Abstract
Taurine (2-aminoethanesulphonic acid), a sulphur-containing amino acid, is found in most mammalian tissues. Although it can be synthesized endogenously, the major source of taurine is from the diet. Taurine was found to exhibit diverse biological actions, including protection against ischemia-reperfusion injury, modulation of intracellular calcium concentration, and antioxidant, antiatherogenic and blood pressure-lowering effects. The present review will address the potential beneficial actions of taurine in congestive heart failure, hypertension, ischemic heart disease, atherosclerosis and diabetic cardiomyopathy. There is a wealth of experimental information and some clinical evidence available in the literature suggesting that taurine could be of benefit in cardiovascular disease of different etiologies. However, double-blind long-term clinical trials need to be conducted before taurine can be unequivocally recommended as a nutritional intervention for the prevention and/or treatment of cardiovascular disease.
Keywords: Angiotensin II, Congestive heart failure, Diabetic cardiomyopathy, Hypertension, Ischemic heart disease, Oxidative stress, Taurine
Taurine is the most abundant intracellular sulphur-containing amino acid (1). Although it can be synthesized from methionine and cysteine in the presence of vitamin B6 (1,2), taurine can be obtained from the diet, predominantly through eggs, meat and seafood. High concentrations of taurine are found in the heart and retina, whereas smaller amounts are found in the brain, kidneys, intestine and skeletal muscle (2). It is now well established that taurine is involved in many diverse biological and physiological functions (1,3). For example, it is known to play a role in bile salt formation and fat digestion. Furthermore, taurine is involved in the maintenance of homeostasis of intracellular Na+ and intracellular Ca2+ concentrations ([Ca2+]i), and in the balance of neurotransmitters (4–6). Taurine deficiency is associated with anxiety, epilepsy, hyperactivity and depression; taurine supplementation can relieve these symptoms (7). Recently, it was shown to be an effective agent in the treatment of alcoholism, fatigue and myotonia (8,9). Taurine has also been reported to protect visual function during diabetes (10) and improve immunocompetence (11). In addition, taurine and its analogues have been observed to exert antineurotoxic and anti-inflammatory effects, and inhibit tumour cell proliferation (10–14). Taurine has also been shown to protect various organs against damage induced by mental and oxidative stress (15–17). Liao et al (18) demonstrated that a taurine transporter is expressed in vascular smooth muscle cells and suggested that it may play an important role in vascular function (19,20). A number of clinical trials revealed beneficial actions of taurine during different pathophysiological conditions (Table 1); however, the mechanisms of these actions are not yet understood. The present review focuses on a discussion of the clinical value and potential of taurine as a nutraceutical for the prevention and treatment of diabetic cardiomyopathy, ischemic heart disease (IHD), hypertension and congestive heart failure (CHF).
TABLE 1.
Clinical studies with taurine
| Taurine dose | Subjects | Results | Reference |
|---|---|---|---|
| 3 g/day for 30 to 45 days | CHF | Decreased left ventricular end-diastolic volume | Jeejeebhoy et al (36) |
| 6 g/day for 4 weeks | CHF | Cardiac function was improved, no side effects | Azuma et al (41) |
| 3 g/day for 7 weeks | Overweight | Decreased TG and body weight | Zhang et al (53) |
| 6 g/day for 3 weeks | High lipid diet | Decreased serum cholesterol and LDL, increased VLDL and TG | Mizushima et al (54) |
| 0.4 g/day for 2 weeks | Healthy volunteer | Decreased platelet aggregation and platelet release | Hayes et al (58) |
| 1.5 g/day for 90 days | Diabetes | Decreased platelet aggregation | Franconi et al (75) |
| 6 g/day for 1 week | Hypertension | Decreased blood pressure | Militante and Lombardini (110) |
CHF Congestive heart failure; LDL Low-density lipoprotein; TG Triglyceride; VLDL Very low-density lipoprotein
Beneficial actions of taurine in ischemia-reperfusion of the heart
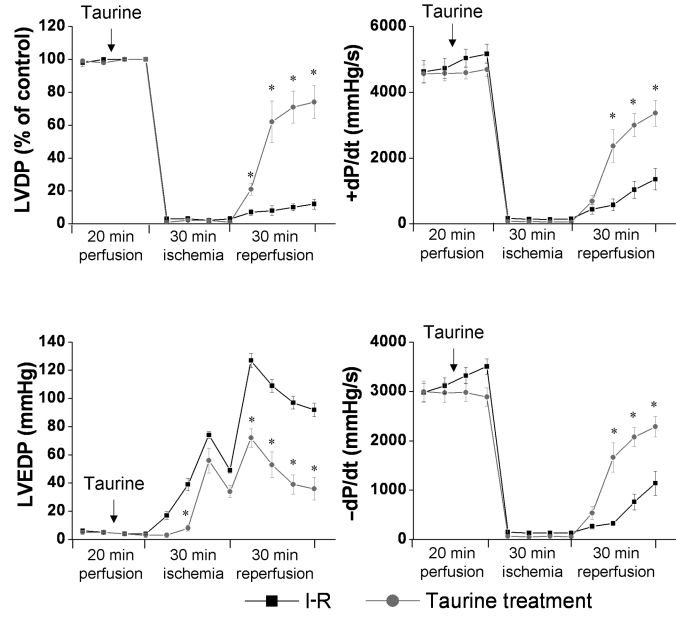
Approximately 12 million individuals visited a physician’s office for IHD in the United States in 2001 (21). Presently, it is estimated that 18.5 million people in the United States suffer from IHD. Data generated from our laboratory (St Boniface Hospital Research Centre, Winnipeg, Manitoba) has demonstrated that taurine protects against loss of functional recovery during ischemia-reperfusion (I-R) of isolated perfused rat hearts (Figure 1). A loss of mechanical function in rat hearts subjected to either the Ca2+ paradox protocol or global I-R has been found to correlate with decreases in myocardial taurine levels (22,23). There was a second phase of taurine release upon reperfusion of the ischemic heart, which exceeded the amount of taurine extruded during the ischemic insult of the heart (24). It has also been recently observed that taurine protects against Ca2+ paradox-induced cardiac injury (25,26) by preventing Ca2+ overload in cardiomyocytes and cell death. Because the increase of intracellular Na+ is a critical step in cardiac damage due to Ca2+ paradox or I-R, taurine supplementation may reduce the intracellular Na+ concentration, and subsequently reduce Ca2+ overload by inhibition of the Na+-Ca2+ exchanger. This effect offers another possible mechanism that explains how taurine protects the heart from I-R-induced damage (26). Furthermore, taurine may provide cardioprotection under conditions of I-R, by virtue of its antioxidant properties (24,27), and may prevent oxidant-mediated damage of the cardiomyocyte membrane and subsequent intracellular Ca2+ overload. It should be pointed out that during acute coronary events (unstable angina and myocardial infarction, often before the onset of ischemic damage), neutrophils are known to secrete proteolytic enzymes in latent forms, which are activated by hypochlorous acid (HOCl) generated by myeloperoxidase (28). Because taurine is a physiological antagonist of HOCl, supplementation with this agent protects the heart by diminishing the inflammatory response in cases of acute coronary artery disease (28). On the other hand, taurine interaction with HOCl produces taurine monochloramine, a less toxic and more stable oxidant that has been reported to activate a cell death pathway involving Bax protein and caspase-9 in lymphocytes (29). While there is no known similar effect in the cardiovascular system, such an interaction would be undesirable.
Figure 1.
The protective effect of taurine on ischemia-reperfusion (I-R) in rat hearts. Isolated rat hearts were perfused in the absence or presence of taurine (10 mM) as described previously (26), and were subjected to 30 min of global ischemia followed by reperfusion. The control value for the left ventricular development pressure (LVDP) was 87±6 mmHg. –dP/dt Rate of left ventricular pressure decay; +dP/dt Rate of left ventricular pressure development; LVEDP Left ventricular end-diastolic pressure
Although the exact mechanisms of the possible beneficial actions of taurine in IHD have yet to be identified, based on the information available in the literature, it can be suggested that the mechanisms could include attenuation of Ca2+ overload, antioxidant effects or membrane-stabilizing effects (30–32). In fact, it would be interesting to investigate whether taurine supplementation could prevent myocardial infarction in atherosclerotic patients. While the aforementioned discussion points to the benefits of taurine supplementation in treating I-R-induced injury, Allo et al (33) reported that taurine depletion protects the heart against I-R-induced injury in the rat model. In this regard, to deplete hearts from control and β-alanine-treated rats of their endogenous levels of taurine produces smaller infarct size-to-risk area ratios after a 45 min ligation of the coronary artery and 2 h of reperfusion. Furthermore, cardioprotection was lost following dietary taurine repletion. Thus, it would appear that the putative beneficial actions of taurine in myocardial I-R warrant further examination.
