SUMMARY
Overnutrition is associated with chronic inflammation in metabolic tissues; however, whether metabolic inflammation compromises the neural regulatory systems and therefore promotes overnutrition-associated diseases remains unexplored. Our results demonstrate that a mediator of metabolic inflammation, IKKβ/NF-κB, normally remains inactive although enriched in the hypothalamic neurons; however, overnutrition atypically activates hypothalamic IKKβ/NF-κB at least through elevated endoplasmic reticulum stress in the hypothalamus. While forced activation of hypothalamic IKKβ/NF-κB interrupts central insulin/leptin signaling and actions, site- or cell-specific suppression of IKKβ either broadly across the brain, or locally within the mediobasal hypothalamus, or specifically in hypothalamic AGRP neurons significantly protects against obesity and glucose intolerance. The involved molecular mechanisms include the control of IKKβ/NF-κB over SOCS3, a core inhibitor of insulin and leptin signaling. In conclusion, the hypothalamic IKKβ/NF-κB program is a general neural mechanism for energy imbalance underlying obesity; suppressing hypothalamic IKKβ/NF-κB represents a new strategy to combat obesity and related diseases.
INTRODUCTION
The hypothalamus is the “headquarters” for regulating energy homeostasis (Elmquist and Flier, 2004; Schwartz and Porte, Jr., 2005). This regulation is primarily mediated in the mediobasal hypothalamus (MBH) by orexigenic AGRP neurons that co-express two neuropeptides—NPY (neuropeptide Y) and AGRP (agouti-related protein)—and anorexigenic POMC neurons that co-express two other neuropeptides—CART (cocaine- and amphetamine-regulated transcript) and POMC (proopiomelanocortin). Leptin and insulin in these neurons control these neuropeptides, leading to normal energy balance and the prevention of obesity. Research has revealed that insulin signaling and leptin signaling in the hypothalamus are integrated through at least PI-3K (Morton et al., 2005; Xu et al., 2005), FoxO1 (Kim et al., 2006; Kitamura et al., 2006), and mTOR (Cota et al., 2006). Recent research has also identified two common inhibitors for insulin and leptin signaling, SOCS3 (Howard and Flier, 2006) and PTP1B (Bence et al., 2006), but their significance in causing disease is poorly understood.
Loss of leptin or insulin signaling in the hypothalamus is sufficient to induce obesity and type 2 diabetes (T2D), as clearly demonstrated in various genetic mouse models with neuronal ablation of insulin signaling (Bruning et al., 2000; Burks et al., 2000; Obici et al., 2002) or leptin signaling (Balthasar et al., 2004; Bates et al., 2003; Lee et al., 1996). In obesity and T2D, along with striking hyperinsulinemia and hyperleptinemia, insulin and leptin levels in the cerebrospinal fluid are elevated, all of which indicate a chronic state of central insulin and leptin resistance. Central administration of insulin or leptin consistently compromises the ability to control food intake in animals during the development of dietary obesity, confirming that hypothalamic (or central) leptin and insulin resistance contribute to the pathophysiology of obesity and T2D. Recent research has also dissociated overnutrition from obesity, demonstrating that overnutrition directly blunts central insulin and leptin sensitivity before the onset of obesity (Wang et al., 2001; Woods et al., 2004). However, how central insulin and leptin resistance are induced by overnutrition and whether core mechanism(s) might be involved are both currently unknown.
IKKβ/NF-κB is a master-switch and central regulator of innate immunity and related functions (Hayden and Ghosh, 2008). In the quiescent state, NF-κB remains inactive in the cytoplasm through binding to the inhibitory protein IκB. Activation of IKKβ by phosphorylation at S177 and S181 induces phosphorylation of its substrate IκBα at S32 and S36, ubiqitination, and subsequent proteosomal degradation. The disappearance of IκBα releases NF-κB to translocate into the nucleus where it mediates the transcription of its target genes. Research during recent decades recognized that overnutrition can induce inflammatory responses in the peripheral metabolic tissues (metabolic inflammation), and therefore cause various metabolic defects in those tissues which underlie T2D (Hotamisligil, 2006; Lehrke and Lazar, 2004). In this context, IKKβ was discovered as a target for an anti-inflammatory therapy that was effective for obesity-associated T2D (Yuan et al., 2001). Our subsequent series of discoveries revealed that IKKβ/NF-κB located in peripheral metabolic tissues affects glucose and protein metabolism in tissue-specific manners (Arkan et al., 2005; Cai et al., 2004; Cai et al., 2005). However, it still remains entirely unexplored whether metabolic inflammation and related mediators could target the metabolic regulatory pathways in the central nervous system (CNS) and then lead to a family of diseases related to overnutrition and obesity. In this study, we pioneered a new direction of research to explore whether IKKβ/NF-κB is the fundamental connection between overnutrition and the dysfunctions of hypothalamic signaling that cause obesity and associated problems.
RESULTS
IKKβ/NF-κB is enriched but suppressed in the hypothalamus
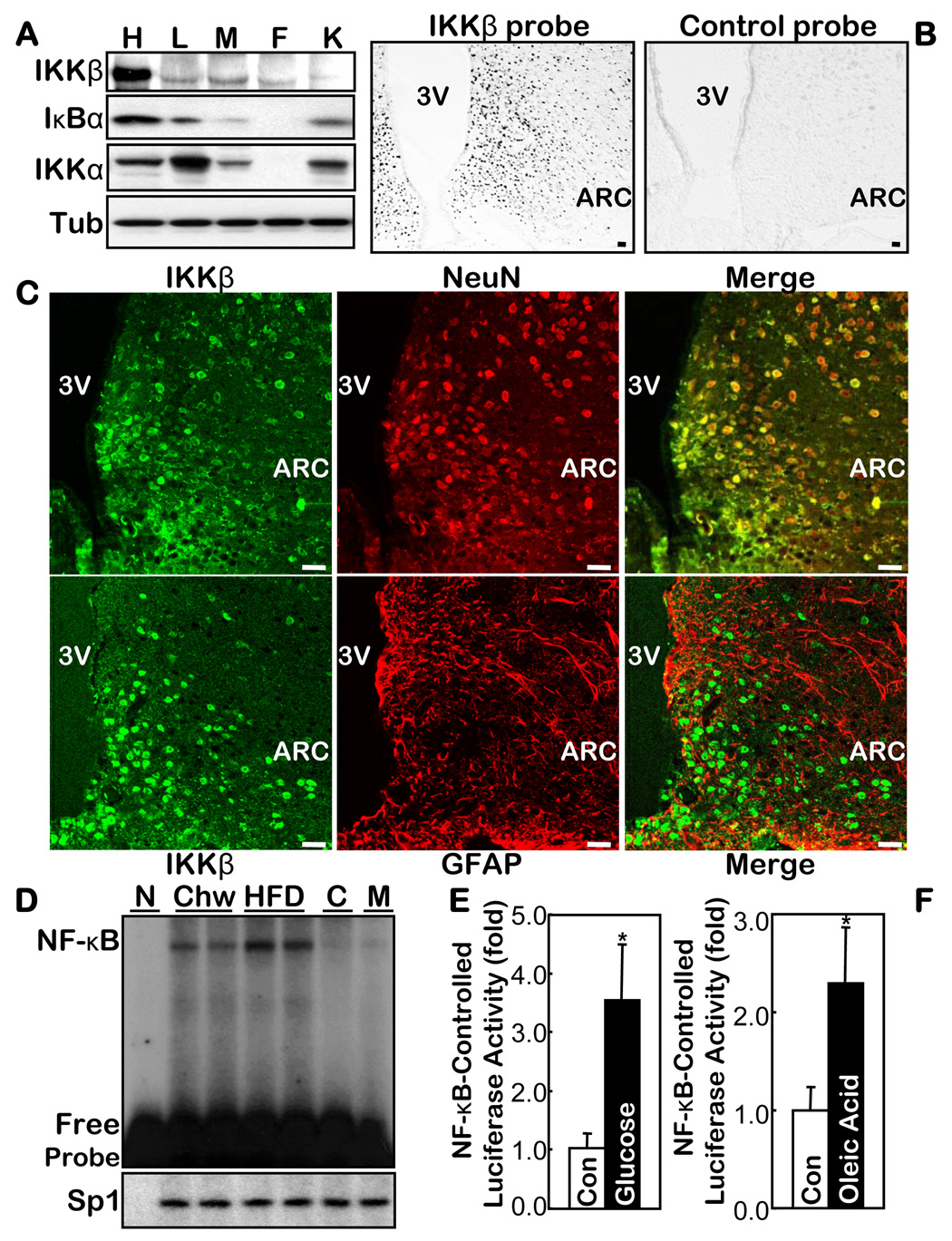
Obesity and associated diseases are characterized by a chronic state of metabolic inflammation. We investigated a possible connection between IKKβ/NF-κB and the central dysregulation of energy balance in the hypothalamus. First, we dissected the hypothalamus (suppl Fig. 1A) and the peripheral organs in normal mice, and observed that the IKKβ protein was highly enriched in the hypothalamus (Fig. 1A). For comparison, IKKα, an IKK isoform that has different signaling and functions from the IKKβ/NF-κB axis, is present at similar levels in the hypothalamus and many of the peripheral tissues (Fig. 1A). Using in situ hybridization, we revealed that IKKβ is expressed predominantly in the MBH (Fig. 1B). After confirming the specificity of IKKβ immunostaining (suppl Figs. 2, 3), we revealed that IKKβ is stained in the neurons but is barely stained in non-neuronal cells (such as astrocytes) in the MBH (Fig. 1C). Thus, IKKβ possesses a unique, distinct pattern in the hypothalamus.
Fig. 1.
