Abstract
BACKGROUND
Diabetes mellitus is an independent risk factor for cardiovascular disease and is also associated with increased susceptibility to cardiovascular complications. It has been suggested that alterations in glucose metabolism and glucose flux via the aldose reductase pathway make the diabetic heart more sensitive to ischemic-reperfusion injury. Previous studies have found sulindac to have inhibitory and anti-inflammatory effects on aldose reductase. The use of aldose reductase inhibitors for the protection of ischemic myocardium is still in an exploratory state.
OBJECTIVES
To evaluate the therapeutic potential of sulindac in an in vivo rat model of acute ischemia (30 min) and reperfusion (4 h) in diabetic and nondiabetic rats.
METHODS
Diabetes was induced in rats by administering streptozotocin (45 mg/kg, intravenously). Myocardial infarction was induced by occlusion of the left anterior descending coronary artery for 30 min followed by 4 h of reperfusion. Infarct size was measured using the staining agent 2,3,5-triphenyltetrazolium chloride. A lead II electrocardiogram was monitored at various intervals throughout the experiment. Sorbitol dehydrogenase levels in heart tissue, as well as lipid peroxide levels in serum and heart tissue, were estimated spectrophotometrically.
RESULTS
Infarct size was increased in diabetic rats in comparison with normal rats. Pretreatment with sulindac significantly reduced infarct size, lipid peroxidation and sorbitol dehydrogenase levels in both diabetic and nondiabetic rats. The degree of cardioprotection was greater in diabetic rats than in nondiabetic rats.
CONCLUSIONS
The present study indicates that the observed cardioprotection provided by sulindac in terms of reducing infarct size in normal rats may be due to its combined antioxidant and anti-inflammatory activities. The inhibition of aldose reductase may be responsible for the enhanced cardioprotection observed in diabetic rats treated with sulindac.
Keywords: Cardioprotection, Diabetes, Polyol pathway, Reperfusion injury, Sulindac
Diabetic patients with coronary artery disease have high morbidity and mortality (1), with the incidence of heart failure after myocardial infarction significantly greater in patients with diabetes than in nondiabetic patients (1,2). Several alterations in myocardial metabolism at the myocyte level may contribute to the development of a number of chronic biochemical changes in diabetic hearts (3). Of the mechanisms proposed to explain the pathogenesis of diabetic complications, increased metabolism of glucose via the polyol pathway has received considerable attention (3). Currently, aldose reductase inhibitors are being developed as potential therapeutic interventions for diabetic complications. Several studies (4,5) have shown modest improvements in neuronal, renal, retinal and cardiac functions in diabetic subjects after treatment with aldose reductase inhibitors. Activation of the polyol pathway triggers inflammatory mediators and contributes to free radical generation, which may also lead to the progression of micro- and macrovascular diabetic complications (6,7). Several studies (8,9) also suggest that anti-inflammatory agents such as sulindac, indomethacin and piroxicam have beneficial effects in streptozotocin (STZ)-induced diabetic neuropathy in rats. In a previous study (10), we demonstrated the protective actions of sulindac in rats with STZ-induced diabetic cardiomyopathy. Beneficial effects observed in those studies may have been due to the combined inhibition of cyclo-oxygenase enzymes and polyol pathway activity. Several experimental studies (11,12) have demonstrated that aldose reductase activity is a key contributor to ischemic injury and aldose reductase-inhibited hearts. When subjected to ischemia-reperfusion, diabetic hearts exhibited less ischemic injury and improved cardiac function compared with nondiabetic hearts. These findings indicate that pharmacological inhibitors of aldose reductase present a novel adjunctive approach for protecting ischemic hearts. There is experimental evidence that a variety of anti-inflammatory drugs have been successfully used in reducing infarct size against ischemia-reperfusion injury (13–15).
Sulindac is a nonselective aldose reductase inhibitor, and is a sulfoxide prodrug that, in vivo, is converted into the metabolites sulindac sulfide and sulfone (16). It also possesses analgesic, antipyretic, anticarcinogenic and anti-inflammatory properties. Although the inhibition of prostaglandin synthesis by inhibiting cyclo-oxygenase constitutes its primary mechanism, free radical scavenging activity for all reactive nitrogen species, reactive oxygen species and hypochlorous acid may strongly contribute to its anti-inflammatory activity (16). Despite the free radical scavenging activity, as well as the inhibitory and anti-inflammatory activities of sulindac on aldose reductase, the cardioprotective effect of sulindac against ischemia-reperfusion injury has so far not been evaluated. Hence, the present study was designed to investigate the protective action of sulindac, a nonselective aldose reductase inhibitor, against ischemia-reperfusion-induced myocardial infarction in diabetic rats.
