and post-sensitization phases revealed a significant genotype × phase interaction for total distance traveled (F2,32=4.89, P<.05). Paired t-tests showed that WT (t=2.87, df=15, P<.01) and D5R KO (t=7.03, df=8, P<.01), but not D1R KO mice, showed a significant increase in total distance traveled across phases (i.e., sensitization) (Figure 2B).
Locomotor stereotypies to a 30 mg/kg sensitizing dose of cocaine
There was a significant genotype × phase interaction for stereotypy scores (F2,28=5.18, P<.05). Paired t-tests showed that there were modest but significant increases in stereotypy scores in WT (t=2.53, df=12, P<.05) and D5R KO (t=2.68, df=10 P<.05), but not D1R KO, which showed a trend for a decrease (Figure 3). Concurrent analysis of the change in locomotor activity between the pre- and post-sensitization phases via videotracking confirmed a significant genotype × phase interaction for total distance traveled (F2,26=11.23, P<.01). Paired t-tests showed that WT (t=5.60, df=11, P<.01) and D5R KO (t=2.99, df=10, P<.05), but not D1R KO mice, showed a significant increase in total distance traveled across phases (i.e., sensitization) (WT acute activity=13.9 ±2.1 meters, WT chronic=22.8 ±1.9, D5R KO acute=16.7 ±3.3, D5R KO chronic=23.3 ±2.9, D1R KO acute=14.4 ±5.9, D1R KO chronic=9.3 ±0.8).
Figure 3.
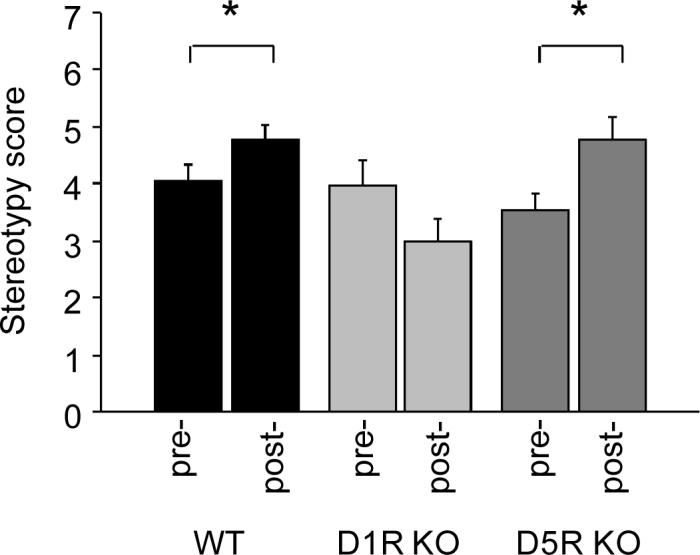
Stereotypy scores in response to cocaine pre- and post-sensitization in D1R KO and D5R KO using a 30 mg/kg sensitizing cocaine dose. Stereotypy scores increased from pre- and post-sensitization in WT and D5R KO, but not D1R KO. n=7−13/genotype. *P<.05 pre- vs. post-sensitization/same genotype. Data are means ±SEM.
Locomotor sensitization to a 20 mg/kg sensitizing dose of cocaine
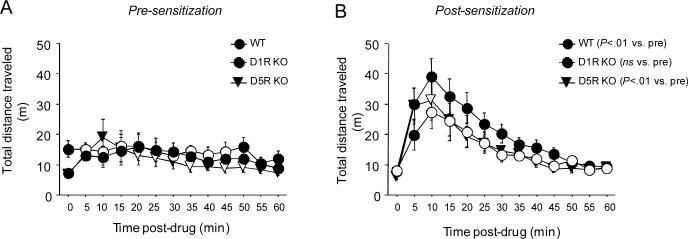
Prior to sensitization, there was a significant effect of time (F12,312=3.66, P<.01) but not of genotype and no genotype × time interaction for total distance traveled following cocaine injection. Simple main effects analysis of time demonstrated a significant effect of time on total distance traveled in WT (F12,114=2.15, P<.05) and D5R KO (F12,96=3.38, P<.01) but not D1R KO (Figure 4A).
Figure 4.