IKKβ/NF-κB in the hypothalamus and its relationship with overnutrition. A. Protein levels of IKKβ, IKKα and IκBα in the hypothalamus (H) vs. the peripheral organs including liver (L), skeletal muscle (M), fat (F), and kidney (K) from normal chow-fed C57BL/6 mice. Tub: tubulin. B & C. Distribution of IKKβ mRNA and protein in the hypothalamus was respectively profiled by probing the brain sections with anti-sense IKKβ oligonucleotides (B) and by co-immunostaining of IKKβ with NeuN or GFAP (C). The labeled sense sequence of IKKβ oligonucleotides was used as the control probe (B, right). Bar = 50 µm. D. DNA binding activity of NF-κB oligonucleotide and an irrelevant control (Sp1) was measured using EMSA in the hypothalamus from HFD- vs. chow-fed C57BL/6 mice. Cold competition (C) with 200-fold excess of unlabelled NF-κB oligo and mutant NF-κB probe (M) were used to assess the specificity of the NF-κB EMSA blots. N: non-specific protein (BSA). E & F. NF-κB-induced luciferase activity was measured in the hypothalamus harvested from the NF-κB reporter mice that received intra-third ventricle infusion of 1 mg glucose (E) or 40 mg oleic acid (F) over 6 hours. The controls (Con) represent the infusion of the empty vehicle or the same concentrations of sorbitol. (n = 4–5 per group, *p<0.05). 3V: third ventricle.
When IKKβ is inactive, its substrate IκBα remains intact, which specifically binds to and inhibits NF-κB. We noted that the levels of IκBα were normally high in the hypothalamus relative to the levels in the peripheral tissues (Fig. 1A), indicating that both IKKβ and NF-κB are suppressed in the hypothalamus in normal physiology with standard nutrition. EMSA further indicated that hypothalamic NF-κB activity was relatively low in normal mice fed standard chow (Fig. 1D). The super-shift results confirmed that hypothalamic NF-κB is the canonical complex composed of p65 (RelA) and p50 (suppl Fig. 4A). We also employed NF-κB reporter mice (Everhart et al., 2006), which report NF-κB activity through the NF-κB-controlled expression of an eGFP-Luciferase fusion protein (NGL mice). We confirmed that hypothalamic NF-κB activity was normally modest by measuring either luciferase activity (suppl Fig. 5A) or eGFP expression (suppl Fig. 5B) in the hypothalamus. In sum, IKKβ/NF-κB remains suppressed, although enriched in the hypothalamus under conditions where nutrition is normal.
Hypothalamic IKKβ/NF-κB is activated by overnutrition
We then explored whether IKKβ/NF-κB in the hypothalamus might be affected by overnutrition, a condition that causes obesity and metabolic inflammation. We observed that a chronic high-fat diet (HFD) feeding up-activated NF-κB in the hypothalamus by ~2-fold (Fig. 1D). NF-κB activity was 5- to 6-fold higher in the hypothalamus of ob+/+ mice (a hyperphagic obesity), even when fed normal chow, than in the controls (WT mice) (suppl Fig. 4B). We then used the NGL mice to examine the MBH, the predominant region that expresses IKKβ. In contrast to the low levels of eGFP expression when the NGL mice were fed a normal chow, a HFD clearly induced eGFP expression in the MBH neurons of the NGL mice (suppl Fig. 5B, C). Thus, the MBH, the first-order brain region for nutrition sensing, represents an important hypothalamic site for the activation of NF-κB through overnutrition. In addition, we observed that mRNA levels of IKKβ and IκBα in the hypothalamus increased both in C57BL/6 mice fed a HFD and in ob+/+ mice fed a normal chow (suppl Fig. 6). These observations further support our understanding that IKKβ/NF-κB is up-activated in the hypothalamus by chronic overnutrition.
We then explored whether the effect of overnutrition on hypothalamic IKKβ/NF-κB could be dissected from obesity. NGL mice fasted overnight received a 6-hour infusion of 20% glucose at the rate of 5 µl/hour through cannula implanted in the third ventricle. The glucose infusion doubled the hypothalamic glucose content, which reached 80% of the levels seen in obese and diabetic db/db mice (suppl Fig. 7). Through measuring NF-κB-driven luciferase activities, we found that the acute overload of glucose significantly increased the NF-κB activity in the hypothalamus (Fig. 1E), but not in the peripheral tissues. We also performed an intra-third ventricle infusion of another nutrient species, oleic acid (OA), using the method and doses established in the recent literature (Lam et al., 2005). OA infusion increased the hypothalamic NF-κB activity in the NF-κB reporter mice (Fig. 1F) in much the same way as did the glucose infusion. These experiments and results indicate that oversupply of general nutrients can acutely activate hypothalamic IKKβ/NF-κB before the onset of obesity. On the other hand, we noted that a physiological supply of nutrients, such as re-feeding following a period of fasting, did not evidently affect hypothalamic NF-κB activity, suggesting that activation of IKKβ/NF-κB is not physiologically required. Our results suggest that chronic overnutrition most likely provides repetitive and persistent signals that up-regulate IKKβ/NF-κB in the hypothalamus.
We then determined whether inflammatory molecules, like those driven by NF-κB typically in immune cells, might be over-produced in the hypothalamus of obese mice. Quantitative RT-PCR revealed normal mRNA levels in the hypothalamus of either HFD-fed or normal chow-fed ob+/+ mice for almost all the cytokines and their receptors that we examined (suppl Table 1). In contrast, quantitative RT-PCR revealed markedly elevated mRNA levels in the NF-κB signaling components, including IKKβ and IκBα, in the hypothalamus (suppl Fig. 7). Consistent with recent literature (Munzberg and Myers, Jr., 2005), SOCS3 mRNA levels in the hypothalamus of HFD-treated mice and normal chow-fed ob+/+ mice were both moderately increased. We therefore suggest that the neuronal activation of IKKβ/NF-κB in the hypothalamus by overnutrition leads to neuron-specific responses mostly associated with non-cytokine program(s).
Targeting IKKβ/NF-κB in the hypothalamus and in the brain to control obesity
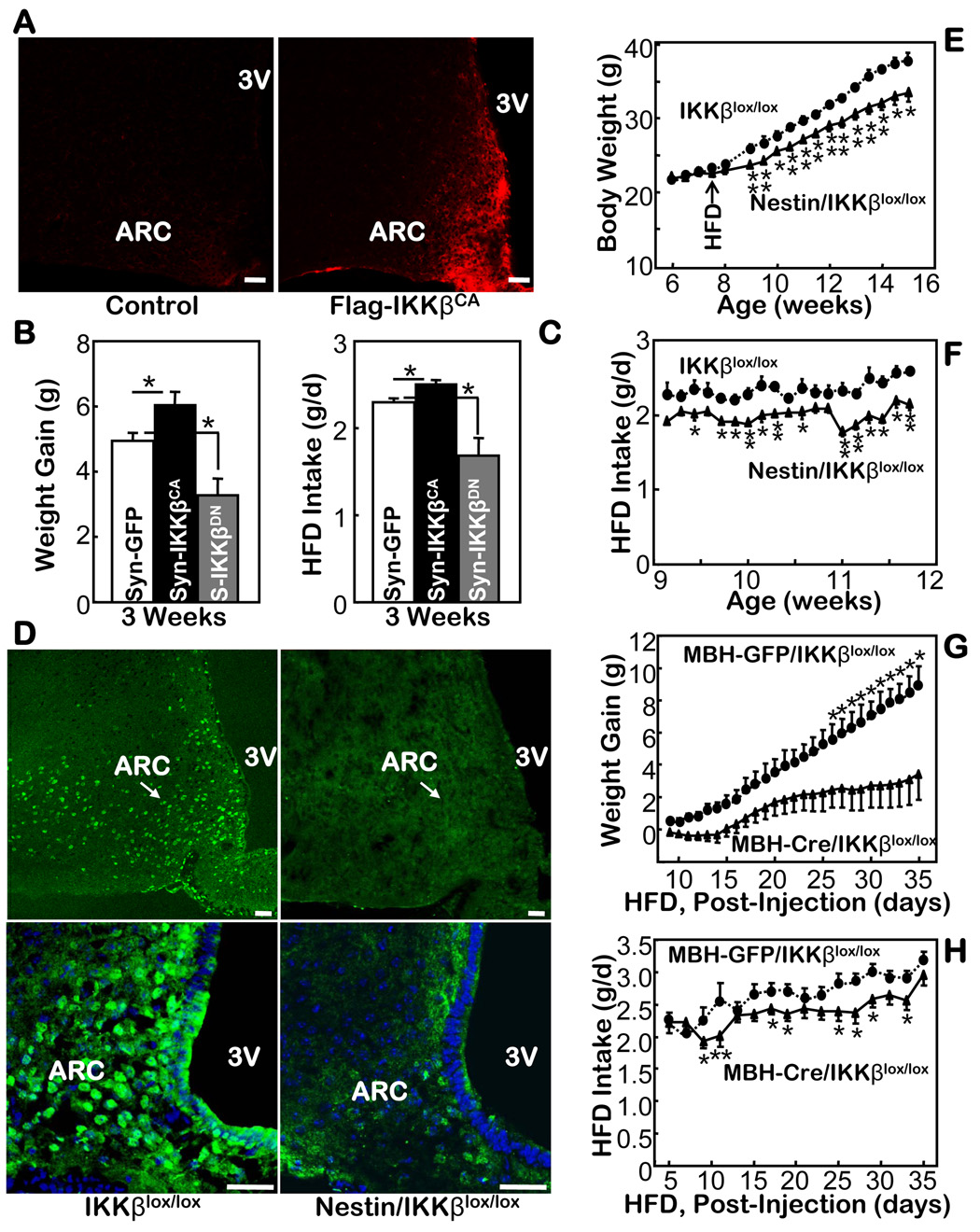
We previously reported that constitutively active IKKβ (IKKβCA) mimics IKKβ phosphorylation, leading to chronic activation of NF-κB (Cai et al., 2004; Cai et al., 2005). Here, we first cloned IKKβCA into a lentiviral vector, in which the synapsin promoter controls the neuron-specific expression of Flag-tagged IKKβCA. In parallel, we also cloned dominant-negative (inhibitory) IKKβ (IKKβDN) into the same viral vector. The same lentiviral vector containing GFP was used as the control. Using the technique of MBH-directed injections (suppl Fig. 1B, C), we delivered each lentivirus bilaterally into the MBH of HFD-fed C57BL/6 mice. While no anatomic lesions were caused, site-specific expression of the targets was confirmed by immunostaining of IKKβ-conjugated Flag tag (Fig. 2A). With low-level but persistent exogenous activation of IKKβ/NF-κB in the MBH by expressed IKKβCA, HFD intake and weight gain of the mice were significantly although mildly promoted (Fig. 2B). Conversely, delivery of IKKβDN in the MBH neurons reduced the levels of HFD-induced weight gain by restricting HFD intake (Fig. 2C).
