Abstract
Ras proteins play a direct causal role in human cancer with activating mutations in Ras occurring in ∼ 30% of tumours. Ras effectors also contribute to cancer, as mutations occur in Ras effectors, notably B-Raf and PI3-K, and drugs blocking elements of these pathways are in clinical development. In 2000, a new Ras effector was identified, RAS-association domain family 1 (RASSF1), and expression of the RASSF1A isoform of this gene is silenced in tumours by methylation of its promoter. Since methylation is reversible and demethylating agents are currently being used in clinical trials, detection of RASSF1A silencing by promoter hypermethylation has potential clinical uses in cancer diagnosis, prognosis and treatment. RASSF1A belongs to a new family of RAS effectors, of which there are currently 8 members (RASSF1–8). RASSF1–6 each contain a variable N-terminal segment followed by a Ras-association (RA) domain of the Ral-GDS/AF6 type, and a specialised coiled-coil structure known as a SARAH domain extending to the C-terminus. RASSF7–8 contain an N-terminal RA domain and a variable C-terminus. Members of the RASSF family are thought to function as tumour suppressors by regulating the cell cycle and apoptosis. This review will summarise our current knowledge of each member of the RASSF family and in particular what role they play in tumourigenesis, with a special focus on RASSF1A, whose promoter methylation is one of the most frequent alterations found in human tumours.
Abbreviations: AP-1, activation protein 1; APC, anaphase-promoting complex; ATM, ataxia telagectasia mutant; C1, protein kinase C conserved region; CIMP, CpG island methylator phenotype; CRC, colorectal cancer; DAG, diacylglycerol; HCC, hepatocellular carcinoma; Hpo, hippo; EBV, human herpes virus; EGF, epidermal growth factor; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus virus; JNK, c-Jun-NH2-kinase; LATS1, Lats/Warts serine/threonine kinase; LOH, loss of heterozygosity; MAP-1, modulator of apoptosis-1; MAPK, mitogen-activated protein kinase; MEF, mouse embryonic fibroblast; MEK, MAPK/ERK kinase; MSP, methylation-specific PCR; MST1, mammalian sterile 20-like kinase-1; NORE1, novel Ras effector 1; NSCLC, non-small cell lung cancer; PI3-K, phosphatidylinositol 3-kinase; PMCA4b, plasma membrane calmodulin-dependent calcium ATPase 4b; RA, RalGDS/AF6 Ras association; RAPL, regulator of adhesion and polarization enriched in lymphocytes; RASSF, Ras-association domain family; RBP1, RASSF1A-binding protein 1; RTK, receptor tyrosine kinase; RSV, respiratory syncytial virus; SAPK/JNK, stress-activated protein kinase/c-Jun N-terminal kinase; SARAH, Sav/RASSF/Hpo; Sav, Salvador; SCLC, small cell lung cancer; SV40, simian virus 40; TCR, T-cell receptor; TNFα, tumour necrosis factor alpha; TRAIL, TNFα-related apoptosis-inducing ligand; TSG, tumour suppressor gene
Keywords: RASSF, Tumour suppressor, Methylation, Cell cycle, Apoptosis, Microtubule
1. RASSF1
1.1. Introduction to Ras and its effectors
The Ras GTPases are a superfamily of molecular switches that regulate a diverse range of functions, including cell proliferation, differentiation, motility and apoptosis in response to extracellular signals. The Ras proteins exist in two states: a GTP-bound active state and a GDP-bound inactive state. In its GTP-bound state, Ras is able to interact with its downstream effectors, and mediate some component of Ras’ cellular actions through complex signal transduction cascades (Fig. 1). Ras effectors are proteins that specifically bind the GTP-bound form of Ras via the Ras protein effector domain. Two of the best-studied Ras effectors are Raf, a serine-threonine kinase that controls the MEK-ERK pathway that activates cellular proliferation [1], and phosphatidylinositol 3-kinase (PI3-K), whose activity is required for activation of the protein kinase B, Akt, which inhibits apoptosis induced by members of the Bcl-family (such as BAD) [2]; see Fig. 1. Raf and PI3-K interact with Ras through their Ras-binding domains (termed RBD and PI3K_rbd, respectively). There is another group of Ras effectors which also share a conserved motif, namely the RalGDS/AF6 Ras association (RA) domain, as defined by sequence homologies between the Ras effectors, Ral guanosine nucleotide-exchange factor (RalGDS; involved in Ras-induced transformation) and the ALL-1 fusion partner from chromosome 6 (AF6; involved in regulating cell adhesion) [3,4]. Recently, new genes encoding the RA domain have been identified, and termed the Ras-association domain family (RASSF), consisting of 8 members to-date, namely RASSF1 (123F2), RASSF2 (Rasfadin/KIAA0168), RASSF3, RASSF4 (AD037), RASSF5 (NORE1), RASSF6, RASSF7 (HRC1) and RASSF8 (Fig. 2). However, although they interact either directly or indirectly with activated Ras, their role in mediating its biological effects remains unclear. What is clear is that they seem to modulate some of the growth inhibitory responses mediated by Ras and may serve as tumour suppressor genes. Thus, RASSF proteins are tumour suppressors, in contrast to traditional Ras effectors such as Raf and PI3-K, which are oncoproteins.
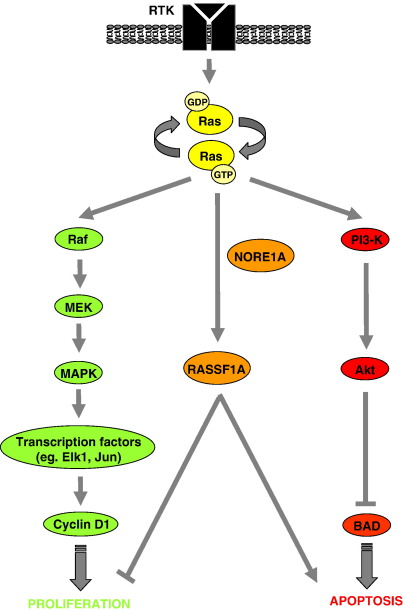
Fig. 1.
Ras signalling pathways. Ras transmits signals from receptor tyrosine kinases (RTK) to the nucleus and regulate a diverse array of biological functions. Ras functions as a molecular switch, being inactive when bound to GDP and active when bound to GTP. Activated Ras acts by regulating the cellular response through distinct Ras effectors proteins and their complex signal transduction cascades, such as mediated by the Raf serine/threonine kinases, the lipid kinase, phosphatidylinositol 3-kinase (PI3-K) and the Ras association domain family 1, RASSF1A. The best-characterised signal transduction pathway of Ras is by the Raf kinases. Activated Raf phosphorylates MAPK/ERK kinase (MEK) and the activated MEK phosphorylates the mitogen-activated protein kinase (MAPK), which becomes activated and translocates to the nucleus where it phosphorylates a set of transcription factors. For example, the activation of Elk-1 leads to the transcription of Fos, which together with the MAPK-activated Jun, forms the activation protein 1 (AP-1), which has been shown to induce cyclin D1 and therefore stimulate proliferation. Another cascade of Ras-activated signalling is by anti-apoptotic PI3-K, which can stimulate the activity of the protein kinase B, Akt. Akt subsequently phosphorylates BAD, a pro-apoptotic member of the Bcl-family, and thus inhibits apoptosis (inactivating BAD enables BCL to promote cell survival by blocking the release of mitochondrial cytochrome c and therefore inhibiting caspase activation). Additionally, Ras regulates a pro-apoptotic pathway by binding to the Ras effectors NORE1 and RASSF1A and RASSF1A can also block cell cycle progression.
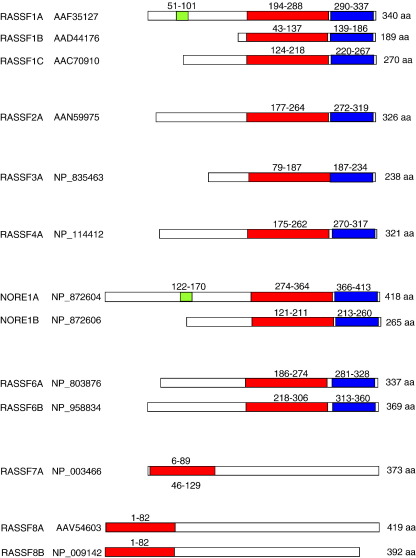
Fig. 2.
Schematic representation of the protein domains of members of the RASSF family described in the literature. Putative DAG-binding (C1, green), Ras association (RA, red) and Sav/RASSF/Hpo interaction (SARAH, blue) domains are shown, predicted using Prosite (release 20.9) [209]. The Genbank ID ‘accession’ number is listed for each protein.
1.2. Identification of RASSF1
Loss of heterozygosity (LOH) studies in lung, breast, and kidney tumours identified several loci on chromosome 3p (namely 3p12, 3p14, 3p21.3, and 3p25–26) that were likely to harbour one or more tumour suppressor genes (TSGs). An important TSG was suspected to reside in 3p21.3 because instability of this region is the earliest and most frequently detected deficiency in lung cancer [5–11]. Overlapping homozygous deletions in lung and breast tumour cell lines reduced the critical region in 3p21.3 to 120 kb in which eight genes resided, namely CACNA2D2, PL6, 101F6, NPRL2/G21, ZMYND10/BLU, RASSF1/123F2, FUS1, and HYAL2 [8,12]. However, despite extensive genetic analysis in lung and breast tumours, none of these candidate genes were frequently mutated.
At the same time, RASSF1 was identified in a yeast two-hybrid screen through its interaction with the human DNA excision repair protein XPA [13]. The nucleotide sequence of the 1.7-kb cDNA identified matched the sequences of human cosmid clones LUCA12 and LUCA13 [14], located in the minimum homozygous deletion region of 120 kb in 3p21.3 [8]. The C-terminus showed high sequence homology (55% identity) with a known murine RAS effector protein (Nore1) [15] and contained a Ras-association (RA) domain. Hence the gene name was changed from 123F2 to Ras-association domain family 1 (RASSF1) gene [13].
1.3. Gene and protein structure of RASSF1
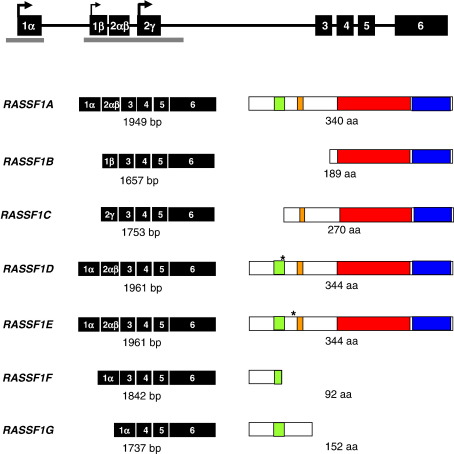
The RASSF1 gene locus is located on chromosome 3p21.3 and consists of eight exons spanning ∼ 11 kb. From this, seven different transcripts are generated (RASSF1A-G) through the use of differential promoters and alternative splicing (Fig. 3) [12,13,16]. RASSF1A and RASSF1C, ubiquitously expressed in normal tissues, are the major isoforms and are transcribed from two different CpG island promoters ∼ 3.5 kb apart. These isoforms have four common C-terminal exons (exons 3–6) which encode a RalGDS/AF6 or Ras-association (RA) domain [3,4] and a Sav/RASSF/Hpo (SARAH) domain (Hippo (Hpo) is the Drosophila homolog of Mst1/2 and together with Salvador (Sav) and Warts (Wts) promotes proper exit from the cell cycle and apoptosis during development) [17]. RA domains mediate interactions with Ras and other small GTPases, and SARAH domains mediate protein–protein interactions crucial in the pathways that induce cell cycle arrest and apoptosis (heterotypic interactions in the case of Sav, RASSF and Hpo, and homotypic interactions in the case of Mst1). Exon 3 also contains a putative ataxia telagectasia mutant (ATM) kinase phosphorylation consensus sequence motif (a peptide containing this sequence is phosphorylated in vitro, suggesting that RASSF1, like p53, may be a substrate for ATM [18]). The 1.9-kb RASSF1A transcript initiates from a promoter located in the first CpG island and transcription initiates with exon 1α followed by exon 2αβ; these exons contain a diacylglycerol/phorbol ester-binding (DAG) domain, also known as the protein kinase C conserved region (C1), which contains a central zinc finger (zinc-binding domain) [19]. The 1.7-kb RASSF1C transcript initiates from a promoter located the second CpG island and transcription initiates with a single N-terminal exon (exon 2γ), the protein sequence of which has no significant similarity to any known protein (Fig. 3).
Fig. 3.
Map of the RASSF1 gene. Through alternative promoter usage and splicing of the exons, 7 transcripts have been reported to be produced from the RASSF1 locus. RASSF1A (Vega: BCM:RASSF1-001, OTTHUMT00000264806), RASSF1B (Vega: BCM:RASSF1-003, OTTHUMT00000264808; Ensembl: Q9NS23-3, ENST00000362008), RASSF1C (Vega: BCM:RASSF1-005 and -006, OTTHUMT00000264808 and OTTHUMT00000264807; Ensembl: Q9NS23-4, ENST00000327761), RASSF1D (Vega: BCM:RASSF1-002, OTTHUMT00000264810; Ensembl: RASF1_HUMAN, ENST00000357043), RASSF1E (Ensembl: Q9NS23-5, ENST00000359365), RASSF1F (Ensembl: Q9NS23-6, ENST00000273611), and RASSF1G (Vega: BCM:RASSF1-004, OTTHUMT00000264809; Ensembl: Q9NS23-7, ENST00000266020). The Vega program also predicts 3 additional transcripts, however, these have yet to be experimentally verified and have not been shown (BCM:RASSF1-007, OTTHUMT00000264812, a 601-bp transcript that produces a 133-amino acid protein with no recognisable domains; BCM:RASSF1-008, OTTHUMT00000264813, a 571-bp transcript that produces a 41-amino acid protein with no recognisable domains; and BCM:RASSF1-009, OTTHUMT00000264814, a 488-bp transcript that produces a 158-amino acid protein containing a C1 domain). UTR regions are depicted by open boxes, exons by black boxes, promoters by black arrows and CpG islands by grey bars (as predicted by Ensembl). The domain structure of the protein products are predicted using Prosite: putative ATM kinase phosphorylation consensus sequence motif (orange), DAG-binding (C1) domain (green), Ras association (RA) domain (red), and Sav/RASSF/Hpo (SARAH) interaction domain (blue) domains. Position of the extra 4 amino acids in RASSF1D and RASSF1E (black asterisk). Ensembl (release 45) [20], Vega (release 24) [165], Prosite (release 20.9) [209].
RASSF1B (also known as the ‘minor’ form or transcript) has the same exon 2αβ as RASSF1A but utilises a different 5′ exon (exon 1β; Fig. 3). RASSF1B is expressed predominantly in haematopoietic cells and the transcript only encodes the RA and SARAH domains [13]. The remaining 4 isoforms (RASSF1D-G) are all splice variants of RASSF1A [16]; the RASSF1D transcript is expressed specifically in cardiac cells and encodes four additional amino acids 5′ of exon 2αβ, the RASSF1E transcript is expressed specifically in pancreatic cells and has an additional four amino acids 3′ of exon 2αβ, the RASSF1F transcript skips exon 2αβ and produces a truncated peptide of 92 amino acids that terminates within the C1 region, and the RASSF1G transcript skips exons 2αβ-3 and produces a truncated peptide of 152 amino acids that terminates just 5′ of the RA domain (Fig. 3). The biological function of these additional transcripts is unknown, however, all RASSF1 isoforms which are transcribed from the first CpG island (namely RASSF1A and RASSF1D-G), are frequently missing in a variety of tumours as a result of epigenetic inactivation of the RASSF1A promoter.
1.4. Orthologues of RASSF1
Orthologues of RASSF1 have been predicted by Ensembl (http://www.ensembl.org/index.html) in a wide range of organisms, including mammals (elephant, cow, monkey, dog, armadillo, opossum, rabbit, rat, mouse), birds (chicken), fish (zebrafish, fugu, medaka, tetraodon, stickleback), worms, flies and sea squirts, although they do not all show multiple transcripts [20]. RASSF1A orthologues are present in many model organisms including Mus musculus (Rassf1; Genbank ID:AF132851), Danio rerio (zgc:92505; Genbank ID:BC081661), and Caenorhabditis elegans (T24F1.3a; Genbank ID:NP_001022361), with each having a C1, RA and SARAH domain. The C. elegans gene product T24F1.3 is a 615 protein containing a unique N-terminal segment, a central C1 zinc finger (aa 165–214), a putative RA domain (aa 396–495), and a C-terminal extension of 65 amino acids relative to NORE and RASSF1A. The C-terminal 300 amino acids of RASSF1A and NORE are ∼ 40% identical (70% similar) in sequence to the central segment of T24F1.3 (containing the C1 and RA domains), suggesting that T24F1.3 is a common precursor to these two mammalian proteins [21]. T24F1.3, like other members of the RASSF family, can bind to the proapoptotic protein mammalian sterile 20-like kinase-1 and -2 (MST1 and MST2) through the SARAH domains of each partner [21]. Experiments feeding worms with RNAi directed against T24F1.3 resulted in “no obvious phenotype” [22,23]. No mutants or targeted knockdowns have been generated for the zebrafish orthologue zgc:92505, however, in situ hybridisation profiles of gene expression in various anatomical regions during the different stages of zebrafish development reveal some stages showing an “expression pattern linked to cell proliferation” (http://mirror.zfin.org/cgi-bin/webdriver%3FMIval%3Daa%1Emarkerview.apg&OID=ZDB-GENE-040912-14). Mice specifically carrying targeted deletions of the Rassf1a isoform of Rassf1 show an increased incidence of both spontaneous and induced tumourigenesis [24,25], consistent with the role of RASSF1A as a tumour suppressor gene. In Drosophila melanogaster there is only one RASSF family member, dRASSF, which is encoded by the CG4656 gene (Genbank ID:AY051923). Like its vertebrate counterparts, dRASSF bears a C-terminal RA and SARAH domain, and although it has an N-terminal LIM domain, this domain shares some similarities with C1 zinc fingers. dRASSF restricts Hpo activity by competing with the scaffold protein Sav for binding to Hpo [26]. In addition, dRASSF was also observed to possess a tumour-suppressor function [26].
1.5. Silencing of RASSF1 in cancer
1.5.1. RASSF1A promoter methylation
Silencing of genes by DNA methylation is a common phenomenon occurring in human cancer cells [27]. It has been reported that promoter methylation plays an essential role in loss of function of certain tumour suppressor genes [28]. Loss of RASSF1A expression is one of the most common events in human cancers, with aberrant promoter methylation reported in at least 37 tumour types, including and abdominal paraganglioma, bladder, brain (neuroblastoma, glioblastoma, medulloblastoma), breast, cervical, cholangiocarcinomas, colon, oesophageal, gastric, head and neck, hepatocellular, Hodgkin’s lymphoma, kidney, lung (small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), malignant mesothelioma), melanoma, nasopharyngeal, osteosarcoma, ovarian, pancreatic, prostate, pheochromocytoma, soft tissue sarcoma (including leiomyosarcoma), testicular germ cell, thyroid and childhood tumours (adrenocortical carcinoma, hepatoblastoma, leukaemia, lymphoma, medulloblastoma, neuroblastoma, pancreatoblastoma, retinoblastoma, rhabdomyosarcoma and Wilms’ tumour) [comprehensively listed in 29,30]. In general, RASSF1A methylation frequency is higher in cancer cell lines compared to the primary tumours, possibly due to the de novo methylation that occurs when cells are kept in culture [31,32], however, treatment with the DNA hypomethylating agent 5-aza-2′-deoxycytidine reactivates the expression of RASSF1A. More recently, hypermethylation of the RASSF1A promoter methylation was also found in human placentas (but not other foetal tissues) during pregnancy (extending the analogy between the primate placenta and malignant tumours to the epigenetic level) [33].
Indeed, RASSF1A, promoter methylation has been demonstrated in epithelial hyperplasia and intraductal papillomas of the breast, as well as cancerous epithelium [34] suggesting that RASSF1A methylation is an early event in breast tumourigenesis. RASSF1A methylation has also been suggested to be an early event in thyroid tumourigenesis [35], childhood neoplasia [37] and endometrial carcinogenesis [38].
1.5.2. RASSF1A mutations
Loss of RASSF1A expression is largely attributed to promoter hypermethylation, as somatic mutations of RASSF1A are uncommon, although several polymorphisms have been detected. In more than 200 samples lung, breast, kidney and nasopharyngeal carcinomas and cell lines analysed, only one frame-shift mutation (at codon 277 in the RA domain) and one missense mutation (at codon 201 in the RA domain) have been identified [13,16,39–41]. However, numerous polymorphisms have been identified in these tumours and cell lines, (NCBI dbSNP build 127 has 43 entries for polymorphisms in RASSF1A [http://www.ncbi.nlm.nih.gov/projects/SNP/]), many of which are located in the functional domains of RASSF1A (five in the C1 domain, four in the ATM phosphorylation site consensus sequence, and five in the RA domain [detailed in 42]), and many of them have proven to encode a functionally impaired mutant RASSF1A. For example, A133S or S131F RASSF1A mutants cannot induce cell cycle arrest by blocking cyclin D1 accumulation (the S131F mutant also shows reduced phosphorylation resulting in less efficient inhibition of cell proliferation) [43], C65R and V211A mutants show reduced growth suppression activity both in vitro and in vivo [44,45], and C65R and R257Q mutants show reduced association with the microtubules [46]. Nevertheless, the functional significance of these alterations in tumourigenesis remains to be determined. For example, one study found frequent alterations at codon 133 in the microtubule association and stabilization domain are preferentially detected in patients with breast carcinoma and fibroadenoma (a benign mammary tumour) compared to control patients with non-tumourous alterations of the breast [47].