Taurine and CHF
The World Health Organization has reported an increase in the incidence of CHF. Between 1968 and 1998, the number of deaths from CHF in the United States rose from about 10,000 to almost 50,000 (34). In fact, approximately five million Americans are currently living with CHF, and 550,000 cases are diagnosed in the United States each year. The number of individuals with CHF is expected to increase as the population ages. The direct and indirect costs of CHF for 2006 were estimated at $29.6 billion in the United States alone (34).
The majority of symptomatic patients with CHF are mal-nourished, and have a relative deficiency of taurine (35). Restoring adequate cardiomyocyte nutrition, including the level of taurine, would seem to be essential to any therapeutic strategy designed to benefit patients with CHF (36). It is well known that CHF is characterized by defects in Ca2+ homeostasis, and because taurine can influence [Ca2+]i, its supplementation could benefit patients with CHF. Indeed, parenteral administration of taurine (200 mg/day for seven days) has been reported to partially protect against myocardial cell necrosis induced by a toxic dose of isoprenaline in chick hearts (37). Taurine was attributed to the prevention of intracellular Ca2+ overload. Jeejeebhoy et al (36) reported that taurine can lower left ventricular end-diastolic pressure in patients with heart failure. Although an increase in cardiac taurine content due to an increase in adrenergic stimulation of taurine influx has been reported in CHF (38,39), oral taurine supplementation (6 g/day for four weeks) in these patients exerted a positive inotropic action and reduced mortality. In Japan, taurine has been used clinically and has been claimed to be beneficial to patients that are unresponsive or resistant to digitalis or diuretics (40,41). In a clinical trial (40), the effect of oral administration of taurine (3 g/day) and coenzyme Q10 (30 mg/day) in 17 patients with CHF secondary to ischemic or idiopathic dilated cardiomyopathy was compared. In the taurine-treated group, unlike the coenzyme Q10-treated group, an improvement of the systolic left ventricular function was observed after six weeks (40). While the mechanisms responsible for this effect need to be defined, Franconi et al (42) reported that the depression of the contractile force of guinea pig ventricular strips in a Ca2+-free or low Ca2+ medium is prevented by taurine. In addition, these investigators blocked the negative inotropic effect of verapamil, a Ca2+ channel blocker, by taurine (42). It was suggested that the positive inotropic effect of taurine could be due to its effect on [Ca2+]i in cardiomyocytes (42). Indeed, other studies showed that Ca2+ is an important factor in mediating the effects of taurine in the heart. For instance, the action of taurine on cardiac function in forced-swim-stressed rats was found to be dependent on the extracellular concentration of Ca2+ (43). Taurine has also been reported to increase [Ca2+]i and contractile force in guinea pig ventricular strips (44). These investigators observed that superfusion of ventricular strips with a taurine-free medium produced a decrease in taurine content at the end of 120 min; this change was restored by superfusion with 10 mM taurine (45).
It is interesting to note that fatal dilated cardiomyopathy in dogs, induced by taurine deficiency, can be prevented by taurine supplementation (46). Also, taurine has been reported to exert an antioxidant effect against isoproterenol-induced myocardial infarction in rats (47). These investigators have suggested that the beneficial action of taurine could be due to a membrane-stabilizing effect. Thus, it would appear, as in the case of IHD, that the beneficial actions of taurine in CHF can be attributed to its effects on [Ca2+]i, its antioxidant properties and its membrane-stabilizating effect. In fact, it may be suggested that taurine can prevent intracellular Ca2+ overload under conditions of oxidative stress, and may improve or prevent defects in Ca2+ handling in cardiomyocytes during CHF. It should also be mentioned that because combination therapy is commonly used in clinical practice, it would be worthwhile to investigate the interaction of taurine with pharmacological agents used to treat CHF.
Antiatherogenic potential of taurine
Atherosclerosis, which affects over 60 million people in the United States alone, has been extensively studied during the past six decades (48). Although low-density lipoproteins (LDL) are known to contribute to the formation of plaque in the arterial wall, oxidized LDL can further exacerbate plaque formation. In hypercholesterolemia, dietary supplementation with taurine has been found to improve the serum lipid profile (49). High cholesterol-fed rats treated with taurine (15 g/kg/day) for five weeks showed a 37% reduction in plasma LDL, a 32% reduction in total cholesterol and a 43% reduction in triglyceride (TG) levels when compared with control rats fed the same diet without taurine (50). Furthermore, rats fed a high taurine diet, in comparison with rats fed a cholesterol-free diet, showed a significant decrease in plasma concentrations of LDL, total cholesterol and TG. In addition, a 43% reduction in hepatic TG and a 77% elevation in free fatty acids in the liver were observed (51). In mice, taurine has been shown to lower serum LDL and very low-density lipoproteins by 44%, while elevating high-density lipoprotein concentrations by 25%; taurine also decreased the concentration of cholesterol in the liver by 19% (51). It was indicated that the ability of taurine to lower cholesterol may be due to its effect on the conversion of cholesterol to bile acids (51). A 31% decrease in aortic lesions in Watanabe heritable hyperlipidemic rabbits given 0.3% taurine in drinking water for 24 weeks has also been reported (52). Clinically, taurine treatment (3 g/day) has been demonstrated to improve lipid metabolism and reduce body weight in overweight subjects, as well as reduce TG levels and the atherogenic index (53). In another clinical study (54), taurine supplementation (6 g/day) in healthy young men consuming a high-fat diet significantly reduced serum total cholesterol and LDL levels. It should be noted that because taurine has an antioxidant effect, it may also reduce the oxidation of LDL (1,2,53) and thereby attenuate the process of atherosclerosis.
Platelet activation, adhesion and aggregation at sites of vascular endothelial disruption caused by atherosclerosis are key events in arterial thrombus formation (55). The status of taurine has been suggested to exert a significant effect on platelet aggregability (56). Platelets from taurine-depleted cats were reported to be twice as sensitive to aggregation as platelets from cats receiving taurine (57). On the other hand, platelets from humans with normal taurine status showed an increase in resistance to aggregation by 30% to 70% when supplemented with taurine at 400 mg/day or 1600 mg/day, respectively (58). Furthermore, it was demonstrated that a decrease in platelet aggregability was associated with an increase in platelet taurine and glutathione concentrations, as well as a decrease in thromboxane release on platelet aggregation (58). Overall, these lines of data demonstrate that taurine, in vivo, stabilizes platelets against aggregation and that during taurine depletion, platelets become overly sensitive to aggregation. This tendency of platelets to aggregate is depressed by taurine.
It should be mentioned that lysophosphatidic acid (LPA) is a major lipid extracted from human atherosclerotic plaques (59). Due to its ability to increase [Ca2+]i, and increase proliferation and migration of vascular smooth muscle cells (59–62), LPA is considered to have an important role in the development of atherosclerosis. While taurine has been shown to inhibit rat vascular smooth muscle cell proliferation (63), preliminary data from our laboratory (St Boniface Hospital Research Centre, Winnipeg, Manitoba) have also revealed that taurine causes concentration-dependent inhibition of the LPA-induced increase in [Ca2+]i in cultured vascular smooth muscle cells (unpublished data). This finding could serve as a mechanism for the reported antiatherosclerotic effects of taurine. In addition, taurine may attenuate the progression of atherosclerosis due to its ability to lower serum lipids and reduce the oxidation of LDL, as well as decrease the risk of arterial thrombus formation by decreasing platelet aggregation.