Fig. 2.
Mouse phenotypes with brain or hypothalamic IKKβ manipulations. A. Immunostaining using anti-Flag antibody for the hypothalamic sections from mice that received intra-MBH injections of Flag-IKKβCA lentivirus and the control lentivirus. Bar = 50 µm. B & C. HFD-fed C57BL/6 mice received intra-MBH injections of a lentivirus in which the synapsin (Syn, S) promoter was employed to direct the neuronal expression of IKKβCA, IKKβDN, and control GFP. Weight gain (B) and food intake (C) were followed after the mice recovered from injections and surgeries. (n = 4–6 per group, *p<0.05). D. Immunostaining of IKKβ in the hypothalamus of Nestin/IKKβlox/lox mice vs. the control IKKβlox/lox mice. Lower panels: the IKKβ staining (green) was merged with the nuclear staining of the MBH cells (blue) by DAPI. Bar = 50 µm. E & F. Body weight (E) and daily food intake (F) were measured for HFD-fed Nestin-IKKβlox/lox mice vs. littermate IKKβlox/lox mice. (n = 5–6 per group, *p<0.05). G & H. Body weight gain (G) and HFD intake (H) were measured in IKKβlox/lox mice that received MBH injections of Cre- or GFP-adenovirus (n = 11–13 per group, *p<0.05).
Considering the disadvantages of a viral approach, such as random infections and immunogenicity of viral particles, we further employed mouse models with genetic suppression of IKKβ in the brain. We first crossed IKKβlox/lox mice (Arkan et al., 2005) with a line of brain-specific Cre transgenic mice, Nestin-Cre mice; both lines had the C57BL/6 background. The compound offspring, Nestin/IKKβlox/lox mice, contained brain-specific ablation of IKKβ (Fig. 2D). Compared with the two types of littermate controls, Nestin-Cre mice and IKKβlox/lox mice, the Nestin/IKKβlox/lox mice demonstrated normal development, growth, body weight, and food intake when fed a chow. When fed a HFD, the Nestin/IKKβlox/lox were significantly protected from the induction of obesity (Fig. 2E). In contrast, neither of the two control groups (Fig. 2E and suppl Fig. 8A) displayed any significant protection. Compared to the control mice, the Nestin/IKKβlox/lox mice consumed a reduced but healthy amount of HFD (Fig. 2F and suppl Fig. 8B), which mainly accounted for the anti-obesity manifestation in the knockout mice.
To further narrow our focus to the hypothalamus, we combined the genetic model with recombinant adenoviruses to selectively ablate IKKβ in the MBH. By delivering Cre-expressing recombinant viruses into the MBH of IKKβlox/lox mice, the floxed IKKβ was excised by Cre expression in the infected MBH cells of IKKβlox/lox mice (MBH-Cre/IKKβlox/lox mice). For the controls, we injected GFP-expressing adenoviruses into the MBH of IKKβlox/lox mice (MBH-GFP/IKKβlox/lox mice). Before the injections, we provided a HFD to both groups of IKKβlox/lox mice for one month to initiate dietary obesity, and following the injections, we continued to treat both mouse groups with the HFD. Using this protocol, we observed that the HFD increased body weight to a much lesser degree in the MBH-Cre/IKKβlox/lox mice than the control mice (Fig. 2G). Consistently, the MBH-IKKβlox/lox mice consumed less HFD than did the controls (Fig. 2H). These results further support that suppression of IKKβ/NF-κB in the MBH offers resistance to obesity under conditions of overnutrition.
The connection of hypothalamic IKKβ/NF-κB with overnutrition by ER stress
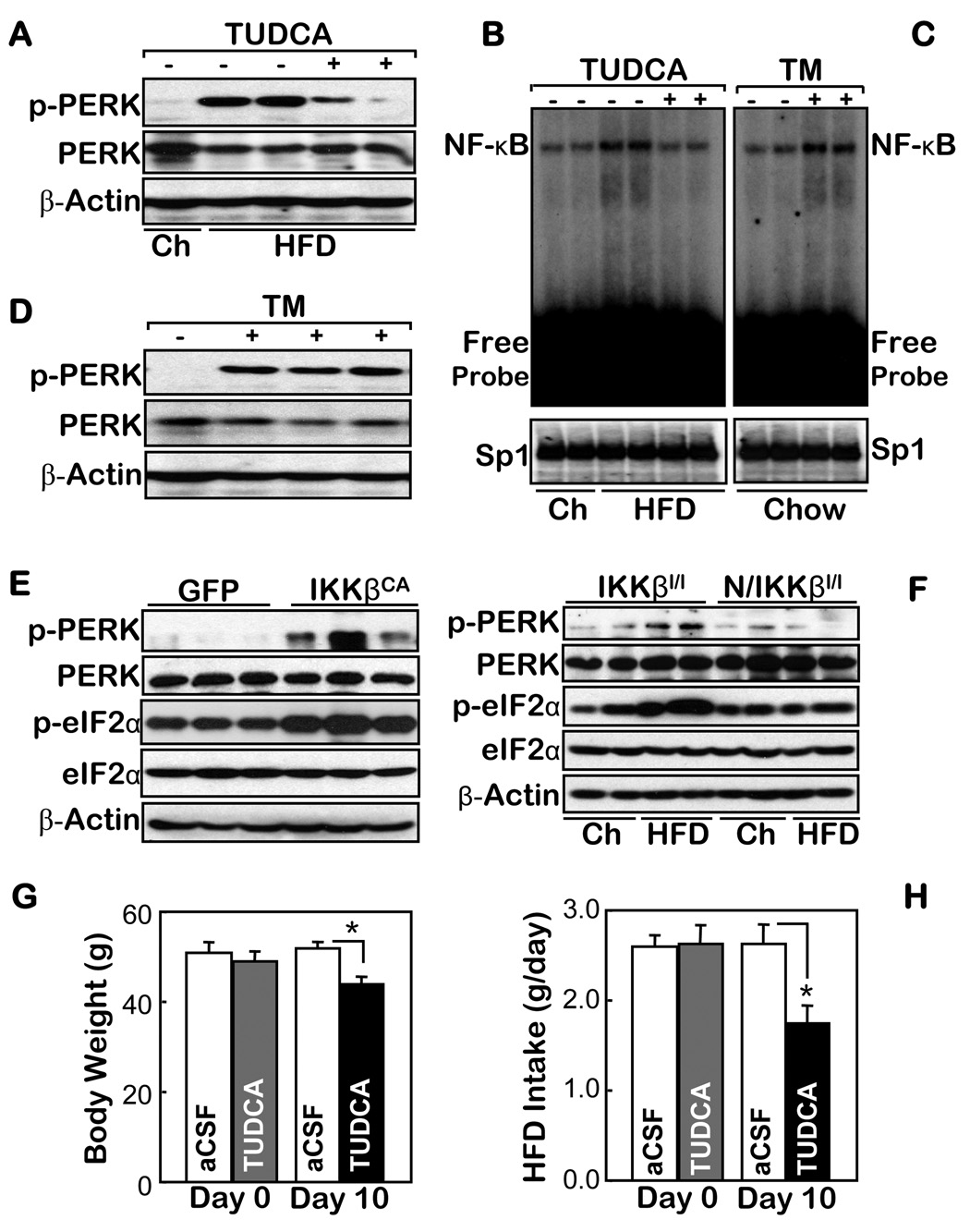
We then explored the intracellular components that could mediate nutritional activation of IKKβ/NF-κB in the hypothalamus and subsequently cause diseases. Recently, ER (endoplasmic reticulum) stress was identified as a critical intracellular event that induces metabolic diseases under conditions of overnutrition (Ozcan et al., 2004; Ozcan et al., 2006). We asked whether ER stress might be involved in the pathways that mediate nutritional activation of hypothalamic IKKβ/NF-κB, and then disease. We first found that ER stress in the hypothalamus, quantitated by the phosphorylation levels of PERK, could be evidently induced by a HFD (Fig. 3A), along with the HFD induction of NF-κB activation in the hypothalamus (Fig. 1D). Furthermore, we revealed that the activation of hypothalamic NF-κB by a HFD was suppressed by an acute intra-third ventricle infusion of an ER stress inhibitor, tauroursodeoxycholic acid (TUDCA) (Fig. 3A, B). In addition, we treated normal chow-fed C57BL/6 mice by an acute intra-third ventricle administration of an ER stress inducer, tunicamycin (TM). Our results revealed that TM activated hypothalamic NF-κB, while it remarkably promoted ER stress in the hypothalamus (Fig. 3 C, D). Thus, ER stress is an intracellular mediator of hypothalamic IKKβ/NF-κB activation by overnutrition.
Figure 3.
The association of ER stress with IKKβ/NF-κB in the hypothalamus. A–D. Normal chow (Ch)-fed mice (A–D) and HFD-fed matched mice (A & B) received intra-third ventricle infusions of (+) 25 µg TUDCA (B) or 3 µg TM (C) vs. the vehicle control (−) over 2 hours (A–D). The markers of ER stress (A & D) and the DNA binding activities of NF-κB and an irrelevant control (Sp1) (B & C) in the hypothalamus of these mice were measured using Western blot and EMSA, respectively. E & F. The ER stress markers were measured in the hypothalamus of normal chow-fed mice that received intra-MBH injections of IKKβCA- vs. GFP-lentivirus (E) and normal chow- vs. HFD-fed Nestin/IKKβlox/lox mice (N/IKKβl/l) and their controls (IKKβlox/lox mice, IKKβl/l) (F). G & H. HFD-fed mice received daily intra-third ventricle injections of TUDCA ((5 µg/d) or the empty vehicle (aCSF) for 10 days. The body weight (G) and average food intake (H) of these mice were measured before (Day 0) and upon the completion of the 10-day therapy. (n = 5 per group; *p<0.05).
