Abstract
Studies showed that the metabolic unlike the neuroendocrine effects of ghrelin could be abrogated by co-administered unacylated ghrelin. The aim was to investigate the interaction between ghrelin and desacyl ghrelin administered intraperitoneally on food intake and neuronal activity (c-Fos) in the arcuate nucleus in non-fasted rats. Ghrelin (13 μg/kg) significantly increased food intake within the first 30 min post injection. Desacyl ghrelin at 64 and 127 μg/kg injected simultaneously with ghrelin abolished the stimulatory effect of ghrelin on food intake. Desacyl ghrelin alone at both doses did not alter food intake. Both doses of desacyl ghrelin injected separately in the light phase had no effects on food intake when rats were fasted for 12 h. Ghrelin and desacyl ghrelin (64 μg/kg) injected alone increased the number of Fos positive neurons in the arcuate nucleus compared to vehicle. The effect on neuronal activity induced by ghrelin was significantly reduced when injected simultaneously with desacyl ghrelin. Double labeling revealed that nesfatin-1 immunoreactive neurons in the arcuate nucleus are activated by simultaneous injection of ghrelin and desacyl ghrelin. These results suggest that desacyl ghrelin suppresses ghrelin-induced food intake by curbing ghrelin-induced increased neuronal activity in the arcuate nucleus and recruiting nesfatin-1 immunopositive neurons.
Keywords: Rat, desacyl ghrelin, ghrelin, food intake, c-Fos, ARC
1. INTRODUCTION
Ghrelin is a 28-amino acid peptide hormone with an n-octanylated serine residue that is predominantly produced by the A/X-like endocrine cells of the gastric oxyntic mucosa in rodents and humans [19]. Ghrelin has been shown to act on the brain-gut axis as the only peripherally produced and centrally active peptide that stimulates food intake in rats [25, 34, 38], mice [2, 36] and humans [37]. Ghrelin exerts its orexigenic action through activation of the functionally active, signaltransducing form of the growth hormone secretagogue receptor type 1a (GHS-R1a), renamed GRLN [9] located in the hypothalamus-pituitary axis [24].
Ghrelin circulates in two major forms: n-octanyl-ghrelin (ghrelin), and des-n-octanyl ghrelin (desacyl ghrelin), which lacks the hydrophobic octanyl group, esterified to Ser3. Acylation is essential for the binding of ghrelin to the GHS-R1a, and although desacyl ghrelin does not bind to the GHS-R1a, it may be biologically active [12, 14, 24]. One group of investigators reported that desacyl ghrelin exerts an inhibitory effect on food intake after intraperitoneal (ip) or central (intracisternal or intracerebroventricular, icv) administration in rodents [8]. Most likely, the effect is mediated by neurons in the arcuate nucleus (ARC) and paraventricular nucleus (PVN) of the hypothalamus [8]. Transgenic mice over-expressing desacyl ghrelin displayed a decrease in food intake and body weight [1, 7]. However, subsequent studies showed that centrally administered desacyl ghrelin stimulates food intake independently from the GHS-R1a while peripheral administration of the peptide had no effects in rodents and humans [32]. There is also evidence suggesting that desacyl ghrelin may counteract ghrelin's role in the control of energy balance and glucose metabolism [1, 11, 12].
Recent reports indicate that the effects of both ghrelin and desacyl ghrelin on food intake are mediated at least in part by distinct brain nuclei, as demonstrated by differential brain Fos expression in response to peripheral administration of each peptide [8, 13]. However, both peptides injected ip induce Fos expression in the ARC of the hypothalamus in rodents [8, 13], and double labeling showed that peripheral injection of ghrelin activates arcuate neurons containing the orexigenic peptides neuropeptide Y (NPY) and Agouti-related protein [36]. However, the neuropeptidergic phenotypes of activated neurons in the ARC induced by peripheral desacyl ghrelin are unknown. Recently, it could be demonstrated that a new peptide called nesfatin-1, derived from the nucleobindin-2 protein (NUCB2) modulates food intake and body weight in rats [27]. In addition, immunohistochemical mapping of the hypothalamus shows that nesfatin-1 immunopositive neurons are - among other areas - localized in the ARC [6, 27]. Therefore, nesfatin-1 was chosen as a candidate for the neurohistochemical analysis performed in this study in response to peripheral simultaneous administration of ghrelin and desacyl ghrelin.
We recently reported that simultaneous administration of peripheral cholecystokinin (CCK) or bombesin together with ghrelin blocks the orexigenic effect of ghrelin and modulates Fos expression in hypothalamic brain nuclei in rats [15, 17]. In contrast, amylin was not able to modulate ghrelin-induced food intake [15]. To further explore the role of desacyl ghrelin in the regulation of food intake and its interaction with ghrelin we investigated whether desacyl ghrelin injected intraperitoneally (ip) influences ghrelin-induced food intake under ad libitum feeding conditions in rats. Additionally, we explore the role of desacyl ghrelin injected alone on food intake in 12-h fasted rats. Furthermore, we assessed changes in neuronal activity in response to peripheral ghrelin, desacyl ghrelin and simultaneous injection of both peptides by mapping the Fos expression in the ARC in rats.
2. METHODS
2.1 Animals
Male Sprague-Dawley rats (Harlan-Winkelmann Co., Borchen, Germany) weighing 250 g were group-housed (4 rats/cage) under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 6:30 a.m./6:30 p.m.), humidity, and temperature (22 ± 2 °C). Animals were fed with a standard rat diet (Altromin®, Lage, Germany) and tap water ad libitum. All animals were trained daily to become accustomed to the experimental conditions for 14 days before starting the experiments. During the handling phase, the back position was practiced to make animals familiar with receiving an intraperitoneal (ip) injection.
Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the state authority for animal research conduct.
2.2 Peptide preparation
Rat desacyl ghrelin (0.5 mg/ml; Peptides International, Louisville, USA) and rat ghrelin (1mg/ml; Peptides International, Louisville, USA) was dissolved in distilled water and stored at −20 °C. Immediately before starting the experiments, peptides were diluted in vehicle solution consisting of sterile 0.15 M NaCl (Braun, Melsungen, Germany) to reach the final concentration of 13 μg (∼4 nmol) ghrelin/kg, and 64 μg (∼20 nmol) or 127 μg (∼40 nmol) desacyl ghrelin/kg-body wt. Peptide solutions were kept on ice for the duration of the experiments. Doses of peptides were selected based on previous studies [8, 17, 32].
2.3 Procedures
2.3.1 Effects of ghrelin and desacyl ghrelin injected ip singly or in combination on food intake
Experiments were performed at 2.5 h after the onset of the light cycle in freely fed rats. This corresponds to their lowest daily food intake. Rats were injected ip simultaneously (final volume: 0.5 ml) with vehicle plus vehicle (n=13), ghrelin (13 μg/kg) plus vehicle (n=12), ghrelin (13 μg/kg) plus 64 μg/kg or 127 μg/kg desacyl ghrelin (n =12/group), or vehicle plus 64 μg/kg (n=13) or 127 μg/kg desacyl ghrelin (n=10). Immediately after the ip injection, each rat was placed in a single housing cage with access to pre-weighed rat chow. Food intake was calculated as the difference between the food weights before and after the feeding period at each time interval (30 min, 1, 2, 3, 4 and 5 h). Cumulative food intake was calculated by summating the values of the different time periods.