1.5.3. Loss of expression of other RASSF1 isoforms
Expression of the RASSF1B isoform was found to be lost in two of four lymphoid tumour cell lines and seven of eight bladder cancer cell lines [13,48]. However, loss of RASSF1B expression was always concomitant with loss of RASSF1A expression and expression of both was recovered following treatment with a demethylating agent [48]. Thus, there have been no reports of samples showing exclusive down-regulation RASSF1B. RASSF1C did not show hypermethylation of its promoter region and was expressed in almost all lung, breast and paediatric tumours and tumour cell lines tested [13,16,49]. However, six of nine transformed ovarian cell lines have been reported to have lost the expression of RASSF1C [50] and RASSF1C expression was almost undetectable in the KRC/Y renal cell carcinoma cell line [40]. Thus RASSF1C may have a tissue-specific effect. Expression of RASSF1F is intimately connected with RASSF1A expression because they share a common promoter region [16,51]. Not surprisingly therefore, re-expression of RASSF1A in 5-aza-2′ deoxycytidine-treated cell lines was coincident with RASSF1F re-expression [49]. Although not reported, expression of RASSF1D, RASSF1E, and RASSF1G is also likely to be linked with expression of RASSF1A as they too share the same promoter region.
1.6. The use of RASSF1A methylation as a tumour biomarker
RASSF1A methylation has the potential to be an ideal cancer biomarker as it occurs at moderate to high frequency in a very wide range of tumour types, yet is comparatively rarely found in normal tissues [34–36]. Thus, methylation of RASSF1A is being considered for use in the clinic as a diagnostic marker, for early tumour detection, and a prognostic marker, to predict the risk of cancer development from benign growths, to predict the prognosis of the patients with a diagnosed tumour, or even as a marker for resistance to some treatments.
1.6.1. Diagnostic marker
It has been demonstrated that cancer patients have increased levels of free DNA in their sera which has been released from the cancer cells [52,53]. Silencing of tumour suppressor genes has established promoter hypermethylation as a common mechanism for tumour suppressor inactivation in human cancers and thus is a promising new target for molecular detection in bodily fluids. Methylation-specific PCR (MSP) can determine the presence or absence of methylation of a gene locus at a sensitivity level of up to 1 methylated allele in 1000 unmethylated alleles, appropriate for the detection of neoplastic cells in a background of normal cells [54]. MSP also allows rapid analysis of multiple gene loci, does not require prior knowledge of epigenetic alteration, and can provide a “yes or no” diagnostic answer.
The value of RASSF1A methylation as a diagnostic marker has been investigated most thoroughly in lung cancer. For example, RASSF1A methylation was found to occur in ∼ 34% of NSCLC tumours, with concomitant methylation observed in the corresponding serum [55,56]. RASSF1A methylation has also been detected in the tumour and corresponding bronchoalveolar lavages in 29% (5/17) of methylated lung cancer cases and when analysed in combination with 5 other tumour-related genes, could diagnose lung cancer in 68% (21/31) of patients [57]. RASSF1A methylation has also been found in the upper aerodigestive tract, bronchial aspirates, plasma and sputum in a significant proportion of current and former smokers [58–61], which correlated with the number of pack years smoked during the lifetime [59]. In general, DNA hypermethylation analysis would be best used as a diagnostic marker in conjunction with conventional diagnostic methods such as cytology and histology. For example, analysis of RASSF1A, p16INK4A and APC methylation in bronchial aspirates showed that cytology, quantitative MSP and histology could detect lung cancer in 44%, 53% and 59% of cases respectively, yet when combined diagnostic sensitivity extended to 81% (69/85) of patients [59].
RASSF1A methylation could also be a useful diagnostic marker for other tumour types, such as breast, bladder and kidney cancer. For example, methylation analysis of RASSF1A, APC and DAPK1 was sufficient to differentiate normal from tumour tissue in 94% of breast cancer cases and 76% of corresponding serum DNA was also positive for methylation [62]. Promoter methylation of APC, RARß2, and RASSF1A in benign breast epithelium is associated with epidemiologic markers of increased breast cancer risk (promoter methylation of RASSF1A and APC occurred more frequently in unaffected women at high-risk for breast cancer, as defined by the Gail model, than in low/intermediate risk women) [63]. RASSF1A, promoter methylation has been demonstrated in epithelial hyperplasia and intraductal papillomas of the breast, as well as cancerous epithelium [34] suggesting that RASSF1A methylation is an early event in breast tumourigenesis. Indeed, RASSF1A methylation has also been suggested to be an early event in thyroid tumourigenesis [35], childhood neoplasia [37] and endometrial carcinogenesis [38].
In breast cancer patients where RASSF1A methylation may be undetectable in the plasma, methylation has been shown to be detectable in tumour DNA eluted from the surface of erythrocytes and leukocytes [64] and in nipple aspirate fluid [65]. Similarly, analysis of urine DNA represents a simple method for kidney and bladder cancer detection. Several studies have reported RASSF1A promoter methylation in urine DNA from patients with kidney tumours, bladder cancer and urothelial cancer but not in normal control samples or patients with cystitis [66–68], although others have found RASSF1A promoter methylation in normal bladder tissues [69,70], although the frequency and extent of methylation appeared to increase with age and malignancy [70]. Thus although MSP has the potential to enhance early detection of bladder cancer using a non-invasive urine test, the lack of tumour specificity in some cases suggests further investigation is required before this test is introduced into clinical practice. Recently, the presence of localized prostate cancer was found to be detectable using quantitative MSP on urinary cells obtained following prostate massage, with the four-gene combination of GSTP1, RASSF1A, RARβ2, and APC best discriminating malignant from non-malignant cases (with 86% sensitivity and 89% accuracy), suggesting that these panel of four genes could stratify patients into low and high risk of having prostate cancer and optimize the need for repeat prostatic biopsies [71].
The diagnostic potential of RASSF1A methylation has also been explored in other cancers. For example, in a study involving ovarian cancer patients the use of 6 genes (RASSF1A, BRCA1, APC, DAPK1, p14ARF and p16INK4A) gave 100% diagnostic coverage with RASSF1A methylation detected in 50% cases; importantly, patient serum or peritoneal fluid was positive for methylation in 88% of the tumour cases analysed, even when the CA-125 (serum marker) levels were low, underscoring the importance of having a reliable tumour marker when early detection is crucial to patient outcome [72]. In a study of DNA collected from tampons, hypermethylation of 3 or more of 5 candidate genes, including RASSF1A, was a significant indicator of endometrial cancer and those patients without endometrial cancer that showed hypermethylation of 3 or more genes were shown to have cervical cancer, endometrial polyps or fibroids [73]. RASSF1A methylation has also been detected in gliomas and corresponding patient serum [55]. Interestingly, RASSF1A methylation in the serum of breast cancer patients was identified as a surrogate marker for the monitoring of response to adjuvant tamoxifen treatment, with persistence of RASSF1A methylation post-surgery and throughout treatment indicating resistance to tamoxifen and loss of methylation indicating a response [74]. However, it is important to note that some studies have shown limited success in the detection of RASSF1A methylation in serum. For example, methylation was detected in 65% (34/52) of Hodgkin’s lymphoma tumours but in only 2/22 corresponding serum [75]. Similarly, a study of nasopharyngeal cancer found that RASSF1A methylation was detected in 67% (20/30) of tumour samples, yet only 37% of mouth and throat rinses, 33% of nasopharyngeal swabs and 3% of plasma samples [76]. Taken together these studies show that RASSF1A methylation can be detected in a range of body fluids from cancer patients, with a sensitivity that compares favourably with conventional diagnostic methods (although care must be put into selecting the correct body fluid). Thus RASSF1A methylation has the potential to be used as a marker for early detection and monitoring (along with a panel of other tumour suppressor genes to ensure 100% coverage) and offers an exciting new approach to cancer diagnosis.
1.6.2. Prognostic marker
For some cancers an association between RASSF1A methylation and adverse patient survival has been observed. In NSCLC patients, some studies have found that RASSF1A methylation correlates with poor survival rate and is associated with poorly differentiated tumours, predominantly with vascular invasion and pleural involvement [16,77,78] although others have found no such correlation [79–81]. However, an association has been observed between RASSF1A methylation, age at which smoking began and a decreased survival rate [82], and RASSF1A methylation and earlier recurrence of lung cancer [51]. Thus although it looks promising, further studies are needed to clarify the prognostic value of RASSF1A methylation in lung cancer.
Methylation of RASSF1A, together with that of other tumour-related genes, may be useful as a marker for tumour progression and metastasis, as many studies have shown this occurs significantly more frequently in tumours of a higher-grade, later stage, more invasive or metastatic tumours, including prostate cancer [83–86], breast cancer [87,88], bladder cancer [48,70,89–92], endometrial cancer [93], neuroendocrine tumours [94,95], melanoma [96], glioma [97], gastric adenocarcinoma [98], salivary adenoid cystic carcinoma [99], pituitary adenoma [100], and malignant pleural effusions [101]. It has recently been proposed that since the RASSF1A methylation index showed a gradual increase from non-lesional liver to regenerative/hyperplastic conditions (chronic liver disease and focal nodular hyperplasia), to preneoplastic lesions to overt tumours, quantitative analysis of RASSF1A gene promoter methylation, rather than the detection of methylation bands per se, might be clinically relevant [102]. It was also recently found that hepatocellular carcinoma (HCC) associated with cirrhosis showed significantly higher frequency of RASSF1A promoter methylation than HCC without cirrhosis [103]. Yet it must be noted that not all studies have found positive correlates with RASSF1A methylation and tumour grade/stage [37,104,105], with some reporting RASSF1A methylation in benign epithelium as well as in the lesions associated with high risk of cancer formation [106] (though it could be argued that this may be a sign of clinically relevant but still benign hyperplasia [107]). However, in general, RASSF1A methylation in tumour tissue and corresponding body fluids often correlates with advanced tumour stage and grade, metastasis, poor tumour differentiation and adverse survival.
1.7. Correlation between RASSF1A methylation and other oncogenic events
Due to the evidence linking RASSF1A to Ras signalling pathways, several studies have looked for correlation between mutation of K-Ras and inactivation of RASSF1A by methylation. An inverse correlation was observed in colorectal cancers [108], pancreatic adenocarcinomas [109], and NSCLC [110]. However, other studies of NSCLC have found no correlation between methylation of RASSF1A and activating mutations in K-Ras [111,112]. Synergy between RASSF1A and members of the Ras signalling pathway has been proposed in melanomas where most tumours and all cell lines with RASSF1A promoter methylation additionally carried B-Raf or N-Ras mutations [113]. Interestingly, in thyroid cancer the situation appears to be reversed as RASSF1A hypermethylation and B-Raf mutations events were mutually exclusive [35]. There is no correlation in the methylation status between RASGRF2 (a Ras guanine nucleotide exchange factor capable of activating Ras) and RASSF1A in NSCLC [114]. These differences between the tissues may simply be a reflection of alterations occurring in other Ras signalling pathways.
Other genes have been reported to show concomitant inactivation with RASSF1A silencing. For example, 90% of undifferentiated thyroid carcinomas examined with p16INK4A inactivation were also silenced for RASSF1A expression [115] and RASSF1A and p16INK4A methylation in stage IIIA lung adenocarcinomas have been shown to be profound indicators of poor survival [78]. A statistically significant association between hypermethylation of RASSF1A and hypermethylation of CASP8 was found in neuroblastic tumours [116]. In hepatocellular carcinoma, a significant association between ‘CpG island methylator phenotype’ (CIMP; in which multiple genes are concurrently methylated in tumours) and methylation of RASSF1A has been reported [117]. However, the significance of these findings, in terms of synergy between these genes and their signalling pathways in tumour formation needs to be further investigated.
Some cancers are associated with infection of the tumour cells by oncogenic viruses, such as human papillomavirus (HPV), human herpes virus (EBV), and simian virus 40 (SV40). For example, cervical cancer and head and neck squamous cell carcinoma (HNSCC) are associated with HPV infection of the tumour cells (the HPV encodes viral proteins (E6 and E7) to subvert control of the cell cycle by inactivating p53 and Rb, respectively). Interestingly, HPV DNA was never found in cervical carcinomas or HNSCCs showing methylation of RASSF1A [118,119], suggesting that the presence of viral proteins abrogated any requirement for RASSF1A inactivation and thus implicating them both in the same pathway. However, no inverse correlation between RASSF1A methylation and HPV infection was found in cervical cancers [120,121]. The EBV is associated with a number of neoplasias, including nasopharyngeal carcinoma, Hodgkin’s lymphoma, and gastric carcinomas. Although RASSF1A methylation is detected in the majority of nasopharyngeal carcinomas [comprehensively reviewed in 29], it is difficult to draw any correlations regarding viral infection of the tumour cells because virtually all cases are EBV positive. However, comparisons between EBV infection and RASSF1A methylation are possible with Hodgkin’s lymphoma and gastric carcinomas as only a subset are EBV positive and although RASSF1A methylation did not correlate with EBV infection in a study of Hodgkin’s lymphoma cases [75], a correlation between EBV infection and RASSF1A methylation was detected in gastric carcinoma [122]. Finally, malignant mesothelioma is frequently associated with SV40 infection, and RASSF1A methylation has been shown to be significantly higher in SV40-positive malignant mesotheliomas than SV40-negative cases [123,124]. Interestingly, a correlation was found in hepatocellular carcinoma tumours between the methylation status of RASSF1A and the presence of DNA damage resulting from aflatoxin B1 (an environmental carcinogen that causes DNA adducts) [125].
1.8. Understanding the mechanism of action for RASSF1’s tumour suppressor function
RASSF1A has been found to be inactivated in more than 40 types of sporadic human cancers, suggesting it plays a key role in tumour prevention. Consistent with this, constitutive over-expression of RASSF1A in various tumour cell lines (NSCLC, prostate, kidney, nasopharyngeal carcinoma, and glioma cell lines) results in cells that are less viable, growth suppressed, less invasive, and show reduced anchorage/substrate independence [13,16,40,44,97,126]. Over-expression or ectopic expression of RASSF1A in lung, kidney, nasopharyngeal and prostate cancer cell lines causes drastic reduction of tumourigenicity both in vitro and in vivo, whereas expression of mutant forms of RASSF1A only showed reduced growth suppression activity [13,16,40,44,45,126,127].
In contrast to RASSF1A, a role for RASSF1C in tumour suppression is not clear as reports of its activities are mixed. For example, ectopic expression of RASSF1C showed no significant effects on growth and induction of apoptosis in the H1299 and A549 cells NSCLC cell lines in vitro and in vivo [127], no suppression of anchorage-independent growth in the H1299 cell line in vitro [16] and no growth inhibitory activity of the U2020 SCLC cell line in vitro [45]. Vos and co-workers found that the growth inhibitory effects of RASSF1C in 293T cells were dependent upon the presence of activated RasG12V [50]. However, over-expression of RASSF1C activated osteoblast cell proliferation (through interaction with IGFBP-5) [128] and a reduction in RASSF1C expression caused decreased lung cancer cell proliferation [129]. In contrast, both RASSF1A and RASSF1C showed similar growth inhibitory activities of the prostate cell line LNCaP and renal cell carcinoma line KRC/Y in vitro, and suppression of tumourigenicity of the KRC/Y cell line in vivo [45]. Mutations in both RASSF1A and RASSF1C were also detected in a gene inactivation test in vivo [45]. More recently, RASSF1A and RASSF1C isoforms have been shown to have opposite effects in controlling the degradation of β-catenin (via regulation of the SCFßTrCP ubiquitin ligase); β-catenin accumulation is promoted by over-expression of RASSF1C or silencing of RASSF1A [130]. This suggests that inhibition of ß-catenin accumulation could be one of the mechanisms by which RASSF1A exerts its tumour suppressor function, and RASSF1C expression in the absence of RASSF1A could play a role in tumourigenesis [130]. Thus additional investigations will be needed to better understand the role of RASSF1C in tumourigenesis, in particular, is it restricted to certain tumour types and are its effects dependent upon it being present in greater amounts than RASSF1A (such as would occur with selective methylation of the RASSF1A promoter in tumourigenesis)?
Mouse models of human cancer have greatly advanced our understanding of tumourigenesis. The first mice lacking Rassf1 were made by Smith and colleagues, who used chromosomal engineering to generate mice carrying a 370-kb deletion (encompassing 12 genes including Rassf1) of the region syntenic to the minimal deletion region on human 3p21.3, frequently deleted in lung tumours [131]. Although homozygous null mice were embryonic lethal, heterozygous mice were viable and fertile, despite being haploinsufficient for 12 genes, and it remains to be seen whether these mice show an increased incidence of tumourigenesis [131]. The generation of knockout mice deficient for only the Rassf1a isoform of Rassf1 [24,25] mimics the situation seen in human tumours in which RASSF1A, but not RASSF1C, is missing. Rassf1a null mice showed an increased incidence of spontaneous tumourigenesis (predominantly lymphomas) and decreased survival rate compared with wild-type mice [24,25]. Rassf1a null mice exposed to physical (irradiation) and chemical (benzo[a]pyrene and urethane) mutagens also showed increased tumour susceptibility relative to controls [24,25]. These data are consistent with the role of a tumour suppressor gene, and suggest that RASSF1A inactivation in combination with other genetic or epigenetic alterations may produce a more severe tumour susceptibility phenotype. However, the mechanisms by which RASSF1A exerts its tumour suppression activities or the pathways it can regulate are not yet fully understood. RASSF1A has been reported to play a role in diverse activities including the regulation of apoptosis and genomic instability as well regulating microtubule dynamics during the cell cycle/mitotic progression, and thus may serve as a node in the integration of signalling pathways controlling a range of critical cellular functions (summarised in Fig. 4).
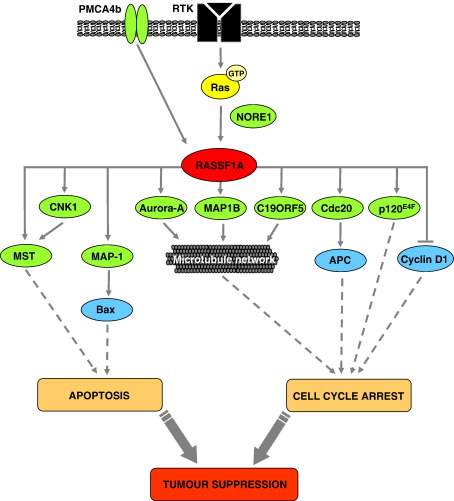
Fig. 4.
A summary of the reported RASSF1A interactions and RASSF1A-mediated biological functions. RASSF1A can regulate the microtubule network, cell cycle progression and apoptosis by recruiting effectors and their signalling pathways. Proteins that directly interact (bind) with RASSF1A are shown in green, with downstream proteins affected by this interaction shown in blue. RASSF1A induces apoptosis through its interaction with Ras, the Ras effector NORE1, the connector enhancer of KSR (CNK1), the pro-apoptotic kinase MST1, and the modulator of apoptosis-1 (MAP-1; activated K-Ras, RASSF1A, and MAP-1 synergize to induce Bax activation and cell death). RASSF1A regulates proliferation through its interactions with the microtubules and Cdc20 (by inhibiting the APC–Cdc20 complex and its degradation of cyclins A and B), the microtubule-associated protein 1B (MAP1B), Aurora-A (which phosphorylates RASSF1A), C19ORF5 (the C19ORF5–RASSF1A interaction at the centrosome is thought to be required for the proper control of the APC–Cdc20 complex during mitosis), the transcription factor p120E4F (RASSF1A-induced G1 cell cycle arrest and S-phase inhibition was enhanced by p120E4F) and inhibition of cyclin D1 accumulation. RASSF1A also inhibits the epidermal growth factor-dependent activation of Erk through the plasma membrane calmodulin-dependent calcium ATPase 4b (PMCA4b). Taken together, these activities all support a tumour suppressor role for RASSF1A.
1.8.1. Microtubule and centrosome binding activities
RASSF1A co-localizes with microtubules in interphase and decorates spindles and centrosomes during mitosis (RASSF1A relocates from the microtubules to the separated centrosomes during prophase, then to the spindle fibres and poles during metaphase and anaphase and finally to the midbody during cytokinesis) [132,133]. Deletion analysis identified the region between amino acids 120 and 185 as the microtubule association domain [134]. Re-expression of RASSF1A in RASSF1A-negative cell lines induces microtubule stabilisation and protects the cells from the actions of microtubule depolymerising agents, such as nocodazole [46,132,134,135]. Two naturally occurring missense mutations in RASSF1A, C65R and R257Q, resulted in RASSF1A mutants that were deficient in their ability to bind microtubules, were less competent at induction of microtubule acetylation and failed to protect against nocodazole-induced polymerisation [46]. These mutants were also deficient in their ability to stop DNA synthesis in NCI-H1299 cells [46], suggesting a link between competency to bind microtubules and ability to induce cell cycle arrest.