We further employed our lentiviral approach and mouse model to dissect the relationship between ER stress and IKKβ/NF-κB in the hypothalamus. Using intra-hypothalamic injections of IKKβCA lentivirus, we found that activating IKKβ/NF-κB elevated ER stress in the hypothalamus, which was reflected by increased levels of both PERK and eIF2α phosphorylation (Fig. 3E). This result indicates that ER stress is both an upstream mediator, and a downstream event, of IKKβ/NF-κB activation in the hypothalamus. This claim was further supported by our findings demonstrating that HFD failed to induce ER stress in the hypothalamus of the Nestin/IKKβlox/lox mice (Fig. 3F). Based on the dual roles of ER stress in the pathological cascade of hypothalamic IKKβ/NF-κB, we assessed the potential disease relevance of ER stress in the CNS. To do this, we provided a chronic intra-third ventricle treatment of TUDCA for mice with obesity induced by a HFD. We found that while the TUDCA treatment did not cause obvious side effects, it significantly decreased the degree of obesity (Fig. 3G), which was associated with the re-balance of food intake in these mice (Fig. 3H). The anti-obesity benefits caused by suppressing ER stress in the CNS resemble the anti-obesity effects from suppressing IKKβ/NF-κB in the CNS (Fig. 2). Thus, under conditions of overnutrition, ER stress and IKKβ/NF-κB in the hypothalamus enhance each other, leading to the central mechanisms of energy imbalance and disease.
Activating hypothalamic IKKβ/NF-κB causes central insulin and leptin resistance
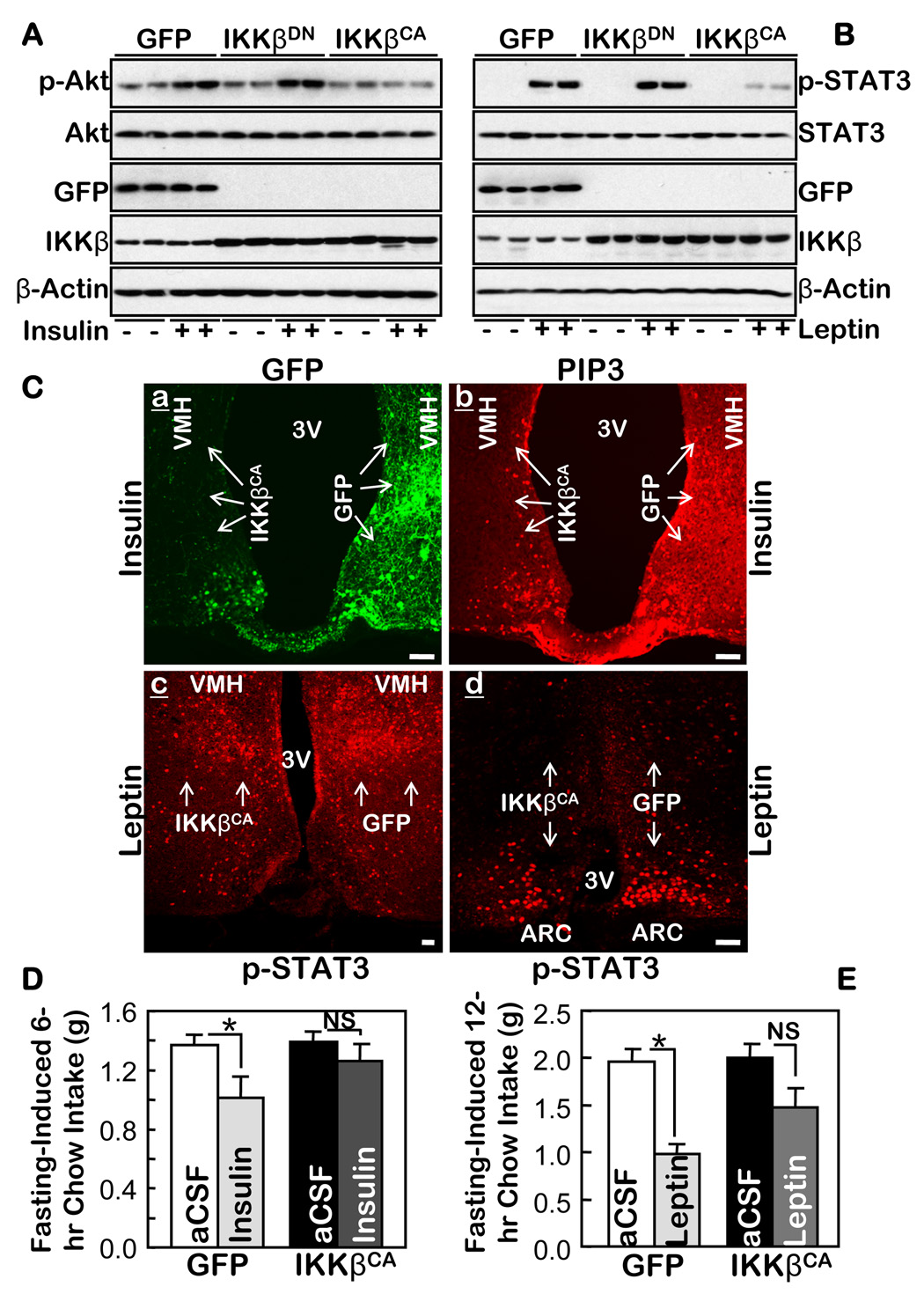
Insulin resistance can be caused in the peripheral tissues by either activating IKKβ/NF-κB (Cai et al., 2005) or ER stress (Ozcan et al., 2004; Ozcan et al., 2006). Since insulin signaling in the hypothalamus is also critical for the central regulation of food and weight balance, we examined whether IKKβ/NF-κB interrupts central insulin signaling in the hypothalamus. We delivered IKKβCA bilaterally into the MBH of adult C57BL/6 mice using an adenoviral vector. As controls, we used the same viral vector in which IKKβCA was replaced by either GFP or IKKβDN. We observed that IKKβCA remarkably impaired Akt activation in response to a third ventricle injection of insulin, but Akt activation was not affected by either IKKβDN or GFP (Fig. 4A). Tyrosine phosphorylation in IR and IRS2 and the binding of IRS2 to PI3 kinase subunit p85 were all suppressed by IKKβCA (suppl Fig. 9), although the effects were somewhat milder. The inhibitory effects of IKKβCA on insulin signaling were also recapitulated in vitro in hypothalamic GT1–7 cells (suppl Fig. 10). These results further reinforce the negative relationship between IKKβ/NF-κB and hypothalamic insulin signaling.
Figure 4.
IKKβ/NF-κB in the MBH mediates central insulin and leptin resistance. A & B. An adenovirus was injected to deliver IKKβCA, IKKβDN, or GFP to both MBH sides of normal C57BL/6 mice. Following surgical recovery, 24-hour-fasted mice received third-ventricle injections of either insulin (A) or leptin (B) (+), or the empty vehicle (−) (A & B). Proteins in the dissected hypothalamus were immunoblotted with the indicated antibodies. C. Adult C57BL/6 mice were injected with adenoviruses to deliver IKKβCA into one side of the MBH and GFP into the other side (a) of the same mice. Recovered mice were fasted 48 hours and then stimulated with insulin (a–b) or leptin (c–d) for 20 min via a third-ventricle cannula. Brain sections across the MBH were directly examined for GFP (a), and immunostained with the indicated primary antibodies (b–d). Arrows indicate the regions in which the injected genes (IKKβCA and GFP) were expressed. Bar = 100 µm. D & E. An adenovirus was injected into adult C57BL/6 mice to deliver either IKKβCA to both sides of the MBH of one group of mice or GFP to both sides of the MBH of another group of mice. Recovered mice fasted for 4 hours received third-ventricle injections of insulin (D), leptin (E), or the vehicle (aCSF) (D & E). Food intake during the indicated time periods following the insulin or leptin injections was measured. (Insulin: n = 12 per group; Leptin: n = 7–8 per group; *p<0.05). NS = non significant.
We also employed immunostaining to directly examine the effects of IKKβCA on insulin signaling in the MBH. After having confirmed the specific immunostaining for insulin-induced PIP3 production in the MBH in over-fasted mice (suppl Fig. 11), we used adenovirus to deliver IKKβCA into one side of the MBH and GFP into the other side of the MBH in the same mice (Fig. 4Ca), which provided the best means of comparison. In response to a third-ventricle injection of insulin, PIP3 was normally induced in the MBH side with GFP; in contrast, very little PIP3 was induced in the MBH side with IKKβCA (Fig. 4Cb), indicating that IKKβCA caused severe insulin insensitivity in the MBH. Following these signaling analyses, we continued to evaluate whether a bilateral delivery of IKKβCA into the MBH was sufficient to affect the ability of central insulin administration to suppress food intake. We found that insulin decreased food intake induced by fasting in the control mice that expressed GFP in the MBH; in contrast, insulin failed to significantly decrease food intake induced by fasting in mice that expressed IKKβCA in the MBH (Fig. 4D). Thus, from a functional standpoint, this result again suggests that local activation of IKKβ/NF-κB in the MBH impairs hypothalamic insulin signaling.
Recent research has established that insulin and leptin signaling function together in the hypothalamus to control energy balance. We explored whether activation of IKKβ/NF-κB in the MBH might also affect hypothalamic leptin signaling. We continued to employ the mouse models in which either IKKβCA or a control was bilaterally delivered into the MBH. Our Western blots (Fig. 4B) and immunostaining (Fig. 4Cc–d, suppl Fig. 11C–D) both revealed that leptin-induced STAT3 phosphorylation in the hypothalamus was reduced by IKKβCA in the MBH. Furthermore, we observed that while leptin reduced fasting-induced food intake by 50.0% in the control mice that expressed GFP bilaterally in the MBH, the reduction was only 26.5% in mice that expressed IKKβCA bilaterally in the MBH (Fig. 4E). To summarize, IKKβ/NF-κB activation in the MBH causes both central insulin and leptin resistance.