2.3.2 Effects of desacyl ghrelin injected ip on food intake in rats fasted for 12 h
Groups of randomized rats deprived of food but not water for 12 h were injected ip (0.5 ml) in the light phase with desacyl ghrelin (64 or 127 μmol/kg, n=9 and 6 respectively) or vehicle (0.15 M NaCl, n=7). Rats were placed in single cages and weighed rat chow was made available to the animals. Food intake was calculated as above.
2.3.3 Effects of ghrelin and desacyl ghrelin injected ip singly or in combination on Fos-immunoreactivity in the ARC
Freely fed rats were injected ip (final volume 0.5 ml) with vehicle plus vehicle (n=4), ghrelin (13 μg/kg) plus vehicle (n=4), vehicle plus 64 μg/kg desacyl ghrelin (n=4) or 13 μg/kg ghrelin plus 64 μg/kg desacyl ghrelin (n=4). Immediately after injection, animals were deprived of food to avoid an influence of ghrelin-induced increase in food intake on Fos expression in the brain but had ad libitum access to water [36]. At 90 min after the ip injection, animals were deeply anesthetized with ip injections of 100 mg/kg ketamine (Ketanest®, Curamed, Karlsruhe, Germany) and 10 mg/kg xylazine (Rompun® 2%, Bayer, Leverkusen, Germany) and anticoagulated with 2 500 U heparin ip (Liquemin®, Hoffmann-La Roche, Grenzach-Whylen, Germany). Transcardial perfusion was performed as described before [16]. For cryoprotection, brain blocks were moved through a sucrose gradient (15% w/v and 27.3% w/v), snap-frozen in hexane at −70 °C, and stored at −80 °C until further processing.
2.4 Immunohistochemistry
2.4.1 Staining for Fos-immunoreactivity (Fos-ir)
Free-floating brain sections (25 μm) were pre-treated with a 1% w/v sodium borohydride solution for 15 min and incubated in a solution containing 3% w/v normal goat serum (Jackson ImmunoResearch Laboratories Inc., Pennsylvania, USA), and 0.3% v/v Triton X-100 in PBS for 60 min to block unspecific antibody binding. Then, the diluted primary antibody solution (rabbit anti-c-Fos; Oncogene Research Products, Boston, USA; 1:8 000 in a solution of 3% w/v normal goat serum, and 0.1% v/v sodium azide in PBS) was applied for 24 h at room temperature. After rinsing the sections in PBS three times and incubation in a solution containing 3% w/v goat serum and 0.1% v/v sodium azide in PBS for 1 h, sections were incubated with the secondary antibody goat biotin-SP-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc.) for 12 h at room temperature (1:2 000 in 3% w/v goat normal serum and 0.1% v/v sodium azide in PBS). After rinsing in PBS three times (sodium azide free), sections were incubated in avidin-biotin peroxidase complex (ABC; 1:1 000; Vector Laboratories, UK) in PBS for 5 h. Subsequently, sections were rinsed in PBS three times again, and then incubated for 10 min in a solution containing 0.6 mg/ml 3,3′-diaminobenzidine, 9.3 mg/ml ammoniumnickelsulfate, 2.3 mg/ml ammonium chloride and 2.3 mg/ml cobalt-(II)-chloride hexahydrate. Peroxidase reaction was started with 0.3% v/v H2O2 and stopped with PBS. Sections were rinsed in PBS three times again and stained with propidium iodide (2.5 μg/ml in PBS) for 15 min to counterstain cell chromatin. Stained sections were mounted on chromium-aluminium-gelatine-coated slides, air-dried, and dehydrated through a graded series of ethanol and xylene. Mounted sections were cover-slipped in Entellan Neu (Merck, Germany) and analyzed using a fluorescence and bright-field microscope (Axiophot 1, Carl Zeiss, Jena, Germany).
2.4.2 Double-staining for Fos- and nesfatin-1-ir in the ARC
Free-floating brain sections (25 μm) were pre-treated with a 1% w/v sodium borohydride solution for 15 min. Subsequently, sections were incubated in a solution containing 3% w/v normal goat serum (Jackson ImmunoResearch Laboratories Inc.), and 0.3% v/v Triton X-100 in PBS for 60 min to block unspecific antibody binding. Afterwards, the diluted primary antibody solution (rabbit anti-c-Fos; Oncogene Research Products, 1:8 000 in a solution of 3% w/v normal goat serum, and 0.1% v/v sodium azide in PBS) was applied for 24 h at room temperature. After rinsing the sections in PBS three times and incubation in a solution containing 3% w/v goat serum and 0.1% v/v sodium azide in PBS for 1 h, sections were incubated with the secondary antibody goat biotin-SP-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc.) for 12 h at room temperature (1:2 000 in 3% w/v goat normal serum, and 0.1% v/v sodium azide in PBS). After rinsing in PBS three times (sodium azide free) sections were incubated in avidin-biotin peroxidase complex (ABC; 1:1 000; Vector Laboratories) in PBS for 5 h. Sections were rinsed in TNT washing buffer (0.1 M Trizma hydrochloride, 0.15 M NaCl and 0.05% v/v Tween 20, pH 7.5) three times, and incubated with Fluorophore Tyramide in amplification solution (PerkinElmer, Waltham, Massachusetts, USA) for 10 min at room temperature. After rinsing sections in TNT washing buffer three times, sections were incubated with the second primary antibody solution (anti-rabbit-nesfatin-1; Phoenix Pharmaceuticals, Burlingame, CA, USA.; 1:500 in PBS containing 1% w/v donkey normal serum) for 24 h at room temperature. After rinsing in PBS for three times, TRITC labeled donkey anti-rabbit IgG (1:200, Jackson ImmunoResearch Laboratories Inc.) in PBS was applied for 12 h at room temperature. After washing in PBS for three times, sections were stained with DAPI (2 μg 4'-6-Diamidino-2-phenylindole/ml in PBS) for 15 min to counterstain cell chromatin. Sections were rinsed in PBS three times again, then embedded in anti-fading solution (100 mg/ml 1,4-Diazabicyclo [2.2.2] octane (Sigma, St. Louis, USA) in 90% v/v glycerine, 10% v/v PBS, pH 7.4) and analyzed using a confocal laser scanning microscope (cLSM 510, Carl Zeiss, Germany).
2.5 Data analysis
The cumulative food intake monitored at the end of the first 30 min, 1, 2, 3, 4 and 5 h after peptide or vehicle injection was expressed as food intake in g/kg body weight [30]. All data were expressed as mean ± SEM and analyzed by Kruskal-Wallis One Way ANOVA. Differences between groups were evaluated by the Dunn's test; p < 0.05 was considered significant.