A yeast two-hybrid screen to identify novel RASSF1A-interacting proteins found 70% of interacting clones had homology to microtubule-associated proteins, including MAP1B and C19ORF5, suggesting that RASSF1A may exert its tumour-suppressive functions through interaction with the microtubules [46]. The interaction of C19ORF5 (also known as BPY2IP1 or MAP1S) and RASSF1A was independently confirmed by other groups [133,136]. C19ORF5 is a ubiquitously expressed member of the MAP1A/B family of microtubule-associated proteins and accumulation of c19ORF5 causes mitochondrial aggregation and cell death [136]. The C19ORF5–RASSF1A interaction at the centrosome is thought to be required for the proper control of the anaphase-promoting complex/Cdc20 complex during mitosis [133]. C19ORF5 can also interact with LRPPRC; C19ORF5 and LRPPRC colocalize with β-tubulin in the cytoplasm, however, in apoptotic cells, they are found to colocalize in the nucleus [136]. An interaction between C19ORF5 and the mitochondrial proteins (NADH-dehydrogenase subunit 1 and cyclooxygenase-1) and LRPPRC associates RASSF1 with mitochondria, an organelle with pivotal functioning in control of apoptosis [136]. Furthermore, depletion of C19ORF5 causes mitotic abnormalities [137]. Thus when taken together with the fact that other microtubule-binding and stabilising proteins are known to also possess tumour suppressor activities (including adenomatous polyposis coli and von Hippel–Lindau proteins), the association of RASSF1A with the microtubules is likely to play an important role in its tumour suppressor activity.
The microtubular association and effect of RASSF1C on stabilization has been reported to be undetectable [133], weaker than [134], or equal to RASSF1A [135], depending on the cell line used. Indeed, Liu and colleagues showed that when expressed alone, RASSF1A and RASSF1C isoforms exhibit identical cellular locations, paclitaxel-like hyperstabilization of microtubules, and paclitaxel-like interference with mitosis [138]. However, when co-expressed with C19ORF5, RASSF1C failed to associate with and promote hyperstabilization of microtubules and it was specifically RASSF1A that caused microtubule hyperstabilization and the accumulation of C19ORF5 on them [138]. This could be the unique property that underpins tumour suppression by only the RASSF1A, not RASSF1C isoform, thus underlying the specific effect of hypermethylation-suppressed RASSF1A in tumour suppression.
1.8.2. Regulating the cell cycle and mitotic progression
Deregulation of the cell cycle is an essential requirement for tumourigenesis. In normal cells, cycling is tightly controlled by a number of protein complexes whose activity is required for the cell to pass through specific checkpoints. RASSF1A has been shown to induce cell cycle arrest by engaging the Rb family cell cycle checkpoint which regulates entry into S phase. Reintroduction of RASSF1A expression in lung and breast cancer cell lines (NCI-H1299 and HME50-hTERT, respectively) results in growth arrest and an inhibition of cyclin D1 protein accumulation (through inhibition of mRNA translation), which can be relieved by ectopic expression of cyclin D1 or other downstream activators of the G1/S-phase transition (such as cyclin A and E7) [43]. Concomitantly, down-regulation of endogenous RASSF1A expression in human epithelial cells resulted in abnormal accumulation of cyclin D1 protein in the absence of detectable changes in cyclin D1 mRNA levels [43]. Consistent with this study, re-expression of RASSF1A in a NSCLC cell line (A549) induced G1 cell cycle arrest and these cells showed a down-regulation of cyclins D1 and D3 [139]. Similarly, over-expression of RASSF1A in the MCF-7 breast cancer cell line exhibited a G1 arrest [135] (although others found no cell cycle arrest [140]). However, the RASSF1A-induced G1 arrest was found to be transient (24–48 hours following transfection) and RASSF1A over-expression also induced a G2/M arrest in these cells (72 hours following transfection) [135]. Interestingly, RASSF1A did not induce G1 arrest in 293T cells (a human embryonic kidney cell line), but rather a more pronounced G2/M arrest was noted [133,135].
In a yeast two-hybrid screen, p120E4F was identified as an interaction partner of RASSF1A (via amino acids 1 to 119 of RASSF1A) [141]. p120E4F is an E1A-regulated transcription factor which interacts with the tumour suppressor genes p14ARF, Rb and p53 and is involved in control of cell cycle arrest near the G1 transition. RASSF1A-induced G1 cell cycle arrest and S-phase inhibition was enhanced by p120E4F [141]. Furthermore, knockdown of endogenous RASSF1A in the breast tumour cell line HB2 and the cervical cancer cell line HeLa leads to a reduction in the binding capacity of p120E4F to the cyclin A2 promoter, whereas the binding capacity is increased in an A549 lung cancer cell line stably expressing RASSF1A [142]. This suggests that cyclin A2, which regulates CDK2 and thereby controls progression through S phase, is the cellular target for RASSF1A through p120E4F, and proposes a transcriptional mechanism for RASSF1A-dependent cell cycle regulation. It has also been proposed that RASSF1A blocks cell cycle arrest at the G1 phase through the c-Jun-NH2-kinase (JNK) pathway, as H1299 lung cancer cells stably transfected with RASSF1A showed reduced JNK and c-Jun phosphorylation, inhibition of JNK activity and down-regulation of cyclin D1 [143].
Over-expression of RASSF1A in 293T and HeLa cells induced stabilization of mitotic cyclins (cyclins A and B) and a mitotic arrest at prometaphase [133]. RASSF1A was also shown to interact with Cdc20, an activator of the anaphase-promoting complex (APC; a protein complex that interacts with ubiquitin-conjugating and activating enzymes to catalyze the ubiquitylation of proteins destined for degradation to allow the cell cycle to progress). After interaction with RASSF1A, Cdc20 is inhibited to activate APC and therefore APC is unable to degrade the mitotic cyclins A and B [133]. Conversely, RNAi-mediated inactivation of RASSF1A in HeLA cells resulted in acceleration of mitotic progression and the premature destruction of cyclins A and B [133]. The RASSF1A-mediated regulation of Cdc20 during mitosis also appeared to be independent of Mad2 and Emi1 (potent negative regulators of Cdc20 during mitotic progression), implying that RASSF1A acts in early prometaphase (after Emi1 destruction and before activation of the Mad2-dependent spindle checkpoint) to prevent the degradation of mitotic cyclins and to delay mitotic progression beyond metaphase (APC is inhibited by sequestration of Cdc20 by Emi1 during S, G2, and prophase, then during prometaphase, RASSF1A takes on the role of the Cdc20 regulator) [133,144]. RASSF1A’s inhibition of APC–Cdc20 activity during mitosis was subsequently shown to be regulated by RASSF1A-binding protein 1 (RBP1; previously termed C19ORF5), as RNAi-mediated depletion of RBP1 prevented both the localization of RASSF1A to the spindle poles and it’s binding to Cdc20, resulting in premature destruction of mitotic cyclins and acceleration of mitotic progression [145]. Thus RASSF1A may mediate its tumour suppressive effects by inducing growth arrest in the G1 and G2/M phases and regulating mitotic progression via regulation of the APC complex and accumulation of cyclins A, B and D1 [133,144,146,147].
However, Rassf1a null mouse embryonic fibroblasts (Rassf1a+/+ MEFs) were recently shown to display evidence of delayed mitosis (taking a longer time to traverse mitosis than Rassf1a+/+ MEFs, with some of them failing to complete mitosis as a result of cytokinesis failure) [148]. Furthermore, this defect was complemented, at least partially, by expression of either RASSF1A or components of the mammalian Hippo pathway, namely MST2, WW45 and LATS1 (recent work in Drosophila has identified a new tumour-suppressor pathway involving the Drosophila MST1 and MST2 ortholog, Hpo, as well as Lats/Warts serine/threonine kinase (LATS1) and Sav, of which WW45 is the human ortholog; RASSF1A, MST1, MST2 and Sav all contain a conserved C-terminal SARAH domain, required for protein–protein interactions) [148]. Furthermore, RASSF1A is a microtubule-binding and stabilising protein (see previous section) and disruption of microtubule dynamics either by microtubule-stabilizing/destabilizing agents or proteins affects cell cycle progression at M phase [reviewed in 149,150]. Indeed, RASSF1A has been shown to interact with the microtubule-associated protein C19ORF5/MAP1S, and siRNA-mediated knockdown C19ORF5 causes mitotic abnormalities and disrupts the microtubule-organizing centre [137]. In addition, Aurora-A interacts with and phosphorylates RASSF1A (residues Thr202 and/or Ser203 located in the microtubule-binding domain), and substitutions of these residues with glutamic acid at both positions, which mimicks constitutive phosphorylation of RASSF1A, disrupt its interactions with microtubules and abolish its ability to induce M-phase cell cycle arrest (Aurora-A overexpression also interferes with RASSF1A-mediated growth suppression) [151]. Thus further studies are needed to elucidate whether the primary cause of RASSF1A-induced mitotic arrest occurs via altered microtubule stabilization or via interactions with Cdc20 or members of the Hippo pathway (or both).
RASSF1 has also been shown to interact with plasma membrane calmodulin-dependent calcium ATPase 4b (PMCA4b) [152]. In a two-hybrid screen, the catalytic domain of PMCA4b was found to interact with RASSF1 (via amino acids 144–193 of RASSF1A or amino acids 74–123 of RASSF1C), with co-expression in cells causing inhibition of the epidermal growth factor (EGF)-dependent activation of the Erk pathway (Erk is a downstream target of the Ras-Raf-MEK signalling cascade that activates cellular proliferation, see Fig. 1) [152].
A role for RASSF1C in cell cycle regulation has been considerably less well-studied (over-expression of RASSF1C induced cell cycle arrest in KRC/Y cells [45], yet over-expression in HeLa cells showed no effect on the cell cycle [133]) and thus remains unclear.
1.8.3. Controlling genomic stability
Given RASSF1A localizes to the mitotic spindle and can complex with the centrosome component γ-tubulin (see Section 1.8.1), and defects in spindle regulation can lead to genomic instability [153], RASSF1A has investigated for its ability to influence genomic stability. Expression of activated Ras has been associated with genomic instability, giving rise to polyploidy, aneuploidy, and derangements of the nuclear structure [134,154,155]. Over-expression of RASSF1A or RASSF1C in the human embryonic kidney cell line 293T or human lung tumour cell line NCI-H1299 blocked the ability of activated Ras to induce genomic instability [134]. Interestingly, a point mutant of RASSF1C, S61F, which was severely defective for stabilizing tubulin, was unable to block the genomic destabilizing effects of Ras (the S61F mutant is equivalent to the S131F mutation of RASSF1A, and these mutations abolish the ATM phosphorylation site in both isoforms) [134]. A substantial proportion of human foreskin fibroblasts depleted of RASSF1A by infection with a retrovirus-based siRNA vector contained more than two centrosomes and showed various mitotic spindle abnormalities including the formation of multipolar spindles, misalignment of chromosomes, and lagging chromosomes, suggestive of chromosome instability [133]. More recently, a significant proportion of cultured fibroblasts from Rassf1a null mouse embryos (Rassf1a–/– MEFs) were found to exhibit delayed mitosis which resulted in cytokinesis failure [148]. However, in vivo analysis of genomic instability by examination of the presence of micronuclei in the blood showed no increase in the percentage of micronuclei from Rassf1a–/– mice compared to wild-type littermates (even in aged mice or mice exposed to DNA damage by low-dose irradiation) [24]. Similarly, Rassf1a–/– MEFs from these mice did not show a significant proportion of cells with more than 2 centrosomes or any gross chromosomal rearrangements by SKY analysis [24]. Thus further detailed analysis will be required to determine whether RASSF1A (or RASSF1C) has a role to play in regulating genomic instability.
1.8.4. Involvement in the pro-apoptotic pathway
Activated Ras is usually associated with enhanced proliferation, transformation and cell survival (see Fig. 1). However Ras can also induce proliferation inhibitory effects and apoptosis. Ras effectors, such as RASSF1, may be specialised to inhibit cell growth and induce cell death, such that inhibition of these pathways, via methylation of the RASSF1A promoter, may be necessary for tumourigenesis. An interaction between RASSF1C and RasG12V was detected during transient expression in mammalian cells by Vos and co-workers [50]. However, others found that neither RASSF1A or RASSF1C (or the C. elegans homolog T24F1.3) showed any significant ability to bind directly to RasG12V or several related GTPases, as determined quantitatively in a yeast two-hybrid assay, by co-transfection in mammalian cells and by binding in vitro (the difference in results was postulated to be because the former study employed much higher amounts of RASSF1 cDNA and higher ratios of RASSF1 to Ras DNA) [21,156]. Instead, it was shown that RASSF1A, unlike RASSF1C, was able to both homodimerise and form heterodimers with the Ras-GTP binding protein, Nore1 (this dimerisation required the amino-terminal 119 amino acids of RASSF1A which are not found in the B and C isoforms) [156]. Thus the ability of RASSF1A to heterodimerise with Nore1 confers an indirect association with Ras-GTP in vivo.
There is quite some uncertainty as to the contribution of apoptosis to the tumour-suppressive actions of RASSF1. Over-expression of RASSF1A in MCF-7 breast cancer cells resulted in morphological and biochemical changes suggestive of apoptosis (cell rounding, increased annexin V staining and appearance of a sub-G1 population) [140] and transient expression of RASSF1C in 293-T cells resulted in substantial apoptosis, that was augmented by co-expression with mutant active Ras [50]. In contrast, others have failed to detect any apoptosis in 293-T cells over-expressing only wild-type RASSF1A or RASSF1C [157]. Thus, the evidence that any isoforms of RASSF1 can initiate apoptosis when over-expressed singly is conflicting. However, there are several lines of evidence to suggest that RASSF1A can participate in pro-apoptotic pathways. For example, RASSF1, its homologue NORE1 and C. elegans orthologue T24F1.3 have all been shown in a yeast two-hybrid assay to specifically bind the pro-apoptotic MST1 (the MST1 binding site is at the C-terminal end of RASSF1A) [21]. MST becomes activated by auto-phosphorylation of threonine (at position 183 of MST1 and 180 of MST2), however, this can be inhibited by co-transfection with RASSF1A or RASSF1C (or NORE1) [158]. Over-expression of mammalian MST1 or MST2 promotes apoptosis, as does over-expression of mutant active Ki-Ras. NORE1A and RASSF1A are constitutively complexed with MST1 and interference with the ability of endogenous MST1/2 to associate with these proteins inhibits Ras-induced apoptosis [158,159]. Thus RASSF1A (and NORE1) may serve as sensory modules to detect pro-apoptotic signals initiated through Ras pathways [21,158]. The RASSF1A–MST1 complex may also indirectly associate with Ras via the scaffold protein CNK1 [157]. CNK1 is a scaffold protein that in Drosophila is required for Ras to activate Raf kinase, and transfection of CNK1 into 293 cells can induce apoptosis [157]. Since CNK1 can bind RASSF1A or RASSF1C (but not NORE1) [157] and RASSF1A can interact with MST1/2 [21], it was proposed that RASSF1A may provide the mechanism linking the two proteins. Indeed, deletion of the C-terminal (RASSF1-interacting) region of MST1 prevented its interaction with CNK1 and despite the fact that both RASSF1A and RASSF1C can interact with CNK1, only RASSF1A is able to augment CNK-induced apoptosis [157].
RASSF1A may also regulate apoptosis via the death receptor signalling pathway. Activated death receptors evoke Bax conformational change, cytochrome c release, and cell death, and RASSF1A has been shown to be required for death receptor-induced Bax conformational change and apoptosis [140]. Stimulation of the death receptors with TNFα (tumour necrosis factor α) or TNFα-related apoptosis-inducing ligand (TRAIL) resulted in recruitment of RASSF1A and modulator of apoptosis-1 (MAP-1) proteins to the receptor complexes and promoted complex formation between RASSF1A and MAP-1; MAP-1 is normally inhibited by an intramolecular interaction, however, the binding of RASSF1A to MAP-1 relieved this inhibitory interaction, resulting in MAP-1 association with Bax [140]. It was subsequently shown that activated K-Ras, RASSF1A, and MAP-1 synergize to induce Bax activation and cell death, with shRNA-mediated inhibition of RASSF1A or use of a tumour-derived point mutant of RASSF1A showing impaired the ability of K-Ras to activate Bax [160].
The recent finding that RASSF1C is a binding partner of Daxx could also allow RASSF1C to play a role in regulating apoptosis. RASSF1C, constitutively anchored by Daxx in promyelocytic leukaemia-nuclear bodies, is released from the nucleus when Daxx is degraded following DNA damage, translocates to the cytoplasmic microtubules and participates in activation of stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway, which responds to a variety of stress stimuli and controls cell fates such as cell cycle entrance, apoptosis and senescence [161].
1.8.5. Controlling cell migration/adhesion
The microtubule stability induced by RASSF1A could have implications for cell adhesion and motility, especially in the light of microarray data showing that genes for cell adhesion and motility such as tropomyosin I and CDH2 were up-regulated in A549 NSCLC cells stably expressing RASSF1A [139]. Indeed, RASSF1A methylation in well-differentiated neuroendocrine tumours (including pancreatic endocrine tumours and carcinoid tumours) [98] and adenoid cystic carcinoma of the salivary gland [103] have been found to be more frequent in tumours showing metastasis. Recently, it was shown that over-expression of RASSF1A diminished the ability of A549 NSCLC cells to migrate either through a transwell filter or to close a wound [162]. In addition, A549 cells stably transfected with RASSF1A exhibited increased cell–cell adhesion, whereas Rassf1a null mouse embryonic fibroblasts and RASSF1A-depleted HeLa cells showed loss of cell–cell adhesion and an increased cell migration that could be partly PI3-K dependent and was associated with increased activation of Rac1 [162]. These findings represent a novel function for RASSF1A, which may help explain its tumour suppression ability independently of its effects on cell cycle and apoptosis.
2. RASSF2
2.1. Gene and protein structure of RASSF2
Located on human chromosome 20p13, RASSF2, originally called Rasfadin, was first identified as a novel gene close to the bovine prion gene and shows a high nucleotide (88%) and amino acid similarity (95%) with a previously described human cDNA, KIAA0168 [163]. In silico characterisation of RASSF2 reported three isoforms (RASSF2A, RASSF2B and RASSF2C), with only the RASSF2A isoform having a 5′ CpG island and predicted promoter region (the open reading frame of RASSF2A was cloned from a brain-specific cDNA library) [164]. Consistent with this, the Vega program [165] predicts 3 transcripts from the RASSF2 locus, with only the RASSF2A transcript (termed RASSF2-001) being translated (see Fig. 5). RASSF2A is a 326-amino acid protein containing an RA domain and acidic coiled-coil SARAH domain (see Fig. 2). RASSF2 lacks the cysteine-rich domain of NORE1 and RASSF1A, however, the RA domain shows 28% identity to that of RASSF1A and 31% identity to that of NORE1.
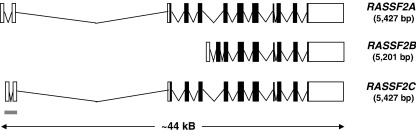
Fig. 5.
Transcripts produced from the RASSF2 locus at chromosome 20p13. Three transcripts are produced from the RASSF2 locus, namely RASSF2A (Vega: RASSF2-001, OTTHUMT00000077828; Ensembl: RASF2_HUMAN, ENST00000379400), RASSF2B (Vega: RASSF2-002, OTTHUMT00000077829), and RASSF2C (Vega: RASSF2-003, OTTHUMT00000253005; Ensembl: novel, ENST00000379376). However, the Vega program only defines RASSF2A as a coding transcript (RASSF2B and RASSF2C are defined as unclassified non-coding transcripts). UTR regions are depicted by open boxes, exons by black boxes and CpG islands by grey bars (as predicted by Ensembl). Ensembl (release 45) [20], Vega (release 24) [165], Prosite (release 20.9) [209].
2.2. Silencing of RASSF2 in cancer
Northern blot analysis revealed a single transcript (5.4 kb) present in most tissues, with the signal being most intense in the brain, peripheral blood, placenta, and lung [166]. Western blot analysis demonstrated that the RASSF2 protein was frequently down-regulated in human lung cancer cell lines [166]. RASSF2A CpG island hypermethylation corresponded with loss of RASSF2A expression in colorectal cancer (CRC) cell lines and treatment with the demethylating agent 5-aza-2-deoxycytidine reactivated RASSF2A expression [164,167]. In addition, single-strand conformation polymorphism analysis and direct sequencing of RASSF2 in 10 CRC cell lines and 140 primary CRCs found only polymorphisms, making it highly unlikely that inactivation of RASSF2 is caused by mutation [167]. RASSF2 promoter methylation was observed in 21/30 (70%) of primary CRC tumours, and this methylation was always tumour-specific (not detected in the matched patient’s DNA from normal mucosa) [164] and occurred more frequently than promoter methylation of any of the other RASSF genes [167]. Methylation of the RASSF2A promoter appears to be an early event in colorectal tumour development as RASSF2A promoter hypermethylation has been reported in a high proportion of colon adenomas, while DNA from matched normal mucosa was unmethylated (interestingly, none of the same colon adenomas demonstrated hypermethylation of the RASSF1A promoter) [164,167]. Several studies have also have reported a positive correlation of RASSF2 promoter methylation with KRAS, BRAF or PIK3CA mutations in these tumours [167–169], although some found these events to be mutually exclusive (and there was also no association between RASSF2A methylation status and RASSF1A or NORE1A methylation status in these tumours).
RASSF2A promoter methylation has also been reported in lung tumour cell lines and primary NSCLC tumours [166,170] (although no positive association with KRAS or EGFR mutations [170]) and is a frequent event in gastric cancer [171] and nasopharyngeal carcinoma, in which it positively correlates with lymph node metastasis [172]. Intriguingly, RASSF2 has been found to be up-regulated in radiation workers (analysis of lymphocytes from three radiation-workers showed RASSF2 was one of several induced genes that could be associated with cell response to ionizing radiation) [173].