IKKβ ablation in hypothalamic AGRP neurons controls obesity
Because IKKβ/NF-κB activation interrupts insulin and leptin signaling, we hypothesized that ablating IKKβ in the insulin- and leptin-sensitive hypothalamic neurons might yield relevant metabolic effects. We targeted AGRP neurons, which are localized mainly in the MBH and are a critical source of hypothalamic insulin and leptin signaling. We bred IKKβlox/lox mice with AGRP-Cre mice in which Cre was located almost exclusively in the targeted MBH neurons (Xu et al., 2005) (suppl Fig. 12). Our data verified the suitability of AGRP-Cre mice for this study by showing that AGRP-Cre mice, when fed either a chow or a HFD, consumed food and gained body weight similarly to wildtype mice (suppl Fig. 8C–D). We refer to offspring with the AGRP neuron-specific knockout of IKKβ (confirmed in Fig. 5A by immunostaining) as AGRP/IKKβlox/lox mice. Littermate AGRP-Cre mice and IKKβlox/lox mice were used as controls. As noted previously (Xu et al., 2005), a fraction of AGRP/IKKβlox/lox mice exhibited partial IKKβ excision in the whole body due to occasional promoter leakage during the embryonic development; we identified these mice using a PCR method and excluded them from the studies.
Figure 5.
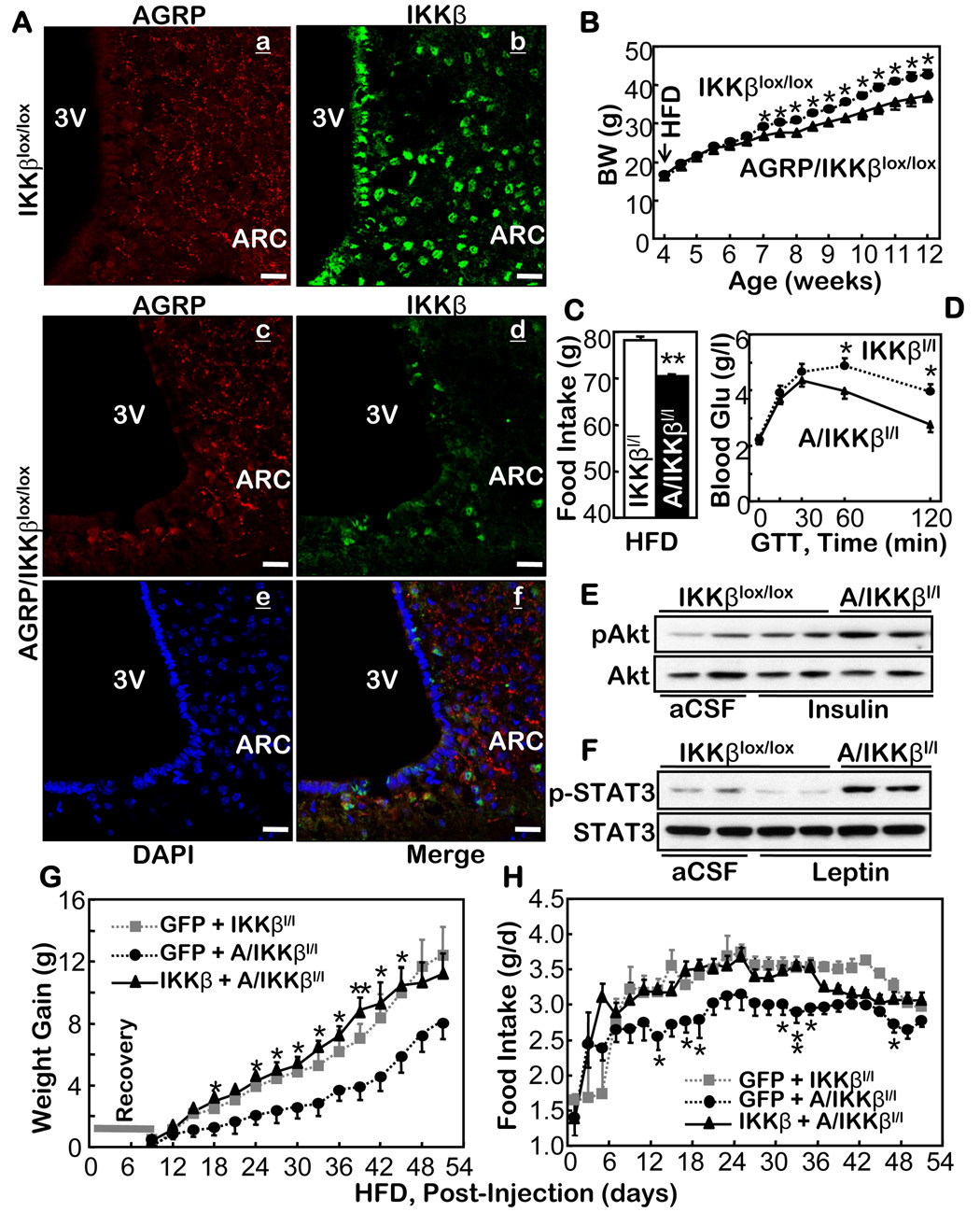
Anti-obesity effects of knocking out IKKβ in the hypothalamic AGRP neurons. A. AGRP (a & c) and IKKβ (b & d) in the hypothalamus of the control mice (IKKβlox/lox mice) (a & b) vs. AGRP-IKKβlox/lox mice (c & d) were immunostained. Immunostaining of AGRP (c) and IKKβ (d) and the nuclear staining by DAPI (e) in the hypothalamus of AGRP-IKKβlox/lox mice were merged to display the absence of IKKβ in AGRP neurons (f). Bar = 25 µm. B–D. Body weight (BW) (B), HFD intake (C), and glucose tolerance (GTT) (D) were tested in HFD-fed AGRP-IKKβlox/lox mice and IKKβlox/lox mice. (n = 12–14 per group, *p<0.05). Glu, Glucose. E & F. HFD-fed AGRP-IKKβlox/lox mice and IKKβlox/lox mice were fasted and received third-ventricle injections of insulin (5 mU) (E), leptin (10 µg) (F), or the vehicle (aCSF) (E & F). Insulin-induced Akt phosphorylation (pAkt) (E) and leptin-induced STAT3 phosphorylation (pSTAT3) (F) were determined using Western blots. G & H. Adenovirus was injected to deliver IKKβ to the both MBH sides of AGRP-IKKβlox/lox mice. For the controls, adenovirus was injected to deliver GFP to the both MBH sides of AGRP-IKKβlox/lox mice and to the both MBH sides of IKKβlox/lox mice. Body weight (G) and food intake (H) in these mice were followed for 8 weeks following the viral injections (n = 5 per group; **p<0.01). C–H. IKKβl/l: IKKβlox/lox mice; A/IKKβl/l: AGRP-IKKβlox/lox mice.
AGRP/IKKβlox/lox mice displayed normal offspring frequency, growth, and activity. When fed normal chow, the body weight and food intake of these mice resembled those of the control mice, including AGRP-Cre, IKKβlox/lox, and wildtype mice. Therefore, the AGRP/IKKβlox/lox mice represent a suitable model for studying the hypothalamic control of energy balance. We treated the AGRP/IKKβlox/lox mice and the matched controls with a HFD post-weaning. After 12 weeks of the HFD, the body weight of AGRP/IKKβlox/lox mice was 13.1% less than that of the controls (Fig. 5B). On average, AGRP/IKKβlox/lox mice ate 10.2% less than the controls (Fig. 5C). The anti-obesity phenotype of the AGRP/IKKβlox/lox mice almost reached the level of the Nestin/IKKβlox/lox mice (Fig. 2), thus suggesting that AGRP neurons are mainly responsible for the neural effects of IKKβ/NF-κB on body weight homeostasis.
We also assessed the anti-obesity characteristics of the AGRP/IKKβlox/lox mice from the aspects of obesity-associated glucose intolerance and central insulin/leptin resistance. HFD-fed AGRP/IKKβlox/lox mice and the matched controls were fasted overnight and subjected to a GTT. As expected, glucose tolerance was impaired in the control mice after 3 months of a HFD. In contrast, HFD failed to induce glucose intolerance in the AGRP/IKKβlox/lox mice (Fig. 5D). We also assessed central insulin and leptin resistance as two biochemical markers of obesity. Compared to mice fed a normal chow, both insulin and leptin signaling were barely induced in the mice fed a HFD, indicating a state of central insulin and leptin resistance that was chronically induced by HFD. In contrast, both insulin and leptin signaling were significantly preserved in the hypothalamus of HFD-fed AGRP/IKKβlox/lox mice (Fig. 5E,F) in which only the AGRP neurons were targeted for IKKβ ablation.
To further confirm that the metabolic phenotypes of AGRP/IKKβlox/lox mice were hypothalamic, we used an adenovirus to bilaterally deliver either IKKβ or control GFP into the MBH of AGRP/IKKβlox/lox mice that had been fed a HFD for 2 months. As further controls, we used the same approach to deliver IKKβ or GFP into the MBH of the littermate IKKβlox/lox mice that were also fed a HFD for 2 months. We observed that the protection against the induction of weight gain by a HFD in AGRP/IKKβlox/lox mice was eliminated by restoring IKKβ in the MBH (Fig. 5G). To reflect such a phenotype reversal, intake of the HFD in AGRP/IKKβlox/lox mice nearly approached the levels displayed by IKKβlox/lox mice during the progression to obesity (Fig. 5H). In sum, the hypothalamic AGRP neurons are critical for the pathological action of IKKβ/NF-κB in the central dysregulation of energy balance.
