Abstract
The frequency of stimulation is one of the primary factors determining the effectiveness of deep brain stimulation (DBS) in relieving tremor. DBS efficacy, however, may depend not only on the average frequency of stimulation, but also on the temporal pattern of stimulation. We conducted intraoperative measurements of the effect of temporally irregular DBS (nonconstant interpulse intervals) on tremor. As the coefficient of variation of irregular high frequency DBS trains increased, they became less effective at reducing tremor (mixed effects regression model, P<0.04).These data provide evidence that the effects of DBS are dependent not only on the average frequency of DBS, but also on the regularity of the temporal spacing of DBS pulses.
Keywords: basal ganglia, deep brain stimulation, movement disorders, subthalamic nucleus, thalamus, tremor, ventral thalamic nuclei
Introduction
Deep brain stimulation (DBS) is an established therapy for the treatment of movement disorders, including essential tremor and Parkinson’s disease. Although the clinical benefits of DBS are well documented, the mechanisms of action remain unclear. The stimulation frequency has a strong impact on outcomes, and maximal reductions in tremor are typically observed only when the frequency is ≥90Hz [1-5]. Recent studies suggest that regularization of neuronal firing underlies the effectiveness of high frequency DBS [6-10], and we hypothesized that temporally irregular stimulus trains would be less effective at suppressing tremor than regular high frequency trains. Here we report tremor responses to DBS trains with the same average frequency but with varying degrees of temporal irregularity.
Methods
Human participants
We conducted experiments on four individuals with DBS-responsive tremor who were having their implantable pulse generator (IPG) surgically replaced due to depleted batteries (Table 1). Irregular stimulus trains cannot be delivered using the IPG (Medtronic Soltera Model 7426 and Kinetra Model 7428, Metronic Inc., Minneapolis, Minnesota, USA), and we used an external stimulator, connected to the implanted DBS lead extension at the time of IPG replacement. Patients participated on a volunteer basis with written informed consent, and the study protocol was approved by the Duke University Institutional Review Board. Patients B and C withheld dopaminergic and/or antitremor medications overnight before the experiment. Patient A did not withhold valproic acid, gabapentin, and azathioprine through the course of the experiment, and patient D did not withhold diazepam. Some patients reported transient paresthesias for some stimulus settings, but there were no adverse events and no incidents of infection.
Table 1.
Demographic characteristics and stimulation settings for each patient
| Patient | Age (sex) |
Disease duration (years) |
Diagnosis | Nucleus | Electrode side |
Tremor typea |
Electrode contacts |
PW (ms) |
Amplitude (V) |
Frequencies tested (Hz) |
Mean IPI (average frequency)c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 41 (Female) | 7 | MS | Vim | Right | Postural, elbow flexed (one block)/intention (two blocks) | 5−6+ | 150 | 4.3 | 0, 23, 46, 93, 185 | 5.4ms (185Hz) |
| B | 50 (Male) | 7 | PD | STN | Left | Rest (four blocks) | 1−2−3+ | 60 | 5.0 (3.0)b | 0, 10, 65, 130, 185 | 7.7ms (130Hz) |
| C | 78 (Male) | 5 | ET | Vim | Left | Postural, wrist extended and elbow relaxed (three blocks) | 1−2+ | 60 | 5.5 (5.0)b | 0, 10, 65, 130, 185 | 7.7ms (130Hz) |
| D | 74 (Female) | Lifelong | MDSwith tremor | Vim | Right | Postural, wrist extended and elbow flexed (more than two blocks) | 4+5− | 90 | 5.5 (2.8)b | 0, 10, 65, 130, 185 | 7.7ms (130Hz) |
IPI, interpulse interval; MDS, myoclonus-dystonia syndrome; MS, multiple sclerosis; PW, pulse width; STN, subthalamic nucleus; Vim, ventral intermediate nucleus of thalamus.
Tremor type was based on presurgical clinical evaluation and posture was adjusted to maximize tremor in the stimulation ‘off’ condition.
Amplitudes in parentheses indicate clinically programmed amplitudes in cases in which they differed from amplitudes used during experiments.
Mean IPI specifies the mean of the IPI distribution used for the irregular IPI trains, with CV=0, 0.1, 0.3, and 0.6.