Assessment of Fos-ir was achieved by counting the number of Fos positive neurons. Cells with black or blue nuclear staining were considered as Fos positive. Every fourth of all consecutive coronal 25 μm sections was counted for Fos-ir positive staining bilaterally in the ventromedial hypothalamic nucleus (VMN) that served as control nucleus (10 sections/rat; Bregma −2.12 to −3.60 mm) and the ARC (10 sections/rat; Bregma −2.12 to −3.60 mm). Anatomic correlations were made according to landmarks given in Paxinos and Watson's stereotaxic atlas [28]. The investigator counting the number of Fos-ir positive cells was blinded to treatments received by the animals. The average number of Fos-ir positive neurons per section for the brain nuclei mentioned above was calculated for four rats per experimental group. Fos data are expressed as mean ± SEM and analyzed by ANOVA. Differences between groups were evaluated by the Fisher LSD method, with p < 0.05 considered significant.
Quantitative assessment of Fos-ir and nesfatin-1-ir double staining in the ARC was achieved by counting the total number of Fos neurons and Fos neurons which are co-localized with nesfatin-1-ir. Thereafter, we calculated the percentage of the Fos-ir neurons which were immunopositive for nesfatin-1 (n=3 per group). Data are presented as mean ± SEM.
3. RESULTS
3.1 Desacyl ghrelin injected ip blocked the stimulation of ghrelin-induced food intake in freely feed rats
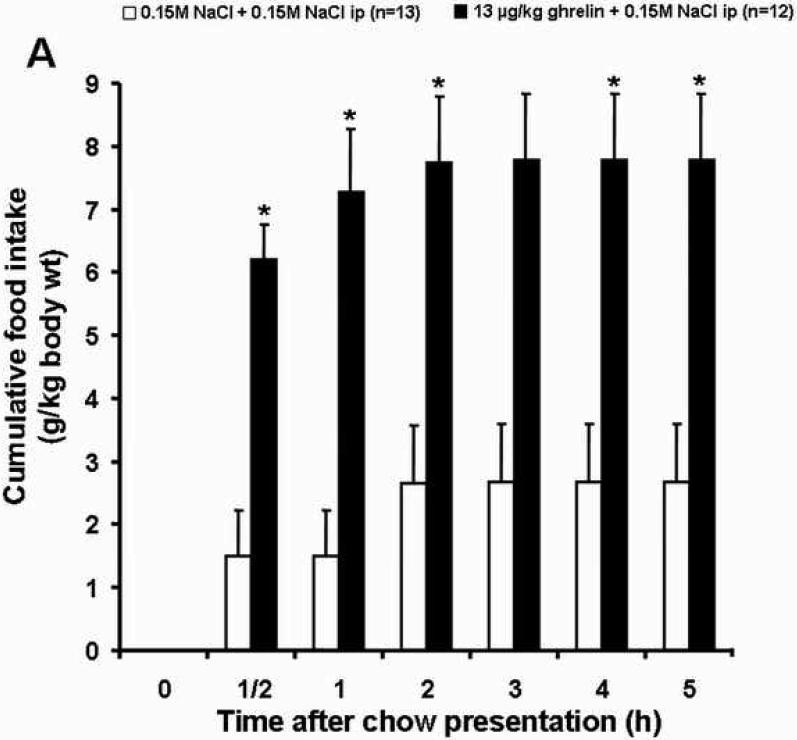
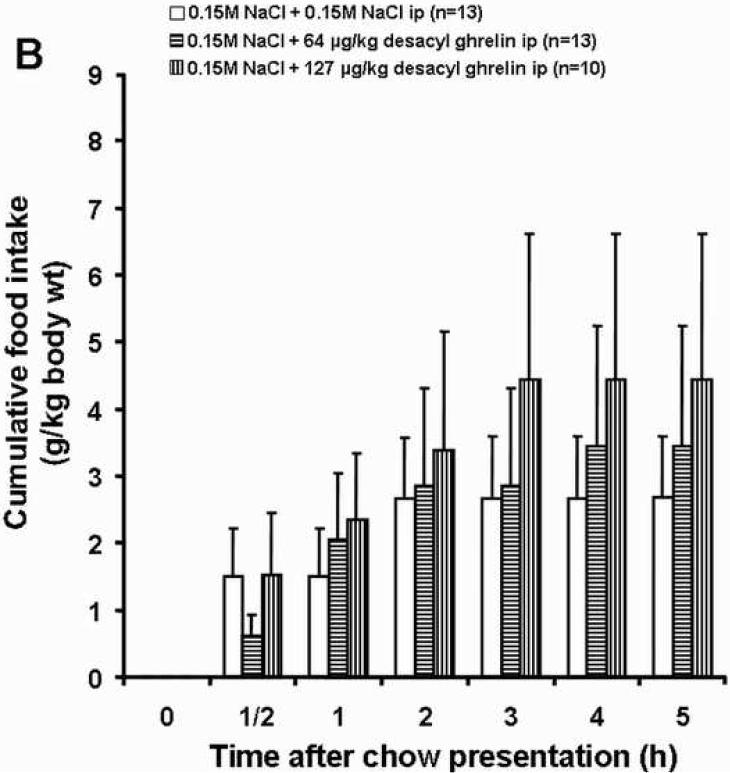
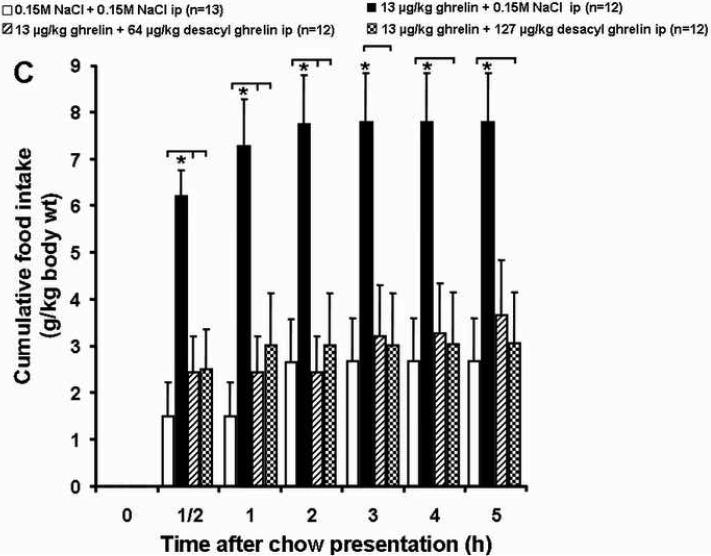
Ghrelin (13 μg /kg, ip) significantly increased food intake within the first half hour after ip injection compared to the vehicle plus vehicle group (6.20 ± 0.56 vs. 1.49 ± 0.72 g/kg-body wt, p<0.05; Fig. 1A and Table 1). Desacyl ghrelin at both doses (64 and 127 μg/kg-body wt) injected simultaneously with ghrelin (13 μg/kg-body wt) abolished the stimulatory effect of ghrelin on food intake (2.44 ± 0.77 and 2.50 ± 0.86 g/kg, respectively; p<0.05; Fig. 1C and Table 1). Desacyl ghrelin at a dose of 64 and 127 μg/kg injected ip alone had no effect on cumulative food intake during the first half hour compared to the vehicle plus vehicle group (0.61 ± 0.32 and 1.53 ± 0.92 g/kg-body wt, respectively; p>0.05; Fig. 1B and Table 1).