2.3. Tumour suppressor activities of RASSF2
RASSF2 binds directly to K-Ras in a GTP-dependent manner via the RA domain, however, only weakly interacts with H-Ras [166]. Two-hybrid screens have found that RASSF2 also interacts/associates with NORE1, MST1 and RASSF3 [158,164]. Over-expression of RASSF2 in A549 human lung cancer cells and RKO CRC cells caused an inhibition of cell growth [166,167]. RASSF2-mediated growth inhibition in 293-T cells was dramatically enhanced by the presence of activated K-Ras, whereas H-Ras had little effect on this activity [166]. The mechanistic basis of RASSF2-mediated growth inhibition has been reported to be due to both apoptosis (as demonstrated by caspase-3 activation, FACS analysis and TUNEL assays) [166,167] and cell cycle arrest (RASSF2-expressing cells showed a ∼ 20% decrease in the G2/M phase of the cell cycle, suggesting the cells tended to arrest in the G0/G1 phase) [166], depending on the cell line used. One study found that introduction of exogenous RASSF2 induced morphologic changes in CRC cell lines (DLD-1 and RKO) associated with altered actin polymerization and suppression of RhoA, which subsequently led to apoptosis [167]. It has been proposed that inhibition of the RAS-signalling pathway sensitizes cancer cells to suspension-dependent apoptosis (anoikis) [174] and consistent with this, RASSF2-induced apoptosis appeared to be caused by loss of adhesion followed by anoikis [167].
Down-regulation of RASSF2 using siRNA has been shown to enhance the ability of K-ras to transform rat kidney cells, suggesting that tumour cells in which negative regulators of K-ras (e.g., members of the RASSF family) are silenced may have a growth advantage during transformation [167]. The idea that silencing (methylation) of RASSF2 plays a key role in KRAS-mediated transformation is supported by reports that KRAS/BRAF mutations are found more frequently in CRCs with RASSF2 methylation than in those without it [167–169].
3. RASSF3
3.1. Gene and protein structure of RASSF3
The RASSF3 gene, located at 12q14.1, is predicted to produce 3 transcripts (RASSF3A, 3B and 3C), due to alternative splicing of the exons (Fig. 6). The isoform described in the literature is that of RASSF3A, which contains five exons and encodes a 238-amino acid protein [175]. The last four exons encode an RA and SARAH domain with a 44% identity (59% homology) to the C-terminus of both RASSF1A and 1C isoforms and 46% identity to the mouse Nore1 protein (Fig. 2). The N-terminal protein sequence of RASSF3A has no similarity to RASSF1A or NORE1A but instead shares high homology with the N-terminus of RASSF1C and NORE1B. The nature of such a conserved sequence domain at the N-terminus of these proteins is unknown, but it might be related to a specific function of the shorter isoforms of this gene family. The RASSF3B and 3C isoforms are shorter than RASSF3A, and do not contain the RA or SARAH domains. The functional significance of these isoforms is unknown and they have not been described in the literature.
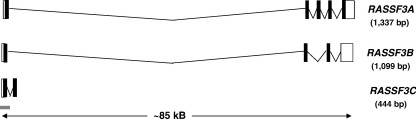
Fig. 6.
Transcripts produced from the RASSF3 locus at chromosome 12q14. Three transcripts are predicted to be produced from the RASSF3 locus. RASSF3A is a 1377-bp transcript composed of 5 exons and translating a 238-residue protein (Vega: RASSF3-001, OTTHUMT00000261784; Ensembl: RASF3_HUMAN, ENST00000336061). RASSF3B is a 1099-bp transcript which splices from exon 2 to exon 4 causing a shift in the reading frame and resulting in a protein containing neither a RA nor SARAH domain (Ensembl: Q86WH2-2, ENST00000283172). Similarly, RASSF3C contains only 2 exons and produces a transcript of 444 bp that translates a 75-residue protein containing neither a RA nor SARAH domain (Vega: RASSF2-002, OTTHUMT00000077829). UTR regions are depicted by open boxes, exons by black boxes and CpG islands by grey bars (as predicted by Ensembl). Protein domains were predicted by Prosite. Ensembl (release 45) [20], Vega (release 24) [165], Prosite (release 20.9) [209].
3.2. Silencing of RASSF3 in cancer
Northern blot analysis showed a 3.8-kb band in all normal tissues (heart, brain, placenta, lung, liver, skeletal muscle, pancreas, small intestine, colon, spleen, thymus, prostate, testis, ovary and peripheral blood leukocytes) and human cancer cell lines (including haematological, colo-rectal, lung, melanoma and cervical cell lines) examined [175]. Although CpG islands have been predicted in the RASSF3 locus (Fig. 6) RASSF3 promoter methylation has not been extensively studied, and there has been no evidence for its methylation in either gliomas (primary tumours or tumour cell lines) [97] or colorectal tumour cell lines [164]. Although a two-hybrid screening of a human lung cDNA library using an MST1 bait yielded multiple copies of NORE1, RASSF1, RASSF2 and RASSF3 (thus showing RASSF3 can interact with MST1) [158], no systematic functional characterisation of RASSF3 has been performed to-date.
4. RASSF4
4.1. Gene and protein structure of RASSF4
RASSF4, also known as AD037, was identified using a bioinformatics-based approach to detect novel RA domain-containing proteins [176]. Alternative splicing of the RASSF4 gene at chromosome 10p11.21 is predicted to result in numerous transcripts (Fig. 7 shows RASSF4A-F although additional variants have been predicted). The only variant that has been described in the literature is the RASSF4A isoform (and is just referred to as RASSF4). RASSF4A is a 1337-bp transcript that produces a protein of 321 amino acids that bears closest homology (∼ 60% identity) to RASSF2. RASSF4 lacks the cysteine-rich domain present in RASSF1A and NORE1 and the putative ATM phosphorylation site present before the RA domain in RASSF1A. However, RASSF4 does contain the RA domain and SARAH motif present in the C-terminus of RASSF1A and other family members (Fig. 2).
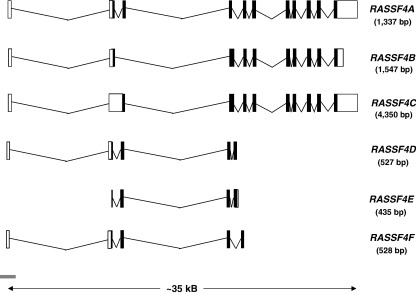
Fig. 7.
Transcripts produced from the RASSF4 locus at chromosome 10q11. Multiple transcripts are predicted from the RASSF4 locus. RASSF4A is a 2472-bp transcript that translates a 321-amino acid protein (Vega: RASSF4-001, OTTHUMT00000047745; Ensembl: RASF4_HUMAN, ENST00000374411 [note: the transcript predicted by Ensembl is only 2344 bp as it does not contain the 5′UTR]). RASSF4B and RASSF4C are very similar to RASSF4A, only differing in the N-terminal exons, with the C-terminal RA and SARAH domains of the translated transcript being identical (Ensembl: Q9H2L5-2 and Q9H2L5-3, ENST00000334940 and ENST00000374417, respectively). RASSF4D (Ensembl: Q5T737_HUMAN, ENST00000374414), RASSF4E (Vega: RASSF-006, OTTHUMT00000047750) and RASSF4F (Vega: RASSF4-008, OTTHUMT00000047752) transcripts are much shorter than the other isoforms due to premature truncation at exon 5, and as such the translated proteins contain no RA or SARAH domains. There are an additional 9 transcripts predicted by the Vega program, however they are alternatively spliced transcripts believed to contain intronic sequence relative to other coding variants or are unclassified non-coding transcripts, so for the sake of clarity these have not been shown. UTR regions are depicted by open boxes, exons by black boxes and CpG islands by grey bars (as predicted by Ensembl). Protein domains were predicted by Prosite. Ensembl (release 45) [20], Vega (release 24) [165], Prosite (release 20.9) [209].
4.2. Silencing of RASSF4 in cancer
RASSF4 has a CpG island spanning the first exon and RASSF4 expression was found to be lost in 12.5% (1/8) of nasopharyngeal carcinoma cell lines/xenografts examined, with bisulfite sequencing analysis revealing dense methylation in the promoter region, and restoration of RASSF4 mRNA observed after treatment with a demethylating agent [177]. RASSF4 is broadly expressed in human tissues (heart, brain, placenta, lung, liver, skeletal muscle and pancreas) but was found to be down-regulated in some human tumour cell lines and primary tumours, with down-regulated expression correlating with methylation of the promoter (which could be reversed upon treatment with the demethylating agent, 5-aza-2′-deoxycytidine) [176]. For example, the CpG island of RASSF4 was frequently hypermethylated in breast, lung, colorectal and kidney tumour cell lines and in primary lung and breast tumours, with no methylation detected in normal samples [176]. In contrast to the RASSF1A promoter which is methylated in 70%–80% of primary SCLCs and 30–34% of NSCLCs (suggesting that RASSF1A methylation is more important for the development of SCLC than NSCLC) [178], the RASSF4 promoter was equally methylated in NSLCs and SCLCs (∼ 21% each) [176]. RASSF4 promoter methylation was also found to only occur rarely in nasopharyngeal carcinoma (1/20 of the samples examined) [177], and in glioma cell lines but not in primary tumours [97]. The coding sequence of RASSF4 was examined for potential inactivating mutations, but none were detected [176].
4.3. Tumour suppressor activities of RASSF4
RASSF4 binds directly to activated, but not wildtype, K-Ras in a GTP-dependent manner via the RA domain [176]. Over-expression of RASSF4 induced cell death in 293-T cells, which was enhanced by the presence of activated K-Ras [176]. Examination of the mechanism by which RASSF4 promotes cell death showed that RASSF4 activated caspases, suggesting that the cell death was apoptotic (RASSF4 was also found to bind proapoptotic MST1 when the two proteins were exogenously expressed) [176]. An established method of activating Ras effectors is to add a C-terminal CAAX membrane localization motif (C, cystein; A, aliphatic amino acid; X, serine or methionine) [179], and RASSF4-mediated apoptosis (caspase activation) in MCF-7 cells was further enhanced by the addition of a Ras-CAAX motif to its C-terminus [176]. Over-expression of RASSF4 also inhibited cell growth (colony formation) in MCF-7 cells and A549 lung cancer cells (but not H1299 lung cancer cells) and the effect again enhanced by the addition of a Ras-CAAX motif to the C-terminus of RASSF4 [176].
5. NORE1
5.1. Gene and protein structure of NORE1
The closest homolog of RASSF1 is novel Ras effector 1 (NORE1), also known as RASSF5. Indeed, mouse Nore1 was actually discovered before human RASSF1; shortly after the identification of Nore1 in 1998 [15], Damman and co-workers in 2000 described a gene located in the short arm of chromosome 3 (one of the most frequently encountered cytogenetic alterations in lung cancer and several other epithelial neoplasms), whose protein was ∼ 50% identical to Nore1 in overall sequence and contained an RA domain in its C-terminal region, hence was named “Ras association domain family 1” (RASSF1) [13]. NORE1 is located on chromosome 1q32.1, and several transcripts have been predicted to be produced from this locus (see Fig. 8). NORE1Aα produces a 418-amino acid protein, NORE1A, containing an RA, SARAH and DAG-binding domain and NORE1B produces a 265-amino acid protein, NORE1B, containing the RA and SARAH domains but not the DAG-binding domain (Fig. 2) [175,180]. Similar to the RASSF1 gene, the two major transcripts, RASSF1A and RASSF1C, encode proteins that exhibit architecture homologous to the NORE1A and NORE1B isoforms, in that they share common RA and SARAH domains in the C-terminus, but have distinct N-termini. Like RASSF1A, NORE1A has a central DAG binding domain N-terminal to the RA domain, whereas NORE1B, like RASSF1C, has a short N-terminal segment containing no identifiable motifs [175]. An additional two transcripts are produced from this locus, namely NORE1Aβ and NORE1Aγ, however, the proteins produced from these transcripts do not encode the SARAH domain and there are no reports of their functional characterisation in the literature.
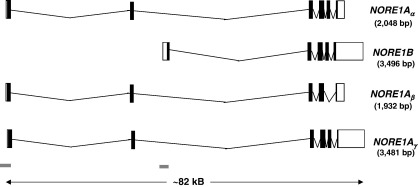
Fig. 8.
Transcripts produced from the RASSF5 locus at chromosome 1q32. Several transcripts are produced from the RASSF5 locus. NORE1Aα is a 2048-bp transcript that translates a 418-amino acid protein (Vega: RASSF5-001, OTTHUMT00000088469; Ensembl: RASF5_HUMAN, ENST00000367118) and NORE1B is a 3496-bp transcript that translates a 265-amino acid protein (Vega: RASSF5-003, OTTHUMT00000088471; Ensembl: Q8WWW0-2, ENST00000304534). additional 2 transcripts are predicted, namely NORE1Aβ (Vega: RASSF5-002, OTTHUMT00000088470; Ensembl: Q8WWW0-3, ENST00000355294), which splices from exon 4 to exon 6 causing a shift in the reading frame that results in premature termination and NORE1Aγ (Vega: RASSF5-004, OTTHUMT00000088472), which has a C-terminal truncation of exon 6, and; translation of these transcripts produces proteins lacking the C-terminal SARAH domain. Additional transcripts have been predicted by the Vega program but as they are non-coding they have not been shown (Vega: RASSF5-005 and -006, OTTHUMT00000088473 and OTTHUMT00000088474, respectively). UTR regions are depicted by open boxes, exons by black boxes and CpG islands by grey bars (as predicted by Ensembl). Protein domains were predicted by Prosite. Ensembl (release 45) [20], Vega (release 24) [165], Prosite (release 20.9) [209].
NORE1 is the human homologue of the mouse Ras effector, Nore1 [15]. Nore1 was discovered in a two-hybrid screen using H-RasG12V as bait; the initial isolate, obtained from a murine T-cell cDNA library, was a partial cDNA encoding the Nore1B isoform, however, the isoform first characterized was a full-length murine Nore1A isolated from brain cDNA [15]. In addition to binding activated Ras, NORE1B (identified as RAPL) was independently isolated by virtue of its ability to bind activated Rap1 in stimulated T cells and to regulate lymphocyte adhesion by associating with the integrin LFA-1 and relocating at the immunological synapse (see Section 5.4). The RA domain of Nore1 binds to Ras-GTP (active Ras) with strong preference over Ras-GDP (inactive Ras) in vitro and endogenous Nore1 binds endogenous Ras in vivo in response to EGF or serum-stimulation [15]. Subsequent yeast two-hybrid studies showed that Nore1 associates with other Ras-like GTPases (including Rap1, Rap2, RRas, RRas2/TC21, and RRas3/MRas) with an affinity comparable to that seen for H- and K-Ras [156]. In addition, it was shown that the hetero-dimerisation of NORE1 with RASSF1A was crucial for the ability of RASSF1A to associate with Ras-like GTPases [156].
5.2. Silencing of NORE1 in cancer
Both NORE1A and NORE1B have separate CpG islands spanning their first exons (see Fig. 8). NORE1A is widely expressed in normal tissues, however, several cancer cell lines (promyelocytic leukaemia HL-60, lymphoblastic leukemia MOLT-4, Burkitt’s lymphoma Raji, lung carcinoma A549 and melanoma G361 cells) expressed very low levels of NORE1A transcript (colorectal adenocarcinoma SW80, myelogenous leukemia K562 and HeLa cells showed transcript levels comparable to those in normal tissues) [175,181]. Similarly, NORE1B also has a wide tissue distribution with absent or down-regulated expression in some cancer cell lines (HeLa, A549, and G361 cells) [175,181]. Aoyama and co-workers surveyed a variety of human tumour cell lines by PCR and also observed that a majority of those examined exhibited very low levels of NORE1A mRNA, with NORE1B expression being more variable [182]. Western blot using an antibody that detected both isoforms of NORE1 found the majority of human lung tumour cell lines examined (11/14) had lost NORE1 expression and expression was also severely reduced or completely absent in the majority of the epithelial-derived adenocarcinomas and SCLCs examined (4/5 of each subtype) [181]. Significantly suppressed NORE1A and RASSF1A mRNA levels have been detected in pheochromocytoma primary tumours compared with normal adrenal medulla, however, methylation of the NORE1A promoter was not observed [99]. Other studies have also found no evidence of methylation of either NORE1A or NORE1B CpG islands in the various tumours examined, including melanomas, nasopharyngeal carcinomas, cervical carcinomas, lung tumours and thyroid tumours and hepatitic livers (those showing chronic hepatitic/cirrhosis, hepatocellular nodules and HCC) [102,175,177,183,184] and deletions or mutations of chromosome 1q32.1–2 in the vicinity of the NORE1 gene is not common in human tumours, except for in renal collecting duct carcinoma (a rare malignant neoplasm of distal nephron origin) [185]. In addition, a family with clear cell renal cell carcinomas (RCC) was found to harbour a translocation between the NORE1 gene on chromosome 1q32.1 and the LSAMP gene on chromosome 3q13.3 that results in haploinactivation of the NORE1 gene [186].
However, there have been reports of evidence of epigenetic inactivation of NORE1 by promoter methylation in tumours. For example, Vos and co-workers found methylation of the NORE1 promoter in some lung cancer cell lines (A549 and H345 cells) and treatment with the demethylating agent, 5-aza-2′-deoxycytidine (but not the deacetylating agent, trichostatin A), caused an increase in NORE1 expression, confirming that promoter silencing by methylation rather than acetylation or gene deletion is the likely mechanism behind the down-regulation of NORE1 expression in lung tumour cells [181]. Methylation of the NORE1A promoter has been reported in various tumour types, including lung, breast, and kidney tumour cell lines and primary tumours (but not in normal tissues) [180,181,186,187], and NORE1A expression in the tumour cell lines was reactivated after treatment with a demethylating agent [180]. Interestingly, in lung cancer this hypermethylation appeared to be histological type specific as 24% (6/25) of primary NSCLC underwent NORE1A methylation, but methylation in SCLC was a rare event (0/22) [180] and epigenetic alteration of NORE1A was confined to NSCLC tumours with a wild-type K-ras (15/17 of the tumours with NORE1 hypermethylation did not harbour a K-ras mutation) [112]. Furthermore, aberrant methylation of NORE1A and SOCS3 promoters has been found to be observed only in a subclass of HCC with poor survival, suggesting that inactivation of these 2 genes might be involved in HCC progression [103]. In contrast, NORE1B promoter was found to be unmethylated in all lung and breast cell lines and primary tumours examined [180] and gliomas (primary tumours or tumour cell lines) [97]. However, methylation of the NORE1B promoter has recently reported in 62% of hepatocellular carcinomas and hepatocarcinoma cell lines examined (compared to only 4% showing NORE1A promoter methylation) [188].
Ras mutations and NORE1A down-regulation have been reported to be mutually exclusive events in follicular thyroid tumours [184], where as others have found no correlation between NORE1 expression and the presence or absence of a Ras mutation (in melanomas, cervical carcinomas, and lung tumours) [181]. Interestingly, one study found although the NORE1A mRNA levels of the majority of the follicular thyroid tumours examined were similar to those in the normal controls, the cases harbouring a PAX8-PPAR11 translocation (n = 6) exhibited dramatically reduced NORE1A expression [184]. Another study found NORE1B and RASSF1A to be epigenetically down-regulated alone in at least 62%, or in combination in 97% of the HCCs studied, in contrast to every third fibrotic or cirrhotic liver only exhibiting silencing of one or both genes, suggesting it may be a critical event allowing HCCs to subverse growth control in the presence of an unaltered Ras (which is rarely mutated in HCCs) [188]. In contrast, no correlation between NORE1A and RASSF1A methylation status was found in NSCLC [180] or renal cell carcinoma and Wilms’ tumour [187].
5.3. Tumour suppressor activities of NORE1
5.3.1. Growth suppression
Re-introduction of NORE1A or NORE1B suppressed the growth of the NORE1-deficient A549 lung cancer cell line and the G361 melanoma cell line (which have disrupted Ras signaling due to a Ras activating mutation or constitutively active B-Raf kinase, respectively) [181,182]. However, when transfected into other NORE1-deficient cell lines with similar disruptions to Ras signalling (the NCI-H460 lung cancer and M14 melanoma cell line), there was no effect on colony formation [182]. Stable expression of NORE1A also impairs the growth of A549 human lung cells and PC12 rat pheochromocytoma cells in soft agar (inhibition of anchorage-independent growth) [99,182]. However, the mechanism behind NORE1-induced growth suppression is not clear. Vos and co-workers found that NORE1-mediated growth suppression occurred in a Ras-dependent manner (NORE1-mediated growth inhibition in 293T cells was increased in the presence of activated H-Ras but decreased in the presence of the H-Ras dominant negative mutant Q61L/C186S), primarily by the induction of apoptosis (the growth suppression was blocked by co-transfection with the anti-apoptotic oncogene, Bcl-2, and caspase activity was detected) [181]. However, another study found that NORE1A-induced growth suppression occurred through a mechanism independent of its ability to bind to activated Ras-like GTPases and the MST1/2 kinases (as determined by transient transfection of a series of deletion mutants of NORE1 into A549 cells), primarily by inducing cell cycle delay rather than apoptosis (expression of NORE1A causes a significant decrease in the number of cells in the S phase and a reciprocal increase of the fraction in the G1 phase, similar to the inhibition of cell cycle progression through G1 reported for RASSF1A [43]) [182]. It was recently proposed that cytoskeletal localization is required for the growth-suppressive effects of NORE1A and that this occurs through the ERK signalling pathway [189].