Intraoperative measurements of tremor
We measured tremor in the limb contralateral to the side of stimulation during unilateral stimulation. Stimuli were delivered with an isolated stimulator (bp isolator, FHC Inc., Bowdoin, Maine, USA) and pulses were controlled by a high-speed digital-to-analog converter through LabView software (National Instruments, Austin, Texas, USA). The regulated voltage waveform was an asymmetric, charge-balanced, biphasic pulse with a large-amplitude short-duration cathodic phase followed by a low-amplitude (10% of cathodic amplitude) and long-duration (10 times the cathodic duration) anodic recharge phase, similar to that used in the IPG. Charge densities were below the manufacturer’s recommended limit of 30 μC/cm2/phase (using conservative estimate of impedance=500Ω).
We tested eight stimulation patterns in each patient: stimulation ‘off’, four constant interpulse interval (IPI=1/instantaneous frequency) trains (Table 1), and three temporally irregular trains with the same mean IPI (Table 1), but with different degrees of irregularity (Fig. 1a and b). Irregular trains were constructed by drawing IPIs from a Gaussian distribution, such that the coefficients of variation (CVs) of the IPI distributions were 0.1, 0.3 and 0.6 (CV=standard deviation of the IPIs/mean of the IPIs). Any random IPIs shorter than the combined duration of the cathodic and anodic phases were lengthened to ensure that charge balancing was complete before the onset of the next pulse (~0–2% of IPIs for CV=0.3 and 4–15% of IPIs for CV=0.6 needed to be lengthened). This caused the effective CVs of the IPIs to decrease slightly in the most irregular trains (CV=0.52–0.56, rather than 0.6). In each block, the stimulation patterns were presented in randomized order, and the patient was blinded to the stimulation parameters. Each patient completed two to four blocks in a single 30–60-min session.
Fig.1.

Effects of stimulus train regularity on relief of postural tremor by deep brain stimulation (DBS). (a) Sample stimulus trains with mean interpulse interval (IPI)=7.7ms (mean frequency=130 Hz), and varying coefficients of variation (CVs). (b) Probability density functions for IPI distributions with a mean IPI=7.7ms (IPI step size of 0.25ms). (c–f) Sample accelerometer recordings (left) and power spectral densities (right) for tremor recorded in patient C during 10 s pre-DBS, 20 s DBS, and 5 s post-DBS. Accelerometer (c) and power spectral densities (d) for regular 130Hz DBS. Accelerometer (e) and power spectral densities (f) for DBS with an average rate of 130Hz and CV=0.6. Scale bars in (c) apply to (e). Log scale axis on power spectral density plots should be noted.
Postural tremor was measured with the wrist extended and/or elbow flexed (Table 1). Rest tremor was measured with the elbow supported and the hand unsupported. Intention tremor was measured as the patients moved their index finger between their nose and an experimenter’s hand. Tremor was recorded continuously during each trial including 5–10 s before turning DBS ‘on’, 10–30 s with DBS ‘on’, and an additional 1–5 s after DBS was switched ‘off’. The patient then relaxed for approximately 30–60 s before the next trial began.
Tremor was measured using an accelerometer taped to the dorsum of the hand (Crossbow CXL04LP3; 5V/4g sensitivity, San Jose, California, USA), and quantified by combining the three accelerometry signals (ax, ay, and az) into one signal We calculated the power spectral density of |a|, which quantifies the power as a function of signal frequency, using the power spectral density (PSD) function (Welch’s averaged periodogram, Hanning window, FFT length=5000) in MATLAB (Mathworks Inc., Natick, Massachusetts, USA). We then defined tremor power as the sum of the power spectral density between 2 and 20Hz, chosen to include the primary and first harmonics of the tremor and to exclude steady state acceleration due to gravity.
Data analysis and statistics
Tremor during DBS was analyzed as log10 of the percent of tremor power in the period preceding DBS onset.
Measurements made across multiple blocks within the same patient were averaged, and measurements from trials in incomplete blocks (one patient) were included to provide the best estimate of the mean for each stimulus condition.