Fig. 1.
Effects of ghrelin and desacyl ghrelin injected alone or simultaneously ip on 5 h-food intake. Freely fed rats were injected ip with vehicle, desacyl ghrelin (64 or 127 μg/kg), ghrelin (13 μg/kg) plus desacyl ghrelin (64 or 127 μg/kg), or ghrelin (13 μg/kg), and cumulative food intake (expressed as g/kg-body weight) was measured at 0.5, 1, 2, 3, 4 and 5 h. Ghrelin significantly increased food intake compared to the vehicle group (A). Desacyl ghrelin at both doses alone had no effect on cumulative food intake (B), and desacyl ghrelin at both doses simultaneously injected with ghrelin abolished the stimulatory effect of ghrelin on food intake (C). Data are expressed as mean and SEM of number of rats indicated in parentheses. * p < 0.05.
Table 1.
Comparison of food intake data between rats treated with ghrelin, vehicle, desacyl ghrelin or ghrelin plus desacyl ghrelin after ip injection. The table gives an overview of the differences in percentage comparing the cumulative food intake data of the different treatment group with the group of animals treated with ghrelin (FI-reduction to ghrelin). The cumulative food intake of the ghrelin group was set to equal 100%.
| Time (h) | Ghrelin | NaCl | Desacyl ghrelin | Ghrelin plus desacyl ghrelin | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 μg/kg | 0.15 M | 64 μg/kg | 127 μg/kg | 13 μg/kg + 64 μg/kg | 13 μg/kg + 127 μg/kg | ||||||
| FI (g/kg) | FI (g/kg) | FI-reduction to ghrelin (%) | FI (g/kg) | FI-reduction to ghrelin (%) | FI (g/kg) | FI-reduction to ghrelin (%) | FI (g/kg) | FI-reduction to ghrelin (%) | FI (g/kg) | FI-reduction to ghrelin (%) | |
| FI = Food intake, values are mean ± SEM of n=10-13 rats/group; p*=<0.05. | |||||||||||
| 0.5 | 6.20±0.56 | 1.49±0.72* | −75.93 | 0.61±0.32 | −90.22* | 1.53±0.92 | −75.37* | 2.44±0.77 | −60.68* | 2.50±0.86 | −59.67* |
| 1 | 7.28±1.00 | 1.49±0.72* | −79.51 | 2.05±0.99 | −71.82* | 2.34±1.00 | −67.77* | 2.44±0.77 | −66.41* | 3.02±1.10 | −58.51* |
| 2 | 7.73±1.06 | 2.65±0.93* | −65.68 | 2.85±1.45 | −63.11* | 3.38±1.77 | −56.27* | 2.44±0.77 | −68.39* | 3.02±1.10 | −60.95* |
| 3 | 7.77±1.06 | 2.66±0.92 | −65.75 | 2.85±1.45 | −63.32* | 4.43±2.18 | −43.06 | 3.21±1.09 | −58.71 | 3.02±1.10 | −61.17* |
| 4 | 7.77±1.06 | 2.66±0.92* | −65.75 | 3.44±1.80 | −55.70* | 4.43±2.18 | −43.06* | 3.26±1.08 | −58.06 | 3.04±1.10 | −60.94* |
| 5 | 7.79±1.05 | 2.68±0.92* | −65.75 | 3.44±1.80 | −55.76* | 4.43±2.18 | −43.14* | 3.65±1.18 | −53.12 | 3.05±1.10 | −65.62* |
One hour after ghrelin administration, the significant increase in cumulative food intake compared to the vehicle plus vehicle group was still observed (7.28 ± 1.0 vs. 1.49 ± 0.72 g/kg, p<0.05; Fig. 1A and Table 1). However, both doses of desacyl ghrelin (64 and 127 μg/kg) injected ip simultaneously with ghrelin (13 μg/kg) blocked the orexigenic effect of ghrelin on food intake (2.44 ± 1.10 g/kg-body wt, p<0.05 and 3.02 ± 1.10 g/kg-body wt, p<0.05, respectively; Fig. 1C and Table 1). Desacyl ghrelin injected ip alone at a dose of 64 or 127 μg/kg-body wt in comparison to the vehicle plus vehicle group did not modify food intake at this point of time (2.05 ± 0.99 and 2.34 ± 1.00 g/kg-body wt, respectively; p>0.05; Fig. 1B and Table 1).
At 2 h after ghrelin injection, the cumulative food intake in the ghrelin plus vehicle group was still significantly higher than in the vehicle plus vehicle group (7.73 ± 1.06 vs. 2.65 ± 0.93 g/kg-body wt, p<0.05; Fig. 1A and Table 1). At this point of time the inhibitory effect of desacyl ghrelin on ghrelin-induced food intake at simultaneous injection of desacyl ghrelin (64 and 127 μg/kg body wt) and ghrelin (13 μg/kg-body wt) was maintained (2.44 ± 0.77 g/kg-body wt, p<0.05 and 3.02 ± 1.10 g/kg-body wt, p<0.05, respectively; Fig. 1C and Table 1). Desacyl ghrelin injected ip alone at a dose of 64 or 127 μg/kg-body wt in comparison to the vehicle plus vehicle group did not have a modulating effect on food intake (2.85 ± 1.45 and 3.38 ± 1.77 g/kg-body wt, respectively; p>0.05; Fig. 1B and Table 1).
At 3, 4, and 5 hours after peptide injection, only few significant differences in feeding behavior between the ghrelin group and the other experimental groups could be observed. (Fig. 1C and Table 1).