Interaction of taurine and angiotensin II
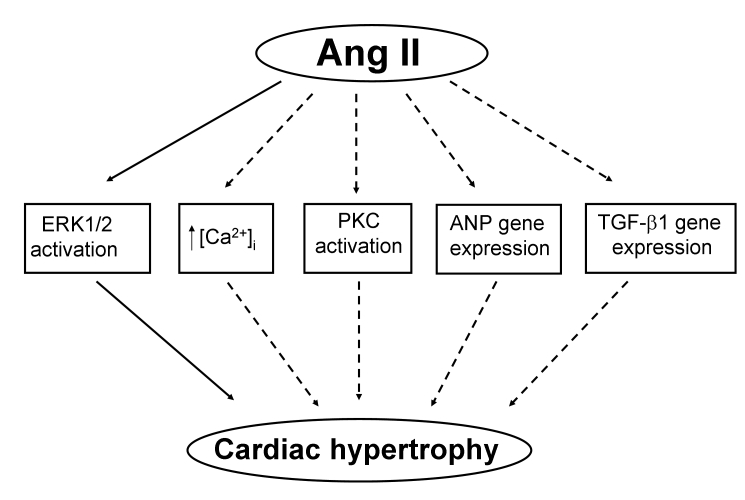
Angiotensin II (Ang II) is an important hormone that plays a key role in the maintenance of cardiovascular homeostasis. Experimental and clinical studies have shown that angiotensin-converting enzyme inhibitors prevent cardiac remodelling, improve heart function and reduce mortality (64). Taurine has also been found to partially block the effects of Ang II (65–68), implying that taurine may interfere with different actions of Ang II in cardiovascular cells (Figure 2). Indeed, taurine has been reported to reduce Ang II-induced cardiac hypertrophy by inhibiting the activation of protein kinase C and mitogen-associated protein kinase (65). Takahashi et al (66,67) reported that 20 mM taurine significantly attenuated Ang II-induced protein synthesis and the increase of [Ca2+]i in neonatal rat cardiomyocytes. Tao et al (68,69) observed that 20 mM taurine significantly reduced Ang II-induced cardiac hypertrophy and arrhythmias. Furthermore, Ang II triggers Na+-Ca2+ exchange, which can be regulated by taurine. This suggests that taurine can be used for the treatment of different cardiovascular abnormalities, where it may reverse the adverse effects of Ang II (70,71). The interaction of taurine and Ang II, with respect to Ca2+ and Na+ homeostasis and salt intake, are summarized in Table 2. However, it should be noted that it is not clear if the blocking effect of taurine on Ang II is due to an inhibitory action on Ang II receptors or other signal transduction mechanisms. Thus, the mechanism of interaction between taurine and Ang II needs to be further investigated.
Figure 2.
The interaction of taurine and angiotensin II (Ang II). Dashed arrows indicate an inhibitory effect caused by taurine. [Ca2+]i Intracellular Ca2+ concentration; ANP Atrial natriuretic peptide; ERK1/2 Extracellularly regulated kinase 1/2; PKC Protein kinase C; TGF-β1 Transforming growth factor β-1
TABLE 2.
Interaction of taurine and angiotensin II (Ang II) in the cardiovascular system
| Taurine | Target | Effects | Reference |
|---|---|---|---|
| 20 mM | Neonatal rat cardiomyocytes | Reduced 29 kDa protein phosphorylation and hypertrophy induced by Ang II | Azuma et al (65) |
| 20 mM | Neonatal rat cardiomyocytes | Blocked Ang II-induced hypertrophy | Takahashi et al (66,67) |
| 20 mM | Neonatal rat cardiomyocytes | Reduced hyperplastic growth and prevented increase in [Ca2+]i | Takahashi et al (66,67) |
| 20 mM | Neonatal rat hypertrophic cardiomyocytes | Inhibited arrhythmia and hypertrophy induced by Ang II | Tao et al (68) |
| 30 mg/kg | Left ventricle in renovascular hypertensive rats | Decreased systolic arterial pressure and weight of left ventricle, reduced blood pressure | Tao and Rao (69) |
| Taurine depletion | Cardiomyocytes and rat heart | Decreased Na+-Ca2+ exchange, prolonged calcium transient, reduced pHi and increase of [Ca2+]i, reduced response to Ang II | Schaffer et al (70) |
| Review | Review | Promoted natriuresis and diuresis, regulated [Na+]i and Na+-Ca2+ exchange and attenuated actions of Ang II | Schaffer et al (71) |
| 3% in H2O | Spontaneously hypertensive rats | Inhibited salt intake induced by renin | Abe et al (107) |
| Dose-dependent | Rat cardiomyocytes | Reduced increase in [Ca2+]i induced by Ang II | Rao and Tao (109) |
[Ca2+]i Intracellular Ca2+ concentration; [Na+]i Intracellular Na+ concentration; pHi Intracellular pH
Taurine and diabetic cardiomyopathy
The epidemic of obesity and sedentary lifestyles is projected to result in diabetes mellitus in over 300 million people worldwide by 2025 (72,73). In 2003, the prevalence of physician-diagnosed diabetes in the United States was about 14.1 million. The 2002 direct and indirect costs of diabetes were approximately $132 billion (34). Cardiovascular disease is responsible for 80% of deaths among diabetic patients (73). In chronic diabetes, intracellular accumulation of sorbitol, resulting from high extracellular levels of glucose, leads to the depletion of intracellular taurine levels, and is associated with the development of diabetic cardiomyopathy (74). The beneficial actions of taurine in both type 1 (insulin-dependent) and type 2 (noninsulin-dependent) diabetes are summarized in Table 3. In type 1 diabetes, platelet aggregation may cause an increased risk of cardiovascular events; it has been demonstrated that platelet taurine levels are lower in diabetic patients. When supplemented with taurine (1.5 g/day for 90 days), both plasma and platelet taurine levels were increased in diabetic patients. These were associated with a decrease in platelet aggregation induced by arachidonic acid (75). An in vitro study (75) showed that taurine inhibited platelet aggregation in a dose-dependent manner in diabetic patients. You and Chang (76) also demonstrated that taurine decreased TG and LDL levels in streptozotocin (STZ)-induced diabetic rats; however, the duration and dose of taurine treatment was an important determinant of the beneficial actions of taurine. Another study (77) reported that supplementation of cholesterol-fed and STZ-induced diabetic mice with taurine reduced serum LDL levels, but did not affect serum glucose levels. The increased level of malondialdehyde, a marker of lipid peroxidation, in the liver and the islet cells of type 1 diabetic mice was observed to be significantly attenuated by taurine supplementation (78). Taurine has also been shown to prevent the occurrence of cardiomyopathy in STZ-induced diabetes in rats due to prevention of the Ang II-mediated apoptosis of cardiomyocytes. Daily supplementation of overweight men, with a genetic predisposition for type 2 diabetes, with taurine (1.5 g/day) for eight weeks was reported to exert no effect on insulin secretion or sensitivity (79). However, the effects of taurine on insulin secretion and action in diabetic patients remains undetermined. It should, however, be noted that taurine accumulation in the pancreas has been reported to suppress insulin secretion in STZ-induced diabetic mice (80), indicating that taurine could exert a negative effect on the regulation of the serum level of glucose. Thus, while taurine supplementation could improve cardiovascular health in diabetic patients, it would also be important that future studies include a determination of tissue taurine content and its impact on their quality of life. Notwithstanding these issues, and the need for a long-term clinical trial in diabetic patients, and assessment of the cardiovascular risks and other complications, it can be suggested that taurine may protect against oxidative stress and lipid peroxidation. It may also improve serum lipid profile, which may slow down the progression of diabetes-induced complications (81–90) without affecting serum glucose levels. The beneficial actions of taurine in diabetes are summarized in Table 3.
TABLE 3.
