Abstract
Steffen Borrmann and colleagues discuss appropriate endpoints and their measurement during phase III trials of new antimalarial drugs.
In 2002, Plasmodium falciparum caused at least 0.5 billion uncomplicated clinical attacks of malaria worldwide, particularly in non-immune young children living in Africa [1]. Because inadequately treated uncomplicated P. falciparum malaria can progress rapidly to life-threatening severe malaria [2,3], mortality from P. falciparum in Africa doubled during the 1990s against a rising frequency of resistance to commonly used drugs, such as chloroquine and sulfadoxine-pyrimethamine [4].
In recent years, the deployment of highly efficacious oral three-day regimens of artemisinin-based combination therapies (ACTs) with parasitological cure rates of 95% or greater in more than 40 endemic countries has radically changed antimalarial treatment [5–8]. This success demands a new approach to the ways in which we assess new antimalarial drugs during clinical development and judge their potential utility for the public health deployment [9]. For example, the ability of slowly eliminated new drugs to delay re-infections and thus secondary malaria episodes for several weeks by suppressing the growth of P. falciparum provides additional public health benefits, especially in high-transmission areas where re-infection rates can exceed 50% in less than six weeks [10,11]. In turn, new drugs with comparatively higher efficacy against primary blood stage infections and/or a shorter elimination half-life minimise morbidity from recrudescent primary infections and may reduce the rate of spread of resistance [9,12], and are thus particularly valuable in situations of low or decreasing transmission rates [13] where the likelihood of re-infection during the relatively short post-treatment prophylactic period is lower (Figure 1A and 1B). There is already a general consensus on the design and interpretation of clinical trials used for monitoring antimalarial drug efficacy by national malaria control programmes [14]. The objective of this paper is to reflect on the design and interpretation of phase III trials.
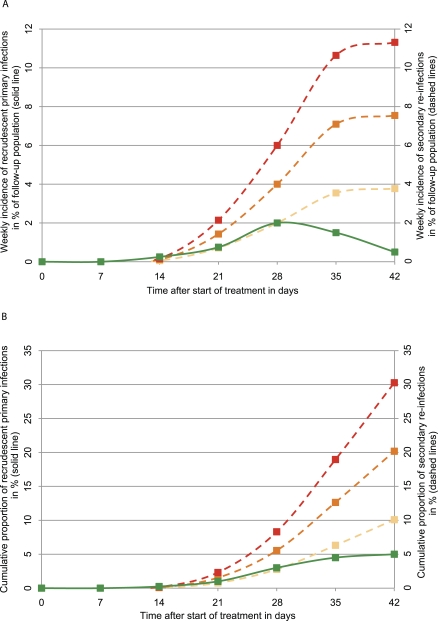
Figure 1. Simulated Plots of Weekly Incidence Rates and Cumulative Proportions for Recrudescent Primary Infections and Re-Infections Following Treatment of Uncomplicated P. falciparum Malaria with a Slowly Eliminated (Partner) Drug (Half-Life of ~1 Week).
The solid line represents recrudescent infections with a cumulative failure rate of 5% by day 42. Dashed lines represent different rates of re-infections corresponding to entomological inoculation rates of two (yellow), four (orange), and six (red) infective mosquito bites/year, respectively. Trailing plasma drug concentrations delay both detectable recrudescent primary and secondary blood stage infections—however, both effects are fading until day 42. Figure 1A illustrates that most recrudescent primary infections are captured by day 42. Extension of follow-up beyond day 28 results in increased ratios of new versus recrudescent infections and hence, elevated risk of outcome misclassifications due to intrinsic limitations of current molecular techniques used to discriminate between primary and secondary infections. The assessment of the total number of recurrent infections as a composite outcome (often denoted the PCR “uncorrected cure rate”) requires some time limits as re-infection occurs eventually in almost everyone after blood concentrations of the drug(s) fall below the MIC (Figure 1B).
Summary Points.
Curing malaria episodes is the key priority for antimalarial treatment; its measurement as primary endpoint in phase III trials provides consistent estimates of the antiparasitic effect of a new regimen.
The delay of secondary malaria episodes by slowly eliminated new drugs provides additional public health benefits in high-transmission areas; it should therefore be measured as key secondary or, preferably, co-primary endpoint.