5.3.2. Apoptosis
The ability of NORE1 on its own to induce apoptosis remains unclear. Studies have found that transient expression of NORE1 induced apoptosis in 293T cells [181] and PC12 rat pheochromocytoma cells [99], whilst others found that NORE over-expression does not promote apoptosis in mammalian cells [21,182]. NORE1 has been shown to bind the pro-apoptotic protein kinase MST1 (via the SARAH domain) [21,190]. Whereas over-expression of MST1 induced apoptosis in NIH3T3 cells, and co-expression with NORE1 had small effect on increasing apoptosis, the addition of a CAAX motif to NORE1 to induce membrane targeting enabled NORE1 alone to induce apoptosis, and NORE1 together with MST1 had a greater pro-apoptotic effect than either protein alone (and this cell death was inhibited by the caspase inhibitor, z-VAD-fmk) [21]. Endogenous NORE1 and MST1 occur in a constitutive complex in vivo that associates with endogenous Ras after serum stimulation [21]. Over-expression of constitutively active Ki-RasG12V promotes apoptosis in a variety of cell lines, however, this is suppressed by over-expression of the C-terminal non-catalytic portion of MST1 (containing auto-inhibitory, dimerisation, and NORE1 binding domains) or by the portion of NORE1 that binds MST1 (H-RasGV12, E37G-mediated apoptosis was also suppressed) [21]. Thus the NORE1–MST1 complex is a novel Ras effector unit that mediates the apoptotic effect of Ras. It was subsequently proposed that NORE1/RASSF1 proteins, in addition to their role in maintaining the low activity of MST1 in vivo, direct MST1 to sites of activation and perhaps co-localization with endogenous substrates [158].
Thus, the weight of evidence indicates that the NORE1 proteins, like the RASSF1 proteins, are inhibitory to cell growth, and the A isoforms of both RASSF1 and NORE1 are likely to function as tumour suppressors that undergo inactivation primarily by epigenetic mechanisms.
5.4. NORE1B in the immune system
In addition to its role as a tumour suppressor gene, NORE1B has also been shown to play an important role in the immune system [reviewed in 191]. NORE1B was first characterised in its ability to mediate the Rap1-induced polarized accumulation and increased affinity of LFA-1 in response to T-cell receptor (TCR) activation, and as a result was named RAPL (‘regulator of adhesion and polarization enriched in lymphocytes’) [192]. Rapl knockout mice were found to be notably impaired in various aspects of their immune response (such as defective adhesion and migration of lymphocytes and dendritic cells) [193]. RAPL has been shown to localise to the microtubules (and activated Rap1 can induce their dissociation from microtubules) [194]. Recently, a yeast two-hybrid screen using an activated RAPL mutant protein as bait identified the proapoptotic kinase MST1 as a binding partner, demonstrating a previously unknown function for MST1 of relaying the Rap1-RAPL signal to induce cell polarity and adhesion of lymphocytes [195]. Endogenous RAPL has also been suggested to modulates Ras signalling outputs triggered by TCR stimulation via recruiting active Ras to the plasma membrane and by contributing to the localization of Carma1 (an essential lipid raft-associated regulator of TCR-induced NF-κB activation) [196].
6. RASSF6
6.1. Gene and protein structure of RASSF6
The RASSF6 gene comprises of 13 exons and is localized at chromosome 4q13.3. As shown in Fig. 9, three transcripts are predicted to be produced from the RASSF6 locus, namely RASSF6A, RASSF6B, and RASSF6C (although the latter isoform has not been described in the literature). RASSF6B has an additional 32 amino acids in the N-terminal region compared to RASSF6A, and RASSF6C has a different N-terminus from both RASSF6A and 6B. However, all isoforms possess the conserved RA and SARAH domains that are characteristic of the RASSF family (Fig. 2). The RA domain of RASSF6 is more similar to those of RASSF2 and RASSF4 (∼ 50% identity) than RASSF1A (∼ 30% identity). The homology of RASSF6 with other members is lower in the SARAH domain (which is ∼ 40% identical to those of RASSF2 and RASSF4, and ∼ 20% identical to those of RASSF1A and NORE1) [197]. Although it possesses an RA domain, similar to the RASSF1 situation [50,156] it is a somewhat controversial issue as to whether RASSF6 directly or indirectly binds Ras proteins; one study reported that RASSF6 interacts directly with K-Ras in a GTP-dependent manner with an affinity comparable to that of other known Ras effectors [198] yet another study reported it does not bind K-Ras, H-Ras, N-Ras, M-Ras, or TC21 under the conditions that NORE1 binds these Ras proteins [197].
Fig. 9.
Transcripts produced from the RASSF6 locus at chromosome 4q13. Three transcripts are produced from the RASSF6 locus. RASSF6A is a 5872-bp transcript that translates a 337-amino acid protein (Vega: RASSF6-001, OTTHUMT00000252278; Ensembl: Q6ZTQ3-2, ENST00000307439; the Ensembl transcript is actually predicted to be slightly smaller at 5123 bp due to a shorter 3′UTR sequence). RASSF6B is a 4331-bp transcript that translates a 369-amino acid protein (Vega: RASSF6-002, OTTHUMT00000252279; Ensembl: RASF6_HUMAN, ENST00000342081; the Ensembl transcript is actually predicted to be slightly larger at 4902 bp due to a longer 3′UTR sequence). RASSF6C is 1360 bp and translates a 325-amino acid protein (Vega: RASSF6-003, OTTHUMT00000252280; Ensembl: Q6ZTQ3-3, ENST00000335049) that differs from RASSF6A and B only at the N-terminus due to use of an alternative exon 2 and splicing around exon 3. UTR regions are depicted by open boxes, and exons by black boxes (no CpG islands were identified; as predicted by Ensembl). Ensembl (release 45) [20], Vega (release 24) [165].
6.2. Silencing of RASSF6 in cancer
RASSF6 transcript was detected in several cancer cell lines including HeLa, MCF-7, U373, A549, and HepG2 cells [197] but reduced levels were found in 30–60% of primary tumour tissues of the breast, colon, kidney, liver, pancreas, stomach and thyroid gland [198]. Interestingly, only 1/7 of the tumour cell lines examined demonstrated partial promoter methylation, and given that the 4q21.21 region has been reported to suffer deletions during tumour development [199], it has been proposed that the loss of RASSF6 expression in primary tumours may involve gene deletions as well as epigenetic mechanisms of silencing [198]. Indeed, whilst some bioinformatics programs predict the positioning of CpG islands within the RASSF6 promoter (CpG Island Searcher [200]), others do not (Ensembl [20]), so further studies are needed to determine whether methylation of the RASSF6 promoter does occur. One study also found that some tumour samples appeared to exhibit elevated levels of RASSF6 compared to the normal tissue, although the reason for this remains as yet unknown [198].
6.3. Tumour suppressor activities of RASSF6
Consistent with a role as a tumour suppressor, over-expression of RASSF6 inhibited the survival of specific tumour cell lines; both RASSF1A and RASSF6 inhibited the growth of MCF-7 human breast tumour cells, RASSF6 was significantly less effective than RASSF1A at inhibiting the growth and survival of A549 human lung tumour cells, and neither RASSF1A nor RASSF6 inhibited the growth of the H1299 human lung tumour cell line [198]. siRNA knockdown of RASSF6 in the H1792 NSCLC cell line enhanced their ability to grow in soft agar, relative to control cells [198]. Similar to RASSF1A, over-expression of RASSF6 induced apoptosis in both the MCF-7 cell line [198] and the HeLa cervical cancer cell line [197]. RASSF6 was found to activate Bax, induce cytochrome c release and trigger both caspase-dependent and caspase-independent pathways of apoptosis [197]. Similar to RASSF1A, RASSF6 could co-immunoprecipitate with MAP-1 and this binding was enhanced by the presence of activated K-Ras (suggesting a potential mechanism by which Ras may activate the pro-apoptotic effects of RASSF6) [198]. RASSF6 knockdown in HeLa cells also partially blocked TNFα-induced cell death (similar to RASSF1A, for which a role in death receptor-mediated apoptosis has been implicated) [197].
6.4. RASSF6 in the immune system
A biological property of RASSF6 that is unique from other members of the RASSF family is its potential to play a role in dictating the degree of inflammatory response to the respiratory syncytial virus (RSV). RASSF6 was discovered within a 250-kb region on chromosome 4q21.21 associated with RSV-induced acute bronchiolitis [201]. One of the major effects of RSV infection is activation of the eukaryotic nuclear factor kb (NF- κB) pathway and this plays a critical role in both promoting inflammation and supporting viral replication by suppressing apoptosis [202]. Recently, RASSF6 expression has been shown to inhibit the basal levels of NF-κB activity in a lung epithelial cell line and this appears to be a specific effect as RASSF2 (the closest family member) exhibited only a modest effect on NF-κB activation [198]. Thus it is possible that defects in RASSF6 may facilitate viral NFkB activation by RSV, and enhance the infection. It is tempting to speculate a potential role of RASSF6 in inflammation in cancer.
7. RASSF7
7.1. Gene and protein structure of RASSF7
First identified in a cluster of three genes within 32 kb upstream of H-RAS1 on chromosome 11p15, RASSF7 was originally termed HRAS1 cluster 1 (HRC1) [203] (and is also known as C11ORF13). Due to alternative splicing, 3 transcripts have been predicted to be produced from this locus, RASSF7A, RASSF7B and RASSF7C (see Fig. 10). These isoforms differ primarily in the C-terminus (although RASSF7A has a truncated N-terminus in comparison to the other isoforms), and each possess an N-terminal RA domain (see Fig. 2) although it is not known whether they directly bind Ras, as the presence of the RA domain does not necessarily signify RAS binding affinity of a protein [204,205]. They also do not possess a SARAH domain, in contrast to RASSF1-6 family members. Thus not surprisingly, RASSF7 shows greatest homology to RASSF8 (e.g. RASSF7A shows 42% similarity to RASSF8A yet only 23% to RASSF1A), which also possesses an N-terminal RA domain and no SARAH domain (see Section 8).
Fig. 10.
Transcripts produced from the RASSF7 locus at chromosome 11p15. Three transcripts are produced from the RASSF7 locus due to use of different N-terminal and C-terminal exons. RASSF7A (Vega: RASSF7-003, OTTHUMT00000254972; Ensembl: RASF7_HUMAN, ENST00000344375) is a 1902-bp transcript that initiates transcription from the ATG present in exon 2, utilises different C-terminal exons from the other RASSF7 transcripts and corresponds to the HRC1 type I transcript identified by Weitzel and colleagues [203]. RASSF7B (Vega: RASSF7-002, OTTHUMT00000254971) uses an alternative first exon to RASSF7A to generate a 1745-bp transcript that translates a 377-amino acid protein. RASSF7C (Vega: RASSF7-001, OTTHUMT00000254970) differs from RASSF7B only in the N-terminal exon and is a 1731-bp transcript that translates a 366-amino acid protein. UTR regions are depicted by open boxes, exons by black boxes and CpG islands by grey bars (as predicted by Ensembl). Each of these proteins contain an N-terminal RA domain (as predicted by Prosite). Ensembl (release 45) [20], Vega (release 24) [165], Prosite (release 20.9) [209].
7.2. The role of RASSF7 in tumourigenesis
Although CpG islands at the RASSF7 locus have been both predicted (see Fig. 10) and experimentally observed [203], it has not yet been reported whether hypermethylation of the RASSF7 promoter occurs in tumours, and indeed whether any of the RASSF7 isoforms play a role in tumourigenesis.
8. RASSF8
8.1. Gene and protein structure of RASSF8
Similar to RASSF7, which is located close to the HRAS1 gene, RASSF8 is located close to the KRAS2 gene (70.8 kb from KRAS2 on chromosome 12p11). RASSF8 is also known as HoJ-1 and C12ORF2. Although Favella and colleagues identified several 5′ UTR variants originating from alternative splicing of exons 1–3 [206], Ensembl [20] and Vega [165] bioinformatics programs primarily predict transcripts which differ due to premature truncation at the C-terminus (exons 4–6). As shown in Fig. 11, 7 transcripts have been predicted from this locus (though most remain to be experimentally verified). RASSF8A and RASSF8B encode 419 and 392 amino acid proteins, respectively which differ only in their C-terminus (due to the use of a different C-terminal exon). Similar to RASSF7A–C, these proteins contain an N-terminal RA domain (see Fig. 2), although it is not known whether they directly bind Ras. RASSF8C–E isoforms are truncated forms of RASSF8A and 8B (due to premature truncation of the transcript in exon 4) and consist primarily of just the RA domain. The RASSF8F and RASSF8G isoforms utilise different exons from RASSF8A and 8B and produce very short proteins of only 24 and 40 amino acids, respectively, which contain no identifiable functional domains, and thus are of unknown significance. Similar to RASSF7A–C, none of the RASSF8 isoforms possess a SARAH domain.
Fig. 11.
Transcripts produced from the RASSF8 locus at chromosome 12p12. Multiple transcripts are predicted to be produced from the RASSF8 locus. RASSF8A (Ensembl: RASF8_HUMAN, ENST00000381352) and RASSF8B (Vega: RASSF8-001, OTTHUMT00000260394; Ensembl: Q8NHQ8-2, ENST00000282884) are very similar, differing only in their C-terminal exon, and both encode proteins containing an N-terminal RA domain. RASSF8C-E transcripts (Vega: RASSF8-003, -004 and -005, OTTHUMT00000260396, OTTHUMT00000260397, and OTTHUMT00000260398, respectively) prematurely terminate at the 5′ end of exon 4 and translate a 91-, 107- and 134-amino acid protein, respectively, that almost consists entirely of the RA domain. RASSF8F (Vega: RASSF8-002, OTTHUMT00000260395) uses an alternative third exon however, as most of this is part of the 5′UTR, the transcript generates a 24-amino acid protein with no recognisable domains. Similarly, RASSF8G (Vega: RASSF8-006, OTTHUMT00000260399) only translates a 40-amino acid protein containing no recognisable domains. UTR regions are depicted by open boxes, exons by black boxes and CpG islands by grey bars (as predicted by Ensembl). Protein domains were predicted by Prosite. Ensembl (release 45) [20], Vega (Release 24) [165], Prosite (Release 20.9) [209].
8.2. The role of RASSF8 in tumourigenesis
Although CpG islands have been predicted for the RASSF8 locus (see Fig. 11), it is not known whether selective methylation of the RASSF8 promoter is found in tumours. However, it was recently reported that common polymorphisms in RASSF8 are not associated with lung adenocarcinoma risk [207]. Favella and colleagues found a decrease in RASSF8A transcript abundance in lung adenocarcinoma as compared to normal lung tissue, raising the possibility that this gene plays a role in the negative control of tumour development [206]. In agreement with this, anchorage-independent growth in soft agar was significantly reduced in RASSF8A-transfected A549 lung cancer cells compared to cells transfected with empty vector [206]. RASSF8 has also been implicated in a complex type of synpolydactyly by the reciprocal chromosomal translocation t(12;22)(p11.2;q13.3), which involves genes RASSF8 and FBLN1 [208].
9. Concluding remarks
As described in this review, the RASSF genes have been shown to have a diverse range of functions including cell cycle regulation and the regulation of microtubule stability in addition to roles in the control of apoptosis and proliferation. This review describes how silencing of RASSF genes, and deregulation of their diverse functions, are likely to be of critical importance in mediating tumour suppression. The exact contribution of each family member to tumour suppression, in each cell type and tissue, is yet to be fully elucidated and the prognostic and diagnostic utility of these genes, compared to other available biomarkers, will require further large systematic clinical studies prior to clinical laboratory tests for these genes becoming commonplace. However, should the newly developed chemotherapeutic methylation inhibitors, such as zebularine and MG98, prove their efficacy in the clinic then it is likely that tests for inactivation of RASSF genes, particularly RASSF1A, will gain importance as a means for assessing therapeutic response.
The majority of what we know about RASSF family members has been derived from detailed in vitro analyses. In the near future the generation of knockout models of all RASSF genes is likely to contribute greatly to our knowledge and understanding of how these genes participate in tumour suppression and will also help us understand the complex interplay between mutations in RASSF genes and other genes in the genome of cancer cells during the genesis of a tumour. In conclusion we still have much to learn about the function of the RASSF gene family members but hopefully we will be able to exploit their secrets as a means for better treating and diagnosing patients in the cancer clinic.
Note added in proof
RASSF1A has been reported to control mitotic progressing by binding and inhibiting Cdc20 [133] (see section 1.8.2), however, a recent study could find no such interaction between RASSF1A and Cdc20 [210].
Acknowledgments
Research in D.J. Adams laboratory funded by Cancer Research UK and The Wellcome Trust.