Locomotor responses to cocaine pre- and post-sensitization in D1R KO and D5R KO using a 20 mg/kg sensitizing cocaine dose. (A) Pre-sensitization, D5R KO and WT, but not D1R KO, showed a locomotor-stimulant response to cocaine. (B) Post-sensitization, all genotypes showed a locomotor-stimulant response to cocaine. The response was greater than at pre-sensitization for WT and D5R KO, but not D1R KO. Filled circles=WT, Open circles=D1R KO, Open triangles=D5R KO. n=7−13/genotype. Data are means ±SEM.
Post-sensitization, there was a significant effect of time (F12,312=27.64, P<.01) but not of genotype and no genotype × time interaction for total distance traveled following cocaine injection. Simple main effects analysis of time demonstrated a significant effect of time on total distance traveled in WT (F12,114=16.63, P<.01), D5R KO (F12,96=11.56, P<.01) and D1R KO (F12,72=5.28, P<.01) (Figure 4B). Examining the 40 minutes after injection on both the pre- and post-repeated treatment phases (as in the 30 mg/kg sensitizing dose experiment) revealed a significant main effect of phase (F1,26=22.55, P<.01), but not of genotype and no phase × genotype interaction. This suggests an increase in locomotor activity across phases (i.e., sensitization) irrespective of genotype. However, it should be noted that the phase × genotype interaction approached significance (F2,26=2.72, P=.0848) and paired t-tests showed that total distance travelled was significantly higher post- than pre-sensitization in WT (t=3.28, df=12, P<.01) and D5R KO (t=5.68, df=8, P<.01), but not D1R KO. Thus, although evident, sensitization was not as robust in D1R KO as in the other genotypes.
Conditioned place preference to cocaine
There was no side preference during habituation in either genotype (WT to-be-saline side=52 ±9% time, WT to-be-cocaine side= 49 ±9% time, KO to-be-saline side=46 ±5% time, KO to-be-cocaine side=54 ±5% time). During testing, there was a significant effect of drug-pairing side (F1,10=42.42, P<.01) but not genotype and no side × genotype interaction for time spent in each side, indicating a significant preference for the drug paired side over the saline paired side, irrespective of genotype (Figure 5A). There was also a significant effect of drug-pairing side (F1,10=11.78, P<.01) but not genotype and no side × genotype interaction for chamber locomotor activity. Mice showed more activity in the drug paired side than the saline paired side regardless of genotype (Figure 5B).
Figure 5.
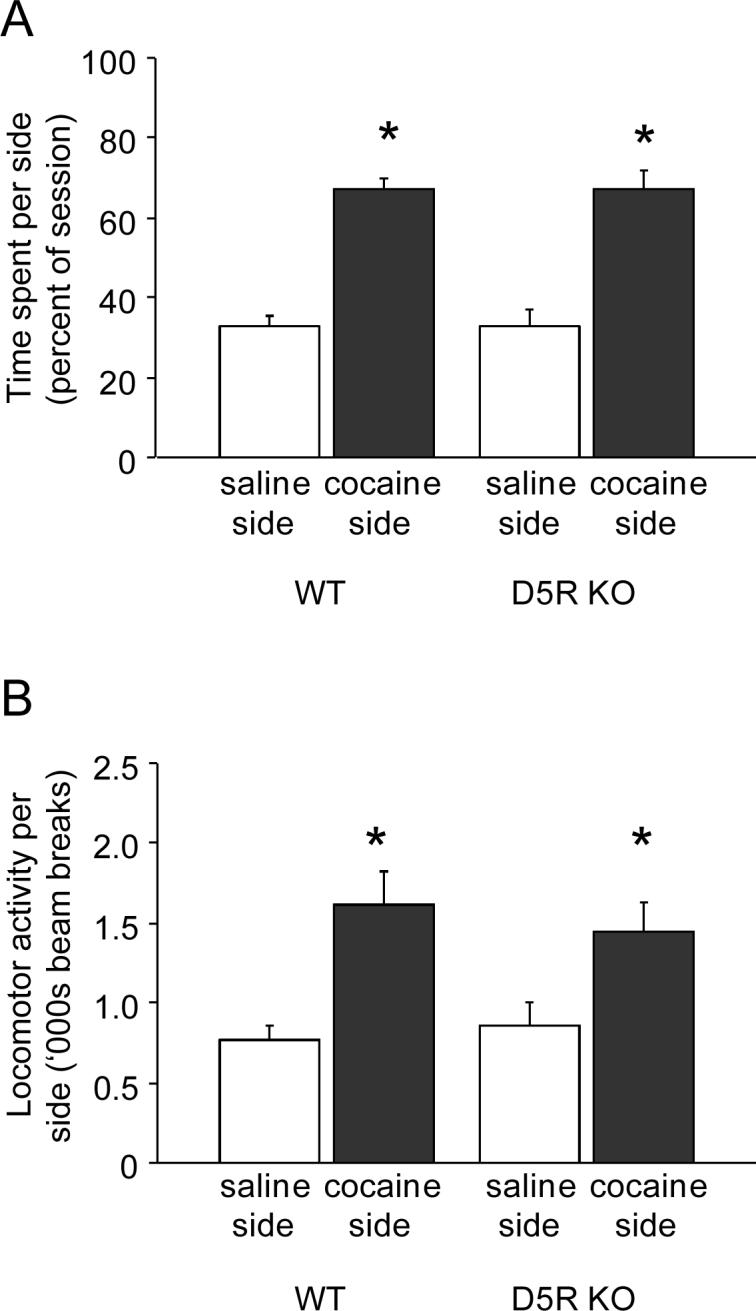
Conditioned place preference to cocaine in D5R KO mice. (A) During CPP testing, mice spent more time in the cocaine-paired side than the saline-paired side regardless of genotype. (B) There was more locomotor activity in the cocaine-paired side than the saline-paired side during CPP testing, irrespective of genotype. n=6/genotype. *P<.05 cocaine-paired vs. saline-paired. Data are means ±SEM.
Discussion