Data were analyzed using two linear mixed-effects regression models, with patient identity as the random effect. The first model included frequency as the fixed effect, and included only tremor measurements recorded during regular (constant IPI) stimulation. The second model included CV of IPIs as the fixed effect, and included only tremor measurements made during stimulation with the same mean IPI, but varying regularity (CV=0, 0.1, 0.3, 0.6). Individual patient intercepts were computed using best linear unbiased predictors from the mixed effects regression model (JMP: SAS Institute, Cary, North Carolina, USA). Two-sided tests were performed on the significance of regression coefficients and statistical significance was defined at α=0.05.
Results
We measured changes in tremor in four patients in response to regular DBS at different frequencies and to irregular DBS with the same average pulse frequency, but different degrees of irregularity in the IPIs. Tremor depended on both the average frequency of stimulation and the regularity of stimulation. Sample accelerometer recordings and power spectra illustrate that tremor was suppressed more during stimulation with regular DBS than during stimulation with irregular DBS (Fig. 1c–f).
Tremor suppression by DBS increased as a function of frequency (Fig. 2a and b). A linear mixed-effects model of log percent tremor as a function of frequency revealed a significant negative correlation between stimulus frequency and tremor (P<0.01, two-sided test on significance of slope, R2=0.42, Fig. 2b).
Fig. 2.
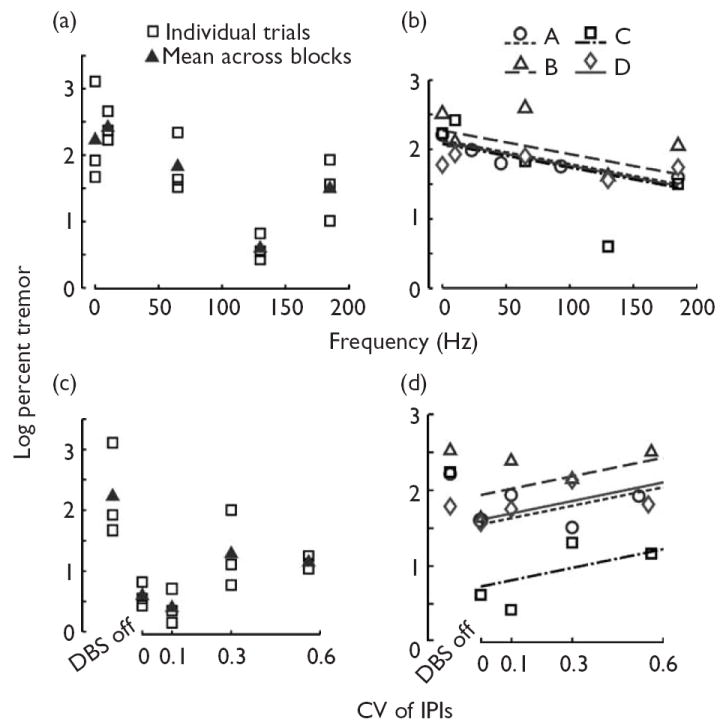
Effects of deep brain stimulation (DBS) frequency and regularity on tremor suppression. (a, b) Log percent tremor power as a function of stimulus frequency in patient C (a) and in all four patients (b). (c, d) Log percent tremor power as a function of stimulus train regularity [coefficient of variation (CV) of the interpulse intervals (IPIs)] in patient C (c), and in all four patients (d). (a, c) The individual tremor measurement replicates are displayed along with their mean. Data represent log10 of 100 times the ratio of tremor power during DBS to tremor power before the application of DBS. (b, d) Means of two to four trials collected with a randomized block design for each patient. Lines represent linear mixed-effects regression model for each patient. (b) The effectiveness of constant IPI DBS in suppressing tremor improved as a function of stimulus frequency (P<0.01, two-sided test on significance of frequency regression coefficient). All lines have the same slope (−0.0034/Hz), but different intercepts [patient A: 2.13, patient B: 2.27, patient C: 2.08, patient D: 2.10 (unitless)]. (d) Although all trains in each patient had the same mean IPI (5.4 ms in patient A, 7.7 ms in patients B–D), the trains with irregular IPIs were less effective at reducing tremor than constant IPI (CV=0) stimulation (P<0.04, two-sided test on significance of CVregression coefficient). All lines have the same slope [0.74 (unitless)], but different intercepts [patient A:1.54, patient B:1.94, patient C: 0.72, patient D:1.61 (unitless)]. Tremor data presented for DBS ‘off’ were not included in the regression model. Legends in (a, b) apply to (c, d), respectively.