Effects of taurine on diabetes and its complications
| Taurine | Target | Effects | Reference |
|---|---|---|---|
| 1.5 g/day for 8 weeks | Overweight men | No significant difference in insulin secretion | Brons et al (79) |
| 2% in diet for 6–12 weeks | Sensory neuron cells from STZ-diabetic rats | Improved Ca2+ homeostasis | Li et al (81) |
| 5% w/w in diet | STZ-induced diabetic rats | Decreased mortality rate, decreased glycemia | Di Leo et al (82) |
| 2.5% in water | Non-obese diabetic mice | Increased pancreatic islet mass and islet cell proliferation, reduced incidences of apoptosis and reduced insulitis | Arany et al (83) |
| 3% in water for 9 weeks | Type 2 diabetic OLETF rats | Decreased part of postprandial glucose oxidation, improved hyperglycemia and insulin resistance, increased muscle glycogen content and increased blood concentration of taurine, electrolytes and fluid volume | Harada et al (84) |
| 5% w/w in diet for 9 weeks | Type 2 diabetic OLETF rats | Decreased serum cholesterol and triacylglycerol, improved insulin sensitivity | Nakaya et al (85) |
| 2% in water for 30 days | High fructose-fed rats | Decreased glucose, glycated protein, glycosylated hemoglobin and fructosamine | Nandhini et al (86) |
| 3% for 14 or 21 days | Type 2 diabetic rats | Increased plasma HDL cholesterol concentration in diabetic rats, decreased cholesterol concentration in both normal and diabetic rats | Nishimura et al (87) |
| 2% w/w or 5% w/w for 16 weeks | STZ-induced diabetes | Decreased retinal conjugated dienes and lipid hyperoxides | Di Leo et al (88) |
| 3% in water for 8 weeks | STZ-treated rats | Prevented the loss of both insulin and adrenergic agonist stimulation | Colivicchi et al (90) |
| 2% in water for 30 days | Fructose-fed rats | Increased urinary kallikrein activity and activity of membrane-bound ATPases, decreased hyperglycemia and insulin resistance | Nandhini and Anuradha (91) |
HDL High-density lipoprotein; OLETF Otsuka Long-Evans Tokushima Fatty; STZ Streptozotocin; w/w Weight/weight
Antihypertensive action of taurine
Taurine (1% to 2% w/v in drinking water) was found to prevent high-fructose diet-induced hypertension in rats (91). Furthermore, this antihypertensive effect was abolished by a kinin B2 receptor antagonist, Hoe 140, indicating the involvement of kinins in the mechanism of taurine action in the development of hypertension in this model (91). In addition, taurine was also found to prevent an increase in platelet [Ca2+]i in high-fructose diet-induced hypertensive rats (92). In salt-dependent hypertensive rats, 3% w/v taurine in drinking water attenuated blood pressure (BP) significantly (93), suggesting that taurine supplementation may have a beneficial effect on hypertension.
An injection of taurine in the anterior hypothalamus area has been reported to decrease BP; this taurine effect seems to be mediated by β-adrenoceptors (94). Thus, the antihypertensive effect of taurine may be partially attributed to its effect on the central nervous system (95). The plasma taurine concentration, and taurine release from the myocardium and aortic wall, were observed to be higher in spontaneously hypertensive rats (SHR) than in Wistar-Kyoto rats (96). Furthermore, taurine content and uptake in the myocardium and aortic wall was lower in SHR, suggesting there was a dysfunction of taurine metabolism in the myocardium and aortic wall in SHR (96), which may have been a contributing factor in hypertension in this rat model. Although taurine deficiency in uninephrectomized rats has been shown to accelerate the development of hypertension in response to high dietary NaCl (97), different responses have been reported with taurine supplementation on salt-induced hypertension in rats (98). In this regard, these investigators observed that while short-term (six weeks) taurine supplementation exerted an antihypertensive effect, long-term (six months) supplementation with taurine had no influence on BP.
A number of experimental studies (99–105) demonstrated that taurine can exert a direct effect on vascular function. The effect of taurine depletion on the cardiovascular responses of rats to vasoactive agents has been examined. For example, Mozaffari and Abebe (99) reported that taurine depletion induced by dietary β-alanine is associated with a reduction in the pressor response to Ang II. Although heart rate was also reduced, it was not significantly different from the control heart rate. These investigators concluded that pressor response to Ang II occurred without impairing baroreflex function. On the other hand, the contractile responses to norepinephrine (NE) and KCl were reported to be attenuated in aortic ring preparations from taurine-treated rats (100). It was also observed that the extent of reduction of contractility was greater in endothelium-intact tissues contracted with NE. While acetylcholine-induced relaxation was augmented in endothelium-intact vessels from rats (100), in another study (101), NE- and KCl-induced contractile responses of endothelium-denuded aortae were found to be enhanced in taurine-depleted rats (101). Furthermore, taurine deficiency was also shown to reduce the relaxant responses of endothelium-intact aortic rings elicited by acetylcholine, an effect attributed to decreased nitric oxide production (102). Interestingly, taurine has recently been reported to reduce the alterations in both contractile and relaxant responses caused by high glucose during diabetes, underscoring the potential of taurine as a therapeutic agent to prevent or ameliorate vascular complications during diabetes (102). Furthermore, taurine has been reported to attenuate acute hyperglycemia-induced endothelial cell apoptosis, as well as leukocyte-endothelial cell interactions and cardiac dysfunction (103), indicating that taurine has the potential to reduce diabetic microvascular inflammatory injury and concomitant cardiac dysfunction. In addition, Kamata et al (77) reported that chronic taurine supplementation improved endothelial-derived relaxation in hypercholesterolemic and diabetic mice, in which nitric oxide was a major factor. The antihypertensive effect of taurine is further indicated by the observation that taurine supplementation decreases BP of stroke-prone SHR (104). These investigators also reported that pretreatment of the mesenteric artery with taurine, in vitro, reduced the NE-induced vasoconstriction. In contrast, in an isolated rabbit ear artery, taurine was shown to have no effect on the NE-induced contraction, but did exert a concentration-dependent vasodilator effect in arteries contracted with a high-potassium medium (105). It should also be noted that endogenous taurine deficiency causes a different inhibitory effect on adenosine receptor-mediated vasorelaxation, depending on the adenosine receptor agonist used (106).
While the mechanisms of the action of taurine on the vasculature need to be further explored, it is evident that the endothelium, and specifically nitric oxide, plays an important role in the vasodilatory action of taurine in response to vasoactive agents. In addition, taurine has been shown to suppress the sympathetic nervous system (54) and block the effects of Ang II (107–109). Thus, taurine may reduce the stress on the cardiovascular system, which could be another mechanism of the antihypertensive action of taurine. It should be noted that clinical studies have demonstrated that supplementation of taurine (6 g/day for seven days) significantly reduced BP in patients with essential hypertension (110). From the aforementioned discussion, taurine can be suggested as a novel treatment for hypertension; however, its long-term effects have not yet been investigated.
CONCLUSIONS
The potential health benefits of taurine in cardiovascular disease are rapidly emerging. Although more research needs to be performed, numerous experimental and several clinical studies demonstrated that taurine helps the cardiovascular system through a variety of mechanisms including an improved lipid profile, modulation of [Ca2+]i, antioxidant effects and antagonism of Ang II action (Figures 3 and 4). Because oxidative stress is known to cause intracellular Ca2+ overload (111), it is likely that the modulation of [Ca2+]i by taurine may be mediated through its antioxidant effects. Furthermore, because Ang II generates reactive oxygen species (112), it can be argued that the antagonism of Ang II actions by taurine may also be a consequence of its antioxidant effects. A recent report (113) demonstrated that taurine can prevent endothelial cell dysfunction induced by high glucose and oxidized LDL. Thus, this action of taurine could be an important mechanism for providing benefits to the cardiovascular system during different pathophysiological conditions. Although it appears that nutritional supplementation with taurine could serve as both a preventative and an adjunct to treatments for patients predisposed to or with existing cardiovascular disease, the effective dose of taurine needs to be established. In addition, changes in the serum levels of taurine in cardiovascular disease need to be measured. As well, toxicological studies related to taurine supplementation need to be performed. Furthermore, double-blind long-term clinical trials are required before the implementation of taurine as a new nutritional intervention for patients at risk of taurine deficiency and cardiovascular disease. An improved therapy would reduce the burden on the public health care system, lead to fewer readmissions and improve quality of life. While no severe side effects were reported with taurine-supplemented beverages or commercially available multivitamin and mineral formulations, some caution should be used when consuming these preparations. Pregnant women should be particularly careful, because it has been reported that maternal taurine supplementation during pregnancy causes insulin resistance and obesity in rat offspring (114).