3.2 Desacyl ghrelin injected ip did not alter the food intake response to a 12-h fast
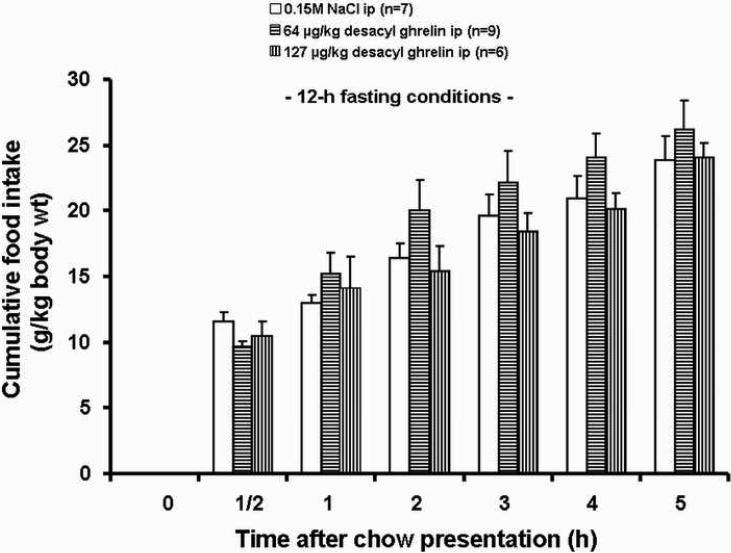
Desacyl ghrelin alone at both doses (64 and 127 μg/kg-body wt, ip) did not suppress the cumulative food intake at any time point throughout the 5-h experiment compared to the vehicle treated rats when injected at the light phase in 12-h fasted rats (Fig. 2). Only a very small trend towards a reduced food intake in the rats could be observed during the first half hour in food-deprived rats.
Fig. 2.
Effects of desacyl ghrelin injected ip on food intake in rats fasted for 12 h. Desacyl ghrelin alone at both doses (64 and 125 μg/kg-body wt, ip) did not suppress cumulative food intake throughout the 5 h experiment compared to vehicle when injected at the light phase in 12 h fasted rats. Data are expressed as mean and SEM of number of rats is indicated in parentheses.
3.3 Modulation of Fos expression in ARC neurons by ghrelin and desacyl ghrelin injected ip singly or in combination
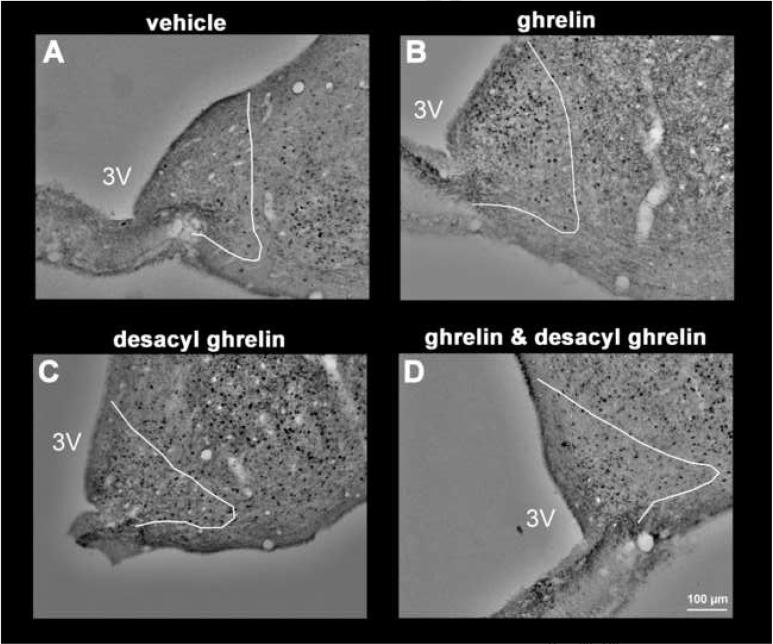
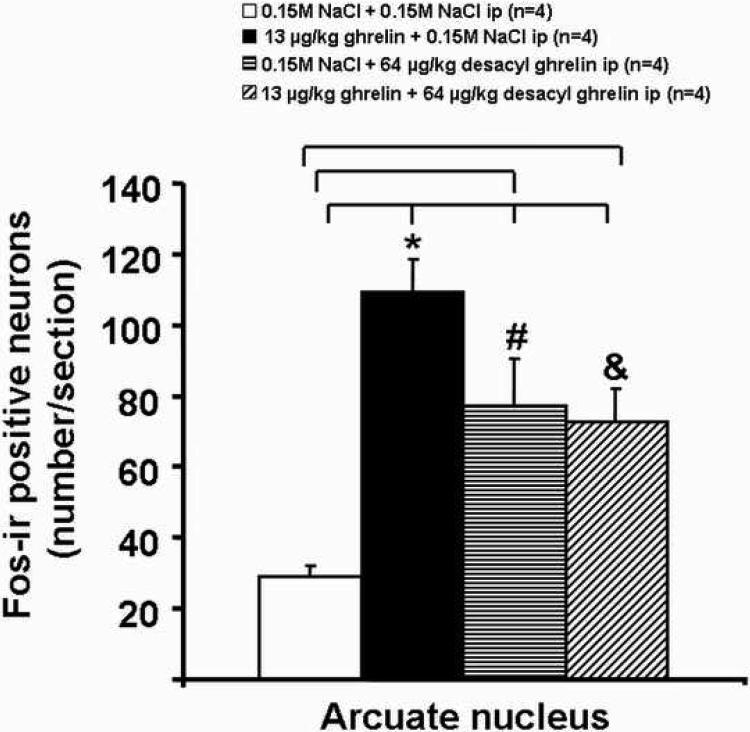
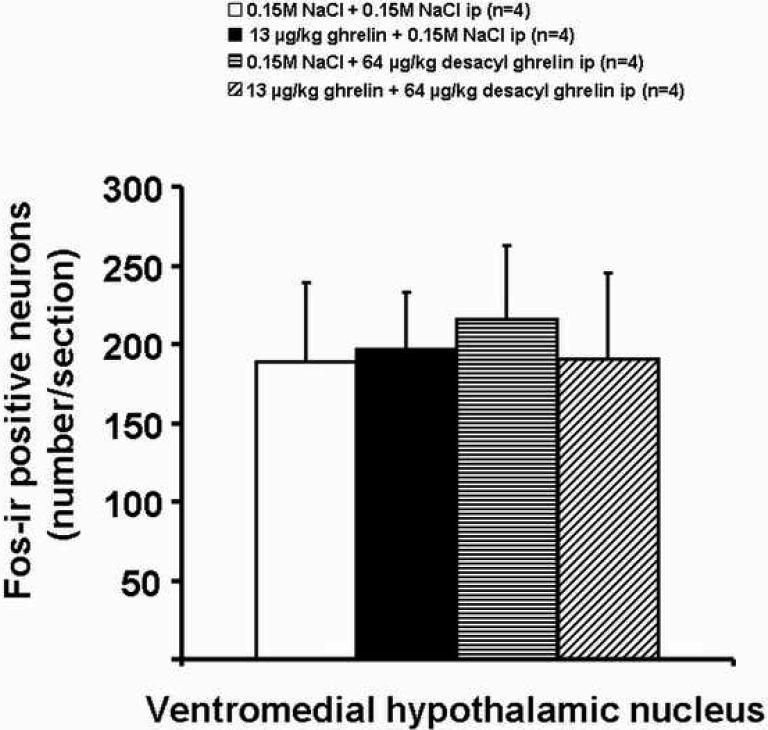
Ghrelin at a dose of 13 μg/kg-body wt administered ip increased the number of Fos-ir positive neurons in the ARC, predominantly in the medial part (Fig. 3) compared to vehicle (mean ± SEM: 109 ± 10 vs. 29 ± 3 neurons/section, p<0.001; Figs. 3 and 4). Likewise, desacyl ghrelin, injected alone, induced neuronal activity localized diffusely over the complete ARC compared to vehicle, as assessed by Fosir, when the peptide was administered at a dose of 64 μg/kg-body wt (77 ± 13, p=0.004; Figs. 3 and 4). The effect of peripheral ghrelin (13 μg ghrelin/kg-body wt) on neuronal activity in the ARC was significantly reduced by simultaneous treatment with desacyl ghrelin injected ip at 64 μg/kg-body wt (72 ± 9 neurons/section, p=0.038 vs. ghrelin alone; Figs. 3 and 4). The Fos-ir positive neurons induced by ip injection of 64 μg/kg desacyl ghrelin alone were localized diffusely over the complete nucleus (Fig. 3). However, both peptides injected ip alone or in combination had no effects on Fos expression in the VMN compared to the vehicle-treated animals (Fig. 5).