References
- 1.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 2000;351:289–305. [PMC free article] [PubMed] [Google Scholar]
- 2.Downward J. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 1998;8:49–54. doi: 10.1016/s0959-437x(98)80061-0. [DOI] [PubMed] [Google Scholar]
- 3.Ponting C.P., Benjamin D.R. A novel family of Ras-binding domains. Trends Biochem. Sci. 1996;21:422–425. doi: 10.1016/s0968-0004(96)30038-8. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T., Taya S., Kaibuchi K. Ras-induced transformation and signaling pathway. J. Biochem. (Tokyo) 1999;126:799–803. doi: 10.1093/oxfordjournals.jbchem.a022519. [DOI] [PubMed] [Google Scholar]
- 5.Sundaresan V., Ganly P., Hasleton P., Rudd R., Sinha G., Bleehen N.M., Rabbitts P. p53 and chromosome 3 abnormalities, characteristic of malignant lung tumours, are detectable in preinvasive lesions of the bronchus. Oncogene. 1992;7:1989–1997. [PubMed] [Google Scholar]
- 6.Hung J., Kishimoto Y., Sugio K., Virmani A., McIntire D.D., Minna J.D., Gazdar A.F. Allele-specific chromosome 3p deletions occur at an early stage in the pathogenesis of lung carcinoma. J. Am. Med. Assoc. 1995;273:558–563. [PubMed] [Google Scholar]
- 7.Kok K., Naylor S.L., Buys C.H. Deletions of the short arm of chromosome 3 in solid tumors and the search for suppressor genes. Adv. Cancer Res. 1997;71:27–92. doi: 10.1016/s0065-230x(08)60096-2. [DOI] [PubMed] [Google Scholar]
- 8.Sekido Y., Ahmadian M., Wistuba I., Latif F., Bader S., Wei M.-H., Duh F.-M., Gazdar A., Lerman M., Minna J. Cloning of a breast cancer homozygous deletion junction narrows the region of search for a 3p21.3 tumor suppressor gene. Oncogene. 1998;16:3151–3157. doi: 10.1038/sj.onc.1201858. [DOI] [PubMed] [Google Scholar]
- 9.Sekido Y., Fong K., Minna J. Progress in understanding the molecular pathogenesis of human lung cancer. Biochim. Biophys. Acta. 1998;1378:21–59. doi: 10.1016/s0304-419x(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 10.Wistuba I., Behrens C., Milchgrub S., Bryant D., Hung J., Minna J.D., Gazdar A.F. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene. 1999;18:643–650. doi: 10.1038/sj.onc.1202349. [DOI] [PubMed] [Google Scholar]
- 11.Wistuba I.I., Behrens C., Virmani A.K., Mele G., Milchgrub S., Girard L., Fondon J.W., III, Garner H.R., McKay B., Latif F., Lerman M.I., Lam S., Gazdar A.F., Minna J.D. High resolution chromosome 3p allelotyping of human lung cancer and preneoplastic/preinvasive bronchial epithelium reveals multiple, discontinuous sites of 3p allele loss and three regions of frequent breakpoints. Cancer Res. 2000;60:1949–1960. [PubMed] [Google Scholar]
- 12.Lerman M.I., Minna J.D. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. Cancer Res. 2000;60:6116–6133. [PubMed] [Google Scholar]
- 13.Dammann R., Li C., Yoon J.H., Chin P.L., Bates S., Pfeifer G.P. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat. Genet. 2000;25:315–319. doi: 10.1038/77083. [DOI] [PubMed] [Google Scholar]
- 14.Wei M.-H., Latif F., Bader S., Kashuba V., Chen J.Y., Duh F.M., Sekido S., Lee C.C., Geil L., Kuzmin I., Zabarovsky E., Klein G., Zbar B., Minna J.D., Lerman M.I. Construction of a 600-kilobase cosmid clone contig and generation of a transcriptional map surrounding the lung cancer tumor suppressor gene (TSG) locus on human chromosome 3p21.3: progress toward the isolation of a lung cancer TSG. Cancer Res. 1996;56:1487–1492. [PubMed] [Google Scholar]
- 15.Vavvas D., Li X., Avruch J., Zhang X.F. Identification of Nore1 as a potential Ras effector. J. Biol. Chem. 1998;273:5439–5442. doi: 10.1074/jbc.273.10.5439. [DOI] [PubMed] [Google Scholar]
- 16.Burbee D.G., Forgacs E., Zochbauer-Muller S., Shivakumar L., Fong K., Gao B., Randle D., Kondo M., Virmani A., Bader S., Sekido Y., Latif F., Milchgrub S., Toyooka S., Gazdar A.F., Lerman M.I., Zabarovsky E., White M., Minna J.D. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J. Natl. Cancer Inst. 2001;93:691–699. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheel H., Hofmann K. A novel interaction motif, SARAH, connects three classes of tumor suppressor. Curr. Biol. 2003;13:R899–R900. doi: 10.1016/j.cub.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Kim S.T., Lim D.S., Canman C.E., Kastan M.B. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 1999;274:37538–37543. doi: 10.1074/jbc.274.53.37538. [DOI] [PubMed] [Google Scholar]
- 19.Newton A.C. Protein kinase C. Seeing two domains. Curr. Biol. 1995;5:973–976. doi: 10.1016/s0960-9822(95)00191-6. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard T.J., Aken B.L., Beal K., Ballester B., Caccamo M., Chen Y., Clarke L., Coates G., Cunningham F., Cutts T., Down T., Dyer S.C., Fitzgerald S., Fernandez-Banet J., Graf S., Haider S., Hammond M., Herrero J., Holland R., Howe K., Howe K., Johnson N., Kahari A., Keefe D., Kokocinski F., Kulesha E., Lawson D., Longden I., Melsopp C., Megy K., Meidl P., Ouverdin B., Parker A., Prlic A., Rice S., Rios D., Schuster M., Sealy I., Severin J., Slater G., Smedley D., Spudich G., Trevanion S., Vilella A., Vogel J., White S., Wood M., Cox T., Curwen V., Durbin R., Fernandez-Suarez X.M., Flicek P., Kasprzyk A., Proctor G., Searle S., Smith J., Ureta-Vidal A., Birney E. Ensembl 2007. Nucleic Acids Res. 2007;35:D610–D617. doi: 10.1093/nar/gkl996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khokhlatchev A., Rabizadeh S., Xavier R., Nedwidek M., Chen T., Zhang X.F., Seed B., Avruch J. Identification of a novel Ras-regulated proapoptotic pathway. Curr. Biol. 2002;12:253–265. doi: 10.1016/s0960-9822(02)00683-8. [DOI] [PubMed] [Google Scholar]
- 22.Kamath R.S., Fraser A.G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D.P., Zipperlen P., Ahringer J. Systemic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 23.Sönnichsen B., Koski L.B., Walsh A., Marschall P., Neumann B., Brehm M., Alleaume A.-M., Artelt J., Bettencourt P., Cassin E., Hewitson M., Holz C., Khan M., Lazik S., Martin C., Nitzsche B., Ruer M., Stamford J., Winzi M., Heinkel R., Röder M., Finell J., Häntsch H., Jones S.J.M., Jones M., Piano F., Gunsalus K.C., Oegema K., Gönczy P., Coulson A., Hyman A.A., Echeverri C.J. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 24.van der Weyden L., Tachibana K.K., Gonzalez M.A., Adams D.J., Ng B.L., Petty R., Venkitaraman A.R., Arends M.J., Bradley A. The RASSF1A isoform of RASSF1 promotes microtubule stability and suppresses tumorigenesis. Mol. Cell. Biol. 2005;25:8356–8367. doi: 10.1128/MCB.25.18.8356-8367.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tommasi S., Dammann R., Zhang Z., Wang Y., Liu L., Tsark W.M., Wilczynski S.P., Li J., You M., Pfeifer G.P. Tumor susceptibility of Rassf1a knockout mice. Cancer Res. 2005;65:92–98. [PubMed] [Google Scholar]
- 26.Polesello C., Huelsmann S., Brown N.H., Tapon N. The Drosophila RASSF homolog antagonizes the hippo pathway. Curr. Biol. 2006;16:2459–2465. doi: 10.1016/j.cub.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones P.A., Baylin S.B. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herman J.B., Baylin S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 29.Dammann R., Schagdarsurengin U., Seidel C., Strunnikova M., Rastetter M., Baier K., Pfeifer G.P. The tumor suppressor RASSF1A in human carcinogenesis: an update. Histol. Histopathol. 2005;20:645–663. doi: 10.14670/HH-20.645. [DOI] [PubMed] [Google Scholar]
- 30.Hesson L.B., Cooper W.N., Latif F. The role of RASSF1A methylation in cancer. Dis. Markers. 2007;23:73–87. doi: 10.1155/2007/291538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990;62:503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- 32.Jones P.A., Wolkowicz M.J., Rideout W.M., III, Gonzales F.A., Marziasz C.M., Coetzee G.A., Tapscott S.J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu R.W., Chim S.S., Wong I.H., Wong C.S., Lee W.S., To K.F., Tong J.H., Yuen R.K., Shum A.S., Chan J.K., Chan L.Y., Yuen J.W., Tong Y.K., Weier J.F., Ferlatte C., Leung T.N., Lau T.K., Lo K.W., Lo Y.M. Hypermethylation of RASSF1A in human and rhesus placentas. Am. J. Pathol. 2007;170:941–950. doi: 10.2353/ajpath.2007.060641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann U., Langer F., Feist H., Glockner S., Hasemeier B., Kreipe H. Quantitative assessment of promoter hypermethylation during breast cancer development. Am. J. Pathol. 2002;160:605–612. doi: 10.1016/S0002-9440(10)64880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing M., Cohen Y., Mambo E., Tallini G., Udelsman R., Ladenson P.W., Sidransky D. Early occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesis. Cancer Res. 2004;64:1664–1668. doi: 10.1158/0008-5472.can-03-3242. [DOI] [PubMed] [Google Scholar]
- 36.Yegnasubramanian S., Kowalski J., Gonzalgo M.L., Zahurak M., Piantadosi S., Walsh P.C., Bova G.S., De Marzo A.M., Isaacs W.B., Nelson W.G. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004;64:1975–1986. doi: 10.1158/0008-5472.can-03-3972. [DOI] [PubMed] [Google Scholar]
- 37.Wong I.H., Chan J., Wong J., Tam P.K. Ubiquitous aberrant RASSF1A promoter methylation in childhood neoplasia. Clin. Cancer Res. 2004;10:994–1002. doi: 10.1158/1078-0432.ccr-0378-3. [DOI] [PubMed] [Google Scholar]
- 38.Pijnenborg J., Dam-de Veen G., Kisters N., Delvoux B., van Engeland M., Herman J., Groothuis P. RASSF1A methylation and K-ras and B-raf mutations and recurrent endometrial cancer. Ann. Oncol. 2007;18:491–497. doi: 10.1093/annonc/mdl455. [DOI] [PubMed] [Google Scholar]
- 39.Astuti D., Agathanggelou A., Honorio S., Dallol A., Martinsson T., Kogner P., Cummins C., Neumann H.P., Voutilainen R., Dahia P., Eng C., Maher E.R., Latif F. RASSF1A promoter region CpG island hypermethylation in phaeochromocytomas and neuroblastoma tumours. Oncogene. 2001;20:7573–7577. doi: 10.1038/sj.onc.1204968. [DOI] [PubMed] [Google Scholar]
- 40.Dreijerink K., Braga E., Kuzmin I., Geil L., Duh F.M., Angeloni D., Zbar B., Lerman M.I., Stanbridge E.J., Minna J.D., Protopopov A., Li J., Kashuba V., Klein G., Zabarovsky E.R. The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7504–7509. doi: 10.1073/pnas.131216298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo K.W., Kwong J., Hui A.B., Chan S.Y., To K.F., Chan A.S., Chow L.S., Teo P.M., Johnson P.J., Huang D.P. High frequency of promoter hypermethylation of RASSF1A in nasopharyngeal carcinoma. Cancer Res. 2001;61:3877–3881. [PubMed] [Google Scholar]
- 42.Dammann R., Schagdarsurengin U., Strunnikova M., Rastetter M., Seidel C., Liu L., Tommasi S., Pfeifer G.P. Epigenetic inactivation of the Ras-association domain family 1 (RASSF1A) gene and its function in human carcinogenesis. Histol. Histopathol. 2003;18:665–677. doi: 10.14670/HH-18.665. [DOI] [PubMed] [Google Scholar]
- 43.Shivakumar L., Minna J., Sakamaki T., Pestell R., White M.A. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol. Cell. Biol. 2002;22:4309–4318. doi: 10.1128/MCB.22.12.4309-4318.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuzmin I., Gillespie J.W., Protopopov A., Geil K, Dreijerink K., Yang Y., Vocke C.D., Duh F.M., Zabarovsky E., Minna J.D., Rhim J.S., Emmert-Buck M.R., Emmert-Buck M.R., Lineham W.M., Lerman M.I. The RASSF1A tumor suppressor gene is inactivated in prostate tumors and suppresses growth of prostate carcinoma cells. Cancer Res. 2002;62:3498–3502. [PubMed] [Google Scholar]
- 45.Li J., Wang F., Protopopov A., Malyukova A., Kashuba V., Minna J.D., Lerman M.I., Klein G., Zabarovsky E. Inactivation of RASSF1C during in vivo tumor growth identifies it as a tumor suppressor gene. Oncogene. 2004;23:5941–5949. doi: 10.1038/sj.onc.1207789. [DOI] [PubMed] [Google Scholar]
- 46.Dallol A., Agathanggelou A., Fenton S.L., Ahmed-Choudhury J., Hesson L., Vos M.D., Clark G.J., Downward J., Maher E.R., Latif F. RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004;64:4112–4116. doi: 10.1158/0008-5472.CAN-04-0267. [DOI] [PubMed] [Google Scholar]
- 47.Schagdarsurengin U., Seidel C., Ulbrich E.J., Kolbl H., Dittmer J., Dammann R. A polymorphism at codon 133 of the tumor suppressor RASSF1A is associated with tumorous alteration of the breast. Int. J. Oncol. 2005;27:185–191. [PubMed] [Google Scholar]
- 48.Lee M.G., Kim H.Y., Byun D.S., Lee S.J., Lee C.H., Kim J.I., Chang S.G., Chi S.G. Frequent epigenetic inactivation of RASSF1A in human bladder carcinoma. Cancer Res. 2001;61:6688–6692. [PubMed] [Google Scholar]
- 49.Harada K., Toyooka S., Maitra A., Maruyama R., Toyooka K.O., Timmons C.F., Tomlinson G.E., Mastrangelo D., Hay R.J., Minna J.D., Gazdar A.F. Aberrant promoter methylation and silencing of the RASSF1A gene in pediatric tumors and cell lines. Oncogene. 2002;21:4345–4349. doi: 10.1038/sj.onc.1205446. [DOI] [PubMed] [Google Scholar]
- 50.Vos M.D., Ellis C.A., Bell A., Birrer M.J., Clark G.J. Ras uses the novel tumor suppressor RASSF1 as an effector to mediate apoptosis. J. Biol. Chem. 2000;275:35669–35672. doi: 10.1074/jbc.C000463200. [DOI] [PubMed] [Google Scholar]
- 51.Endoh H., Yatabe Y., Shmizu S., Tajima K., Kuwano H., Takahashi T., Mitsudomi T. RASSF1A gene inactivation in non-small cell lung cancer and its clinical implication. Int. J. Cancer. 2003;106:45–51. doi: 10.1002/ijc.11184. [DOI] [PubMed] [Google Scholar]
- 52.Leon S.A., Shapiro B., Sklaroff D.M., Yaros M.J. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 53.Stroun M., Anker P., Maurice P., Lyautey J., Lederrey C., Beljanski M. Neoplastic characteristics of the DNA found in the plasma of cancer patients. Oncology. 1989;46:318–332. doi: 10.1159/000226740. [DOI] [PubMed] [Google Scholar]
- 54.Herman J.G., Graff J.R., Myöhänen S., Nelkin B.D., Baylin S.B. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramirez J.L., Taron M., Balana C., Sarries C., Mendez P., de Aguirre I., Nunez L., Roig B., Queralt C., Botia M., Rosell R. Serum DNA as a tool for cancer patient management. Rocz. Akad. Med. Bialymst. 2003;48:34–41. [PubMed] [Google Scholar]
- 56.Wang Y., Yu Z., Wang T., Zhang J., Hong L., Chen L. Identification of epigenetic aberrant promoter methylation of RASSF1A in serum DNA and its clinicopathological significance in lung cancer. Lung Cancer. 2007;56:289–294. doi: 10.1016/j.lungcan.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Topaloglu O., Hoque M.O., Tokumaru Y., Lee J., Ratovitski E., Sidransky D., Moon C.S. Detection of promoter hypermethylation of multiple genes in the tumor and bronchoalveolar lavage of patients with lung cancer. Clin. Cancer Res. 2004;10:2284–2288. doi: 10.1158/1078-0432.ccr-1111-3. [DOI] [PubMed] [Google Scholar]
- 58.Zochbauer-Muller S., Lam S., Toyooka S., Virmani A.K., Toyooka K.O., Seidl S., Minna J.D., Gazdar A.F. Aberrant methylation of multiple genes in the upper aerodigestive tract epithelium of heavy smokers. Int. J. Cancer. 2003;107:612–616. doi: 10.1002/ijc.11458. [DOI] [PubMed] [Google Scholar]
- 59.Schmiemann V., Bocking A., Kazimirek M., Onofre A.S.C., Gabbert H.E., Kappes R., Gerharz C.D., Grote H.J. Methylation assay for the diagnosis of lung cancer on bronchial aspirates: a cohort study. Clin. Cancer Res. 2005;11:7728–7734. doi: 10.1158/1078-0432.CCR-05-0999. [DOI] [PubMed] [Google Scholar]
- 60.Belinsky S.A., Klinge D.M., Dekker J.D., Smith M.W., Bocklage T.J., Gilliland F.D., Crowell R.E., Karp D.D., Stidley C.A., Picchi M.A. Gene promoter methylation in plasma and sputum increases with lung cancer risk. Clin. Cancer Res. 2005;11:6505–6511. doi: 10.1158/1078-0432.CCR-05-0625. [DOI] [PubMed] [Google Scholar]
- 61.Honorio S., Agathanggelou A., Schuermann M., Pankow W., Viacava P., Maher E.R., Latif F. Detection of RASSF1A aberrant promoter hypermethylation in sputum from chronic smokers and ductal carcinoma in situ from breast cancer patients. Oncogene. 2003;22:147–150. doi: 10.1038/sj.onc.1206057. [DOI] [PubMed] [Google Scholar]
- 62.Dulaimi E., Hillinck J., Ibanez de Caceres I., Al-Saleem T., Cairns P. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin. Cancer Res. 2004;10:6189–6193. doi: 10.1158/1078-0432.CCR-04-0597. [DOI] [PubMed] [Google Scholar]
- 63.Lewis C.M., Cler L.R., Bu D.W., Zöchbauer-Müller S., Milchgrub S., Naftalis E.Z., Leitch A.M., Minna J.D., Euhus D.M. Promoter hypermethylation in benign breast epithelium in relation to predicted breast cancer risk. Clin. Cancer Res. 2005;11:166–172. [PubMed] [Google Scholar]
- 64.Rykova E.Y., Laktionov P.P., Skvortsova T.E., Starikov A.V., Kuznetsova N.P., Vlassov V.V. Extracellular DNA in breast cancer—Cell-surface-bound, tumor-derived extracellular DNA in blood of patients with breast cancer and non-malignant tumors. Ann. N. Y. Acad. Sci. 2004;1022:217–220. doi: 10.1196/annals.1318.033. [DOI] [PubMed] [Google Scholar]
- 65.Krassenstein R., Sauter E., Dulaimi E., Battagli C., Ehya H., Klein-Szanto A., Cairns P. Detection of breast cancer in nipple aspirate fluid by CpG island hypermethylation. Clin. Cancer Res. 2004;10:28–32. doi: 10.1158/1078-0432.ccr-0410-3. [DOI] [PubMed] [Google Scholar]
- 66.Battagli C., Uzzo R.G., Dulaimi E., Ibanez de Caceres I., Krassenstein R., Al-Saleem T., Greenberg R.E., Cairns P. Promoter hypermethylation of tumor suppressor genes in urine from kidney cancer patients. Cancer Res. 2003;63:8695–8699. [PubMed] [Google Scholar]
- 67.Chan M.W.Y., Chan L.W., Tang N.L.S., Lo K.W., Tong J.H.M., Chan A.W.H., Cheung H.Y., Wong W.S., Chan P.S.F., Lai F.M.M., To K.F. Frequent hypermethylation of promoter region of RASSF1A in tumor tissues and voided urine of urinary bladder cancer patients. Int. J. Cancer. 2003;104:611–616. doi: 10.1002/ijc.10971. [DOI] [PubMed] [Google Scholar]
- 68.Dulaimi E., Uzzo R.G., Greenberg R.E., Al-Saleem T., Cairns P. Detection of bladder cancer in urine by a tumor suppressor gene hypermethylation panel. Clin. Cancer Res. 2004;10:1887–1893. doi: 10.1158/1078-0432.ccr-03-0127. [DOI] [PubMed] [Google Scholar]
- 69.Friedrich M.G., Weisenberger D.J., Cheng J.C., Chandrasoma S., Siegmund K.D., Gonzalgo M.L., Toma M.I., Huland H., Yoo C., Tsai Y.C., Nichols P.W., Bochner B.H., Jones P.A., Liang G. Detection of methylated apoptosis-associated genes in urine sediments of bladder cancer patients. Clin. Cancer Res. 2004;10:7457–7465. doi: 10.1158/1078-0432.CCR-04-0930. [DOI] [PubMed] [Google Scholar]
- 70.Yates D.R., Rehman I., Meuth M., Cross S.S., Hamdy F.C., Catto J.W. Methylational urinalysis: a prospective study of bladder cancer patients and age stratified benign controls. Oncogene. 2006;25:1984–1988. doi: 10.1038/sj.onc.1209209. [DOI] [PubMed] [Google Scholar]
- 71.Roupret M., Hupertan V., Yates D.R., Catto J.W., Rehman I., Meuth M., Ricci S., Lacave R., Cancel-Tassin G., de la Taille A., Rozet F., Cathelineau X., Vallancien G., Hamdy F.C., Cussenot O. Molecular detection of localized prostate cancer using quantitative methylation-specific PCR on urinary cells obtained following prostate massage. Clin. Cancer Res. 2007;13:1720–1725. doi: 10.1158/1078-0432.CCR-06-2467. [DOI] [PubMed] [Google Scholar]
- 72.Ibanez de Caceres I., Battagli C., Esteller M., Herman J.G., Dulaimi E., Edelson M.I., Bergman C., Ehya H., Eisenberg B.L., Cairns P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64:6476–6481. doi: 10.1158/0008-5472.CAN-04-1529. [DOI] [PubMed] [Google Scholar]