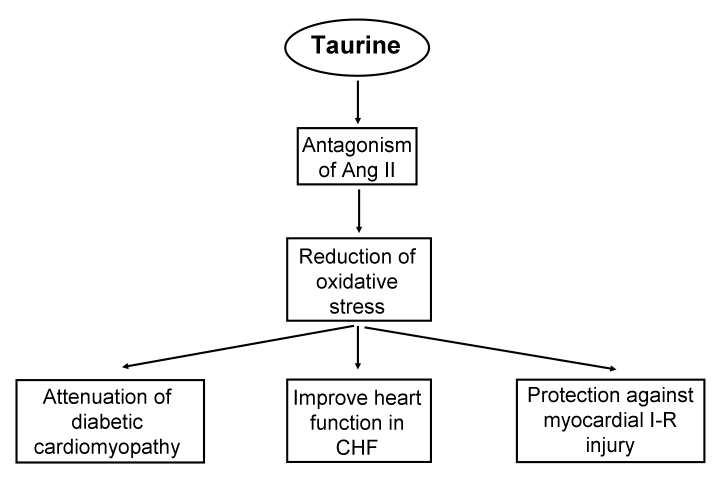
Figure 3.
Mechanism of taurine action involving angiotensin II (Ang II) antagonism in atherosclerosis, ischemic heart disease, congestive heart failure (CHF) and diabetic cardiomyopathy. I-R Ischemia-reperfusion
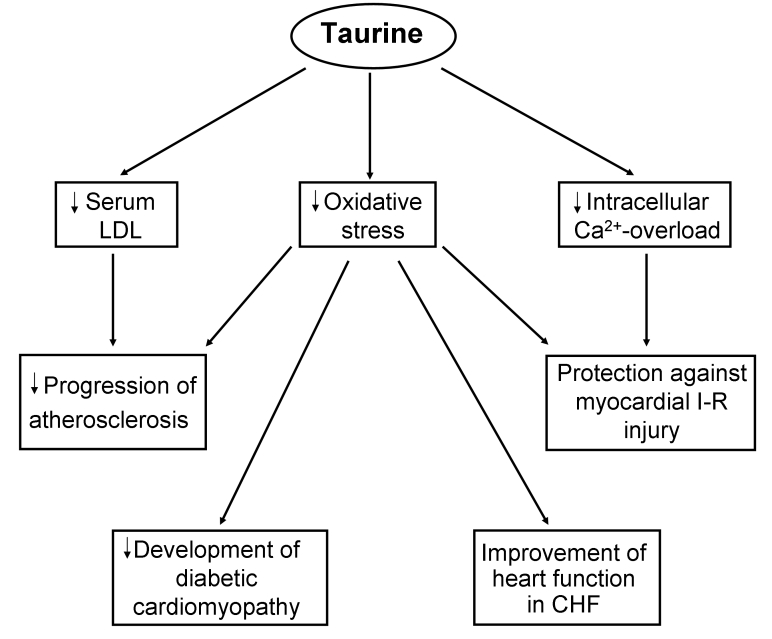
Figure 4.
Mechanisms of taurine action involving reductions in oxidative stress, serum low density lipoproteins (LDL) and intracellular Ca2+ overload in diverse cardiovascular abnormalities. CHF Congestive heart failure; I-R Ischemia-reperfusion
Acknowledgments
The work reported in the present article was supported by a grant from the Canadian Institutes of Health Research.
REFERENCES
- 1.Boucknooghe T, Remacle C, Reusens B. Is taurine a functional nutrient? Curr Opin Clin Nutr Metab Care. 2006;9:728–33. doi: 10.1097/01.mco.0000247469.26414.55. [DOI] [PubMed] [Google Scholar]
- 2.Birdsall TC. Therapeutic applications of taurine. Altern Med Rev. 1998;3:128–36. [PubMed] [Google Scholar]
- 3.Warskulat U, Heller-Stilb B, Oermann E, et al. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007;428:439–58. doi: 10.1016/S0076-6879(07)28025-5. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RC, Win T, Bittner S. Taurine analogues; a new class of therapeutics: Retrospect and prospects. Curr Med Chem. 2005;12:2021–39. doi: 10.2174/0929867054546582. [DOI] [PubMed] [Google Scholar]
- 5.Parcell S. Sulfur in human nutrition and applications in medicine. Altern Med Rev. 2002;7:22–44. [PubMed] [Google Scholar]
- 6.Guizouarn H, Motais R, Garcia-Romeu, Borgese F. Cell volume regulation: The role of taurine loss in maintaining membrane potential and cell pH. J Physiol. 2000;523:147–54. doi: 10.1111/j.1469-7793.2000.t01-1-00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong WX, Chen SW, Li YL, et al. Effects of taurine on rat behaviors in three anxiety models. Pharmacol Biochem Behav. 2006;83:271–6. doi: 10.1016/j.pbb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Soyka M, Roesner S. New pharmacological approaches for the treatment of alcoholism. Expert Opin Pharmacother. 2006;7:2341–53. doi: 10.1517/14656566.7.17.2341. [DOI] [PubMed] [Google Scholar]
- 9.Trip J, Drost G, van Engelen BG, Faber CG. Drug treatment for myotonia. Cochrane Database Syst Rev. 2006:CD004762. doi: 10.1002/14651858.CD004762.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X, Xu Z, Mi M, et al. Dietary taurine supplementation ameliorates diabetic retinopathy via anti-excitotoxicity of glutamate in streptozotocin-induced Sprague-Dawley rats. Neurochem Res. 2008;33:500–7. doi: 10.1007/s11064-007-9465-z. [DOI] [PubMed] [Google Scholar]
- 11.Grimble RF. The effects of sulfur amino acid intake on immune function in humans. J Nutr. 2006;136(6 Suppl):1660S–5S. doi: 10.1093/jn/136.6.1660S. [DOI] [PubMed] [Google Scholar]
- 12.Klusa V, Klimaviciusa L, Duburs G, Poikans J, Zharkovsky A. Anti-neurotoxic effects of tauropyrone, a taurine analogue. Adv Exp Med Biol. 2006;583:499–508. doi: 10.1007/978-0-387-33504-9_56. [DOI] [PubMed] [Google Scholar]
- 13.Petrovic L, Schlegel KA, Ries J, et al. In vitro effect of taurolidine on squamous cell carcinoma in the oral cavity. Mund Kiefer Gesichtschir. 2003;7:102–7. doi: 10.1007/s10006-003-0452-5. [DOI] [PubMed] [Google Scholar]
- 14.Marcinkiewicz J, Kurnyta M, Biedron R, Bobek M, Kontny E, Maslinski W. Anti-inflammatory effects of taurine derivatives (taurine chloramine, taurine bromamine, and taurolidine) are mediated by different mechanisms. Adv Exp Med Biol. 2006;583:481–92. doi: 10.1007/978-0-387-33504-9_54. [DOI] [PubMed] [Google Scholar]
- 15.Zeybek A, Sağlam B, Cikler E, Cetinel S, Ercan F, Sener G. Taurine ameliorates stress-induced degeneration of the urinary bladder. Acta Histochem. 2007;109:208–14. doi: 10.1016/j.acthis.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Zeybek A, Ercan F, Cetinel S, Cikler E, Sağlam B, Sener G. Taurine ameliorates water avoidance stress-induced degenerations of gastrointestinal tract and liver. Dig Dis Sci. 2006;51:1853–61. doi: 10.1007/s10620-006-9425-5. [DOI] [PubMed] [Google Scholar]
- 17.Zeybek A, Sağlam B, Cikler E, Cetinel S, Ercan F, Sener G. Protective effects of taurine on protamine sulfate induced bladder damage. World J Urol. 2006;24:438–44. doi: 10.1007/s00345-006-0106-y. [DOI] [PubMed] [Google Scholar]
- 18.Liao XB, Zhou XM, Li JM, et al. Taurine transporter is expressed in vascular smooth muscle cells. Amino Acids. 2007;33:639–43. doi: 10.1007/s00726-006-0486-8. [DOI] [PubMed] [Google Scholar]
- 19.Allard ML, Jeejeebhoy KN, Sole MJ. The management of conditioned nutritional requirements in heart failure. Heart Fail Rev. 2006;11:75–82. doi: 10.1007/s10741-006-9195-3. [DOI] [PubMed] [Google Scholar]
- 20.Tan B, Jiang DJ, Huang H, et al. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the DDAH/ADMA pathway. Vascul Pharmacol. 2007;46:338–45. doi: 10.1016/j.vph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Hing E, Middleton K. National Hospital Ambulatory Medical Care Survey: 2001 outpatient department summary. Adv Data. 2003;338:1–26. [PubMed] [Google Scholar]
- 22.Kramer JH, Chovan JP, Schaffer SW. Effect of taurine on calcium paradox and ischemic heart failure. Am J Physiol. 240:H238–46. doi: 10.1152/ajpheart.1981.240.2.H238. [DOI] [PubMed] [Google Scholar]
- 23.Schaffer SW, Pastukh V, Solodushko V, Kramer J, Azuma J. Effect of ischemia, calcium depletion and repletion, acidosis and hypoxia on cellular taurine content. Amino Acids. 2002;23:395–400. doi: 10.1007/s00726-002-0201-3. [DOI] [PubMed] [Google Scholar]
- 24.Kingston R, Kelly CJ, Murray P. The therapeutic role of taurine in ischaemia-reperfusion injury. Curr Pharm Des. 2004;10:2401–10. doi: 10.2174/1381612043384015. [DOI] [PubMed] [Google Scholar]
- 25.Xu YJ, Saini HK, Zhang M, Elimban V, Dhalla NS. MAPK activation and apoptotic alterations in hearts subjected to calcium paradox are attenuated by taurine. Cardivas Res. 2006;72:163–74. doi: 10.1016/j.cardiores.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi-Takihara K, Azuma J, Kishimoto S, Onishi S, Sperelakis N. Taurine prevention of calcium paradox-related damage in cardiac muscle. Its regulatory action on intracellular cation contents. Biochem Pharmacol. 1988;37:2651–8. doi: 10.1016/0006-2952(88)90259-6. [DOI] [PubMed] [Google Scholar]