Fig. 3.
Effects of ghrelin and desacyl ghrelin injected ip singly or in combination on Fos-immunoreactivity (Fos-ir) in the ARC. 13 μg/kg ghrelin ip induced an increase in the number of Fos-ir positive neurons (black staining) in the ARC (B). Desacyl ghrelin (64 μg/kg) injected alone induced a little less neuronal activity in the ARC (C). The effect of peripheral ghrelin (13 μg/kg) on neuronal activity in the ARC was significantly reduced by simultaneous injection with desacyl ghrelin (D) (64 μg/kg-body wt). After saline injection only few Fos-ir positive neurons were found in the ARC (A). The white line delineates the area of the arcuate nucleus in accordance with landmarks from the Paxinos and Watson rat brain atlas [28]. The white scale bar represents 100 μm. 3V=third ventricle.
Fig. 4.
Effects of ghrelin, desacyl ghrelin, ghrelin and desacyl ghrelin injected ip on the number of Fos-ir positive neurons in the ARC. Freely fed rats were injected ip with vehicle (A), ghrelin (B), desacyl ghrelin (C), ghrelin plus desacyl ghrelin (D), and were euthanized 90 min later to process the brains for Fos-ir. Data are expressed as mean and SEM of number of rats is indicated in parenthesis. * p < 0.05 vs. vehicle, vs. desacyl ghrelin, and vs. ghrelin plus desacyl ghrelin; # p < 0.05 vs. vehicle; & p < 0.05 vs. vehicle.
Fig. 5.
Effects of ghrelin and desacyl ghrelin injected ip singly or in combination on Fos-immunoreactivity (Fos-ir) in the VMN. All peptides and combinations injected ip had no effects on Fos expression in the VMN compared to vehicle. Data are expressed as mean and SEM of number of rats is indicated in parentheses.
3.3.1 Double labeling with Fos and nesfatin-ir
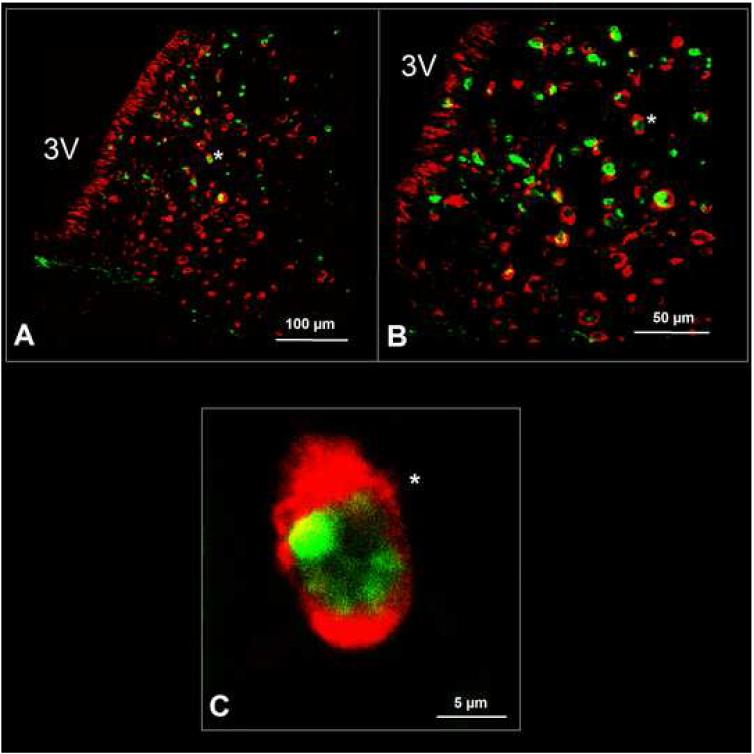
The nesfatin-1 immunoreactive neurons were not localized in one particular aspect of the nucleus, for example the lateral part (Fig. 6A). The double staining procedure for nesfatin-1 and Fos revealed that 55 ± 7% of the Fos-positive neurons in the ARC were immunopositive for nesfatin-1 after simultaneous peripheral administration of ghrelin and desacyl ghrelin (Figs. 6A and B). Furthermore, we observed that 43 ± 4% of the Fos positive neurons induced by peripheral desacyl ghrelin alone were nesfatin-1 immunopositive, and that 18 ± 3% of the Fos-ir neurons induced by ghrelin alone also showed immunoreactivity for nesfatin-1.
Fig. 6.
Nesfatin-1 immunoreactive neurons could be found in the whole ARC. The nesfatin-1 immunopositive neurons were not localized in one particular part of the nucleus, for example the lateral part (A). The double staining for nesfatin-1 (red staining) and Fos (green staining) revealed that numerous Fos-positive neurons in the ARC were also immunopositive for nesfatin-1 (asterisk) after simultaneous peripheral administration of ghrelin and desacyl ghrelin (A and B). Notably, nesfatin-1 immunoreactivity could only be detected in the cytoplasm, whereas the transcription factor Fos was - as expected - localized within the nucleus (C). 3V=third ventricle.
Notably, nesfatin-1 could only be detected in the cytoplasm, whereas the transcription factor Fos was - as expected - localized within the nucleus (Fig. 6C).
4. DISCUSSION
The present study provides evidence that desacyl ghrelin reduces the stimulatory effect of ip ghrelin on food intake and Fos expression in the hypothalamic arcuate nucleus when injected ip in freely fed rats at a desacyl ghrelin:ghrelin ratio of 5:1.