WHO suggests aiming to achieve parasitological cure rates of ≥95%; if adopted for drug development this sets a high bar and may lead to the premature rejection of potentially valuable new drugs.
Since small differences in cure rates of ≥95% may be outweighed by advantages in cost, dosing, or tolerability, new drugs can be examined in non-inferiority trials with a proposed difference margin of ≤5%.
We recommend survival analysis of the primary endpoint.
Defining the Primary Endpoint
In the era of highly efficacious ACTs there is considerable debate among experts on what, exactly, constitutes the most relevant property of a new antimalarial drug [9,15]. In other words, in phase III trials, should we ask:
-
How efficacious is the new drug in curing primary blood stage infections (chemotherapeutic efficacy)?
or
-
How efficacious is the new regimen in curing primary infections and in preventing secondary infections (composite chemotherapeutic and post-treatment prophylactic efficacy)?
or
How efficacious is the new drug in reducing the post-treatment incidence of malaria and its complications (clinical risk reduction)?
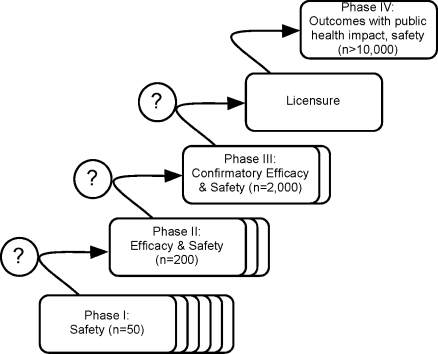
Whilst the scientific question being asked determines the definition of the primary endpoint, it is important to understand that the measure of drug efficacy by any of these three endpoints is, to a variable extent, determined by factors unrelated to the intrinsic antiparasitic effects of antimalarial treatment regimens. Extensive inter-population variation in levels of acquired host immunity [16] and parasite re-infection rates [10,17] complicates the interpretation and comparison of results from different geographical areas or between sites in multicentre phase III trials [18]. Pharmacogenetic differences have not been shown to play a major role in the variation in therapeutic response (although there are relatively few studies), but pharmacokinetic differences related to age and pregnancy have been large, and clinically important for several antimalarials [19]. This is a concern for malaria control programmes in endemic countries and agencies that fund drug purchases, both of whom require comparability of clinical trial outcomes to weigh the relative advantages of new antimalarial drugs as they emerge from the clinical development pipeline (phases I–IV; Figure 2).
Figure 2. Simplified Outline of the Iterative Process of the Clinical Development of New Antimalarial Candidate Drugs or Drug Combinations.
Phase III trials are designed to provide pivotal efficacy and safety data for obtaining regulatory approval. The indicated numbers of study participants are approximations of the magnitude of required total sample sizes in different transmission settings.
1. Cure of primary malaria episodes and infections.
The chemotherapeutic efficacy of an antimalarial drug against primary malaria episodes can be estimated by the established “in vivo test” methodology [20]. This test observes two key events. The first criterion is the alleviation of clinical symptoms and the suppression of the density of the pathogenic asexual blood stage parasites in the peripheral blood below the light-microscopic detection threshold (around 20–50 parasites/μl) within the first few days (avoiding “early treatment failure”). The second event is the potential recrudescence of persistent asexual blood stage parasites after one week (“late parasitological treatment failure”) (Figure 1A), which may or may not be associated with clinical symptoms of malaria (“late clinical treatment failure”) [18,20]. In the absence of re-infection, and assuming adequate drug absorption, the incidence of and the time to the microscopic detection of recrudescent blood stage infections has been shown to be primarily a function of (1) variability in pharmacodynamic variables, i.e., parasite susceptibility and initial parasite biomass; (2) pharmacokinetic parameters, i.e., drug elimination kinetics; and also (3) drug-unrelated parameters, primarily the patients' immune status [16,20–23].