- 27.Ueno T, Iguro Y, Yotsumoto G, et al. Taurine at early reperfusion significantly reduces myocardial damage and preserves cardiac function in the isolated rat heart. Resuscitation. 2007;73:287–95. doi: 10.1016/j.resuscitation.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 28.McCarty MF. A taurine-supplemented vegan diet may blunt the contribution of neutrophil activation to acute coronary events. Med Hypotheses. 2004;63:419–25. doi: 10.1016/j.mehy.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 29.Emerson DK, McCormick ML, Schmidt JA, Knudson CM. Taurine monochloramine activates a cell death pathway involving Bax and Caspase-9. J Biol Chem. 2005;280:3233–41. doi: 10.1074/jbc.M411672200. [DOI] [PubMed] [Google Scholar]
- 30.Frank JS, Langer GA, Nudd LM, Seraydarian K. The myocardial cell surface, its histochemistry, and the effect of sialic acid and calcium removal on its structure and cellular ionic exchange. Circ Res. 1977;41:702–14. doi: 10.1161/01.res.41.5.702. [DOI] [PubMed] [Google Scholar]
- 31.Gross GJ, Kersten JR, Warltier DC. Mechanisms of postischemic contractile dysfunction. Ann Thorac Surg. 1999;68:1898–904. doi: 10.1016/s0003-4975(99)01035-8. [DOI] [PubMed] [Google Scholar]
- 32.Park JL, Lucchesi BR. Mechanisms of myocardial reperfusion injury. Ann Thorac Surg. 1999;68:1905–12. doi: 10.1016/s0003-4975(99)01073-5. [DOI] [PubMed] [Google Scholar]
- 33.Allo SN, Bagby L, Schaffer SW. Taurine depletion, a novel mechanism for cardioprotection from regional ischemia. Am J Physiol. 1997;273:H1956–61. doi: 10.1152/ajpheart.1997.273.4.H1956. [DOI] [PubMed] [Google Scholar]
- 34.Thom T, Haase N, Rosamond W, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 35.Sole MJ, Jeejeebhoy KN. Conditioned nutritional requirements and the pathogenesis and treatment of myocardial failure. Curr Opin Clin Nutr Metab Care. 2000;3:417–24. doi: 10.1097/00075197-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Jeejeebhoy F, Keith M, Freeman M, et al. Nutritional supplementation with MyoVive repletes essential cardiac myocyte nutrients and reduces left ventricular size in patients with left ventricular dysfunction. Am Heart J. 2002;143:1092–100. doi: 10.1067/mhj.2002.121927. [DOI] [PubMed] [Google Scholar]
- 37.Ohta H, Azuma J, Onishi S, Awata N, Takihara K, Kishimoto S. Protective effect of taurine against isoprenaline-induced myocardial damage. Basic Res Cardiol. 1986;81:473–81. doi: 10.1007/BF01907753. [DOI] [PubMed] [Google Scholar]
- 38.Huxtable RJ, Chubb J, Azari J. Physiological and experimental regulation of taurine content in the heart. Fed Proc. 1980;39:2685–90. [PubMed] [Google Scholar]
- 39.Huxtable R, Bressler R. Taurine concentrations in congestive heart failure. Science. 1974;184:1187–8. doi: 10.1126/science.184.4142.1187. [DOI] [PubMed] [Google Scholar]
- 40.Azuma J, Hasegawa H, Sawamura A, et al. Taurine for treatment of congestive heart failure. Int J Cardiol. 1982;2:303–4. doi: 10.1016/0167-5273(82)90052-3. [DOI] [PubMed] [Google Scholar]
- 41.Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J. 1992;56:95–9. doi: 10.1253/jcj.56.95. [DOI] [PubMed] [Google Scholar]
- 42.Franconi F, Stendardi I, Matucci R, et al. Inotropic effect of taurine in guinea-pig ventricular strips. Eur J Pharmacol. 1984;102:511–4. doi: 10.1016/0014-2999(84)90572-7. [DOI] [PubMed] [Google Scholar]
- 43.Satoh H, Nakatani T, Tanaka T, Haga S. Cardiac functions and taurine’s actions at different extracellular calcium concentrations in forced swimming stress-loaded rats. Biol Trace Elem Res. 2002;87:171–82. doi: 10.1385/BTER:87:1-3:171. [DOI] [PubMed] [Google Scholar]
- 44.Franconi F, Martini F, Stendardi I, Matucci R, Zilletti L, Giotti A. Effect of taurine on calcium levels and contractility in guinea-pig ventricular strips. Biochem Pharmacol. 1982;31:3181–5. doi: 10.1016/0006-2952(82)90547-0. [DOI] [PubMed] [Google Scholar]
- 45.Franconi F, Stendardi I, Martini F, Zilletti L, Giotti A. Interaction between organic calcium-channel blockers and taurine in vitro and in vivo. J Pharm Pharmacol. 1982;34:329–30. doi: 10.1111/j.2042-7158.1982.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 46.Belanger MC, Ouellet M, Queney G, Moreau M. Taurine-deficient dilated cardiomyopathy in a family of golden retrievers. J Am Anim Hosp Assoc. 2005;41:284–91. doi: 10.5326/0410284. [DOI] [PubMed] [Google Scholar]
- 47.Shiny KS, Kumar SH, Farvin KH, Anandan R, Devadasan K. Protective effect of taurine on myocardial antioxidant status in isoprenaline-induced myocardial infarction in rats. J Pharm Pharmacol. 2005;57:1313–7. doi: 10.1211/jpp.57.10.0010. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez ER, Kannewurf BS. Atherosclerosis: A unifying disorder with diverse manifestations. Am J Health Syst Pharm. 1998;55:S4–7. doi: 10.1093/ajhp/55.suppl_1.S4. [DOI] [PubMed] [Google Scholar]
- 49.Ito T, Azuma J. Taurine is a possible anti-atherosclerotic agent. Nippon Yakurigaku Zasshi. 2004;123:311–7. doi: 10.1254/fpj.123.311. [DOI] [PubMed] [Google Scholar]
- 50.Park T, Lee K. Dietary taurine supplementation reduces plasma and liver cholesterol and triglyceride levels in rats fed a high-cholesterol or a cholesterol-free diet. Adv Exp Med Biol. 1998;442:319–25. doi: 10.1007/978-1-4899-0117-0_40. [DOI] [PubMed] [Google Scholar]
- 51.Murakami S, Kondo-Ohta Y, Tomisawa K. Improvement in cholesterol metabolism in mice given chronic treatment of taurine and fed a high-fat diet. Life Sci. 1999;64:83–91. doi: 10.1016/s0024-3205(98)00536-0. [DOI] [PubMed] [Google Scholar]
- 52.Murakami S, Kondo Y, Sakurai T, Kitajima H, Nagate T. Taurine suppresses development of atherosclerosis in Watanabe heritable hyperlipidemic (WHHL) rabbits. Atherosclerosis. 2002;163:79–87. doi: 10.1016/s0021-9150(01)00764-x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang M, Bi LF, Fang JH, et al. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–71. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 54.Mizushima S, Nara Y, Sawamura M, Yamori Y. Effects of oral taurine supplementation on lipids and sympathetic nerve tone. Adv Exp Med Biol. 1996;403:615–22. doi: 10.1007/978-1-4899-0182-8_68. [DOI] [PubMed] [Google Scholar]
- 55.Steinhubl SR, Moliterno DJ. The role of the platelet in the pathogenesis of atherothrombosis. Am J Cardiovasc Drugs. 2005;5:399–408. doi: 10.2165/00129784-200505060-00007. [DOI] [PubMed] [Google Scholar]
- 56.Ji Y, Tao L, Xu HL, Rao MR. Effects of taurine and enalapril on blood pressure, platelet aggregation and the regression of left ventricular hypertrophy in two-kidney-one-clip renovascular hypertensive rats. Yao Xue Xue Bao. 1995;30:886–90. [PubMed] [Google Scholar]
