Abstract
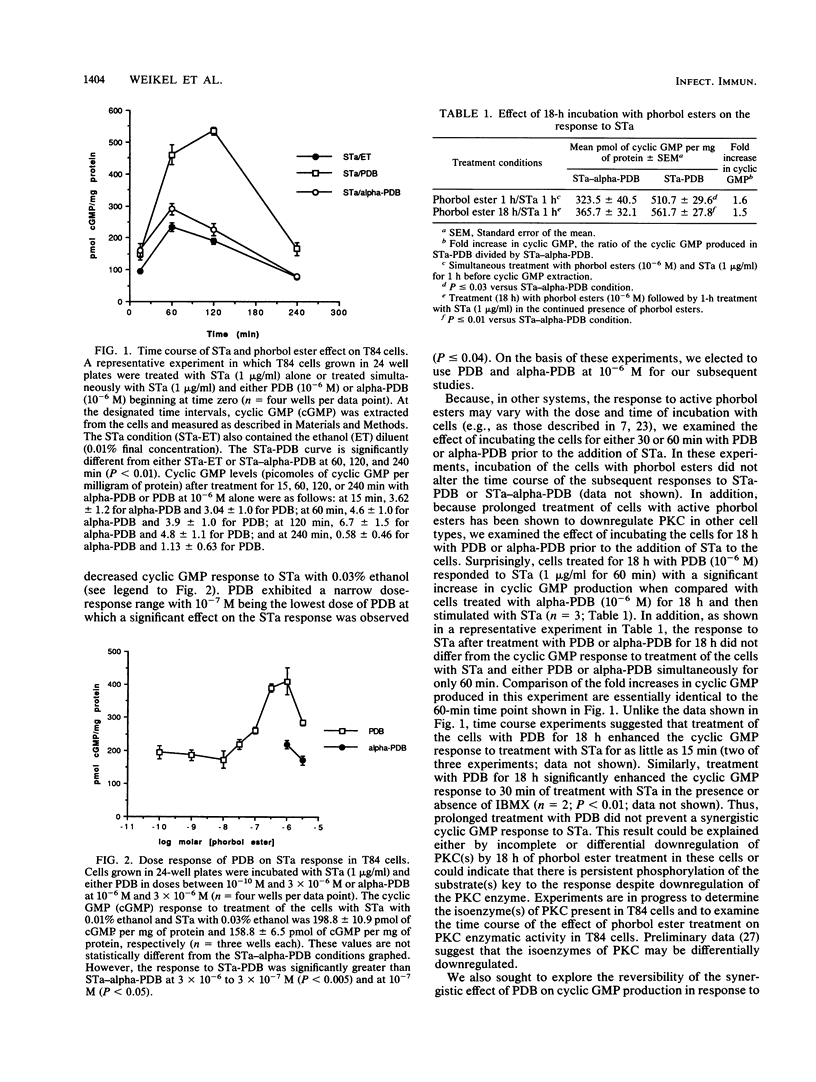
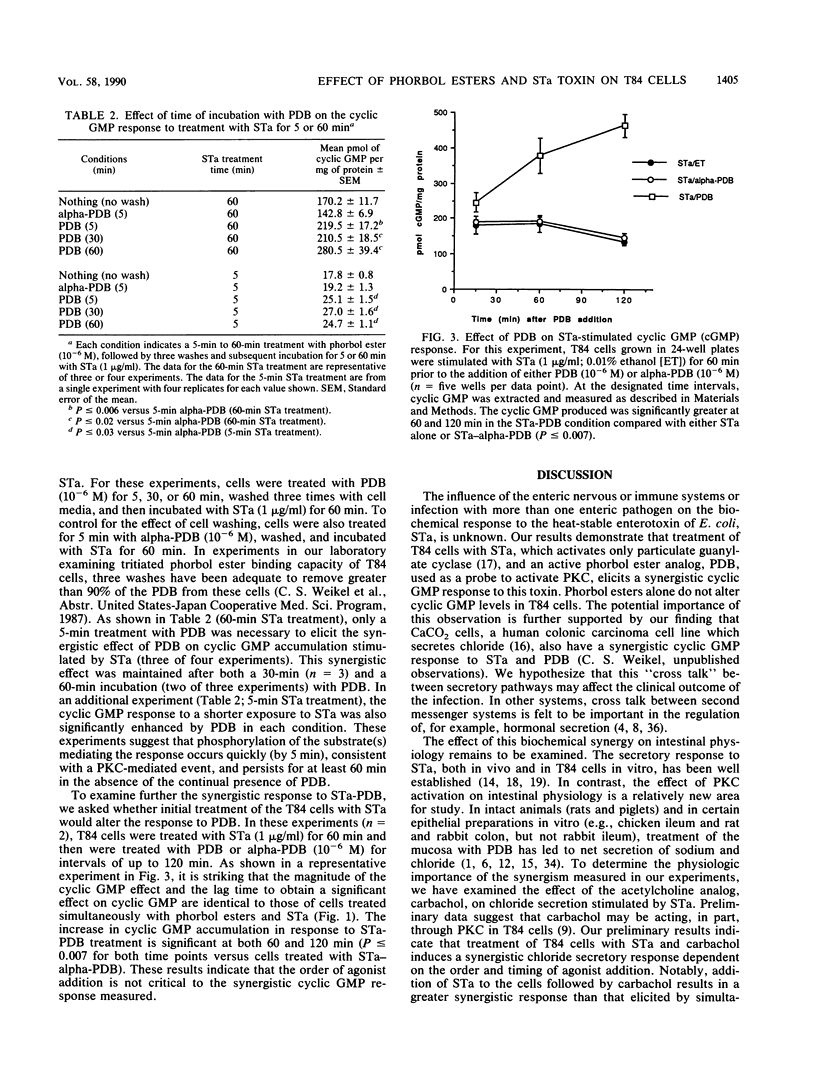
We examined the effect of protein kinase C (PKC) activation on the cyclic GMP response to heat-stable enterotoxin (STa) in a colonic carcinoma intestinal epithelial cell line, T84 cells. Our results demonstrate that the active phorbol ester analog, phorbol dibutyrate, but not the inactive alpha-phorbol dibutyrate, acts synergistically with STa to elevate cyclic GMP in intact T84 cells. The effect is observed in the absence or presence of the phosphodiesterase inhibitor, isobutylmethylxanthine, which suggests that phorbol dibutyrate modifies cyclic GMP synthesis rather than cyclic GMP degradation. In contrast to several systems in which prolonged treatment with phorbol ester desensitizes PKC-mediated responses, the cyclic GMP response in T84 cells is not diminished by prolonged treatment of T84 cells with phorbol dibutyrate. Also, transient treatment of T84 cells with phorbol dibutyrate enhances subsequent STa-stimulated cyclic GMP accumulation. These observations suggest that PKC activation produces a long-lived signal in T84 cells which enhances cyclic GMP accumulation in response to STa. Second messenger "cross talk" [T. Yoshimasa, D. R. Sibley, M. Bouvier, R. J. Lefkowitz, and M. G. Caron, Nature (London) 327:67-70, 1987] may be important in the pathogenesis of diarrheal disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn J., Chang E. B., Field M. Phorbol ester inhibition of Na-H exchange in rabbit proximal colon. Am J Physiol. 1985 Nov;249(5 Pt 1):C527–C530. doi: 10.1152/ajpcell.1985.249.5.C527. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooker G., Terasaki W. L., Price M. G. Gammaflow: a completely automated radioimmunoassay system. Science. 1976 Oct 15;194(4262):270–276. doi: 10.1126/science.184530. [DOI] [PubMed] [Google Scholar]
- Brostrom M. A., Brostrom C. O., Brotman L. A., Green S. S. Regulation of Ca2+-dependent cyclic AMP accumulation and Ca2+ metabolism in intact pituitary tumor cells by modulators of prolactin production. Mol Pharmacol. 1983 Mar;23(2):399–408. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chang E. B., Wang N. S., Rao M. C. Phorbol ester stimulation of active anion secretion in intestine. Am J Physiol. 1985 Sep;249(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1985.249.3.C356. [DOI] [PubMed] [Google Scholar]
- Coffey R. G., Hadden J. W. Phorbol myristate acetate stimulation of lymphocyte guanylate cyclase and cyclic guanosine 3':5'-monophosphate phosphodiesterase and reduction of adenylate cyclase. Cancer Res. 1983 Jan;43(1):150–158. [PubMed] [Google Scholar]