As many drugs have no effects on pre-erythrocytic (liver stage) development, new blood stage infections may become patent as early as one week after blood concentrations of the antimalarial drug fall below the minimum inhibitory concentration (MIC) [24]. This situation requires molecular fingerprinting techniques to determine the likely origin of the parasite strain(s) in the recurrent infection and hence, to separate chemotherapeutic failures (recrudescence of the primary infection) from reinfection (Figure 1A) [14]. Since even a single misclassification can significantly change the risk difference between test and reference arms at cure rate estimates of at least 95%, the issue of accuracy of parasite strain genotyping (“PCR correction”) has attracted considerable attention [9,25–29]. The controversy centres on the very real risk that a variable proportion of recurrent infections may either (1) be misclassified leading to underestimation or (less likely) overestimation of cure rates or (2) remain indeterminate (unclassifiable) [29,30], especially in high-transmission areas when follow-up periods extend past day 28 (Figure 1A) [25–28]. There are also unresolved issues related to relapses of P. vivax infections in Asia [31]. Even so, re-infection-adjusted parasitological cure rates provide demonstrably consistent estimates of the antiparasitic effect of an antimalarial regimen across different transmission settings [10,17], and the World Health Organization (WHO) endorses PCR-corrected primary endpoints for monitoring antimalarial drug efficacy in endemic countries [14,32]. At the same time, the exclusion or censoring of all reinfections limits the clinical relevance of chemotherapeutic endpoints in high-transmission areas (Figure 1B).
2. Cure of primary infections and prevention of re-infections.
Of course, any recurrent infection can be considered a failure, as even new infections reflect a post-treatment “chemoprophylaxis” breakthrough (Figure 1A) [33]. Frequently, this effect has been assessed by using the proportion of the total number of recurrences as a composite endpoint without distinguishing between recrudescent primary and secondary infections (or for that matter, curative and preventive effects) [15,34]. But since re-infection occurs eventually in almost everyone after blood concentrations of the drug(s) fall below the MIC, the measurement of the composite endpoint requires some time limits.
In high-transmission areas, the composite effect size will largely be determined by the post-treatment prophylactic efficacy against secondary infections [10]. The duration of this protection depends on the ability of the drug to suppress the clinically silent intra-hepatocytic parasite development and on the elimination half-life of the drug (Figure 1A) [33]. The problem with assessing the composite outcome (often denoted the PCR “uncorrected cure rate”) alone is that it combines two effects (curative activity and post-treatment prophylaxis) that, although related, are not directly proportional to each other (Figure 1B). To illustrate this point, the reported crude, PCR-uncorrected parasitological day 28 failure rates in two trials of the recently registered six-dose regimen of artemether-lumefantrine can be compared. These rates differed by 25% whereas the corresponding parasitological cure rate estimates adjusted for the difference in re-infection rates varied by only 7% [10,17]. The interpretation of such large inter-site variations poses a problem in rigorous and costly phase III programs where the reproducibility of what the phase III trial set out to measure, i.e., the antiparasitic effect of the new regimen, is a prime concern [14].
3. Post-treatment reduction of clinical risk.
Recrudescent primary infections, as well as re-infections, entail the risk of secondary malaria and/or hematological complications requiring re-treatment [34,35]. Clinical endpoints, which measure the reduction of these clinical events, provide important public health–relevant information above parasitologically defined endpoints [15,36,37].
The risk that a patient with recurrent infection will be symptomatic at detection or succumb subsequently to secondary malaria during follow-up is related to the individual level of specific, if imperfect, acquired immunity (“semi-immunity”). For example, an analysis of antimalarial treatment trials in high-transmission areas found that children below one year were at least three times as likely to experience symptomatic recurrences than children aged more than three years [34]. This dependence of clinical risks on the prevalence of acquired immunity in the study population or age group [18,34] (on top of large variation introduced by different reinfection/recurrence rates) confounds the estimation of the antiparasitic efficacy of an antimalarial drug by clinical endpoints, and therefore undermines the comparability of study results between different endemic areas, leading to large inter-site variation in multicentre phase III trials [18]. Alternatively, preliminary data from study sites on key confounding factors could, in conjunction with normalisation provided by a standard comparator treatment, be used to adjust for inter-site differences in trials with a primary clinical endpoint. The feasibility of such an adjusted primary analysis approach in regulatory phase III trials needs to be explored further.
Based on the above considerations, whilst it is clear that, in areas of very high transmission, multiplicity of infection confers a significant and irreducible error in genotyping [38], parasite “strain typing” with the highest possible resolution power is required for separating curative and preventive effects in regulatory phase III trials of new antimalarial drugs. Chemotherapeutic efficacy against primary malaria episodes and infections should be the primary endpoint of phase III trials; protective efficacy against secondary infections and clinical episodes should be either key secondary endpoints, or in high-transmission areas, possibly co-primary endpoints. A broad consultation on the utility, classification, and respective merits of alternative, especially PCR-uncorrected, composite endpoints in phase III trials of new antimalarial drugs, including measurement of the delay instead of proportions of recurrent infections, should be undertaken.