- 73.Fiegl H., Gattringer C., Widschwendter A., Schneitter A., Ramoni A., Sarlay D., Gaugg I., Goebel G., Muller H.M., Mueller-Holzner E., Marth C., Widschwendter M. Methylated DNA collected by tampons—A new tool to detect endometrial cancer. Cancer Epidemiol. Biomark. Prev. 2004;13:882–888. [PubMed] [Google Scholar]
- 74.Fiegl H., Millinger S., Mueller-Holzner E., Marth C., Ensinger C., Berger A., Klocker H., Goebel G., Widschwendter M. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 2005;65:1141–1145. doi: 10.1158/0008-5472.CAN-04-2438. [DOI] [PubMed] [Google Scholar]
- 75.Murray P.G., Qiu G.H., Fu L., Waites E.R., Srivastava G., Heys D., Agathanggelou A., Latif F., Grundy R.G., Mann J.R., Starczynski J., Crocker J., Parkes S.E., Ambinder R.F., Young L.S., Tao Q. Frequent epigenetic inactivation of the RASSF1A tumor suppressor gene in Hodgkin’s lymphoma. Oncogene. 2004;23:1326–1331. doi: 10.1038/sj.onc.1207313. [DOI] [PubMed] [Google Scholar]
- 76.Chang H.W., Chan A., Kwong D.L.W., Wei W.I., Sham J.S.T., Yuen A.P.W. Evaluation of hypermethylated tumor suppressor genes as tumor markers in mouth and throat rinsing fluid, nasopharyngeal swab and peripheral blood of nasopharygeal carcinoma patient. Int. J. Cancer. 2003;105:851–855. doi: 10.1002/ijc.11162. [DOI] [PubMed] [Google Scholar]
- 77.Tomizawa Y., Kohno T., Kondo H., Otsuka A., Nishioka M., Niki T., Yamada T., Maeshima A., Yoshimura K., Saito R., Minna J.D., Yokota J. Clinicopathological significance of epigenetic inactivation of RASSF1A at 3p21.3 in stage I lung adenocarcinoma. Clin. Cancer Res. 2002;8:2362–2368. [PubMed] [Google Scholar]
- 78.Wang J., Lee J.J., Wang L., Liu D.D., Lu C., Fan Y.-H., Hong W.K., Mao L. Value of p16(INK4a) and RASSF1A promoter hypermethylation in prognosis of patients with resectable non-small cell lung cancer. Clin. Cancer Res. 2004;10:6119–6125. doi: 10.1158/1078-0432.CCR-04-0652. [DOI] [PubMed] [Google Scholar]
- 79.Toyooka S., Suzuki M., Maruyama R., Toyooka K.O., Tsukuda K., Fukuyama Y., Iizasa T., Aoe M., Date H., Fujisawa T., Shimizu N., Gazdar A.F. The relationship between aberrant methylation and survival in non-small-cell lung cancers. Br. J. Cancer. 2004;91:771–774. doi: 10.1038/sj.bjc.6602013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maruyama R., Sugio K., Yoshino L., Maehara Y., Gazdar A.F. Hypermethylation of FHIT as a prognostic marker in nonsmall cell lung carcinoma. Cancer. 2004;100:1472–1477. doi: 10.1002/cncr.20144. [DOI] [PubMed] [Google Scholar]
- 81.Choi N., Son D.S., Song I., Lee H.S., Lim Y.S., Song M.S., Lim D.S., Lee J., Kim H., Kim J. RASSF1A is not appropriate as an early detection marker or a prognostic marker for non-small cell lung cancer. Int. J. Cancer. 2005;115:575–581. doi: 10.1002/ijc.20916. [DOI] [PubMed] [Google Scholar]
- 82.Kim D.H., Kim J.S., Ji Y.I., Shim Y.M., Kim H., Han J.H., Park J. Hypermethylation of RASSF1A promoter is associated with the age at starting smoking and a poor prognosis in primary non-small cell lung cancer. Cancer Res. 2003;63:3743–3746. [PubMed] [Google Scholar]
- 83.Kang G.H., Lee S., Lee H.J., Hwang K.S. Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia. J. Pathol. 2004;202:233–240. doi: 10.1002/path.1503. [DOI] [PubMed] [Google Scholar]
- 84.Liu L.M., Yoon J.H., Dammann R., Pfeifer G.P. Frequent hypermethylation of the RASSF1A gene in prostate cancer. Oncogene. 2002;21:6835–6840. doi: 10.1038/sj.onc.1205814. [DOI] [PubMed] [Google Scholar]
- 85.Maruyama R., Toyooka S., Toyooka K.O., Virmani A.K., Zochbauer-Muller S., Farinas A.J., Minna J.D., McConnell J., Frenkel E.P., Gazdar A.F. Aberrant promoter methylation profile of prostate cancers and its relationship to clinicopathological features. Clin. Cancer Res. 2002;8:514–519. [PubMed] [Google Scholar]
- 86.Jeronimo C., Henrique R., Hoque M.O., Mambo E., Ribeiro F.R., Varzim G., Oliveira J., Teixeira M.R., Lopes C., Sidransky D. A quantitative promoter methylation profile of prostate cancer. Clin. Cancer Res. 2004;10:8472–8478. doi: 10.1158/1078-0432.CCR-04-0894. [DOI] [PubMed] [Google Scholar]
- 87.Muller H.M., Widschwendter A., Fiegl H., Ivarsson L., Goebel G., Perkmann E., Marth C., Widschwendter M. DNA methylation in serum of breast cancer patients: an independent prognostic marker. Cancer Res. 2003;63:7641–7645. [PubMed] [Google Scholar]
- 88.Mehrotra J., Vali M., McVeigh M., Kominsky S.L., Fackler M.J., Lahti-Domenici J., Polyak K., Sacchi N., Garrett-Mayer E., Argani P., Sukumar S. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin. Cancer Res. 2004;10:3104–3109. doi: 10.1158/1078-0432.ccr-03-0118. [DOI] [PubMed] [Google Scholar]
- 89.Maruyama R., Toyooka S., Toyooka K.O., Harada K., Virmani A.K., Zöchbauer-Müller S., Farinas A.J., Vakar-Lopez F., Minna J.D., Sagalowsky A., Czerniak B., Gazdar A.F. Aberrant promoter methylation profile of bladder cancer and its relationship to clinicopathological features. Cancer Res. 2001;61:8659–8663. [PubMed] [Google Scholar]
- 90.Catto J.W., Azzouzi A.R., Rehman I., Feeley K.M., Cross S.S., Amira N., Fromont G., Sibony M., Cussenot O., Meuth M., Hamdy F.C. Promoter hypermethylation is associated with tumor location, stage, and subsequent progression in transitional cell carcinoma. J. Clin. Oncol. 2005;23:2903–2910. doi: 10.1200/JCO.2005.03.163. [DOI] [PubMed] [Google Scholar]
- 91.Marsit C.J., Karagas M.R., Schned A., Kelsey K.T. Carcinogen exposure and epigenetic silencing in bladder cancer. Ann. N. Y. Acad. Sci. 2006;1076:810–821. doi: 10.1196/annals.1371.031. [DOI] [PubMed] [Google Scholar]
- 92.Dhawan D., Hamdy F.C., Rehman I., Patterson J., Cross S.S., Feeley K.M., Stephenson Y., Meuth M., Catto J.W. Evidence for the early onset of aberrant promoter methylation in urothelial carcinoma. J. Pathol. 2006;209:336–343. doi: 10.1002/path.1991. [DOI] [PubMed] [Google Scholar]
- 93.Jo H., Kim J.W., Kang G.H., Park N.H., Song Y.S., Kang S.B., Lee H.P. Association of promoter hypermethylation of the RASSF1A gene with prognostic parameters in endometrial cancer. Oncol. Res. 2006;16:205–209. doi: 10.3727/000000006783981125. [DOI] [PubMed] [Google Scholar]
- 94.Liu L., Broaddus R.R., Yao J.C., Xie S., White J.A., Wu T.T., Hamilton S.R., Rashid A. Epigenetic alterations in neuroendocrine tumors: methylation of RAS-association domain family 1, isoform A and p16 genes are associated with metastasis. Mod. Pathol. 2005;18:1632–1640. doi: 10.1038/modpathol.3800490. [DOI] [PubMed] [Google Scholar]
- 95.Geli J., Kiss N., Lanner F., Foukakis T., Natalishvili N., Larsson O., Kogner P., Hoog A., Clark G.J., Ekstrom G.J., Backdahl M., Farnebo F., Larsson C. The Ras effectors NORE1A and RASSF1A are frequently inactivated in pheochromocytoma and abdominal paraganglioma. Endocr. Relat. Cancer. 2007;14:125–134. doi: 10.1677/ERC-06-0031. [DOI] [PubMed] [Google Scholar]
- 96.Hoon D.S.B., Spugnardi M., Kuo C., Huang S.K., Morton D.L., Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23:4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hesson L., Bieche I., Krex D., Criniere E., Hoang-Xuan K., Maher E.R., Latif F. Frequent epigenetic inactivation of RASSF1A and BLU genes located within the critical 3p21.3 region in gliomas. Oncogene. 2004;23:2408–2419. doi: 10.1038/sj.onc.1207407. [DOI] [PubMed] [Google Scholar]
- 98.Byun D.S., Lee M.G., Chae K.S., Ryu B.G., Chi S.G. Frequent epigenetic inactivation of RASSF1A by aberrant promoter hypermethylation in human gastric adenocarcinoma. Cancer Res. 2001;61:7034–7038. [PubMed] [Google Scholar]
- 99.Li J., El-Naggar A., Mao L. Promoter methylation of p16INK4a, RASSF1A, and DAPK is frequent in salivary adenoid cystic carcinoma. Cancer. 2005;104:771–776. doi: 10.1002/cncr.21215. [DOI] [PubMed] [Google Scholar]
- 100.Qian Z.R., Sano T., Yoshimoto K., Ishizuka A., Mizusawa N., Horiguchi H., Hirokawa M., Asa S.L. Inactivation of RASSF1A tumor suppressor gene by aberrant promoter hypermethylation in human pituitary adenomas. Lab. Invest. 2005;85:464–473. doi: 10.1038/labinvest.3700248. [DOI] [PubMed] [Google Scholar]
- 101.Katayama H., Hiraki A., Aoe K., Fujiwara K., Matsuo K., Maeda T., Murakami T., Toyooka S., Sugi K., Ueoka H., Tanimoto M. Aberrant promoter methylation in pleural fluid DNA for diagnosis of malignant pleural effusion. Int. J. Cancer. 2007;120:2191–2195. doi: 10.1002/ijc.22576. [DOI] [PubMed] [Google Scholar]
- 102.Di Gioia S., Bianchi P., Destro A., Grizzi F., Malesci A., Laghi L., Levrero M., Morabito A., Roncalli M. Quantitative evaluation of RASSF1A methylation in the non-lesional, regenerative and neoplastic liver. BMC Cancer. 2006;6:89. doi: 10.1186/1471-2407-6-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Calvisi D.F., Ladu S., Gorden A., Farina M., Conner E.A., Lee J.S., Factor V.M., Thorgeirsson S.S. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;30:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 104.Woodson K., Gillespie J., Hanson J., Emmert-Buck M., Phillips J.M., Linehan W.M., Tangrea J.A. Heterogeneous gene methylation patterns among pre-invasive and cancerous lesions of the prostate: a histopathologic study of whole mount prostate specimens. Prostate. 2004;60:25–31. doi: 10.1002/pros.20013. [DOI] [PubMed] [Google Scholar]
- 105.Woodson K., Hanson J., Tangrea J. A survey of gene-specific methylation in human prostate cancer among black and white men. Cancer Lett. 2004;205:181–188. doi: 10.1016/j.canlet.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 106.Aitchison A., Warren A., Neal D., Rabbitts P. RASSF1A promoter methylation is frequently detected in both pre-malignant and non-malignant microdissected prostatic epithelial tissues. Prostate. 2007;67:638–644. doi: 10.1002/pros.20475. [DOI] [PubMed] [Google Scholar]
- 107.Bastian P.J., Ellinger J., Wellmann A., Wernert N., Heukamp L.C., Muller S.C., von Ruecker A. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin. Cancer Res. 2005;11:4097–4106. doi: 10.1158/1078-0432.CCR-04-1832. [DOI] [PubMed] [Google Scholar]
- 108.van Engeland M., Roemen G., Brink M., Pachen M.M., Weijenberg M.P., de Bruine A.P., Arends J.W., van den Brandt P.A., de Goeij A.F., Herman J.G. K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene. 2002;21:3792–3795. doi: 10.1038/sj.onc.1205466. [DOI] [PubMed] [Google Scholar]
- 109.Dammann R., Schagdarsurengin U., Liu L.M., Otto N., Gimm O., Dralle H., Boehm B.O., Pfeifer G.P., Hoang-Vu C. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003;22:3806–3812. doi: 10.1038/sj.onc.1206582. [DOI] [PubMed] [Google Scholar]
- 110.Li J., Zhang Z.Q., Dai Z.Y., Popkie A.P., Plass C., Morrison C., Wang Y., You M. RASSF1A promoter methylation and Kras2 mutations in non small cell lung cancer. Neoplasia. 2003;5:362–366. doi: 10.1016/S1476-5586(03)80029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim D.H., Kim J.S., Park J.H., Lee S.K., Ji Y.I., Kwon Y.M., Shim Y.M., Han J., Park J. Relationship of Ras association domain family 1 methylation and K-ras mutation in primary non-small cell lung cancer. Cancer Res. 2003;63:6206–6211. [PubMed] [Google Scholar]
- 112.Irimia M., Fraga M.F., Sanchez-Cespedes M., Esteller M. CpG island promoter hypermethylation of the Ras-effector gene NORE1A occurs in the context of a wild-type K-ras in lung cancer. Oncogene. 2004;23:8695–8699. doi: 10.1038/sj.onc.1207914. [DOI] [PubMed] [Google Scholar]
- 113.Reifenberger J., Knobbe C.B., Sterzinger A.A., Blaschke B., Schulte K.W., Ruzicka T., Reifenberger G. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int. J. Cancer. 2004;109:377–384. doi: 10.1002/ijc.11722. [DOI] [PubMed] [Google Scholar]
- 114.Chen H., Suzuki M., Nakamura Y., Ohira M., Ando S., Iida T., Nakajima T., Nakagawara A., Kimura H. Aberrant methylation of RASGRF2 and RASSF1A in human non-small cell lung cancer. Oncol. Rep. 2006;15:1281–1285. [PubMed] [Google Scholar]
- 115.Schagdarsurengin U., Gimm O., Hoang-Vu C., Dralle H., Pfeifer G.P., Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer Res. 2002;62:3698–3701. [PubMed] [Google Scholar]
- 116.Lazcoz P., Munoz J., Nistal M., Pestana A., Encio I., Castresana J.S. Frequent promoter hypermethylation of RASSF1A and CASP8 in neuroblastoma. BMC Cancer. 2006;6:254. doi: 10.1186/1471-2407-6-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang C., Li Z., Cheng Y., Jia F., Li R., Wu M., Li K., Wei L. CpG island methylator phenotype association with elevated serum alpha-fetoprotein level in hepatocellular carcinoma. Clin. Cancer Res. 2007;13:944–952. doi: 10.1158/1078-0432.CCR-06-2268. [DOI] [PubMed] [Google Scholar]
- 118.Kuzmin I., Liu L.M., Dammann R., Geil L., Stanbridge E.J., Wilczynski S.P., Lerman M.I., Pfeifer G.P. GP, Inactivation of RAS association domain family 1A gene in cervical carcinomas and the role of human papillomavirus infection. Cancer Res. 2003;63:1888–1893. [PubMed] [Google Scholar]
- 119.Dong S.M., Sun D.I., Benoit N.E., Kuzmin I., Lerman M.I., Sidransky D. Epigenetic inactivation of RASSF1A in head and neck cancer. Clin. Cancer Res. 2003;9:3635–3640. [PubMed] [Google Scholar]
- 120.Cohen Y., Singer G., Lavie O., Dong S.M., Beller U., Sidransky D. The RASSF1A tumor suppressor gene is commonly inactivated in adenocarcinoma of the uterine cervix. Clin. Cancer Res. 2003;9:2981–2984. [PubMed] [Google Scholar]
- 121.Yu M.Y., Tong J.H.M., Chan P.K.S., Lee T.L., Chan M.W., Chan A.W., Lo K.W., To K.F. Hypermethylation of the tumor suppressor gene Rassf1a and frequent concomitant loss of heterozygosity at 3p21 in cervical cancers. Int. J. Cancer. 2003;105:204–209. doi: 10.1002/ijc.11051. [DOI] [PubMed] [Google Scholar]
- 122.Kang G.H., Lee S., Kim W.H., Lee H.W., Kim J.C., Rhyu M.G., Ro J.Y. Epstein–Barr virus-positive gastric carcinoma demonstrates frequent aberrant methylation of multiple genes and constitutes CpG island methylator phenotype-positive gastric carcinoma. Am. J. Pathol. 2002;160:787–794. doi: 10.1016/S0002-9440(10)64901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toyooka S., Toyooka K.O., Maruyama R., Virmani A.K., Girard L., Miyajima K., Harada K., Ariyoshi Y., Takahashi T., Sugio K., Brambilla E., Gilcrease M., Minna J.D., Gazdar A.F. DNA methylation profiles of lung tumors. Mol. Cancer Ther. 2001;1:61–67. [PubMed] [Google Scholar]
- 124.Toyooka S., Carbone M., Toyooka K.O., Bocchetta M., Shivapurkar N., Minna J.D., Gazdar A.F. Progressive aberrant methylation of the RASSF1A gene in simian virus 40 infected human mesothelial cells. Oncogene. 2002;21:4340–4344. doi: 10.1038/sj.onc.1205381. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y.J., Ahsan H., Chen Y., Lunn R.M., Wang L.Y., Chen S.Y., Lee P.H., Chen C.J., Santella R.M. High frequency of promoter hypermethylation of RASSF1A and p16 and its relationship to aflatoxin B1-DNA adduct levels in human hepatocellular carcinoma. Mol. Carcinog. 2002;35:85–92. doi: 10.1002/mc.10076. [DOI] [PubMed] [Google Scholar]
- 126.Chow L.S., Lo K.W., Kwong J., To K.F., Tsang K.S., Lam C.W., Dammann R., Huang D.P. RASSF1A is a target tumor suppressor from 3p21.3 in nasopharyngeal carcinoma. Int. J. Cancer. 2004;109:839–847. doi: 10.1002/ijc.20079. [DOI] [PubMed] [Google Scholar]
- 127.Ji L., Nishizaki M., Gao B., Burbee D., Kondo M., Kamibayashi C., Xu K., Yen N., Atkinson E.N., Fang B., Lerman M.I., Roth J.A., Minna J.D. Expression of several genes in the human chromosome 3p21.3 homozygous deletion region by an adenovirus vector results in tumor suppressor activities in vitro and in vivo. Cancer Res. 2002;62:2715–2720. [PMC free article] [PubMed] [Google Scholar]
- 128.Amaar Y.G., Baylink D.J., Mohan S. Ras-association domain family 1 protein, RASSF1C, is an IGFBP-5 binding partner and a potential regulator of osteoblast cell proliferation. J. Bone Miner. Res. 2005;20:1430–1439. doi: 10.1359/JBMR.050311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Amaar Y.G., Minera M.G., Hatran L.K., Strong D.D., Mohan S., Reeves M.E. Ras association domain family 1C protein stimulates human lung cancer cell proliferation. Am. J. Physiol., Lung Cell. Mol. Physiol. 2006;291:L1185–L1190. doi: 10.1152/ajplung.00072.2006. [DOI] [PubMed] [Google Scholar]
- 130.Estrabaud E., Lassot I., Blot G., Le Rouzic E., Tanchou V., Quemeneur E., Daviet L., Margottin-Goguet F., Benarous R. RASSF1C, an isoform of the tumor suppressor RASSF1A, promotes the accumulation of beta-catenin by interacting with betaTrCP. Cancer Res. 2007;67:1054–1061. doi: 10.1158/0008-5472.CAN-06-2530. [DOI] [PubMed] [Google Scholar]
- 131.Smith A.J.H, Xian J., Richardson M., Johnstone K.A, Rabbitts P.H. Cre-loxP chromosome engineering of a targeted deletion in the mouse corresponding to the 3p21.3 region of homozygous loss in human tumours. Oncogene. 2002;21:4521–4529. doi: 10.1038/sj.onc.1205530. [DOI] [PubMed] [Google Scholar]
- 132.Liu L., Tommasi S., Lee D.H., Dammann R., Pfeifer G.P. Control of microtubule stability by the RASSF1A tumor suppressor. Oncogene. 2003;22:8125–8136. doi: 10.1038/sj.onc.1206984. [DOI] [PubMed] [Google Scholar]
- 133.Song M.S., Song S.J., Ayad N.G., Chang J.S., Lee J.H., Hong H.K., Lee H., Choi N., Kim J., Kim H., Kim J.W., Choi E-J., Kirschner M.W., Lim D-S. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC–Cdc20 complex. Nat. Cell Biol. 2004;6:129–137. doi: 10.1038/ncb1091. [DOI] [PubMed] [Google Scholar]
- 134.Vos M.D., Martinez A., Elam C., Dallol A., Taylor B.J., Latif F., Clark G.J. A role for the RASSF1A tumor suppressor in the regulation of tubulin polymerization and genomic stability. Cancer Res. 2004;64:4244–4250. doi: 10.1158/0008-5472.CAN-04-0339. [DOI] [PubMed] [Google Scholar]
- 135.Rong R., Jin W., Zhang J.M., Sheikh M.S., Huang Y. Tumor suppressor RASSF1A is a microtubule-binding protein that stabilizes microtubules and induces G2/M arrest. Oncogene. 2004;23:8216–8230. doi: 10.1038/sj.onc.1207901. [DOI] [PubMed] [Google Scholar]
- 136.Liu L., Amy V., Liu G., McKeehan W.L. Novel complex integrating mitochondria and the microtubular cytoskeleton with chromosome remodeling and tumor suppressor RASSF1 deduced by in silico homology analysis, interaction cloning in yeast, and colocalization in cultured cells. In Vitro Cell Dev. Biol. Anim. 2002;38:582–594. doi: 10.1290/1543-706x(2002)38<582:ncimat>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dallol A., Cooper W.N., Al-Mulla F., Agathanggelou A., Maher E.R., Latif F. Depletion of the Ras association domain family 1, isoform A-associated novel microtubule-associated protein, C19ORF5/MAP1S, causes mitotic abnormalities. Cancer Res. 2007;67:492–500. doi: 10.1158/0008-5472.CAN-06-3604. [DOI] [PubMed] [Google Scholar]
- 138.Liu L., Vo A., McKeehan W.L. Specificity of the methylation-suppressed A isoform of candidate tumor suppressor RASSF1 for microtubule hyperstabilization is determined by cell death inducer C19ORF5. Cancer Res. 2005;65:1830–1838. doi: 10.1158/0008-5472.CAN-04-3896. [DOI] [PubMed] [Google Scholar]
- 139.Agathanggelou A., Bieche I., Ahmed-Choudhury J., Nicke B., Dammann R., Baksh S., Gao B., Minna J.D., Downward J., Maher E.R., Latif F. Identification of novel gene expression targets for the Ras association domain family 1 (RASSF1A) tumor suppressor gene in non-small cell lung cancer and neuroblastoma. Cancer Res. 2003;63:5344–5351. [PMC free article] [PubMed] [Google Scholar]