METHODS
Animals
Sprague Dawley rats of either sex, weighing 200 g to 250 g, were selected. Rats were maintained under standard laboratory conditions at 25±2°C, relative humidity of 50±15%, and normal photoperiod of 12 h light and 12 h darkness. A commercial pellet diet (Rayan Biotechnologies Pvt Ltd, India) and water were provided ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee and by the animal regulatory body of the government (Regd No 516/01/A/CPCSEA).
Chemicals
STZ and sulindac were purchased from Sigma Chemicals Ltd (USA). 2,3,5-triphenyltetrazolium chloride (TTC) was purchased from BDH chemicals Ltd (United Kingdom) and 1,1,3,3-tetraethoxypropane was purchased from Sigma Chemicals Ltd (USA). All other chemicals and reagents used were of analytical grade.
Induction of diabetes
STZ was dissolved in citrate buffer (pH 4.5) and administered at a dose of 45 mg/kg with a single injection into the tail vein of animals lightly anesthetized with ether. After 48 h, diabetes was confirmed by estimates of serum glucose levels using the GOD-POD method (17). Animals were observed for three weeks. Rats showing glucose levels of 19.42 mmol/L to 22.2 mmol/L were used in the experiment.
Experimental design
A total of 48 rats (24 normal and 24 diabetic surviving rats) were used. The rats were divided into eight groups (four normal and four diabetic groups), each group consisting of six animals. Sulindac was dissolved in dimethyl sulfoxide and administered as an intraperitoneal injection 10 min before reperfusion. Treatment groups were as follows: group 1, normal sham control group treated with vehicle; group 2, normal control group treated with vehicle; groups 3 and 4, normal groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, respectively; group 5, diabetic sham control group treated with vehicle; group 6, diabetic control group treated with vehicle; groups 7 and 8; diabetic groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, respectively. All groups were subjected to ischemia-reperfusion except groups 1 and 5.
Surgical preparation
Rats were anesthetized with thiopentone sodium (30 mg/kg, intraperitoneally), tracheotomized and ventilated with room air using a Techno positive pressure mechanical respirator (Crampton Parkinson Ltd., United Kingdom). A left thoracotomy and pericardiotomy were performed, and the left coronary artery was dissected free above the first diagonal branch and was ligated just below the origin of the left circumflex artery with the help of a silk thread (6.0). The artery was occluded for 30 min by a knot. The silk thread was removed after 30 min with the help of two knot releasers to allow reperfusion of the heart for 4 h. A lead II electrocardiogram was monitored at various intervals throughout the experiment by using Cardiart 408 (BPL Ltd, India) with 20 mm/mV sensitivity at a paper speed of 50 mm/s.
Quantification of infarct size
After sacrificing the animals in all the groups by injecting 2.56 M KCl directly into the left ventricle, the heart was immediately excised from the thorax and the greater vessels were removed. The left ventricle was separated from the heart and weighed. It was sliced parallel to the atrioventricular groove in 2 mm to 3 mm thick sections, and the slices were incubated in 1% TTC solution prepared in pH 7.4 phosphate buffer for 30 min at 37°C (18). In viable myocardium, TTC is converted by dehydrogenase enzymes to formazan, a red pigment that stains tissue dark red (19). Nonviable infarcted myocardium that does not take TTC stain remains pale in colour. The pale necrotic tissue was separated from the stained portions and weighed on an electronic balance (Dhona 200D, Dhona Instruments Pvt Ltd, India). Infarct size was calculated as the percentage fraction of nonviable myocardium of the left ventricle.
Determination of lipid peroxide levels in serum
Before sacrificing animals at the end of the 4 h reperfusion, a 2 mL blood sample was collected from the left ventricle for estimating lipid peroxide levels in blood serum. Serum lipid peroxide levels were estimated by the method developed by Yagi (20). Tetraethoxypropane (in amounts of 0.5 nmol, 1 nmol, 2 nmol, 4 nmol, 6 nmol, 8 nmol and 10 nmol) served as an external standard. Lipid peroxide levels in serum were expressed as nmol/mL.