Defining the Benchmark for Efficacy
Optimal target profiles for new antimalarial drugs have been described elsewhere and used as guiding principles from early discovery through clinical development [39,40]. In an ideal world, antimalarial treatments would be 100% efficacious; in the real world, WHO suggests aiming to achieve parasitological cure rate point estimates of at least 95% (excluding re-infections) [41]. To establish with 95% confidence that a new treatment can demonstrate a cure rate of at least 95% in a phase III trial with 500 patients per group, the true (unknown) cure rate would have to be at least 97%. This sets a high bar for evaluating new candidate drugs and might lead to the premature rejection of new products because of suboptimal dosing, formulation, or the play of chance. A greater than 90% parasitological cure rate (lower boundary of the 95% confidence interval) represents an alternative, more realistic initial target for new treatments (requiring only a true cure rate of at least 93% under the same sample size assumption).
To date, there is no guidance on the benchmark for the protective efficacy of a new antimalarial drug in preventing secondary infections, or the composite efficacy of curative and preventive effects. The establishment of a uniform benchmark is challenging: in addition to clearing more than 90% of primary infections, should a new drug prevent 30%, 40%, or more of secondary infections? Over which time frame—four, six, or more weeks? Or, put more simply, for how long on average should re-infections or, more generally, recurrent infection be delayed ? Should the new drug be superior to or “just as good as” the reference treatment? Or to use a hypothetical example, in times of continued shortage of viable therapeutic options, is it desirable to turn down a new once-daily regimen with a composite efficacy comparable to the recently introduced twice-daily regimen of artemetherlumefantrine, but which may be inferior to dihydroartemisinin-piperaquine because piperaquine is exceptionally slowly eliminated [10,42,43]? All this needs to be considered in a context of declining malaria transmission as effective control measures are rolled out. There is currently no consensus on these issues.
Duration of Follow-Up
Figure 1A illustrates how trailing plasma drug concentrations of slowly eliminated antimalarial drugs delay detectable recrudescent primary and secondary blood stage infections alike. These effects fade during follow-up beyond day 28.
The current use of day 28 estimates provides a single benchmark period to compare all new drugs, but we feel there is a need to revisit this single time-point for phase III trial endpoints, particularly for new investigational drugs or combinations containing at least one component with an intrinsically long plasma half-life.
Superiority or Non-Inferiority?
In phase III, the new candidate drug is compared in a randomised controlled trial [44] to a standard treatment that retains the desired “control effect size” [44], e.g., the targeted more than 90% parasitological cure rate (excluding reinfections). Historically, when existing recommended treatments were failing, antimalarial phase III drug trials have been designed as superiority trials [9,45], but there is a dramatic increase in the sample sizes required to demonstrate superiority of a potential future replacement for a drug with currently very high parasitological cure rates [9].
Small differences in parasitological cure rates demonstrated in superiority trials, especially between 95% and 100%, are important in reducing the emergence and spread of resistance [9,12], but from a pragmatic perspective such differences may be outweighed by advantages in cost, dosing, shelf lives, or tolerability, or importantly, by the gain of a therapeutic alternative should parasite resistance to the current first-line drug emerge. An alternative to the superiority criterion for a new drug is whether it is “no worse than” the standard treatment [9]. Non-inferiority trials test if the observed difference between treatment arms falls below a difference Δ margin; if not, the new treatment is considered non-inferior [46]. The Δ margin is selected to ensure that a drug with clinically meaningful inferiority to the comparator treatment is rejected [47]. We propose to use a Δ margin of 5%, or its equivalent as hazard ratio limit, within the limits of cure rates exceeding 90%.
The superiority of the post-treatment prophylactic efficacy of a new drug with comparatively higher anti-liver stage activity or prolonged plasma half-life can be best examined in areas with high re-infection rates because of the postulated differential impact on re-infection and/or secondary malaria rates [33]. The comparison to a reference treatment with similar pharmacodynamic and pharmacokinetic characteristics, however, requires inflated sample sizes in high-transmission areas.
Linked Perspective.