Desacyl ghrelin (64 and 127 μg/kg-body wt ip) abolished the ghrelin-induced increase in food intake at 30 min after injection as shown by the non-significant difference between control (vehicle plus vehicle) and desacyl ghrelin plus ghrelin groups while ghrelin injection results in a 4.2-fold increase in food intake. During the experiment under ad libitum feeding conditions, both doses of desacyl ghrelin administered simultaneously with ghrelin resulted in a ∼60% suppression of food intake over the 5-h post injection period compared to the ghrelin group (Table 1). The observed orexigenic effect of ghrelin injected ip in fed rats is in agreement with previous studies of our and other groups [17, 25, 34]. The inhibitory effect of ip injected desacyl ghrelin is observed at doses that had no effect on food intake in freely fed rats monitored during the light phase. Likewise, previous studies performed in the light phase showed that ip desacyl ghrelin did not alter the 30 min or 2 h cumulative food intake in fasted rats [7] or mice [26] in the fed state. However desacyl ghrelin decreased the dark-phase feeding response in rats [7, 8] and transgenic mice over-expressing desacyl ghrelin displayed a decrease in food intake and body weight [1]. Consistent with present observations in rats, both ip and icv desacyl ghrelin injection prior to ghrelin has been reported to block the orexigenic effect induced by ghrelin alone while having no effect of its own in goldfish (Carassius auratus) [23] indicative that interaction between desacyl ghrelin and ghrelin can be observed across species.
The pathway through which ip desacyl ghrelin initiates its inhibitory action seems to involve a direct action in the brain. This is supported by the ability of the peptide to cross the blood brain barrier in a non-saturable way due to the missing octanylation [4, 8]. In addition, there is evidence that the anorexic effect induced by ip desacyl ghrelin is not modulated by capsaicin treatment and not associated with Fos expression in the nucleus tractus solitarii of the brainstem in rats [8].
The present study also showed that the inhibitory action of desacyl ghrelin on ghrelin-induced food intake is selective to exogenous ghrelin since the peptide did not influence the refeeding response to a fast. Likewise in fasted mice desacyl ghrelin injected ip at a dose of 7.5 nmol/mouse was also found to have no effect on food intake [26] which contrasts with initial reports showing an inhibitory effect of desacyl ghrelin on food intake in fasted rats at a similar dose [8] or mice at a large dose 274 μg/kg [1], Neary et al. were also unable to reproduce these findings in fasted mice. The reason of these discrepancies remains unclear.
To demonstrate the effect of ghrelin on food intake, our experiments were carried out during the light phase. At this time, the animals are already satiated. Only under these conditions it is possible to assess potential pharmacological interactions between an orexigenic and a satiety peptide. It is rather difficult to detect the effect of a satiety peptide during this phase with the respective metabolic condition and ad libitum feeding. Our data indicate that animals treated solely with desacyl ghrelin take in similar amounts of food as compared to vehicle treated animals, since these animals are already satiated. This offers an explanation why desacyl ghrelin does not exert a satiating effect under these experimental conditions. We recently reported a similar phenomenon for cholecystokinin (CCK-8S) administered ip to ad libitum fed animals during the light phase [18]. The findings of Sánchez et al. also support this idea [31]. They found endogenous plasma levels of ghrelin to be very low at this point of time, which could be the reason for the missing inhibitory effect of desacyl ghrelin when administered singly.
It remains remarkable that there is no effect of desacyl ghrelin during the light phase under fasted conditions. Our experiments were all carried out at the beginning of the light cycle. At this time, ghrelin levels in animals after a 12 h fast are elevated and decrease immediately after food intake [5]. We acknowledge that the point of time of the administration of desacyl ghrelin immediately before beginning to measure food intake was not optimal, because at the beginning of the light phase, plasma ghrelin levels are highly induced by 12 h of fasting. Maybe ghrelin induced neuronal processing in the brain cannot be blocked any further at this point.
Mechanisms through which ghrelin increases food intake are well characterized to be linked with the activation of NPY neurons in the ARC. According to recent studies, peripheral injection of ghrelin induces a significant increase of Fos expression in arcuate neurons in rats and mice especially in the ventromedial part of the nucleus [13, 33, 36]. Furthermore, it has been revealed that a population of Fos-ir positive neurons contains the orexigenic peptide NPY [36] and blockade of NPY impaired ghrelin's orexigenic effect [25]. Thus, one could speculate that NPY neurons in the ARC are involved in the central mechanisms that mediate meal initiation by peripheral ghrelin. Our data showing increased Fos expression induced by desacyl ghrelin in ARC neurons scattered diffusely over the complete nucleus bear similarity with those reported in freely fed lean mice and rats which showed an increased number of Fos-ir positive neurons in the ARC after ip injection of desacyl ghrelin at similar doses [1, 8]. Furthermore, it has been described that desacyl ghrelin increased the amount of cocaine-amphetamine-regulated transcript (CART) mRNA in the mouse hypothalamus [1]. The neuropeptide CART is widely distributed in the rodent brain, being expressed in the ARC, PVN, and dorsomedial hypothalamus among others [3, 10, 20, 21] and reduces food intake when micro-injected into the lateral brain ventricle [22, 35].
A recent study indicates that nesfatin-1 derived from the NUCB2 protein decreases food intake and body weight in rodents after icv injection [27]. In addition, an immunohistochemical study showed that nesfatin-1 immunopositive neurons are also located in ARC neurons, and co-localize with POMC/CART-neurons but not with NPY/AgRP-neurons [6, 27]. Double labeling performed in the present study revealed that nesfatin-1 immunoreactive neurons in the ARC are activated by simultaneous injection of ghrelin and desacyl ghrelin. In this study we found that ∼55% of the neurons that were positive for Fos after simultaneous administration of both peptides were also immunopositive for nesfatin-1, whereas after injection of desacyl ghrelin alone, ∼43% of the Fos positive neurons in the ARC were nesfatin-1 immunoreactive. These findings provide novel neuroanatomical evidence that may support new pathways of peripherally administered desacyl ghrelin by blocking ghrelin-activated NPY/AgRP-neurons in the ARC via nesfatin-1 immunopositive neurons. It has been reported that nesfatin-1 inhibits NPY-neurons as evidenced in whole cell current clamp recordings in the rat ARC which would be consistent with such neuronal mechanisms [29]. In the present study we observed that after peripheral injection of ghrelin, ∼18% of the Fos-positive neurons in the ARC were also immunoreactive for nesfatin-1. The reason or the physiological relevance of this interesting observation remains unclear and needs further investigation.
In conclusion, the present data show that desacyl ghrelin injected ip blocked the orexigenic effect induced by peripheral ghrelin in freely fed rats and attenuates Fos expression induced by ghrelin in the ventromedial part of the ARC. Double staining revealed that the effect of desacyl ghrelin on the inhibition of ghrelin-activated neurons in the ARC may be mediated via nesfatin-1 immunopositive neurons.