IKKβ/NF-κB promotes SOCS3 expression
Our results led to the consideration of how IKKβ/NF-κB causes hypothalamic insulin and leptin resistance. Recent research shows that SOCS3 is a critical inhibitor for both insulin and leptin signaling in the hypothalamus (Howard et al., 2004; Mori et al., 2004; Ueki et al., 2004). Using luciferase driven by the mouse SOCS3 promoter in the pGL3 plasmid (SOCS3-pGL3), we tested whether NF-κB regulates the promoter activity of SOCS3 in cultured cells. This SOCS3 promoter contains two putative NF-κB-DNA binding motifs at −94 and −754 bp. Our results revealed that IKKβCA expression increased SOCS3 promoter activity (Suppl Fig. 13A). This increase was abolished by expressing the IκBα super-repressor (IκBαSR) (Suppl Fig. 13B), which selectively inhibited NF-κB but not the upstream IKKβ. In addition to the promoter assay, we also found that both mRNA and protein levels of SOCS3 were stimulated by TNF-α (an activator of IKKβ/NF-κB) in HEK 293 cells, and these effects were reversed by expressing IκBαSR in these cells (suppl Fig. 14A–B). All these results indicate that IKKβ/NF-κB possesses the ability to activate the SOCS3 gene.
We then screened various stimuli that increase SOCS3 promoter activity in cultured cells, and further tested whether addition of IκBαSR could inhibit the increase. In HEK 293 cells stably expressing leptin receptor, leptin increased SOCS3 promoter activity, but interestingly, insulin also increased its activity (suppl Fig. 13B). Further, IκBαSR reduced the effects of leptin and insulin in stimulating the SOCS3 promoter activity (suppl Fig. 13B). Therefore, SOCS3, classically produced by STAT3 which can be activated through leptin, is also controlled by NF-κB. To further support this hypothesis, we deleted each of the two putative NF-κB-DNA binding motifs in the SOCS3-pGL3 plasmid. The activity of the SOCS3 promoter was substantially reduced by deleting either NF-κB-DNA binding motif (suppl Fig. 13C). These results further suggest that IKKβ/NF-κB is involved in SOCS3 gene activation. In addition, using RT-PCR and Western blot analyses (suppl Fig. 14C–D), we confirmed that a virus-mediated delivery of IKKβCA into the MBH significantly elevated SOCS3 mRNA and protein in the hypothalamus of mice fed a normal chow. The induction of SOCS3 by IKKβCA in the hypothalamus is relatively modest, but nonetheless correlates with a similar increase of hypothalamic SOCS3 under conditions of overnutrition (suppl Table 1). Further, the modest increase of SOCS3 is reminiscent of the “low-grade levels” of metabolic inflammation present in obesity and related diseases, and could be pathogenically relevant to the development of these diseases.
SOCS3 mediates the effects of hypothalamic IKKβ/NF-κB on energy balance
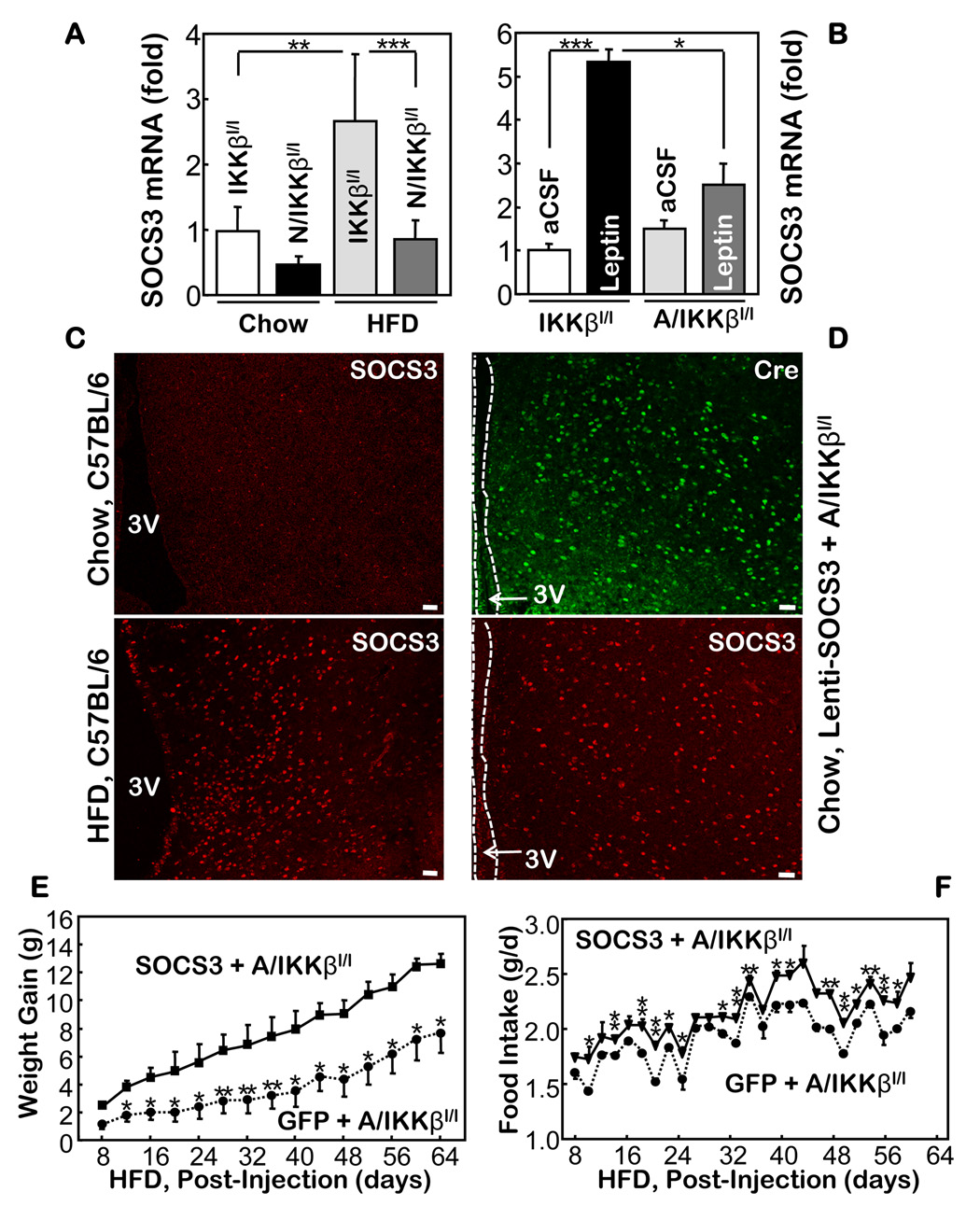
Following our observation showing that a HFD failed to increase the hypothalamic levels of SOCS3 mRNA in Nestin-IKKβlox/lox mice (Fig. 6A), we further examined whether the acute promotion of hypothalamic SOCS3 mRNA by leptin could be impaired by IKKβ ablation. We tested this question using AGRP-IKKβlox/lox mice, since we have demonstrated that the hypothalamic AGRP neurons are mainly responsible for the anti-obesity effect created by IKKβ ablation. Our results revealed that hypothalamic SOCS3 mRNA levels in the AGRP-IKKβlox/lox mice were elevated by leptin to lesser degrees than in the control mice (Fig. 6B). These data suggest that SOCS3 might be involved in the hypothalamic IKKβ/NF-κB’s dysregulation of energy balance.
Figure 6.
The role of SOCS3 for the anti-obesity phenotype of AGRP-IKKβlox/lox mice. A. Relative mRNA levels of SOCS3 in the hypothalamus of HFD- and chow-fed Nestin-IKKβlox/lox mice and IKKβlox/lox mice. B. Relative mRNA levels of SOCS3 in the hypothalamus of chow-fed IKKβlox/lox mice and AGRP-IKKβlox/lox mice that received third-ventricle injections of leptin. (n = 4–6 per group; *p<0.05, **p<10−2, ***p<10−3). C. SOCS3 immunostaining in the MBH of chow- vs. HFD-fed C57BL/6 mice. D. Immunostaining of Cre (green) and SOCS3 (red) in chow-fed AGRP-IKKβlox/lox mice following intra-MBH injections of SOCS3 lentivirus. E & F. SOCS3 lentivirus (SOCS3) or GFP lentivirus (GFP) was injected into the MBH of HFD-fed AGRP-IKKβlox/lox mice; body weight (E) and HFD intake (F) of injected mice were recorded. (n = 4–8 per group; *p<0.05, **p<0.01). Bar = 50 µm. A–F. IKKβl/l: IKKβlox/lox mice; N/IKKβl/l: Nestin-IKKβlox/lox mice; A/IKKβl/l: AGRP-IKKβlox/lox mice.
Indeed, after confirming the specificity of SOCS3 immunostaining (suppl Figs. 15, 16), we revealed that a HFD significantly induced SOCS3 in the MBH (Fig. 6C). We also noted that the induction of SOCS3 in the MBH by overnutrition appeared to be limited to the neurons, which evidently differs from the massive induction of SOCS3 in many glial cells in response to third ventricle injections of cytokines such as LIF (suppl Fig. 16B). The neuronal activation of SOCS3 by HFD aligns well with our model showing that IKKβ/NF-κB possesses neuronal, non-cytokine program(s) for mediating the dysregulation of energy balance during overnutrition. To further examine the role of SOCS3 in the pathogenic cascade of hypothalamic IKKβ/NF-κB during overnutrition, we employed a rescue experiment to determine whether adding SOCS3 to the MBH neurons of AGRP-IKKβlox/lox mice could reduce the anti-obesity phenotype in these mice. We created a SOCS3 lentivirus controlled by a neuron-specific (synapsin) promoter. For the controls, we used the same lentivirus but with SOCS3 replaced by GFP. Following the intra-MBH injections of lentiviruses (Fig. 6D), the mice were maintained on a HFD. Our study revealed that the exogenous expression of SOCS3 in the MBH neurons significantly decreased the anti-obesity and feeding phenotypes of AGRP-IKKβlox/lox mice (Fig. 6E, F). Therefore, from a mechanistic point, these results further support our hypothesis that suppressing IKKβ/NF-κB in hypothalamic neurons represents a promising strategy for counteracting obesity and associated diseases during overnutrition.