The main findings of the current study were that C57BL/6J-congenic D5R KO showed normal acute and sensitized locomotor responses to cocaine and developed normal cocaine CPP, whereas C57BL/6J-congenic D1R KO showed no acute cocaine response but failed to sensitize to cocaine in a dose-dependent fashion.
Analysis of locomotor exploration during undrugged exposure to a novel open field showed that while genotypes did not differ in initial activity, D1R KO were generally more active than WT over the latter part of the session. D1R KO mice also showed markedly more exploration of the aversive center of the open field than WT controls, further indicating the disorganization of exploratory behavior in D1R KO. This basal open field profile is largely consistent with previous studies of D1R KO, although some discrepancies exist (reviewed in (Holmes et al. 2004; Waddington et al. 2005)). In contrast to the D1R phenotype, D5R KO showed marginally quicker habituation to the open field as evidenced by lesser activity than WT during the latter part of the test, but were otherwise normal. This concurs with previous observations of moderately depressed locomotor activity in D5R KO mice (Holmes et al. 2001; O'Sullivan et al. 2005).
A well-replicated finding is that D1R KO mice (Drago et al. 1996; Xu et al. 1994), including those tested on a congenic C57BL/6J background (Karasinska et al. 2005), fail to respond to the hyperactivity-inducing effects of acute treatment with D1R/D5R agonists or psychostimulants including cocaine. D1R KO consistently showed negligible locomotor responses to acute cocaine treatment across three separate experiments in our study. On the other hand, D5R KO responded to acute cocaine in a manner indistinguishable from WT controls. This latter finding contrasts somewhat with an earlier observation of a modestly diminished locomotor response to acute cocaine in D5R KO mice (Elliot et al. 2003). The use of a 129/SvJ1 × C57BL/6J hybrid genetic background in the earlier study, versus a congenic C57BL/6J background in the present study, provides the most likely explanation for this difference given the ability of genetic background to influence dopamine receptor KO phenotypes (Holmes et al. 2004; Kelly et al. 1998; Waddington et al. 2005), although other methodological factors may also have contributed. Notwithstanding, the finding that, unlike loss of D1R, loss of D5R does not abolish the acute locomotor response to cocaine extends evidence that D5R KO does not affect the discriminative-stimulus effects of cocaine (Elliot et al. 2003). Thus, D5R appears to play a dispensable role in mediating various acute behavioral effects of cocaine. By extension this suggests that the ability of mixed D1R/D5R antagonists to attenuate the reinforcing properties of cocaine across various experimental animal paradigms (Barrett et al. 2004; Self 2004) and in human volunteers (Romach et al. 1999) are likely principally D1R mediated.