Acknowledgments
This work was supported by grants from the German Research Foundation (DFG: KO 3864/1-1) and the Charité-Universitätsmedizin Berlin (UFF 2008-251), and grants to Y.T. (Research Career Scientist Award, Department of Veterans Affairs and NIHDK R01 33061).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut. 2005;54:18–24. doi: 10.1136/gut.2004.038737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–45. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- [3].Balkan B, Koylu E, Pogun S, Kuhar MJ. Effects of adrenalectomy on CART expression in the rat arcuate nucleus. Synapse. 2003;50:14–9. doi: 10.1002/syn.10213. [DOI] [PubMed] [Google Scholar]
- [4].Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–7. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- [5].Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr., Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- [6].Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–94. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- [7].Chen CY, Chao Y, Chang FY, Chien EJ, Lee SD, Doong ML. Intracisternal desacyl ghrelin inhibits food intake and non-nutrient gastric emptying in conscious rats. Int J Mol Med. 2005;16:695–9. [PubMed] [Google Scholar]
- [8].Chen CY, Inui A, Asakawa A, Fujino K, Kato I, Chen CC, Ueno N, Fujimiya M. Des-acyl Ghrelin Acts by CRF Type 2 Receptors to Disrupt Fasted Stomach Motility in Conscious Rats. Gastroenterology. 2005;129:8–25. doi: 10.1053/j.gastro.2005.04.015. [DOI] [PubMed] [Google Scholar]
- [9].Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, Spedding M, Kojima M, Kangawa K. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57:541–6. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- [10].Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–81. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gauna C, Delhanty PJ, Hofland LJ, Janssen JA, Broglio F, Ross RJ, Ghigo E, van der Lely AJ. Ghrelin stimulates, whereas des-octanoyl ghrelin inhibits, glucose output by primary hepatocytes. J Clin Endocrinol Metab. 2005;90:1055–60. doi: 10.1210/jc.2004-1069. [DOI] [PubMed] [Google Scholar]
- [12].Heijboer AC, van den Hoek AM, Parlevliet ET, Havekes LM, Romijn JA, Pijl H, Corssmit EP. Ghrelin differentially affects hepatic and peripheral insulin sensitivity in mice. Diabetologia. 2006;49:732–8. doi: 10.1007/s00125-006-0138-2. [DOI] [PubMed] [Google Scholar]
- [13].Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047–9. doi: 10.1046/j.1365-2826.2000.00584.x. [DOI] [PubMed] [Google Scholar]
- [14].Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–13. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- [15].Kobelt P, Goebel M, Stengel A, Schmidtmann M, van dV I, Tebbe JJ, Veh RW, Klapp BF, Wiedenmann B, Wang L, Taché Y, Mönnikes H. Bombesin but not amylin blocks the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2006;291:R903–13. doi: 10.1152/ajpregu.00681.2005. [DOI] [PubMed] [Google Scholar]
- [16].Kobelt P, Tebbe JJ, Tjandra I, Bae HG, Rüter J, Klapp BF, Wiedenmann B, Mönnikes H. Two immunocytochemical protocols for immunofluorescent detection of c-Fos positive neurons in the rat brain. Brain Res Brain Res Protoc. 2004;13:45–52. doi: 10.1016/j.brainresprot.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [17].Kobelt P, Tebbe JJ, Tjandra I, Stengel A, Bae HG, Andresen V, van dV I, Veh RW, Werner CR, Klapp BF, Wiedenmann B, Wang L, Taché Y, Mönnikes H. CCK inhibits the orexigenic effect of peripheral ghrelin. Am J Physiol Regul Integr Comp Physiol. 2005;288:R751–58. doi: 10.1152/ajpregu.00094.2004. [DOI] [PubMed] [Google Scholar]
- [18].Kobelt P, Wisser AS, Stengel A, Goebel M, Bannert N, Gourcerol G, Inhoff T, Noetzel S, Wiedenmann B, Klapp BF, Taché Y, Mönnikes H. Peripheral obestatin has no effect on feeding behavior and brain Fos expression in rodents. Peptides. 2008;29:1018–27. doi: 10.1016/j.peptides.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- [20].Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–32. [PubMed] [Google Scholar]
- [21].Koylu EO, Weruaga E, Balkan B, Alonso JR, Kuhar MJ, Pogun S. Colocalization of cart peptide immunoreactivity and nitric oxide synthase activity in rat hypothalamus. Brain Res. 2000;868:352–7. doi: 10.1016/s0006-8993(00)02290-3. [DOI] [PubMed] [Google Scholar]
- [22].Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–6. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- [23].Matsuda K, Miura T, Kaiya H, Maruyama K, Shimakura SI, Uchiyama M, Kangawa K, Shioda S. Regulation of food intake by acyl and des-acyl ghrelins in the goldfish. Peptides. 2006;27:2321–5. doi: 10.1016/j.peptides.2006.03.028. [DOI] [PubMed] [Google Scholar]
- [24].Muccioli G, Baragli A, Granata R, Papotti M, Ghigo E. Heterogeneity of Ghrelin/Growth Hormone Secretagogue Receptors. Toward the Understanding of the Molecular Identity of Novel Ghrelin/GHS Receptors. Neuroendocrinology. 2007;86:147–164. doi: 10.1159/000105141. [DOI] [PubMed] [Google Scholar]
- [25].Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- [26].Neary NM, Druce MR, Small CJ, Bloom SR. Acylated ghrelin stimulates food intake in the fed and fasted states but desacylated ghrelin has no effect. Gut. 2006;55:135. [PMC free article] [PubMed] [Google Scholar]
- [27].Oh I, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- [28].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Third ed. Academic Press; San Diego: 1997. [Google Scholar]
- [29].Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res. 2008;1230:99–106. doi: 10.1016/j.brainres.2008.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rüter J, Kobelt P, Tebbe JJ, Avsar Y, Veh R, Wang L, Klapp BF, Wiedenmann B, Taché Y, Mönnikes H. Intraperitoneal injection of ghrelin induces Fos expression in the paraventricular nucleus of the hypothalamus in rats. Brain Res. 2003;991:26–33. doi: 10.1016/j.brainres.2003.07.005. [DOI] [PubMed] [Google Scholar]
- [31].Sanchez J, Oliver P, Pico C, Palou A. Diurnal rhythms of leptin and ghrelin in the systemic circulation and in the gastric mucosa are related to food intake in rats. Pflugers Arch. 2004;448:500–6. doi: 10.1007/s00424-004-1283-4. [DOI] [PubMed] [Google Scholar]
- [32].Toshinai K, Yamaguchi H, Sun Y, Smith RG, Yamanaka A, Sakurai T, Date Y, Mondal MS, Shimbara T, Kawagoe T, Murakami N, Miyazato M, Kangawa K, Nakazato M. Des-acyl Ghrelin Induces Food Intake by a Mechanism Independent of the Growth Hormone Secretagogue Receptor. Endocrinology. 2006;147:2306–14. doi: 10.1210/en.2005-1357. [DOI] [PubMed] [Google Scholar]
- [33].Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA. Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol. 2002;14:580–6. doi: 10.1046/j.1365-2826.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- [34].Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–13. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- [35].Wang C, Billington CJ, Levine AS, Kotz CM. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. Neuroreport. 2000;11:3251–5. doi: 10.1097/00001756-200009280-00040. [DOI] [PubMed] [Google Scholar]
- [36].Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- [37].Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- [38].Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–8. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]