DISCUSSION
Overnutrition subverts the innate immune response of hypothalamic IKKβ/NF-κB
Obesity and its co-morbidities represent the greatest epidemic and public health problems facing our society. Relatively recent environmental changes, especially overnutrition, are clearly responsible for the high prevalence of these diseases. Faced with the energy abundance typical of today’s developed world, our control systems have yet to evolve to readily regulate the body’s energy balance. This newly emerged challenge to the control systems in our bodies has led to excessive energy stores (fat) and ultimately to disease. While the CNS control systems, like the hypothalamus, might conceivably be essential for this regulatory failure, the participating molecular program(s) remains unknown.
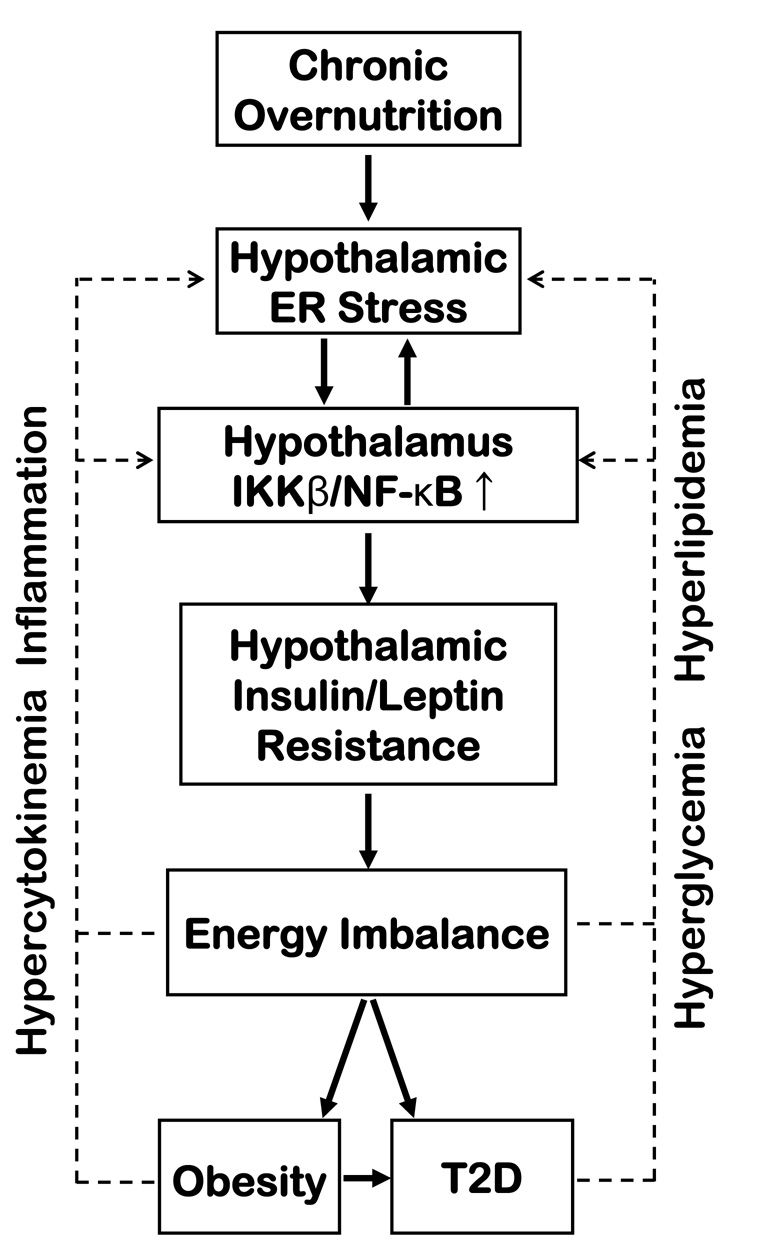
We discovered that the “master switch” innate immunity regulator, IKKβ/NF-κB, while highly enriched in the hypothalamic neurons, is normally not activated. Importantly, this pathway can be activated in the hypothalamic neurons by the chronic overnutrition that causes obesity. Such activation can even be mimicked without obesity by an acute but extreme oversupply of various nutrients to the CNS. Our findings indicate that persistent signals to the CNS under conditions of overnutrition can atypically stimulate an innate immunity-like response directed by IKKβ/NF-κB in the hypothalamic neurons; this process affects the neuronal regulation of energy balance (Fig. 7). Overall, our findings align with the emerging view that evolution integrated some regulatory pathways that govern the body’s reactions to both pathogens and nutrition (Hotamisligil, 2006). We postulate that the hypothalamus might have developed the unique property of responding to extreme environmental stimuli (previously pathogens, now overnutrition) by using IKKβ/NF-κB to affect vital systems. Thus, although the near-instantaneous signaling of hypothalamic IKKβ/NF-κB might once have been critical for survival in a pathogen-filled environment by helping innate immunity (an energy-intensive process), this signaling—as our study demonstrates—might now be very responsive and truly detrimental in today’s near-constant calorie-rich environment. Hypothalamic IKKβ/NF-κB could underlie the entire family of modern diseases induced by overnutrition and obesity.
Figure 7.
Schematic of the proposed role of hypothalamic IKKβ/NF-κB and ER stress in obesity-related disease pathways. We propose that IKKβ/NF-κB connects with ER stress in the hypothalamus and translates overnutrition into central insulin and leptin resistance, leading to the development of obesity and T2D (solid arrows). We also further postulate that, as obesity and T2D progress, IKKβ/NF-κB participates in cumulative feedback loops (broken arrows).
The hypothalamus: a key target for metabolic inflammation via IKKβ/NF-κB
Obesity and its associated diseases are characterized by chronic metabolic inflammation (Hotamisligil, 2006; Lehrke and Lazar, 2004). This type of inflammation has been considered peripheral and a consequence of obesity. Our previous research established that IKKβ/NF-κB promotes metabolic inflammation in peripheral tissues (Cai et al., 2004; Cai et al., 2005). In this study, our research originated in an exploration of the potential metabolic relevance of the pro-inflammatory “master switch” IKKβ/NF-κB in the hypothalamus. We discovered that IKKβ/NF-κB in the hypothalamic neurons responds to the metabolic signals produced by overnutrition, and is a general cause of multiple neural disease pathways, and thus contributes to diseases related to overnutrition. Therefore, while obesity induces metabolic inflammation, our discovery indicates that metabolic inflammation could also promote energy imbalance and obesity by interrupting regulatory signaling in the CNS. It is also noteworthy that the hypothalamic dysregulation of energy balance by overnutrition involves a neuron-specific, non-cytokine program through IKKβ/NF-κB. This program significantly differs from the inflammatory reactions seen in the CNS of certain chronic, cachectic diseases (such as cancer, AIDS, and infectious diseases) which are often associated with chaotic releases and powerful actions of many inflammatory cytokines in non-neuronal cells, such as astrocytes, oligodendrocytes and microglial cells.
A relevant question therefore concerns how overnutrition activates IKKβ/NF-κB in the hypothalamus. We demonstrated that an acute, local oversupply of either glucose or lipid in the CNS recapitulated the hypothalamic activation of IKKβ/NF-κB by chronic overnutrition. This separation of chronic overnutrition from obesity suggests that overnutrition might at least primarily activate IKKβ/NF-κB in the hypothalamus. The effects of the individual nutrient species in the hypothalamus agree with many other studies showing that both glucose and lipid can activate IKKβ/NF-κB in the peripheral metabolic tissues and cultured cells. A recent report found that Toll-like receptor 4 (TLR4) mediates lipid-induced insulin resistance (Shi et al., 2006). Since TLR4 is an activator of IKKβ/NF-κB in various circumstances (Hayden and Ghosh, 2008), TLR4 could be a link between overnutrition and IKKβ/NF-κB activation at the cell membrane level, but whether this potential relationship occurs in the hypothalamus remains to be explored. In this paper, we have revealed that ER stress and IKKβ/NF-κB promote each other in the hypothalamus during overnutrition. This finding indicates that overnutrition could activate IKKβ/NF-κB at the intracellular level in the hypothalamic neurons. Overall, this profile of hypothalamic IKKβ/NF-κB reinforces recent research in which ER stress was implicated in metabolic inflammation (Hotamisligil, 2006). Furthermore, in addition to the primary induction of hypothalamic IKKβ/NF-κB by enriched nutrients, we postulate that the pathophysiological changes during the progress of obesity and T2D, such as hyperlipidemia, hyperglycemia, hyperinsulinemia, hyperleptinemia, and elevated inflammatory substances in the peripheral tissues and circulation, might secondarily, but significantly, promote ER stress and IKKβ/NF-κB in the hypothalamus and accelerate disease progression (Fig. 7).
IKKβ/NF-κB: a core mechanism for hypothalamic insulin and leptin resistance
Insulin and leptin signaling in the hypothalamus are critical for sensing the availability of fuel in the body and preventing excessive energy stores, and thus obesity. Recent research discovered that insulin and leptin signaling are integrated and function together to regulate energy balance. The PI-3K/Akt, FoxO1, MAP kinase, and mTOR pathways have recently been identified as molecular links between insulin and leptin signaling. Recent research has also demonstrated that overnutrition induces hypothalamic insulin and leptin resistance, but the underlying basis remains unknown. In this research, we discovered that IKKβ/NF-κB in the hypothalamus mediates both hypothalamic insulin and leptin resistance. Thus, for the first time, our findings posit IKKβ/NF-κB as a core disease pathway that universally mediates hypothalamic insulin and leptin resistance that leads to obesity and T2D.
Recent research has revealed that SOCS3 is a common inhibitor for leptin and insulin signaling (Howard and Flier, 2006). Indeed, forced overexpression of SOCS3 decreases both leptin-induced phosphorylation of JAK2, and insulin-induced phosphorylation of IR, IRS, and Akt. Selectively ablating the SOCS3 gene in hypothalamic neurons can enhance hypothalamic leptin signaling, which is sufficient to protect against dietary obesity (Howard et al., 2004; Kievit et al., 2006). In this research, we discovered that the pathogenic induction of hypothalamic SOCS3 during overnutrition also requires NF-κB. More important, suppression of IKKβ/NF-κB weakens the promotion of SOCS3 expression by various in vitro and in vivo conditions known to desensitize insulin and leptin signaling. While other mechanisms remain to be explored, SOCS3 is an essential mediator for the pathological actions of hypothalamic IKKβ/NF-κB in causing central leptin and insulin resistance that underlie obesity and T2D.