Loss of D5R failed to alter the development of cocaine sensitization in three replications using two different sensitizing doses - i.e., after receiving repeated cocaine injections, D5R KO showed an augmented locomotor response to cocaine challenge that was no different from the augmented response produced in WT controls. Extending these data to another model of cocaine's reinforcing effects D5R KO also developed normal cocaine CPP. In this context, previous studies have demonstrated intact cocaine CPP in mice with D1R KO or loss of Fos in D1R-expressing neurons (Karasinska et al. 2005; Miner et al. 1995; Zhang et al. 2006). This contrasts with the ability of systemic or intra-accumbel administration of the D1R/D5R antagonist SCH23390 to prevent cocaine CPP (Baker et al. 1998; Cervo and Samanin 1995; Hnasko et al. 2007; Pruitt et al. 1995). Taken together with recent evidence that constitutive loss of dopamine itself does not prevent cocaine CPP (Hnasko et al. 2007), these data indicate that KO of dopamine signaling through either D1R or D5R is not sufficient to prevent cocaine CPP.
As noted in the Introduction a previous study had demonstrated that D1R KO did not develop locomotor sensitization to cocaine (Xu et al. 2000). Subsequent studies found that mice lacking function of either the NR1 N-methyl-D-aspartate receptor or the immediate-early gene Fos in D1R-expressing neurons showed attenuated cocaine locomotor sensitization (Heusner and Palmiter 2005; Zhang et al. 2006). Consistent with these studies, D1R KO failed to display sensitization after repeated treatment with 30 mg/kg cocaine. Nonetheless, the possibility remained that the absence of a sensitization effect in D1R KO had actually been a false negative caused by increased sensitization to this relatively high sensitizing dose. This could have resulted in stereotypies in D1R KO and a consequent reduction in the principle measure of sensitization, total distance traveled. We addressed this issue in a replicate experiment in which stereotypies were measured, and found that although D1R KO again failed to show sensitization-related increase in total distance travelled there was no indication of excessive stereotypies.
An interesting and somewhat surprisingly finding was that, when tested with a 20 mg/kg sensitizing dose, D1R KO displayed evidence of a partial sensitization to cocaine. Specifically, despite again showing no acute locomotor response to cocaine, D1R KO clearly showed a cocaine-induced increase in locomotor activity following repeated treatment with a 20 mg/kg dose. However, unlike the pre- versus post-sensitization locomotor increase in WT and D5R KO the cocaine-induced locomotor response in D1R KO was not significantly increased across the phases. Nonetheless, these data suggest that D1R KO can exhibit locomotor sensitization under certain conditions and as such are seemingly at odds with current assumptions regarding the necessary contribution of D1R to cocaine sensitization. The reasons for the apparent discrepancy between our findings and those of Xu et al (Xu et al. 2000) remain to be ascertained. Genetic background is one obvious difference which, as discussed above in the context of D5R KO, could also be an influence on phenotypic outcomes in the D1R KO. Sensitizing dose per se does not appear to be the defining difference, as divergent effects were found at the same 20 mg/kg dose. Of possible critical importance however is the fact that our study used a sensitization protocol in which mice were treated once daily, while Xu et al treated twice daily. Therefore, the sensitizing regimen used by Xu et al may have more closely approximated to our 30 mg/kg cocaine sensitizing experiment, in which D1R KO showed no sensitization. This is one way to reconcile the findings of the two studies. In turn, it suggests a refinement of the D1R sensitization model in which the effects of D1R KO are dependent upon the intensity of sensitization regimen employed, with higher daily exposure to cocaine associated with a clear loss-of-sensitization phenotype.