This Policy Forum is further discussed in a PLoS Medicine Perspective by Colin J. Sutherland:
Sutherland CJ (2008) Comparing highly efficacious antimalarial drugs. PLoS Med 5(11): e228. doi:10.1371/journal.pmed.0050228
Analytical Strategies
Intention-to-treat versus per-protocol approach.
The transition from superiority to non-inferiority trial designs has implications for the choice of the primary analysis population [48]. Non-inferiority trials lack internal controls for assessment accuracy, a conservative analysis approach, and protection from bias by blinding [9]. Technical and methodological inadequacies, e.g., missing or uninterpretable PCR data in an intention-to-treat analysis (considered as conservative in superiority trials), or simple mistakes can blur true differences in treatment effects and thus lead to inadvertent conclusion of non-inferiority when in fact the new drug is inferior (type I error) [46,49]. On the other hand, indiscriminate classification of defaulting patients as failures in the intention-to-treat analysis (“worst-case scenario”), particularly losses to follow-up or re-infections when recrudescent rates or time to recrudescence are the primary endpoint, reduces the power of non-inferiority trials and thus leads to incorrect finding of inferiority when in fact the new drug is not inferior (type II error). If the primary endpoint is measured as proportional point estimate (i.e., not by survival analysis), we recommend basing the analysis on the per-protocol population, which includes only patients with observed treatment responses and other informative outcomes, e.g., intake of outside-protocol antimalarial medication. The preferred method for comparing antimalarial drug efficacy, however, is survival analysis [9].
Survival analysis.
Survival analysis techniques are increasingly used to assess failure rates in randomised phase III trials [49]. The key advantages of this approach for antimalarial, or any other anti-infective drug studies, are: (1) the statistical model reflects specific biological processes (delay of recrudescence or re-infection [20]) and (2) the analytical procedure deals specifically with incomplete but informative data, i.e., patients with incomplete follow-up contribute to the assessment, whereas they are excluded in per-protocol analyses [9,50]. Reinfections, losses to follow-up, and protocol deviations can be censored at the time of defaulting. Analogous to tests of the risk difference of point estimates [37], the non-inferiority hypothesis of survival estimates can be examined by using a modified confidence interval (CI) approach [51].
Interpretation of Trial Results
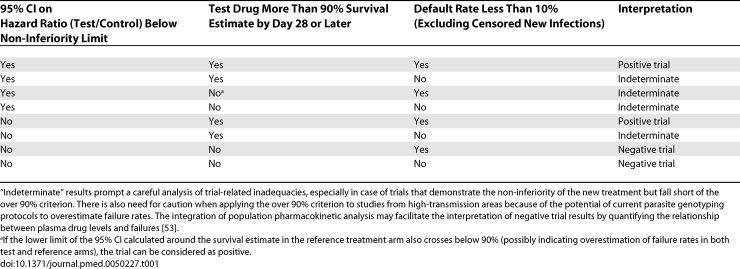
The interpretation of a non-inferiority trial with a survival estimate of recrudescent infections can be based on two criteria: (1) the hypothesis test result, e.g., the 95% CI of the proportional hazard ratio test/control remains below the non-inferiority limit on the hazard ratio to support claims of efficacy [51]; and (2) the day 28 and later survival rate estimate in the test drug arm. Table 1 lists possible combinations of trial results and recommendations for their interpretation. The standardised reporting of key baseline and outcome variables of antimalarial trials using a hierarchical system will greatly facilitate further detailed post-hoc analyses and interpretations [18].
Table 1.
Potential Outcome Scenarios of Antimalarial Phase III Trials with a Chemotherapeutic Endpoint and Their Proposed Interpretation
Conclusion
Consensus-agreed regulatory guidelines on how phase III trials of antimalarial drugs for uncomplicated P. falciparum malaria are designed and interpreted are only now being developed [52]. This review intends to stimulate an informed discussion on the utility of the different primary endpoints in future phase III antimalarial trials and proposes a comparative framework for the interpretation of results from ongoing trials. We hope that consensus between academia, public health professionals, industry, and regulators on the design and particularly the primary endpoint of phase III trials allows earlier appraisal of the potential advantages or equivalence of a new treatment in comparison with existing therapies ahead of more extensive phase IV programmes.
Acknowledgments
We would like to acknowledge the contributions of Carl Craft and Lise Riopel, who kindly provided comments on the manuscript. This paper is published with the permission of the director of the Kenyan Medical Research Institute. The authors would like to acknowledge the valuable input of Kasia Stepniewska during the development of this paper.