Hypothalamic IKKβ: a new, promising target to counter obesity and T2D
Our work also marks the first attempt to study whether inhibiting an innate immune pathway in the hypothalamus could help to calibrate the set point of nutritional balance and therefore aid in counteracting energy imbalance and diseases induced by overnutrition. These results reveal that dietary obesity can be largely prevented by inhibiting IKKβ/NF-κB either virally or genetically, whether broadly throughout the CNS, locally in the related hypothalamic regions, or precisely in a defined subpopulation of hypothalamic neurons. Altogether, our results reveal an entirely new therapeutic strategy for combating the ever-increasing spread of obesity and associated diseases. We recognize that the significance of this strategy has yet to be realized in clinical practice; currently, most anti-inflammatory therapies have limited direct effects on IKKβ/NF-κB and limited capacity to be concentrated in the CNS. Nonetheless, our discoveries offer outstanding potential for treating these serious diseases. Because we also revealed that IKKβ/NF-κB normally remains inactive in the CNS, suppressing IKKβ/NF-κB in the hypothalamus is likely a safe approach. However, challenges still remain, such as finding methods to selectively suppress this pathway in the CNS, that will certainly inspire future research explorations.
EXPERIMENTAL PROCEDURES
Animal Models and Chemicals
NGL mice (Everhart et al., 2006), IKKβlox/lox mice (Arkan et al., 2005), and AGRP-Cre mice (Xu et al., 2005) have been described previously. Nestin-Cre mice, ob+/+ mice, db+/+ mice, and C57BL/6 mice were obtained from Jackson Laboratory. All the mouse lines were in C57BL/6, except for AGRP-Cre mice, which were originally in the FVB background. We backcrossed AGRP-Cre mice to C57BL/6 background for 5 generations. We identified and excluded a small fraction of whole-body IKKβ knockout offspring from breeding AGRP-Cre mice with IKKβlox/lox mice. These mice were identified by using a PCR method to detect the excised IKKβ gene in the genomes isolated from various peripheral and brain tissues. The UW-Madison IACUC approved the procedures. The HFD and normal chow were from Research Diets, Inc. TUDCA and TM were from Calbiochem, insulin was from Novartis, and leptin from R & D Systems.
Third-ventricle and Intra-hypothalamic Injections
The bilateral injections to the MBH were directed using an ultra-precise stereotax (Kopf Instruments) to the coordinates of 1.5 mm posterior to the bregma, 5.8 mm below the surface of the skull, and 0.4 mm lateral to midline. Purified lentivirus or adenovirus in artificial cerebrospinal fluid (aCSF) was injected over 10 min through a 26-gauge guide cannula and a 33-gauge injector connected to a Hamilton Syringe and an infusion pump. At the end of the experiments, the mice were assessed for intra-MBH deliveries by immunostaining the markers and the targets. Our final analyses included only those animals in which target induction was limited to the MBH and in which more than 70% of the targeted MBH cells were infected (suppl Fig. 1 B & C). For third-ventricle cannulation, we implanted a guide cannula into the ventral third ventricle at the midline coordinates of 1.8 mm posterior to the bregma and 5.0 mm below the surface of the skull.
Acute Food Intake Studies and GTT
The individually housed mice were first measured for food intake induced by fasting. Four hours before the designated ‘night time’, we removed food and injected 1–2 µl of the vehicle (aCSF) into the third ventricle of each mouse. Food was then returned to the mice immediately before the designated ‘night time’. Food intake for each mouse was measured during the various following periods. This procedure was subsequently performed for the same set of mice, but using instead an injection of insulin (5–10 mU) or leptin (5–15 µg). For GTT, overnight-fasted mice were IP injected with glucose (2 g/kg body weight). Blood glucose at various time points was measured using a Glucometer Elite (Bayer, Elkhart, IN).
Plasmids and Recombinant Viruses
We introduced the cDNA of IKKβCA (S177/181E), IKKβDN (either S177/181A or K44A) or SOCS3 (provided by J. Seufert) into the lentiviral vector Lox-Syn-Syn (provided by G. Francisco) in which the synapsin promoter controlled neuron-specific expression of the introduced genes. The lentiviruses were produced from HEK293T cells through co-transfecting the target plasmid with package plasmids using CaCl2. Lentiviruses were purified by ultracentrifugation and then quantitated. Ad5-CMV-driven Cre adenovirus and matched controls were provided by S. Albelda. Ad5-CMV-driven IKKβ adenovirus and Ad5-CMV-driven GFP or LacZ were produced in our lab or in a core facility at UW-Madison.
Tissue harvest, Immunoprecipitation, and Western Blots
The hypothalamus was dissected according to the established method (Kim et al., 2006) (suppl Fig. 1A). Animal tissues were homogenized, and the proteins were dissolved in a lysis buffer, and Western blots were performed as previously described (Cai et al., 2004; Cai et al., 2005). Proteins dissolved in a lysis buffer or isolated by immunoprecipitation were separated by SDS/PAGE and identified by immunoblotting. Primary antibodies included rabbit anti-p-STAT3, anti-STAT3, anti-p85, anti-p-Akt, anti-Akt, anti-IKKβ, anti-IKKα, anti-eIF2α, anti-p-eIF2α, anti-p-ERK, and anti-β-Actin (Cell Signaling); rabbit anti-IκBα, anti-PERK, anti-GFP, and anti-tubulin (Santa Cruz). Secondary antibodies included HRP-conjugated anti-rabbit and anti-mouse antibodies (Pierce).
Quantitative RT-PCR and EMSA
We extracted total RNA from homogenized tissues using TRIzol (Invitrogen), synthesized cDNA using the Advantage RT-for PCR kit (BD Biosciences), and performed real-time PCR using SYBR®Green PCR Master Mix (Applied Biosystems). Results were normalized against TATA box-binding protein (TBP) or GAPDH gene expression. We performed EMSA using the protocol detailed in our previous publications (Cai et al., 2004; Cai et al., 2005). Briefly, NF-κB oligonucleotide (Santa Cruz) and two control oligonucleotides, Sp1 and Oct1 (Promega), were labeled with [γ-32P] ATP. The animal tissues were homogenized in a lysis buffer, and the lysate was then incubated with the probe. For negative controls, we used both mutant NF-κB gel-shift oligonucleotide (Santa Cruz) and cold competition with 200-fold excess of unlabelled NF-κB gel-shift oligonucleotide. The NF-κB-probe complex was separated in un-denatured 4% PAGE, and the radioactivity was then detected on X-ray film.
Heart Perfusion, immunostaining, and in situ Hybridization
Mice under anesthesia were transcardially perfused with 4% PFA, and the brains were removed, post-fixed in 4% PFA, and infiltrated with 20–30% sucrose. Brain sections across the MBH (15-µm thickness) were blocked with serum of appropriate species, penetrated with 0.2% Triton-X 100, treated with anti-Flag (Stratagene), anti-IKKβ (Santa Cruz), anti-NeuN (Chemicon), anti-GFAP (Dako), anti-pSTAT3 (Cell Signaling), anti-PIP3 (Echelon), anti-SOCS3 (IBL), or anti-AGRP (Phoenix Pharmaceutical Inc) antibodies, and then subjected to reaction with fluorescence-conjugated secondary antibodies (Jackson). Naïve IgGs of the appropriate species were used as negative controls. For in situ hybridization, free-floating slices were digested with proteinase K, pre-hybridized, and then hybridized with digoxigenin-labeled antisense IKKβ nucleotide probe, and followed by color development using anti-digoxigenin antibody (Fab)-conjugated with alkaline phosphatase (Roche) and NBT/BCIP substrates.
Statistical Analyses
Data are presented as mean ± SEM. Student’s t tests were used for statistical analysis. P<0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank G. Barsh and A. Xu for AGRP-Cre mice; T. Blackwell for NGL mice; P. Mellon for GT1-7 cells; L. Rui for HEK 293-lepR cells; J. Seufert for SOCS3 cDNA; R. Bruick for SOCS3-pGL3; S. Albelda for Cre adenovirus; G. Francisco for synpasin promoter-controlled lentiviral vector; and S. Miyamoto for p65 antibody. We thank S. Shoelson, M. Hendrickson, A. Attie, D. Sun, C. Berridge, and Cai lab members for suggestions and technical assistance. This study was supported by NIH grants (R56 and R01 DK078750), American Diabetes Association Junior Faculty Award (1-07-JF-09), and UW-Madison Start-up funds (all to D. Cai).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arkan VC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. Nat. Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burks DJ, Font de MJ, Schubert M, Withers DJ, Myers MG, Towery HH, Altamuro SL, Flint CL, White MF. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature. 2000;407:377–382. doi: 10.1038/35030105. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat. Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- Everhart MB, Han W, Sherrill TP, Arutiunov M, Polosukhin VV, Burke JR, Sadikot RT, Christman JW, Yull FE, Blackwell TS. Duration and intensity of NF-kappaB activity determine the severity of endotoxin-induced acute lung injury. J. Immunol. 2006;176:4995–5005. doi: 10.4049/jimmunol.176.8.4995. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat. Med. 2004;10:734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol. Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat. Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat. Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat. Neurosci. 2005;8:579–584. doi: 10.1038/nn1456. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. Inflamed about obesity. Nat. Med. 2004;10:126–127. doi: 10.1038/nm0204-126. [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat. Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat. Neurosci. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Obici S, Morgan K, Barzilai N, Feng Z, Rossetti L. Overfeeding rapidly induces leptin and insulin resistance. Diabetes. 2001;50:2786–2791. doi: 10.2337/diabetes.50.12.2786. [DOI] [PubMed] [Google Scholar]
- Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–578. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.