A number of caveats to this and the other conclusions of the present study should be acknowledged. These mainly relate to the inherent limitations of the constitutive KO approach. First, irreversible receptor KO cannot dissociate a failure to develop sensitization from a failure to express sensitization to the higher dose of cocaine in D1R KO. Suggesting that a failure to show sensitization in D1R KO may be due an inability to express the behavior, treatment with a D1R/D5R antagonist during sensitizing phase does not typically prevent the subsequent expression of sensitization (Fontana et al. 1993; Kalivas and Stewart 1991; Mattingly et al. 1996; Steketee 1998; White et al. 1998). On the other hand, the sensitization process is clearly paralleled by changes in D1R/D5R function (Kalivas and Stewart 1991). The second limitation stems from the potential for constitutive KO of a gene to produce compensatory alterations, especially during development, that mask the normal function of a dopamine receptor (Holmes et al. 2004; Waddington et al. 2005). In this context, it would be premature to completely exclude a role for D5R in reward-related behavioral adaptations until additional techniques for selectively inactivating D5R (e.g., RNA interference) can be brought to bear upon the question. The same qualification applies to our findings regarding extant low-dose locomotor sensitization in D1R KO. For example, compensatory changes may have somehow mitigated the consequences of D1R loss for sensitization to the low, but not high, sensitizing cocaine dose. Of note in this context is that while attenuated D1R KO locomotor sensitization to amphetamine has been found in some but not all studies (Crawford et al. 1997; Karper et al. 2002; McDougall et al. 2005; Xu et al. 2000), Karper et al found that the D1R/D5R antagonist SCH23390 blocked amphetamine sensitization in WT but not D1R KO (Karper et al. 2002). This suggests the uncoupling of the D1R contribution to amphetamine sensitization in the KO mice via the recruitment of alternative mechanisms (Karper et al. 2002). Indeed, there is evidence of abnormal cortical neuronal morphology and changes in gene expression of for example G-protein signaling related (Rgs family) proteins in D1R KO (Stanwood et al. 2005; 2006; Zhang et al. 2005). Whether these or other changes support the partial maintenance of cocaine sensitization observed in D1R KO remains to be determined.
In summary, present findings further extend the phenotypic characterization of the consequences of KO of D1R or D5R for basal locomotor exploration, acute responses to cocaine and sensitization to repeated cocaine administration. During undrugged exposure to a novel open field, congenic D1R KO mice showed locomotor hyperactivity relative to WT, while congenic D5R KO were normal with the exception of marginally quicker habituation. The locomotor hyperactivity-inducing effects of acute cocaine treatment were unaltered in D5R KO but lost in D1R KO. Locomotor sensitization to repeated cocaine treatment was normal in D5R KO, as was conditioned place preference to cocaine. D1R KO prevented sensitization to a high but not low sensitizing dose of cocaine, without causing excessive locomotor stereotypies. Taken together, these data demonstrate that constitutive KO of D5R does not abolish two behavioral measures of cocaine's reinforcement-related effects, and that KO of D1R is not sufficient to block cocaine sensitization under all conditions.
Acknowledgements
We are very grateful to David Cabrera for maintenance of the breeding colonies. Research supported by the Intramural Research Programs of the National Institute of Alcohol Abuse and Alcoholism and National Institute of Neurological Disease and Stroke.
References
- Baker DA, Fuchs RA, Specio SE, Khroyan TV, Neisewander JL. Effects of intraaccumbens administration of SCH-23390 on cocaine-induced locomotion and conditioned place preference. Synapse. 1998;30:181–93. doi: 10.1002/(SICI)1098-2396(199810)30:2<181::AID-SYN8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–73. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS. Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci. 1995;15:7821–36. doi: 10.1523/JNEUROSCI.15-12-07821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J Neurosci. 2002;22:5817–22. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006;187:455–66. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Wiedholz LM, Millstein RA, Carroll J, Murphy DL, Daws LC, Holmes A. Ethanol-related behaviors in serotonin transporter knockout mice. Alcohol Clin Exp Res. 2006;30:1957–65. doi: 10.1111/j.1530-0277.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- Cabib S, Castellano C, Cestari V, Filibeck U, Puglisi-Allegra S. D1 and D2 receptor antagonists differently affect cocaine-induced locomotor hyperactivity in the mouse. Psychopharmacology (Berl) 1991;105:335–9. doi: 10.1007/BF02244427. [DOI] [PubMed] [Google Scholar]
- Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavon N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci. 2003;23:8506–12. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–50. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S, Gnegy ME. C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacol Biochem Behav. 2007 doi: 10.1016/j.pbb.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Nash N, Heilman C, Sunahara R, Hartney A, Tiberi M, Rye DB, Caron MG, Niznik HB, Levey AI. Dopamine D(5) receptor immunolocalization in rat and monkey brain. Synapse. 2000;37:125–45. doi: 10.1002/1098-2396(200008)37:2<125::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Valjent E, Pascoli V, Robin A, Stipanovich A, Luedtke RR, Belluscio L, Girault JA, Herve D. Quantitative changes in Galphaolf protein levels, but not D1 receptor, alter specifically acute responses to psychostimulants. Neuropsychopharmacology. 2007;32:1109–21. doi: 10.1038/sj.npp.1301230. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Drago J, Watson JB, Levine MS. Effects of repeated amphetamine treatment on the locomotor activity of the dopamine D1A-deficient mouse. Neuroreport. 1997;8:2523–7. doi: 10.1097/00001756-199707280-00021. [DOI] [PubMed] [Google Scholar]
- Creese I, Iversen SD. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39:345–57. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- Daunais JB, McGinty JF. Acute and chronic cocaine administration differentially alters striatal opioid and nuclear transcription factor mRNAs. Synapse. 1994;18:35–45. doi: 10.1002/syn.890180106. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91:12564–8. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Westphal H, Steiner H. D1 dopamine receptor-deficient mouse: cocaine-induced regulation of immediate-early gene and substance P expression in the striatum. Neuroscience. 1996;74:813–23. doi: 10.1016/0306-4522(96)00145-5. [DOI] [PubMed] [Google Scholar]
- Elliot EE, Sibley DR, Katz JL. Locomotor and discriminative-stimulus effects of cocaine in dopamine D5 receptor knockout mice. Psychopharmacology (Berl) 2003;169:161–8. doi: 10.1007/s00213-003-1494-y. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana D, Post RM, Weiss SR, Pert A. The role of D1 and D2 dopamine receptors in the acquisition and expression of cocaine-induced conditioned increases in locomotor behavior. Behav Pharmacol. 1993;4:375–387. [PubMed] [Google Scholar]
- Grandy DK, Zhang YA, Bouvier C, Zhou QY, Johnson RA, Allen L, Buck K, Bunzow JR, Salon J, Civelli O. Multiple human D5 dopamine receptor genes: a functional receptor and two pseudogenes. Proc Natl Acad Sci U S A. 1991;88:9175–9. doi: 10.1073/pnas.88.20.9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl) 2007;191:311–22. doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258:882–90. [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15:6287–99. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevents cocaine sensitization and decreases cocaine preference. J Neurosci. 2005;25:6651–7. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–8. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci. 2002;22:10801–10. doi: 10.1523/JNEUROSCI.22-24-10801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR, Crawley JN. Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci. 2001;115:1129–44. [PubMed] [Google Scholar]
- Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004;47:1117–34. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy DL, Gold E, Crawley JN. Abnormal anxiety-related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–80. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Karasinska JM, George SR, Cheng R, O'Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur J Neurosci. 2005;22:1741–50. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- Karper PE, De la Rosa H, Newman ER, Krall CM, Nazarian A, McDougall SA, Crawford CA. Role of D1-like receptors in amphetamine-induced behavioral sensitization: a study using D1A receptor knockout mice. Psychopharmacology (Berl) 2002;159:407–14. doi: 10.1007/s00213-001-0936-7. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–9. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Gutierrez A, Martin R, Penafiel A, Rivera A, de la Calle A. Dopamine D5 receptors of rat and human brain. Neuroscience. 2000;100:689–99. doi: 10.1016/s0306-4522(00)00274-8. [DOI] [PubMed] [Google Scholar]