- Cronin M. J., Summers S. T., Sortino M. A., Hewlett E. L. Protein kinase C enhances growth hormone releasing factor (1-40)-stimulated cyclic AMP levels in anterior pituitary. Actions of somatostatin and pertussis toxin. J Biol Chem. 1986 Oct 25;261(30):13932–13935. [PubMed] [Google Scholar]
- Dharmsathaphorn K., Cohn J., Beuerlein G. Multiple calcium-mediated effector mechanisms regulate chloride secretory responses in T84-cells. Am J Physiol. 1989 Jun;256(6 Pt 1):C1224–C1230. doi: 10.1152/ajpcell.1989.256.6.C1224. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Pandol S. J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986 Feb;77(2):348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Cheng H. Y., Sharp G. W. Effects of phorbol esters on sodium and chloride transport in rat colon. Am J Physiol. 1986 Oct;251(4 Pt 1):G509–G517. doi: 10.1152/ajpgi.1986.251.4.G509. [DOI] [PubMed] [Google Scholar]
- Dreyfus L. A., Frantz J. C., Robertson D. C. Chemical properties of heat-stable enterotoxins produced by enterotoxigenic Escherichia coli of different host origins. Infect Immun. 1983 Nov;42(2):539–548. doi: 10.1128/iai.42.2.539-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset E., Bernabeu J., Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol. 1985 May;248(5 Pt 1):C410–C418. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Hughes J. M., Chang B., Robertson D. C., Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980 Aug;142(2):220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Huott P. A., Liu W., McRoberts J. A., Giannella R. A., Dharmsathaphorn K. Mechanism of action of Escherichia coli heat stable enterotoxin in a human colonic cell line. J Clin Invest. 1988 Aug;82(2):514–523. doi: 10.1172/JCI113626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal R. K., Jaiswal N., Sharma R. K. Negative regulation of atrial natriuretic factor receptor coupled membrane guanylate cyclase by phorbol ester. Potential protein kinase C regulation of cyclic GMP signal in isolated adrenocortical carcinoma cells of rat. FEBS Lett. 1988 Jan 18;227(1):47–50. doi: 10.1016/0014-5793(88)81411-x. [DOI] [PubMed] [Google Scholar]
- Kennedy D. J., Greenberg R. N., Dunn J. A., Abernathy R., Ryerse J. S., Guerrant R. L. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect Immun. 1984 Dec;46(3):639–643. doi: 10.1128/iai.46.3.639-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss Z., Steinberg R. A. Phorbol ester-mediated protein phosphorylations in S49 mouse lymphoma cells. Cancer Res. 1985 Jun;45(6):2732–2740. [PubMed] [Google Scholar]