Determination of lipid peroxide levels in myocardium
Lipid peroxide levels in the myocardium were measured by the method developed by Ohkawa et al (21). Briefly, the infarcted left ventricular tissues were homogenized in 1.15% KCl (10% weight/volume). The assay mixture, consisting of 0.1 mL of tissue homogenate, 0.2 mL of 8.1% sodium dodecyl sulphate, 1.5 mL of 20% acetic acid (adjusted to pH 3.5 with NaOH) and 1.5 mL of 0.8% aqueous solution of thiobarbituric acid, was heated for 60 min at 95°C. Thereafter, the mixture was cooled and extracted with a 5 mL mixture of n-butanol and pyridine (15:1 volume/volume). After centrifugation at 4000 rpm for 10 min, the organic phase was assayed spectrophotometrically at 532 nm. Tetraethoxypropane (in amounts of 2 nmol, 4 nmol, 6 nmol and 8 nmol served as an external standard. Malondialdehyde levels in myocardium were expressed as nmol/g of tissue.
Determination of sorbitol dehydrogenase in heart tissue
Sorbitol dehydrogenase was assayed by the method of Urlich (22). To 1.6 mL of triethanolamine buffer, 0.1 mL of NADH was added followed by addition of 1.0 mL of tissue homogenate. The mixture was incubated for 30 min at 25°C until the extinction was constant. Then 0.3 mL of D-fructose was added and mixed to start the reaction. The extinction was read at 60 s intervals for 5 min to 8 min (until the absorbance was stabilized) at 365 nm. A mixture containing reagents without tissue homogenate served as a control. Enzyme activity was calculated by multiplying 882 with the change in extinction (882 × change in extinction). The enzyme activity was expressed as units/g protein. Protein was measured by the method developed by Lowry et al (23).
Statistical analysis
The results are expressed as mean ± SEM. Differences in infarct size, serum and tissue lipid peroxide levels, and sorbitol dehydrogenase levels were determined using one-way ANOVA. Individual groups were compared using Tukey’s post hoc test. Differences with P<0.001 were considered to be statistically significant.
RESULTS
Effect of sulindac on myocardial infarct size
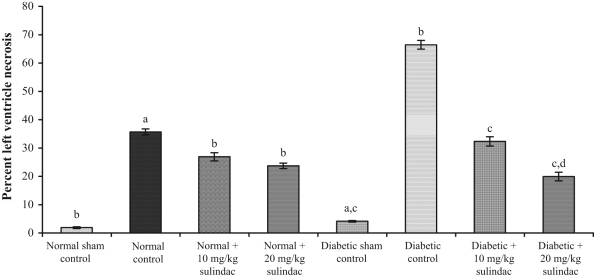
In the normal control group, the per cent left ventricle necrosis was 35.69±1.02%, which was statistically significant compared with the normal sham control group (1.9±0.3%) (Figure 1). In the normal groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, infarct size was significantly (P<0.001) decreased to 26.92±1.43% and 23.74±0.96%, respectively. In the diabetic groups, infarct size was 66.44±1.55%, which was statistically significant compared with the diabetic sham control group (4.16±0.25%). In the diabetic groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, infarct size was 32.34±1.61% and 19.94±1.51%, respectively.
Figure 1.