Glossary
Abbreviations
- ACT
artemisinin-based combination therapy
- CI
confidence interval
- MIC
minimum inhibitory concentration
- WHO
World Health Organization
Footnotes
Steffen Borrmann is with the Kenya Medical Research Institute/Wellcome Trust Research Programme, Centre for Geographical Medicine Research –Coast, Kilifi, Kenya; and the University of Heidelberg School of Medicine, Institute of Hygiene, Heidelberg, Germany. Tim Peto is with the Nuffield Department of Clinical Medicine, John Radcliffe Hospital, Headington, Oxford, United Kingdom. Robert W. Snow is with the Malaria Public Health and Epidemiology Group, Centre for Geographic Medicine, Kenya Medical Research Institute/Wellcome Trust Research Programme, Nairobi, Kenya; and the Centre for Vaccinology and Tropical Medicine, University of Oxford, Churchill Hospital, Headington, Oxford, United Kingdom. Win Gutteridge is with the Medicines for Malaria Venture, Geneva, Switzerland. Nicholas J. White is with the Centre for Vaccinology and Tropical Medicine, University of Oxford, Churchill Hospital, Headington, Oxford, United Kingdom; and the Mahidol - Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Funding: SB is funded by a German Research Foundation (DFG) Junior Group grant (SFB 544, A7). RWS and NJW are Wellcome Trust Principal Research Fellows. SB and RWS acknowledge the support of the Kenyan Medical Research Institute. NJW is part of the Mahidol Oxford Tropical Medicine Research Unit funded by the Wellcome Trust of Great Britain. The funding sources played no role in the decision to submit the article or in its preparation.
Competing Interests: WG is chair of the Medicines for Malaria Expert Scientific Advisory Committee. The remaining authors declare that they have no competing interests.
Provenance: Not commissioned; externally peer reviewed
References
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- Snow RW, Marsh K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–264. doi: 10.1016/s0065-308x(02)52013-3. [DOI] [PubMed] [Google Scholar]
- Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–597. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- Snow RW, Eckert E, Teklehaimanot A. Estimating the needs for artesunate-based combination therapy for malaria case-management in Africa. Trends Parasitol. 2003;19:363–369. doi: 10.1016/s1471-4922(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Mutabingwa TK. Artemisinin-based combination therapies (ACTs): Best hope for malaria treatment but inaccessible to the needy! Acta Trop. 2005;95:305–315. doi: 10.1016/j.actatropica.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bathurst I, Hentschel C. Medicines for Malaria Venture: Sustaining antimalarial drug development. Trends Parasitol. 2006;22:301–307. doi: 10.1016/j.pt.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Amin AA, Zurovac D, Kangwana BB, Greenfield J, Otieno DN, et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar J. 2007;6:72. doi: 10.1186/1475-2875-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska K, White NJ. Some considerations in the design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria. Malar J. 2006;5:127. doi: 10.1186/1475-2875-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, et al. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: A randomized trial. PLoS Clin Trials. 2007;2:e20. doi: 10.1371/journal.pctr.0020020. doi: 10.1371/journal.pctr.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedke SG, Mpimbaza A, Kamya MR, Nzarubara BK, Dorsey G, et al. Combination treatments for uncomplicated falciparum malaria in Kampala, Uganda: Randomised clinical trial. Lancet. 2004;364:1950–1957. doi: 10.1016/S0140-6736(04)17478-3. [DOI] [PubMed] [Google Scholar]
- Uhlemann AC, McGready R, Ashley EA, Brockman A, Singhasivanon P, et al. Intrahost selection of Plasmodium falciparum pfmdr1 alleles after antimalarial treatment on the northwestern border of Thailand. J Infect Dis. 2007;195:134–141. doi: 10.1086/509809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai A, Ali AS, Kachur SP, Martensson A, Abbas AK, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Methods and techniques for clinical trials on antimalarial drug efficacy. 2008. Available: http://www.who.int/malaria/docs/drugresistance/MalariaGenotyping.pdf. Accessed 10 October 2008.