- Lai W. S., el-Fakahany E. E. Phorbol ester-induced inhibition of cyclic GMP formation mediated by muscarinic receptors in murine neuroblastoma cells. J Pharmacol Exp Ther. 1987 May;241(2):366–373. [PubMed] [Google Scholar]
- Moss J., Burns D. L., Hsia J. A., Hewlett E. L., Guerrant R. L., Vaughan M. NIH conference. Cyclic nucleotides: mediators of bacterial toxin action in disease. Ann Intern Med. 1984 Nov;101(5):653–666. doi: 10.7326/0003-4819-101-5-653. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ono Y., Fujii T., Ogita K., Kikkawa U., Igarashi K., Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988 May 15;263(14):6927–6932. [PubMed] [Google Scholar]
- Patel A., Linden J. Purification of 125I-labeled succinyl cyclic nucleotide tyrosine methyl esters by high-performance liquid chromatography. Anal Biochem. 1988 Feb 1;168(2):417–420. doi: 10.1016/0003-2697(88)90338-7. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. Calcium and cAMP in stimulus-response coupling. Ann N Y Acad Sci. 1980;356:346–353. doi: 10.1111/j.1749-6632.1980.tb29622.x. [DOI] [PubMed] [Google Scholar]
- Snyder J. D., Merson M. H. The magnitude of the global problem of acute diarrhoeal disease: a review of active surveillance data. Bull World Health Organ. 1982;60(4):605–613. [PMC free article] [PubMed] [Google Scholar]
- Walsh J. A., Warren K. S. Selective primary health care: an interim strategy for disease control in developing countries. N Engl J Med. 1979 Nov 1;301(18):967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- Warhurst G., Higgs N. B., Lees M., Tonge A., Turnberg L. A. Activation of protein kinase C attenuates prostaglandin E2 responses in a colonic cell line. Am J Physiol. 1988 Jul;255(1 Pt 1):G27–G32. doi: 10.1152/ajpgi.1988.255.1.G27. [DOI] [PubMed] [Google Scholar]
- Weikel C. S., Nellans H. N., Guerrant R. L. In vivo and in vitro effects of a novel enterotoxin, STb, produced by Escherichia coli. J Infect Dis. 1986 May;153(5):893–901. doi: 10.1093/infdis/153.5.893. [DOI] [PubMed] [Google Scholar]
- Weikel C. S., Sando J. J., Guerrant R. L. Stimulation of porcine jejunal ion secretion in vivo by protein kinase-C activators. J Clin Invest. 1985 Dec;76(6):2430–2435. doi: 10.1172/JCI112258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weymer A., Huott P., Liu W., McRoberts J. A., Dharmsathaphorn K. Chloride secretory mechanism induced by prostaglandin E1 in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1828–1836. doi: 10.1172/JCI112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]
- Zwiller J., Revel M. O., Malviya A. N. Protein kinase C catalyzes phosphorylation of guanylate cyclase in vitro. J Biol Chem. 1985 Feb 10;260(3):1350–1353. [PubMed] [Google Scholar]