Tremor suppression by high frequency DBS decreased as the stimulus train became more irregular (Fig. 2c and d). A linear mixed-effects model of log percent tremor as a function of stimulus irregularity (CV of the IPIs) revealed a significant positive correlation between stimulus irregularity and tremor power (P<0.03, two-sided test on significance of slope, R2=0.83, Fig. 2d). The magnitude of the slope [0.83 (unitless); 95% confidence interval: 0.10–1.55 (unitless)] indicated that as the CV of the stimulus increased from 0 to 0.6, there was a 3.1-fold increase in the median percent tremor (not logged), approximately the same as the proportional increase in the median percent tremor that occurred between 130Hz DBS and DBS ‘off’ in the frequency regression model.
Discussion
Our results demonstrate that tremor reduction by DBS depends not only on the rate, but also on the temporal regularity of stimulation. High frequency stimulation provided better symptom relief than low frequency stimulation [1-5], and the effectiveness of high frequency DBS decreased as the degree of irregularity of stimulation increased. These results reinforce the importance of the pattern of neuronal firing in movement disorders, and support the hypothesis that regularization of neuronal firing is required for effective DBS [6-10].
We theorize three candidate mechanisms for why the temporally irregular stimulation was less effective than regular stimulation. First, the decreased effectiveness of irregular DBS in relieving tremor may be due to long IPIs in the trains inducing burst responses in thalamus. Computational [11] and experimental [12] results indicate that irregular stimulus trains, per se, do not lead to thalamic bursting, but rather pauses between spikes in trains of thalamic input that exceed 20 ms (<50Hz) lead to burst responses in thalamus. Bursting in the thalamus has been associated with both essential tremor and Parkinson’s disease [13,14], and DBS-induced rebound bursts may increase tremor.
Second, the decreased effectiveness of irregular DBS in relieving tremor may be due to long IPIs providing an opportunity for intrinsic pathological activity to recover and propagate through the thalamus before the arrival of the next stimulus pulse. Such a mechanism explains the similar ineffectiveness of the irregular stimulus trains and regular low frequency DBS (<~100Hz).
Third, the decreased effectiveness of irregular DBS in relieving tremor may be due to the irregularity, per se, of the stimulation patterns. Under this hypothesis, even if irregular DBS is able to override pathological bursting [14,15], it may be unable to drive regular firing patterns, and thus is clinically ineffective. Neurons downstream of the stimulated nucleus may adapt to ignore regularized inputs from the stimulated nucleus, because constant rate firing provides no new information to the downstream neurons. Such an explanation provides a basis for understanding the similar effects of high frequency DBS and surgical lesioning of the target nucleus [10].
A limitation of this study is the heterogeneity of the patient population. Although the specific mechanism(s) by which DBS exerts its effects may vary across the diseases and target nuclei examined in this study, it is nonetheless remarkable that the effects of stimulus rate and regularity were evident in a heterogeneous population of patients. Such findings speak to the robustness of the effect of stimulus rate and regularity on tremor. Another limitation is the short duration of DBS before assessment of tremor and the short interval between trials. Longer trials, however, would result in the experiment becoming too long to conduct during an operative procedure and there are presently no other settings in which to conduct these studies. Similarly, short trial lengths have been used in studies of parameter settings [3,5,16], as tremor reduction following onset of DBS occurs ‘within a few seconds’ [17] (Fig. 1c and e). The short onset and offset of tremor during DBS allowed us to rule out plasticity mechanisms, which occur over much longer time scales than our trial duration. Finally, our experiments have the limitation that the effects of DBS on tremor in the patient with myoclonus–dystonia syndrome were not exclusively distinguishable from effects on myoclonus. Excluding the data from this patient, however, still results in a significant slope in the irregularity regression model (P<0.05).