- Laufer MK, Djimde AA, Plowe CV. Monitoring and deterring drug-resistant malaria in the era of combination therapy. Am J Trop Med Hyg. 2007;77:160–169. [PubMed] [Google Scholar]
- Borrmann S, Matsiegui PB, Missinou MA, Kremsner PG. Effects of Plasmodium falciparum parasite population size and patient age on early and late parasitological outcomes of antimalarial treatment in children. Antimicrob Agents Chemother. 2008;52:1799–1805. doi: 10.1128/AAC.00755-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, et al. Supervised versus unsupervised intake of six-dose artemether-lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: A randomised trial. Lancet. 2005;365:1467–1473. doi: 10.1016/S0140-6736(05)66416-1. [DOI] [PubMed] [Google Scholar]
- Price RN, Dorsey G, Ashley EA, Barnes KI, Baird JK, et al. World Antimalarial Resistance Network I: Clinical efficacy of antimalarial drugs. Malar J. 2007;6:119. doi: 10.1186/1475-2875-6-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KI, Watkins WM, White NJ. Antimalarial dosing regimens and drug resistance. Trends Parasitol. 2008;24:127–134. doi: 10.1016/j.pt.2007.11.008. [DOI] [PubMed] [Google Scholar]
- White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djimde AA, Doumbo OK, Traore O, Guindo AB, Kayentao K, et al. Clearance of drug-resistant parasites as a model for protective immunity in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:558–563. [PubMed] [Google Scholar]
- Stepniewska K, Taylor WR, Mayxay M, Price R, Smithuis F, et al. In vivo assessment of drug efficacy against Plasmodium falciparum malaria: Duration of follow-up. Antimicrob Agents Chemother. 2004;48:4271–4280. doi: 10.1128/AAC.48.11.4271-4280.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RN, Uhlemann AC, van Vugt M, Brockman A, Hutagalung R, et al. Molecular and pharmacological determinants of the therapeutic response to artemether-lumefantrine in multidrug-resistant Plasmodium falciparum malaria. Clin Infect Dis. 2006;42:1570–1577. doi: 10.1086/503423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JE, Rao S, Williams F, Freilich D, Luke T, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: An update. J Infect Dis. 2007;196:145–154. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- Guthmann JP, Pinoges L, Checchi F, Cousens S, Balkan S, et al. Methodological issues in the assessment of antimalarial drug treatment: Analysis of 13 studies in eight African countries from 2001 to 2004. Antimicrob Agents Chemother. 2006;50:3734–3739. doi: 10.1128/AAC.01618-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Yeka A, Nsobya SL, Dokomajilar C, Rosenthal PJ, et al. Complexity of Plasmodium falciparum infections and antimalarial drug efficacy at 7 sites in Uganda. J Infect Dis. 2006;193:1160–1163. doi: 10.1086/501473. [DOI] [PubMed] [Google Scholar]
- Slater M, Kiggundu M, Dokomajilar C, Kamya MR, Bakyaita N, et al. Distinguishing recrudescences from new infections in antimalarial clinical trials: Major impact of interpretation of genotyping results on estimates of drug efficacy. Am J Trop Med Hyg. 2005;73:256–262. [PubMed] [Google Scholar]
- Gatton ML, Cheng Q. Can estimates of antimalarial efficacy from field studies be improved. Trends Parasitol. 2008;24:68–73. doi: 10.1016/j.pt.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WJ, Greenhouse B, Rosenthal PJ, Dorsey G. The use of genotyping in antimalarial clinical trials: A systematic review of published studies from 1995–2005. Malar J. 2006;5:122. doi: 10.1186/1475-2875-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnert A, Arez AP, Babiker HA, Beck HP, Benito A, et al. Genotyping of Plasmodium falciparum infections by PCR: A comparative multicentre study. Trans R Soc Trop Med Hyg. 2001;95:225–232. doi: 10.1016/s0035-9203(01)90175-0. [DOI] [PubMed] [Google Scholar]
- Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, et al. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. 2003. Available: http://www.who.int/malaria/docs/ProtocolWHO.pdf. Accessed 10 October 2008.