- 140.Baksh S., Tommasi S., Fenton S., Yu V.C., Martins L.M., Pfeifer G.P., Latif F., Downward J., Neel B.G. The tumor suppressor RASSF1A and MAP-1 link death receptor signalling to bax conformational change and cell death. Mol. Cell. 2005;18:637–650. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 141.Fenton S.L., Dallol A., Agathanggelou A., Hesson L., Ahmed-Choudhury J., Baksh S., Sardet C., Dammann R., Minna J.D., Downward J., Maher E.R., Latif F. Identification of the E1A-regulated transcription factor p120E4F as an interacting partner of the RASSF1A candidate tumor suppressor gene. Cancer Res. 2004;64:102–107. doi: 10.1158/0008-5472.can-03-2622. [DOI] [PubMed] [Google Scholar]
- 142.Ahmed-Choudhury J., Agathanggelou A., Fenton S.L., Ricketts C., Clark G.J., Maher E.R., Latif F. Transcriptional regulation of cyclin A2 by RASSF1A through the enhanced binding of p120E4F to the cyclin A2 promoter. Cancer Res. 2005;65:2690–2697. doi: 10.1158/0008-5472.CAN-04-3593. [DOI] [PubMed] [Google Scholar]
- 143.Whang Y.M., Kim Y.H., Kim J.S., Yoo Y.D. RASSF1A suppresses the c-Jun-NH2-kinase pathway and inhibits cell cycle progression. Cancer Res. 2005;65:3682–3690. doi: 10.1158/0008-5472.CAN-04-2792. [DOI] [PubMed] [Google Scholar]
- 144.Song M.S., Lim D.S. Control of APC–Cdc20 by the tumor suppressor RASSF1A. Cell Cycle. 2004;3:574–576. [PubMed] [Google Scholar]
- 145.Song M.S., Chang J.S., Song S.J., Yang T.H., Lee H., Lim D.S. The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J. Biol. Chem. 2005;280:3920–3927. doi: 10.1074/jbc.M409115200. [DOI] [PubMed] [Google Scholar]
- 146.Mathe E. RASSF1A, the new guardian of mitosis. Nat. Genet. 2004;36:117–118. doi: 10.1038/ng0204-117. [DOI] [PubMed] [Google Scholar]
- 147.Jackson P.K. Linking tumor suppression, DNA damage and the anaphase-promoting complex. Trends Cell Biol. 2004;14:331–334. doi: 10.1016/j.tcb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Guo C., Tommasi S., Liu L., Yee J.K., Dammann R., Pfeifer G.P. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr. Biol. 2007;17:700–705. doi: 10.1016/j.cub.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 149.Bhalla K.N. Microtubule-targeted anticancer agents and apoptosis. Oncogene. 2003;22:9075–9086. doi: 10.1038/sj.onc.1207233. [DOI] [PubMed] [Google Scholar]
- 150.Walczak C.E. Microtubule dynamics and tubulin interacting proteins. Curr. Opin. Cell Biol. 2000;12:52. doi: 10.1016/s0955-0674(99)00056-3. [DOI] [PubMed] [Google Scholar]
- 151.R. Rong, L.Y. Jiang, M.S. Sheikh, Y. Huang, Mitotic kinase Aurora—A phosphorylates RASSF1A and modulates RASSF1A-mediated microtubule interaction and M-phase cell cycle regulation. Oncogene Jun 11 (2007) [Epub ahead of print, doi:10.1038/sj.onc.1210575]. [DOI] [PubMed]
- 152.Armesilla A.L., Williams J.C., Buch M.H., Pickard A., Emerson M., Cartwright E.J., Oceandy D., Vos M.D., Gillies S., Clark G.J., Neyses L. Novel functional interaction between the plasma membrane Ca2+ pump 4b and the proapoptotic tumor suppressor Ras-associated factor 1 (RASSF1) J. Biol. Chem. 2004;23:31318–31328. doi: 10.1074/jbc.M307557200. [DOI] [PubMed] [Google Scholar]
- 153.Wassmann K., Benezra R. Mitotic checkpoints: from yeast to cancer. Curr. Opin. Genet. Dev. 2001;11:83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- 154.Denko N.C., Giaccia A.J., Stringer J.R., Stambrook P.J. The human Ha-ras oncogene induces genomic instability in murine fibroblasts within one cell cycle. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5124–5128. doi: 10.1073/pnas.91.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saavedra H.I., Knauf J.A., Shirokawa J.M., Wang J., Ouyang B., Elisei R., Stambrook P.J., Fagin J.A. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene. 2000;19:3948–3954. doi: 10.1038/sj.onc.1203723. [DOI] [PubMed] [Google Scholar]
- 156.Ortiz-Vega S., Khokhlatchev A., Nedwidek M., Zhang X.F., Dammann R., Pfeifer G.P., Avruch J. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene. 2002;21:1381–1390. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- 157.Rabizadeh S., Xavier R.J., Ishiguro K., Bernabeortiz J., Lopez-Ilasaca M., Khokhlatchev A., Mollahan P., Pfeifer G.P., Avruch J., Seed B. The scaffold protein CNK1 interacts with the tumor suppressor RASSF1A and augments RASSF1A-induced cell death. J. Biol. Chem. 2004;279:29247–29254. doi: 10.1074/jbc.M401699200. [DOI] [PubMed] [Google Scholar]
- 158.Praskova M., Khoklatchev A., Ortiz-Vega S., Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Avruch J., Praskova M., Ortiz-Vega S., Liu M., Zhang X.F. Nore1 and RASSF1 regulation of cell proliferation and of the MST1/2 kinases. Methods Enzymol. 2005;407:290–310. doi: 10.1016/S0076-6879(05)07025-4. [DOI] [PubMed] [Google Scholar]
- 160.Vos M.D., Dallol A., Eckfeld K., Allen N.P., Donninger H., Hesson L.B., Calvisi D., Latif F., Clark G.J. The RASSF1A tumor suppressor activates Bax via MOAP-1. J. Biol. Chem. 2006;281:4557–4563. doi: 10.1074/jbc.M512128200. [DOI] [PubMed] [Google Scholar]
- 161.Kitagawa D., Kajiho H., Negishi T., Ura S., Watanabe T., Wada T., Ichijo H., Katada T., Nishina H. Release of RASSF1C from the nucleus by Daxx degradation links DNA damage and SAPK/JNK activation. EMBO J. 2006;25:3286–3297. doi: 10.1038/sj.emboj.7601212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dallol A., Agathanggelou A., Tommasi S., Pfeifer G.P., Maher E.R., Latif F. Involvement of the RASSF1A tumor suppressor gene in controlling cell migration. Cancer Res. 2005;65:7653–7659. doi: 10.1158/0008-5472.CAN-05-0247. [DOI] [PubMed] [Google Scholar]
- 163.Comincini S., Castiglioni B.M., Foti G.M., Del Vecchio I., Ferretti L. Isolation and molecular characterization of rasfadin, a novel gene in the vicinity of the bovine prion gene. Mamm. Genome. 2001;12:150–156. doi: 10.1007/s003350010239. [DOI] [PubMed] [Google Scholar]
- 164.Hesson L.B., Wilson R., Morton D., Adams C., Walker M., Maher E.R., Latif F. CpG island promoter hypermethylation of a novel Ras-effector gene RASSF2A is an early event in colon carcinogenesis and correlates inversely with K-ras mutations. Oncogene. 2005;24:3987–3994. doi: 10.1038/sj.onc.1208566. [DOI] [PubMed] [Google Scholar]
- 165.Loveland J. VEGA, the genome browser with a difference. Brief Bioinfom. 2005;6:189–193. doi: 10.1093/bib/6.2.189. [DOI] [PubMed] [Google Scholar]
- 166.Vos M.D., Ellis C.A., Elam C., Ulku A.S., Taylor B.J., Clark G.J. RASSF2 is a novel K-Ras-specific effector and potential tumor suppressor. J. Biol. Chem. 2003;278:28045–28051. doi: 10.1074/jbc.M300554200. [DOI] [PubMed] [Google Scholar]
- 167.Akino K., Toyota M., Suzuki H., Mita H., Sasaki Y., Ohe-Toyota M., Issa J.P., Hinoda Y., Imai K., Tokino T. The Ras effector RASSF2 is a novel tumor-suppressor gene in human colorectal cancer. Gastroenterology. 2005;129:156–169. doi: 10.1053/j.gastro.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 168.Nosho K., Yamamoto H., Takahashi T., Mikami M., Taniguchi H., Miyamoto N., Adachi Y., Arimura Y., Itoh F., Imai K., Shinomura Y. Genetic and epigenetic profiling in early colorectal tumors and prediction of invasive potential in pT1 (early invasive) colorectal cancers. Carcinogenesis. 2007;28:1364–1370. doi: 10.1093/carcin/bgl246. [DOI] [PubMed] [Google Scholar]
- 169.Park H.W., Kang H.C., Kim I.J., Jang S.G., Kim K., Yoon H.J., Jeong S.Y., Park J.G. Correlation between hypermethylation of the RASSF2A promoter and K-ras/BRAF mutations in microsatellite-stable colorectal cancers. Int. J. Cancer. 2007;120:7–12. doi: 10.1002/ijc.22276. [DOI] [PubMed] [Google Scholar]
- 170.Kaira K., Sunaga N., Tomizawa Y., Yanagitani N., Ishizuka T., Saito R., Nakajima T., Mori M. Epigenetic inactivation of the RAS-effector gene RASSF2 in lung cancers. Int. J. Oncol. 2007;31:169–173. [PubMed] [Google Scholar]
- 171.Endoh M., Tamura G., Honda T., Homma N., Terashima M., Nishizuka S., Motoyama T. RASSF2, a potential tumour suppressor, is silenced by CpG island hypermethylation in gastric cancer. Br. J. Cancer. 2005;93:1395–1399. doi: 10.1038/sj.bjc.6602854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang Z., Sun D., Van do N., Tang A., Hu L., Huang G. Inactivation of RASSF2A by promoter methylation correlates with lymph node metastasis in nasopharyngeal carcinoma. Int. J. Cancer. 2007;120:32–38. doi: 10.1002/ijc.22185. [DOI] [PubMed] [Google Scholar]
- 173.Sakamoto-Hojo E.T., Mello S.S., Pereira E., Fachin A.L., Cardoso R.S., Junta C.M., Sandrin-Garcia P., Donadi E.A., Passos G.A. Gene expression profiles in human cells submitted to genotoxic stress. Mutat. Res. 2003;544:403–413. doi: 10.1016/j.mrrev.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 174.Eckert L.B., Repasky G.A., Ulku A.S., McFall A., Zhou H., Sartor C.I., Der C.J. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–4592. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 175.Tommasi S., Dammann R., Jin S.G., Zhang X.F., Avruch J., Pfeifer G.P. RASSF3 and NORE1: identification and cloning of two human homologues of the putative tumor suppressor gene RASSF1. Oncogene. 2002;21:2713–2720. doi: 10.1038/sj.onc.1205365. [DOI] [PubMed] [Google Scholar]
- 176.Eckfeld K., Hesson L., Vos M.D., Bieche I., Latif F., Clark G.J. RASSF4/AD037 is a potential ras effector/tumor suppressor of the RASSF family. Cancer Res. 2004;64:8688–8693. doi: 10.1158/0008-5472.CAN-04-2065. [DOI] [PubMed] [Google Scholar]
- 177.Chow L.S., Lo K.W., Kwong J., Wong A.Y., Huang D.P. Aberrant methylation of RASSF4/AD037 in nasopharyngeal carcinoma. Oncol. Rep. 2004;12:781–787. [PubMed] [Google Scholar]
- 178.Agathanggelou A., Honorio S., Macartney D.P., Martinez A., Dallol A., Rader J., Fullwood P., Chauhan A., Walker R., Shaw J.A., Hosoe S., Lerman M.I., Minna J.D., Maher E.R., Latif F. Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene. 2001;22:1509–1518. doi: 10.1038/sj.onc.1204175. [DOI] [PubMed] [Google Scholar]
- 179.Reuther G.W., Buss J.E., Quilliam L.A., Clark G.J., Der C.J. Analysis of function and regulation of proteins that mediate signal transduction by use of lipid-modified plasma membrane-targeting sequences. Methods Enzymol. 2000;327:331–350. doi: 10.1016/s0076-6879(00)27288-1. [DOI] [PubMed] [Google Scholar]
- 180.Hesson L., Dallol A., Minna J.D., Maher E.R., Latif F. NORE1A, a homologue of RASSF1A tumour suppressor gene is inactivated in human cancers. Oncogene. 2003;22:947–954. doi: 10.1038/sj.onc.1206191. [DOI] [PubMed] [Google Scholar]
- 181.Vos M.D., Martinez A., Ellis C.A., Vallecorsa T., Clark G.J. The pro-apoptotic Ras effector Nore1 may serve as a Ras-regulated tumor suppressor in the lung. J. Biol. Chem. 2003;278:21938–21943. doi: 10.1074/jbc.M211019200. [DOI] [PubMed] [Google Scholar]
- 182.Aoyama Y., Avruch J., Zhang X.F. Nore1 inhibits tumor cell growth independent of Ras or the MST1/2 kinases. Oncogene. 2004;23:3426–3433. doi: 10.1038/sj.onc.1207486. [DOI] [PubMed] [Google Scholar]
- 183.Nakamura N., Carney J.A., Jin L., Kajita S., Pallares J., Zhang H., Qian X., Sebo T.J., Erickson L.A., Lloyd R.V. RASSF1A and NORE1A methylation and BRAFV600E mutations in thyroid tumors. Lab. Invest. 2005;85:1065–1075. doi: 10.1038/labinvest.3700306. [DOI] [PubMed] [Google Scholar]
- 184.Foukakis T., Au A.Y.M., Wallin G., Geli J., Forsberg L., Clifton-Bligh R., Robinson B.G., Lui W.-O., Zedenius J., Larsson C. The Ras effector NORE1A is suppressed in follicular thyroid carcinomas with a PAX8-PPARγ fusion. J. Clin. Endocrinol. Metab. 2006;91:1143–1149. doi: 10.1210/jc.2005-1372. [DOI] [PubMed] [Google Scholar]
- 185.Steiner G., Cairns P., Polascik T.J., Marshall F.F., Epstein J.I., Sidransky D., Schoenberg M. High-density mapping of chromosomal arm 1q in renal collecting duct carcinoma: region of minimal deletion at 1q32.1–32.2. Cancer Res. 1996;56:5044–5046. [PubMed] [Google Scholar]
- 186.Chen J., Lui W.O., Vos M.D., Clark G.J., Takahashi M., Schoumans J., Khoo S.K., Petillo D., Lavery T., Sugimura J., Astuti D., Zhang C., Kagawa S., Maher E.R., Larsson C., Alberts A.S., Kanayama H.O., The B.T. The t(1;3) breakpoint-spanning genes LSAMP and NORE1 are involved in clear cell renal cell carcinomas. Cancer Cell. 2003;4:405–413. doi: 10.1016/s1535-6108(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 187.Morris M.R., Hesson L.B., Wagner K.J., Morgan N.V., Astuti D., Lees R.D., Cooper W.N., Lee J., Gentle D., Macdonald F., Kishida T., Grundy R., Yao M., Latif F., Maher E.R. Multigene methylation analysis of Wilms’ tumour and adult renal cell carcinoma. Oncogene. 2003;22:6794–6801. doi: 10.1038/sj.onc.1206914. [DOI] [PubMed] [Google Scholar]
- 188.Macheiner D., Heller G., Kappel S., Bichler C., Stättner S., Ziegler B., Kandioler D., Wrba F., Schulte-Hermann R., Zöchbauer-Müller S. NORE1B, a candidate tumor suppressor, is epigenetically silenced in human hepatocellular carcinoma. J. Hepatol. 2006;45:81–89. doi: 10.1016/j.jhep.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 189.Moshnikova A., Frye J., Shay J.W., Minna J.D., Khokhlatchev A.V. The growth and tumor suppressor NORE1A is a cytoskeletal protein that suppresses growth by inhibition of the ERK pathway. J. Biol. Chem. 2006;281:8143–8152. doi: 10.1074/jbc.M511837200. [DOI] [PubMed] [Google Scholar]
- 190.Hwang E., Ryu K.S., Paakkonen K., Guntert P., Cheong H.K., Lim D.S., Lee J.O., Jeon Y.H., Cheong C. Structural insight into dimeric interaction of the SARAH domains from Mst1 and RASSF family proteins in the apoptosis pathway. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9236–9241. doi: 10.1073/pnas.0610716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kinashi T., Katagiri K. Regulation of immune cell adhesion and migration by regulator of adhesion and cell polarization enriched in lymphoid tissues. Immunology. 2005;116:164–171. doi: 10.1111/j.1365-2567.2005.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Katagiri K., Maeda A., Shimonaka M., Kinashi T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 2003;4:741–748. doi: 10.1038/ni950. [DOI] [PubMed] [Google Scholar]
- 193.Katagiri K., Ohnishi N., Kabashima K., Iyoda T., Takeda N., Shinkai Y., Inaba K., Kinashi T. Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat. Immunol. 2004;5:1045–1051. doi: 10.1038/ni1111. [DOI] [PubMed] [Google Scholar]
- 194.Fujita H., Fukuhara S., Sakurai A., Yamagishi A., Kamioka Y., Nakaoka Y., Masuda M., Mochizuki N. Local activation of Rap1 contributes to directional vascular endothelial cell migration accompanied by extension of microtubules on which RAPL, a Rap1-associating molecule, localizes. J Biol. Chem. 2005;280:5022–5031. doi: 10.1074/jbc.M409701200. [DOI] [PubMed] [Google Scholar]
- 195.Katagiri K., Imamura M., Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat. Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 196.Ishiguro K., Avruch J., Landry A., Qin S., Ando T., Goto H., Xavier R. Nore1B regulates TCR signaling via Ras and Carma1. Cell. Signal. 2006;18:1647–1654. doi: 10.1016/j.cellsig.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ikeda M., Hirabayashi S., Fujiwara N., Mori H., Kawata A., Iida J., Bao Y., Sato Y., Iida T., Sugimura H., Hata Y. Ras-association domain family protein 6 induces apoptosis via both caspase-dependent and caspase-independent pathways. Exp. Cell Res. 2007;313:1484–1495. doi: 10.1016/j.yexcr.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 198.N.P. Allen, H. Donninger, M.D. Vos, K. Eckfeld, L. Hesson, L. Gordon, M.J. Birrer, F. Latif, G.J. Clark, RASSF6 is a novel member of the RASSF family of tumor suppressors, Oncogene (2007) Advance online publication 2 April 2007 (doi:10.1038/sj.onc.1210440). [DOI] [PubMed]
- 199.Diep C.B., Teixeira M.R., Thorstensen L., Wiig J.N., Eknaes M., Nesland J.M., Giercksky K.E., Johansson B., Lothe R.A. Genome characteristics of primary carcinomas, local recurrences, carcinomatoses, and liver metastases from colorectal cancer patients. Mol. Cancer. 2004;3:6. doi: 10.1186/1476-4598-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Takai D., Jones P.A. The CpG island searcher: a new WWW resource. In Silico Biol. 2003;3:235–240. [PubMed] [Google Scholar]
- 201.Hull J., Rowlands K., Lockhart E., Sharland M., Moore C., Hanchard N., Kwiatkowski D.P. Haplotype mapping of the bronchiolitis susceptibility locus near IL8. Hum. Genet. 2004;114:272–279. doi: 10.1007/s00439-003-1038-x. [DOI] [PubMed] [Google Scholar]
- 202.Bitko V., Garmon N.E., Cao T., Estrada B., Oakes J.E., Lausch R.N., Barik S. Activation of cytokines and NF-kappa B in corneal epithelial cells infected by respiratory syncytial virus: potential relevance in ocular inflammation and respiratory infection. BMC Microbiol. 2004;4:28. doi: 10.1186/1471-2180-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Weitzel J.N., Kasperczyk A., Mohan C., Krontiris T.G. The HRAS1 gene cluster: two upstream regions recognizing transcripts and a third encoding a gene with a leucine zipper domain. Genomics. 1992;14:309–319. doi: 10.1016/s0888-7543(05)80221-6. [DOI] [PubMed] [Google Scholar]
- 204.Kiel C., Wohlgemuth S., Rousseau F., Schymkowitz J., Ferkinghoff-Borg J., Wittinghofer F., Serrano L. Recognizing and defining true Ras binding domains II: in silico prediction based on homology modelling and energy calculations. J. Mol. Biol. 2005;348:759–775. doi: 10.1016/j.jmb.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 205.Wohlgemuth S., Kiel C., Kramer A., Serrano L., Wittinghofer F., Herrmann C. Recognizing and defining true Ras binding domains I: biochemical analysis. J. Mol. Biol. 2005;348:741–758. doi: 10.1016/j.jmb.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 206.Falvella F.S., Manenti G., Spinola M., Pignatiello C., Conti B., Pastorino U., Dragani T.A. Identification of RASSF8 as a candidate lung tumor suppressor gene. Oncogene. 2006;25:3934–3938. doi: 10.1038/sj.onc.1209422. [DOI] [PubMed] [Google Scholar]
- 207.Falvella F.S., Spinola M., Manenti G., Conti B., Pastorino U., Skaug V., Haugen A., Dragani T.A. Common polymorphisms in D12S1034 flanking genes RASSF8 and BHLHB3 are not associated with lung adenocarcinoma risk. Lung Cancer. 2007;56:1–7. doi: 10.1016/j.lungcan.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 208.Debeer P., Schoenmakers E.F., Twal W.O., Argraves W.S., De Smet L., Fryns J.P., Van De Ven W.J. The fibulin-1 gene (FBLN1) is disrupted in a t(12;22) associated with a complex type of synpolydactyly. J. Med. Genet. 2002;39:98–104. doi: 10.1136/jmg.39.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hulo N., Bairoch A., Bulliard V., Cerutti L., De Castro E., Langendijk-Genevaux P.S., Pagni M., Sigrist C.J.A. The PROSITE database. Nucleic Acids Res. 2006;34:D227–D230. doi: 10.1093/nar/gkj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu L., Baier K., Dammann R., Pfeifer G.P. The tumor suppressor RASSF1A does not interact with Cdc20, an activator of the anaphase-promoting complex. Cell Cycle. 2007;6:1663–1665. doi: 10.4161/cc.6.13.4435. [DOI] [PubMed] [Google Scholar]