- Lachowicz JE, Sibley DR. Molecular characteristics of mammalian dopamine receptors. Pharmacol Toxicol. 1997;81:105–13. doi: 10.1111/j.1600-0773.1997.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Rowlett JK, Ellison T, Rase K. Cocaine-induced behavioral sensitization: effects of haloperidol and SCH 23390 treatments. Pharmacol Biochem Behav. 1996;53:481–6. doi: 10.1016/0091-3057(95)02101-9. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Marsden CA. Cocaine-induced behaviour: dopamine D1 receptor antagonism by SCH 23390 prevents expression of conditioned sensitisation following repeated administration of cocaine. Neuropharmacology. 1993;32:387–91. doi: 10.1016/0028-3908(93)90161-u. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Reichel CM, Cyr MC, Karper PE, Nazarian A, Crawford CA. Importance of D(1) receptors for associative components of amphetamine-induced behavioral sensitization and conditioned activity: a study using D(1) receptor knockout mice. Psychopharmacology (Berl) 2005;183:20–30. doi: 10.1007/s00213-005-0146-9. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Miner LL, Drago J, Chamberlain PM, Donovan D, Uhl GR. Retained cocaine conditioned place preference in D1 receptor deficient mice. Neuroreport. 1995;6:2314–6. doi: 10.1097/00001756-199511270-00011. [DOI] [PubMed] [Google Scholar]
- O'Sullivan GJ, Kinsella A, Sibley DR, Tighe O, Croke DT, Waddington JL. Ethological resolution of behavioural topography and D1-like versus D2-like agonist responses in congenic D5 dopamine receptor mutants: identification of D5:D2-like interactions. Synapse. 2005;55:201–11. doi: 10.1002/syn.20107. [DOI] [PubMed] [Google Scholar]
- Phillips TJ. Behavior genetics of drug sensitization. Crit Rev Neurobiol. 1997;11:21–33. doi: 10.1615/critrevneurobiol.v11.i1.20. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Post RM, Rose H. Increasing effects of repetitive cocaine administration in the rat. Nature. 1976;260:731–2. doi: 10.1038/260731a0. [DOI] [PubMed] [Google Scholar]
- Pruitt DL, Bolanos CA, McDougall SA. Effects of dopamine D1 and D2 receptor antagonists on cocaine-induced place preference conditioning in preweanling rats. Eur J Pharmacol. 1995;283:125–31. doi: 10.1016/0014-2999(95)00309-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Romach MK, Glue P, Kampman K, Kaplan HL, Somer GR, Poole S, Clarke L, Coffin V, Cornish J, O'Brien CP, Sellers EM. Attenuation of the euphoric effects of cocaine by the dopamine D1/D5 antagonist ecopipam (SCH 39166). Arch Gen Psychiatry. 1999;56:1101–6. doi: 10.1001/archpsyc.56.12.1101. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006;26:8549–58. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Kane S, Stewart CL, Ho A, Kreek MJ. Locomotion, stereotypy, and dopamine D1 receptors after chronic “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2003;75:123–31. doi: 10.1016/s0091-3057(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47(Suppl 1):242–55. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sibley DR. New insights into dopaminergic receptor function using antisense and genetically altered animals. Annu Rev Pharmacol Toxicol. 1999;39:313–41. doi: 10.1146/annurev.pharmtox.39.1.313. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Parlaman JP, Levitt P. Anatomical abnormalities in dopaminoceptive regions of the cerebral cortex of dopamine D1 receptor mutant mice. J Comp Neurol. 2005;487:270–82. doi: 10.1002/cne.20548. [DOI] [PubMed] [Google Scholar]
- Stanwood GD, Parlaman JP, Levitt P. Genetic or pharmacological inactivation of the dopamine D1 receptor differentially alters the expression of regulator of G-protein signalling (Rgs) transcripts. Eur J Neurosci. 2006;24:806–18. doi: 10.1111/j.1460-9568.2006.04970.x. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav Pharmacol. 1998;9:69–76. [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–91. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–5. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Godinot N, Bertrand L, Yang-Feng TL, Fremeau RT, Jr., Caron MG. Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci U S A. 1991;88:7491–5. doi: 10.1073/pnas.88.17.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Waddington JL, O'Tuathaigh C, O'Sullivan G, Tomiyama K, Koshikawa N, Croke DT. Phenotypic studies on dopamine receptor subtype and associated signal transduction mutants: insights and challenges from 10 years at the psychopharmacology-molecular biology interface. Psychopharmacology (Berl) 2005;181:611–38. doi: 10.1007/s00213-005-0058-8. [DOI] [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu XT. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, Lipp HP. Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002;25:336–40. doi: 10.1016/s0166-2236(02)02192-6. [DOI] [PubMed] [Google Scholar]
- Xu M, Guo Y, Vorhees CV, Zhang J. Behavioral responses to cocaine and amphetamine administration in mice lacking the dopamine D1 receptor. Brain Res. 2000;852:198–207. doi: 10.1016/s0006-8993(99)02258-1. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu XT, Cooper DC, Moratalla R, Graybiel AM, White FJ, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–55. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang L, Tang Y, Zhang Q, Lou D, Sharp FR, Zhang J, Xu M. Repeated cocaine administration induces gene expression changes through the dopamine D1 receptors. Neuropsychopharmacology. 2005;30:1443–54. doi: 10.1038/sj.npp.1300680. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–96. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]