- White NJ. How antimalarial drug resistance affects post-treatment prophylaxis. Malar J. 2008;7:9. doi: 10.1186/1475-2875-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro P, Pinoges L, Checchi F, Vaillant M, Guthmann JP. Risk associated with asymptomatic parasitaemia occurring post-antimalarial treatment. Trop Med Int Health. 2008;13:83–90. doi: 10.1111/j.1365-3156.2007.01977.x. [DOI] [PubMed] [Google Scholar]
- Smith T, Schellenberg JA, Hayes R. Attributable fraction estimates and case definitions for malaria in endemic areas. Stat Med. 1994;13:2345–2358. doi: 10.1002/sim.4780132206. [DOI] [PubMed] [Google Scholar]
- Staedke SG, Kamya MR, Dorsey G, Gasasira A, Ndeezi G, et al. Amodiaquine, sulfadoxine/pyrimethamine, and combination therapy for treatment of uncomplicated falciparum malaria in Kampala, Uganda: A randomised trial. Lancet. 2001;358:368–374. doi: 10.1016/S0140-6736(01)05557-X. [DOI] [PubMed] [Google Scholar]
- Zongo I, Dorsey G, Rouamba N, Tinto H, Dokomajilar C, et al. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: A randomised non-inferiority trial. Lancet. 2007;369:491–498. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- Greenhouse B, Dokomajilar C, Hubbard A, Rosenthal PJ, Dorsey G. Impact of transmission intensity on the accuracy of genotyping to distinguish recrudescence from new infection in antimalarial clinical trials. Antimicrob Agents Chemother. 2007;51:3096–3103. doi: 10.1128/AAC.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- Medicines for Malaria Venture. Target product profile. 2008. Available: http://www.mmv.org/IMG/pdf/PRODUCT_PROFILE_with_logo.pdf. Accessed 10 October 2008.
- World Health Organization. Guidelines for the treatment of malaria. 2006. Available: http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf. Accessed 10 October 2008.
- Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung RM, et al. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: An open-label randomised comparison. Lancet. 2007;369:757–765. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarning J, Lindegardh N, Annerberg A, Singtoroj T, Day NP, et al. Pitfalls in estimating piperaquine elimination. Antimicrob Agents Chemother. 2005;49:5127–5128. doi: 10.1128/AAC.49.12.5127-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Grimes DA. Generation of allocation sequences in randomised trials: Chance, not choice. Lancet. 2002;359:515–519. doi: 10.1016/S0140-6736(02)07683-3. [DOI] [PubMed] [Google Scholar]
- Myint HY, Tipmanee P, Nosten F, Day NP, Pukrittayakamee S, et al. A systematic overview of published antimalarial drug trials. Trans R Soc Trop Med Hyg. 2004;98:73–81. doi: 10.1016/s0035-9203(03)00014-2. [DOI] [PubMed] [Google Scholar]
- Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: The importance of rigorous methods. BMJ. 1996;313:36–39. doi: 10.1136/bmj.313.7048.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency. Guideline on the choice of the non-inferiority margin. 2005. Available: http://www.sctweb.org/presentations/2008/GuidelineontheChoiceoftheNon-inferiorityMargin.pdf. Accessed 10 October 2008.
- European Medicines Agency. Points to consider on switching between superiority and non-inferiority. 2000. Available: http://www.emea.europa.eu/pdfs/human/ewp/048299en.pdf. Accessed 10 October 2008. [DOI] [PMC free article] [PubMed]
- Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ. Reporting of noninferiority and equivalence randomized trials: An extension of the CONSORT statement. JAMA. 2006;295:1152–1160. doi: 10.1001/jama.295.10.1152. [DOI] [PubMed] [Google Scholar]
- Machekano R, Dorsey G, Hubbard A. Efficacy studies of malaria treatments in Africa: Efficient estimation with missing indicators of failure. Stat Methods Med Res. 2008;17:191–206. doi: 10.1177/0962280207078202. [DOI] [PubMed] [Google Scholar]
- Rothmann M, Li N, Chen G, Chi GY, Temple R, et al. Design and analysis of non-inferiority mortality trials in oncology. Stat Med. 2003;22:239–264. doi: 10.1002/sim.1400. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administartion. Guidance for industry. Antibacterial drug products: Use of noninferiority studies to support approval. 2007. Available: http://www.fda.gov/Cder/guidance/7884dft.htm. Accessed 10 October 2008.
- Price RN, Hasugian AR, Ratcliff A, Siswantoro H, Purba HL, et al. Clinical and pharmacological determinants of the therapeutic response to dihydroartemisinin-piperaquine for drug-resistant malaria. Antimicrob Agents Chemother. 2007;51:4090–4097. doi: 10.1128/AAC.00486-07. [DOI] [PMC free article] [PubMed] [Google Scholar]