The IPG replacement surgery provides a unique opportunity for direct connection to implanted DBS leads under stable conditions. In contrast to investigations conducted using externalized leads between the implant of the DBS lead and subsequent implantation of the IPG [7], the present approach eliminates the confounding effects of focal acute brain edema (i.e., microlesion) caused by the insertion of the lead. Furthermore, direct connection to the implanted lead eliminates the substantial limitations on the range of experimental stimuli possible with the IPG, and enabled this first-time assessment of the effects of the temporal regularity of stimulation. Our results demonstrate the feasibility and utility of conducting intraoperative experiments during IPG replacement surgeries, and open the door for testing other experimental stimuli.
Conclusion
The effects of DBS are dependant not only on the average frequency of DBS, but also on the regularity of the temporal spacing of DBS pulses.
Acknowledgments
This study was supported by NIH R21-NS055320, NIH K25-NS0535444, and a National Science Foundation Graduate Research Fellowship. Authors report no conflicts of interest.
References
- 1.Ushe M, Mink JW, Revilla FJ, Wernle A, Schneider Gibson P, McGee-Minnich L, et al. Effect of stimulation frequency on tremor suppression in essential tremor. Mov Disord. 2004;19:1163–1168. doi: 10.1002/mds.20231. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- 3.Kuncel AM, Cooper SE, Wolgamuth BR, Clyde MA, Snyder SA, Montgomery EB, Jr, et al. Clinical response to varying the stimulus parameters in deep brain stimulation for essential tremor. Mov Disord. 2006;21:1920–1928. doi: 10.1002/mds.21087. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery EB, Jr, Baker KB, Kinkel RP, Barnett G. Chronic thalamic stimulation for the tremor of multiple sclerosis. Neurology. 1999;53:625–628. doi: 10.1212/wnl.53.3.625. [DOI] [PubMed] [Google Scholar]
- 5.Moro E, Esselink RJ, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson’s disease of electrical parameter settings in stn stimulation. Neurology. 2002;59:706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 6.Kuncel AM, Cooper SE, Wolgamuth BR, Grill WM. Amplitude- and frequency-dependent changes in neuronal regularity parallel changes in tremor with thalamic deep brain stimulation. IEEE Trans Neural Syst Rehabil Eng. 2007;15:190–197. doi: 10.1109/TNSRE.2007.897004. [DOI] [PubMed] [Google Scholar]
- 7.Birdno MJ, Cooper SE, Rezai AR, Grill WM. Pulse-to-pulse changes in the frequency of deep brain stimulation affect tremor and modeled neuronal activity. J Neurophysiol. 2007;98:1675–1684. doi: 10.1152/jn.00547.2007. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Gad I, Elias S, Vaadia E, Bergman H. Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J Neurosci. 2004;24:7410–7419. doi: 10.1523/JNEUROSCI.1691-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. NeuroReport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 11.Babadi B. Bursting as an effective relay mode in a minimal thalamic model. J Comput Neurosci. 2005;18:229–243. doi: 10.1007/s10827-005-6560-5. [DOI] [PubMed] [Google Scholar]
- 12.Person AL, Perkel DJ. Unitary IPSPS drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46:129–140. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Lenz FA, Kwan HC, Martin RL, Tasker RR, Dostrovsky JO, Lenz YE. Single unit analysis of the human ventral thalamic nuclear group. Tremor-related activity in functionally identified cells. Brain. 1994;117(Pt 3):531–543. doi: 10.1093/brain/117.3.531. [DOI] [PubMed] [Google Scholar]
- 14.Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93:117–127. doi: 10.1152/jn.00527.2004. [DOI] [PubMed] [Google Scholar]
- 15.Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96:549–564. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- 16.O’Suilleabhain PE, Frawley W, Giller C, Dewey RB., Jr Tremor response to polarity, voltage, pulse width and frequency of thalamic stimulation. Neurology. 2003;60:786–790. doi: 10.1212/01.wnl.0000044156.56643.74. [DOI] [PubMed] [Google Scholar]
- 17.Beuter A, Titcombe MS. Modulation of tremor amplitude during deep brain stimulation at different frequencies. Brain Cogn. 2003;53:190–192. doi: 10.1016/s0278-2626(03)00107-6. [DOI] [PubMed] [Google Scholar]