- 57.Welles EG, Boudreaux MK, Tyler JW. Platelet, antithrombin, and fibrinolytic activities in taurine-deficient and taurine-replete cats. Am J Vet Res. 1993;54:1235–43. [PubMed] [Google Scholar]
- 58.Hayes KC, Pronczuk A, Addesa AE, Stephan ZF. Taurine modulates platelet aggregation in cats and humans. Am J Clin Nutr. 1989;49:1211–6. doi: 10.1093/ajcn/49.6.1211. [DOI] [PubMed] [Google Scholar]
- 59.Kaneyuki U, Ueda S, Yamagishi S, et al. Pitavastatin inhibits lysophosphatidic acid-induced proliferation and monocyte chemoattractant protein-1 expression in aortic smooth muscle cells by suppressing Rac-1-mediated reactive oxygen species generation. Vascul Pharmacol. 2007;46:286–92. doi: 10.1016/j.vph.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendis MR, Dhalla NS. Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol. 2003;19:1525–36. [PubMed] [Google Scholar]
- 61.Damirin A, Tomura H, Komachi M, et al. Role of lipoprotein-associated lysophospholipids in migratory activity of coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292:H2513–22. doi: 10.1152/ajpheart.00865.2006. [DOI] [PubMed] [Google Scholar]
- 62.Ninio E. Phospholipid mediators in the vessel wall: Involvement in atherosclerosis. Curr Opin Clin Nutr Metab Care. 2005;8:123–31. doi: 10.1097/00075197-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X, Tenner TE, Jr, Lombardini JB. Inhibition of rat vascular smooth muscle cell proliferation by taurine and taurine analogues. Biochem Pharmacol. 1999;57:1331–9. doi: 10.1016/s0006-2952(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 64.Schmieder RE. Mechanisms for the clinical benefits of angiotensin II receptor blockers. Am J Hypertens. 2005;18:720–30. doi: 10.1016/j.amjhyper.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 65.Azuma M, Takahashi K, Fukuda T, et al. Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eur J Pharmacol. 2000;403:181–8. doi: 10.1016/s0014-2999(00)00483-0. [DOI] [PubMed] [Google Scholar]
- 66.Takahahsi K, Azuma M, Baba A, Schaffer S, Azuma J. Taurine improves angiotensin II-induced hypertrophy of cultured neonatal rat heart cells. Adv Exp Med Biol. 1998;442:129–35. doi: 10.1007/978-1-4899-0117-0_17. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi K, Azuma M, Taira K, et al. Effect of taurine on angiotensin II-induced hypertrophy of neonatal rat cardiac cells. J Cardiovasc Pharmacol. 1997;30:725–30. doi: 10.1097/00005344-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Tao L, Wang HX, Rao MR. Effect of angiotensin and taurine on arrhythmia in cultured neonatal rat hypertrophic heart myocytes. Yao Xue Xue Bao. 1996;31:326–30. [PubMed] [Google Scholar]
- 69.Tao L, Rao MR. Effects of enalapril and taurine on left ventricular hypertrophy and arrhythmia in renovascular hypertensive rat. Yao Xue Xue Bao. 1996;31:891–6. [PubMed] [Google Scholar]
- 70.Schaffer SW, Ballard-Croft C, Takahashi K, Azuma J. Effect of taurine depletion on angiotensin II-mediated modulation of myocardial function. Adv Exp Med Biol. 1998;442:145–52. doi: 10.1007/978-1-4899-0117-0_19. [DOI] [PubMed] [Google Scholar]
- 71.Schaffer SW, Lombardini JB, Azuma J. Interaction between the actions of taurine and angiotensin II. Amino Acids. 2000;18:305–18. doi: 10.1007/pl00010320. [DOI] [PubMed] [Google Scholar]
- 72.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 73.Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: Mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107:539–57. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- 74.Hansen SH. The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev. 2001;17:330–46. doi: 10.1002/dmrr.229. [DOI] [PubMed] [Google Scholar]
- 75.Franconi F, Bennardini F, Mattana A, et al. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplementation. Am J Clin Nutr. 1995;61:1115–9. doi: 10.1093/ajcn/61.4.1115. [DOI] [PubMed] [Google Scholar]
- 76.You J, Chang KJ. Effects of taurine supplementation on lipid peroxidation, blood glucose and blood lipid metabolism in streptozotocin-induced diabetic rats. Adv Exp Med Biol. 1998;442:163–8. doi: 10.1007/978-1-4899-0117-0_21. [DOI] [PubMed] [Google Scholar]
- 77.Kamata K, Sugiura M, Kojima S, Kasuya Y. Restoration of endothelium-dependent relaxation in both hypercholesterolemia and diabetes by chronic taurine. Eur J Pharmacol. 1996;303:47–53. doi: 10.1016/0014-2999(96)00094-5. [DOI] [PubMed] [Google Scholar]
- 78.Lim E, Park S, Kim H. Effect of taurine supplementation on the lipid peroxide formation and the activities of glutathione-related enzymes in the liver and islet of type I and II diabetic model mice. Adv Exp Med Biol. 1998;442:99–103. doi: 10.1007/978-1-4899-0117-0_13. [DOI] [PubMed] [Google Scholar]
- 79.Brons C, Spohr C, Storgaard H, Dyerberg J, Vaag A. Effect of taurine treatment on insulin secretion and action, and on serum lipid levels in overweight men with a genetic predisposition for type II diabetes mellitus. Eur J Clin Nutr. 2004;58:1239–47. doi: 10.1038/sj.ejcn.1601955. [DOI] [PubMed] [Google Scholar]
- 80.Tokunaga H, Yoneda Y, Kuriyama K. Streptozotocin-induced elevation of pancreatic taurine content and suppressive effect of taurine on insulin secretion. Eur J Pharmacol. 1983;87:237–43. doi: 10.1016/0014-2999(83)90333-3. [DOI] [PubMed] [Google Scholar]
- 81.Li F, Obrosova IG, Abatan O, et al. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am J Physiol Endocrinol Metab. 2005;288:E29–36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 82.Di Leo MA, Santini SA, Silveri NG, Giardina B, Franconi F, Ghirlanda G. Long-term taurine supplementation reduces mortality rate in streptozotocin-induced diabetic rats. Amino Acids. 2004;27:187–91. doi: 10.1007/s00726-004-0108-2. [DOI] [PubMed] [Google Scholar]
- 83.Arany E, Strutt B, Romanus P, Remacle C, Reusens B, Hill DJ. Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia. 2004;47:1831–7. doi: 10.1007/s00125-004-1535-z. [DOI] [PubMed] [Google Scholar]
- 84.Harada N, Ninomiya C, Osako Y, et al. Taurine alters respiratory gas exchange and nutrient metabolism in type 2 diabetic rats. Obes Res. 2004;12:1077–84. doi: 10.1038/oby.2004.135. [DOI] [PubMed] [Google Scholar]
- 85.Nakaya Y, Minami A, Harada N, Sakamoto S, Niwa Y, Ohnaka M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am J Clin Nutr. 2000;71:54–8. doi: 10.1093/ajcn/71.1.54. [DOI] [PubMed] [Google Scholar]
- 86.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand. 2004;181:297–303. doi: 10.1111/j.1365-201X.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- 87.Nishimura N, Umeda C, Ona H, Yokogoshi H. The effect of taurine on plasma cholesterol concentration in genetic type 2 diabetic GK rats. J Nutr Sci Vitaminol (Tokyo) 2002;48:483–90. doi: 10.3177/jnsv.48.483. [DOI] [PubMed] [Google Scholar]
- 88.Di Leo MA, Ghirlanda G, Gentiloni Silveri N, Giardina B, Franconi F, Santini SA. Potential therapeutic effect of antioxidants in experimental diabetic retina: A comparison between chronic taurine and vitamin E plus selenium supplementations. Free Radic Res. 2003;37:323–30. doi: 10.1080/1071576021000055271. [DOI] [PubMed] [Google Scholar]
- 89.Nandhini AT, Anuradha CV. Taurine modulates kallikrein activity and glucose metabolism in insulin resistant rats. Amino Acids. 2002;22:27–38. doi: 10.1007/s726-002-8199-3. [DOI] [PubMed] [Google Scholar]
- 90.Colivicchi MA, Raimondi L, Bianchi L, Tipton KF, Pirisino R, Della Corte L. Taurine prevents streptozotocin impairment of hormone-stimulated glucose uptake in rat adipocytes. Eur J Pharmacol. 2004;495:209–15. doi: 10.1016/j.ejphar.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 91.Nandhini AT, Anuradha CV. Hoe 140 abolishes the blood pressure lowering effect of taurine in high fructose-fed rats. Amino Acids. 2004;26:299–303. doi: 10.1007/s00726-003-0003-2. [DOI] [PubMed] [Google Scholar]
- 92.Harada H, Tsujino T, Watari Y, Nonaka H, Emoto N, Yokoyama M. Oral taurine supplementation prevents fructose-induced hypertension in rats. Heart Vessels. 2004;19:132–6. doi: 10.1007/s00380-003-0757-1. [DOI] [PubMed] [Google Scholar]
- 93.Hagiwara K, Kuroki G, Yuan PX, et al. The effect of taurine on the salt-dependent blood pressure increase in the voltage-dependent calcium channel beta 3-subunit-deficient mouse. J Cardiovasc Pharmacol. 2003;41(Suppl 1):S127–31. [PubMed] [Google Scholar]
- 94.Chen L, Li RJ, Zhou YB, Chen JJ. Effect of microinjection of taurine into anterior hypothalamic area on blood pressure in rats. Sheng Li Xue Bao. 1999;51:593–6. [PubMed] [Google Scholar]
- 95.Yoshioka M, Takasugi Y, Koga Y. Central hypotensive effect involving neurotransmitters of long-term administration of taurine to stroke-prone spontaneously hypertensive rat. Masui. 2007;56:139–47. [PubMed] [Google Scholar]
- 96.Shi YR, Qi YF, Bu DF, et al. Dysfunction of myocardial and vascular taurine transport in spontaneously hypertensive rats. Sheng Li Xue Bao. 2002;54:359–64. [PubMed] [Google Scholar]
- 97.Mozaffari MS, Patel C, Abdelsayed R, Schaffer SW. Accelerated NaCl-induced hypertension in taurine-deficient rat: Role of renal function. Kidney Int. 2006;70:329–37. doi: 10.1038/sj.ki.5001503. [DOI] [PubMed] [Google Scholar]
- 98.Mozaffari MS, Miyata N, Schaffer SW. Effects of taurine and enalapril on kidney function of the hypertensive glucose-intolerant rat. Am J Hypertens. 2003;16:673–80. doi: 10.1016/s0895-7061(03)00915-4. [DOI] [PubMed] [Google Scholar]
- 99.Mozaffari MS, Abebe W. Cardiovascular responses of the taurine-depleted rat to vasoactive agents. Amino Acids. 2000;19:625–34. doi: 10.1007/s007260070012. [DOI] [PubMed] [Google Scholar]
- 100.Abebe W, Mozaffari MS. Effects of chronic taurine treatment on reactivity of the rat aorta. Amino Acids. 2000;19:615–23. doi: 10.1007/s007260070011. [DOI] [PubMed] [Google Scholar]
- 101.Abebe W, Mozaffari MS. Taurine depletion alters vascular reactivity in rats. Can J Physiol Pharmacol. 2003;81:903–9. doi: 10.1139/y03-088. [DOI] [PubMed] [Google Scholar]
- 102.Abebe W. Effects of taurine on the reactivity of aortas from diabetic rats. Life Sci. 2008;82:279–89. doi: 10.1016/j.lfs.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 103.Casey RG, Gang C, Joyce M, Bouchier-Hayes DJ. Taurine attenuates acute hyperglycaemia-induced endothelial cell apoptosis, leucocyte-endothelial cell interactions and cardiac dysfunction. J Vasc Res. 2007;44:31–9. doi: 10.1159/000097893. [DOI] [PubMed] [Google Scholar]
- 104.Li N, Sawamura M, Nara Y, Ikeda K, Yamori Y. Direct inhibitory effects of taurine on norepinephrine-induced contraction in mesenteric artery of stroke-prone spontaneously hypertensive rats. Adv Exp Med Biol. 1996;403:257–62. doi: 10.1007/978-1-4899-0182-8_27. [DOI] [PubMed] [Google Scholar]
- 105.Franconi F, Giotti A, Manzini S, Martini F, Stendardi I, Zilletti L. The effect of taurine on high potassium-and noradrenaline-induced contraction in rabbit ear artery. Br J Pharmacol. 1982;75:605–12. doi: 10.1111/j.1476-5381.1982.tb09180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abebe W, Mozaffari MS. Effect of taurine deficiency on adenosine receptor-mediated relaxation of the rat aorta. Vascul Pharmacol. 2003;40:219–28. doi: 10.1016/j.vph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 107.Abe M, Shibata K, Matsuda T, Furukawa T. Inhibition of hypertension and salt intake by oral taurine treatment in hypertensive rats. Hypertension. 1987;10:383–9. doi: 10.1161/01.hyp.10.4.383. [DOI] [PubMed] [Google Scholar]
- 108.Zhu DN, Moriguchi A, Mikami H, Higaki J, Ogihara T. Central amino acids mediate cardiovascular response to angiotensin II in the rat. Brain Res Bull. 1998;45:189–97. doi: 10.1016/s0361-9230(97)00338-9. [DOI] [PubMed] [Google Scholar]
- 109.Rao MR, Tao L. Effects of taurine on signal transduction steps induced during hypertrophy of rat heart myocytes. Adv Exp Med Biol. 1998;442:137–43. doi: 10.1007/978-1-4899-0117-0_18. [DOI] [PubMed] [Google Scholar]
- 110.Militante JD, Lombardini JB. Treatment of hypertension with oral taurine: Experimental and clinical studies. Amino Acids. 2002;23:381–93. doi: 10.1007/s00726-002-0212-0. [DOI] [PubMed] [Google Scholar]
- 111.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 112.Guo X, Saini HK, Wang J, Gupta SK, Goyal RK, Dhalla NS. Prevention of remodeling in congestive heart failure due to myocardial infarction by blockade of the renin-angiotensin system. Expert Rev Cardiovasc Ther. 2005;3:717–32. doi: 10.1586/14779072.3.4.717. [DOI] [PubMed] [Google Scholar]
- 113.Ulrich-Merzenich G, Zeitler H, Vetter H, Bhonde RR. Protective effects of taurine on endothelial cells impaired by high glucose and oxidized low density lipoproteins. Eur J Nutr. 2007;46:431–8. doi: 10.1007/s00394-007-0682-7. [DOI] [PubMed] [Google Scholar]
- 114.Hultman K, Alexanderson C, Mannerås L, Sandberg M, Holmäng A, Jansson T. Maternal taurine supplementation in the late pregnant rat stimulates postnatal growth and induces obesity and insulin resistance in adult offspring. J Physiol. 2007;579:823–33. doi: 10.1113/jphysiol.2006.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]