Effect of sulindac on per cent left ventricle necrosis of experimental group animals. Values are expressed as mean ± SEM (n=6 animals per group). aP<0.001 versus normal sham control; bP<0.001 versus normal control; cP<0.001 versus diabetic control; dP<0.001 versus diabetic + 10 mg/kg sulindac
Effect of sulindac on lipid peroxide levels in serum and heart tissue
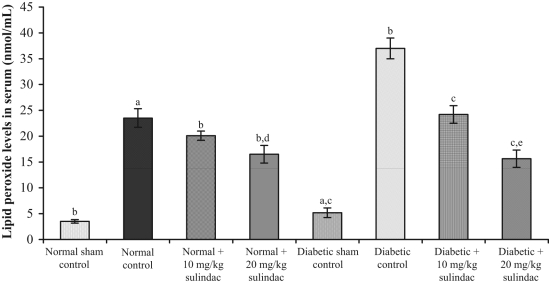
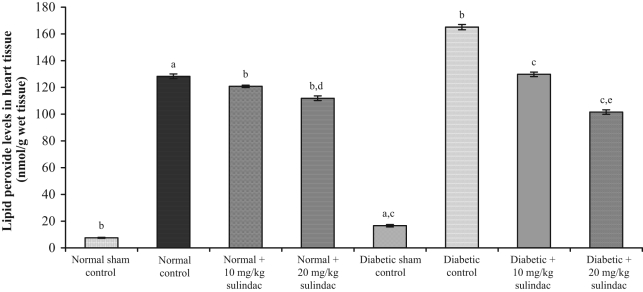
In the normal sham control group, lipid peroxide levels in serum and heart tissue were 3.51±0.44 nmol/mL and 7.53±0.34 nmol/g tissue, respectively. Lipid peroxide levels in serum and heart tissue of control animals were 23.5±1.7 nmol/mL (Figure 2) and 128.3±1.8 nmol/g tissue (Figure 3), respectively. In the normal groups treated with sulindac at a dose of 10 mg/kg, lipid peroxide levels in serum and heart tissue were 20.1±0.9 nmol/mL and 120.8±0.9 nmol/g tissue, respectively. In the normal groups treated with sulindac at a dose of 20 mg/kg, the lipid peroxide levels in serum and heart tissue were 16.5±1.7 nmol/mL and 111.9±1.7 nmol/g tissue, respectively. In the diabetic sham control group, lipid peroxide levels in serum and heart tissue were 5.20±0.75 nmol/mL and 16.60±0.93 nmol/g tissue, respectively. In the diabetic control group, lipid peroxide levels in serum and heart tissue were 37.0±1.4 nmol/mL and 165.1±2.0 nmol/g tissue, respectively. In the diabetic groups treated with sulindac at a dose of 10 mg/kg, lipid peroxide levels in serum and heart tissue were 24.24±1.49 nmol/mL and 129.84±1.70 nmol/g tissue, respectively. In the diabetic groups treated with sulindac at a dose of 20 mg/kg, lipid peroxide levels in serum and heart tissue were 15.64±1.72 nmol/mL and 101.61±1.68 nmol/g tissue, respectively.
Figure 2.
Effect of sulindac on lipid peroxide levels in serum of experimental group animals. Values are expressed as mean ± SEM (n=6 animals per group). aP<0.001 versus normal sham control; bP<0.001 versus normal control; cP<0.001 versus diabetic control; dP<0.001 versus normal + 10 mg/kg sulindac; eP<0.001 versus diabetic + 10 mg/kg sulindac
Figure 3.
Effect of sulindac on lipid peroxide levels in heart tissue of experimental group animals. Values are expressed as mean ± SEM (n=6 animals per group). aP<0.001 versus normal sham control; bP<0.001 versus normal control; cP<0.001 versus diabetic control; dP<0.001 versus normal + 10 mg/kg sulindac; eP<0.001 versus diabetic + 10 mg/kg sulindac
Effect of sulindac on sorbitol dehydrogenase levels in heart tissue
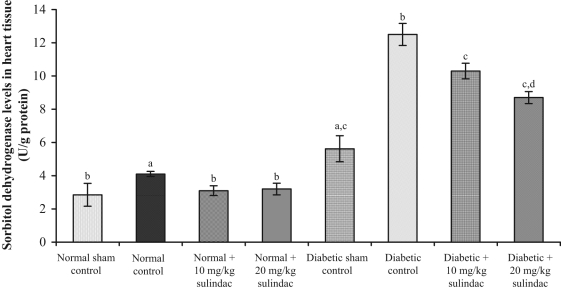
In the normal control group, sorbitol dehydrogenase level was 4.1±0.16 U/g protein, which was statistically significant compared with normal sham control group (2.85±0.69 U/g protein) (Figure 4). In normal groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, sorbitol dehydrogenase levels were 3.1±0.29 U/g protein and 3.2±0.35 U/g protein, respectively. In the diabetic control group, sorbitol dehydrogenase level was 12.5±0.66 U/g protein, which was statistically significant compared with diabetic sham control group (5.62±0.78 U/g protein). In diabetic groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, sorbitol dehydrogenase levels were 10.3±0.47 U/g protein and 8.7±0.36 U/g protein, respectively.
Figure 4.
Effect of sulindac on sorbitol dehydrogenase levels in heart tissue of experimental group animals. Values are expressed as mean ± SEM (n=6 animals per group). aP<0.001 versus normal sham control; bP<0.001 versus normal control; cP<0.001 versus diabetic control; dP<0.001 versus diabetic + 10 mg/kg sulindac
Effect of sulindac on heart rate
Heart rate was recorded at various time intervals during the experiment for all groups (Table 1).
TABLE 1.
Heart rate at various stages of occlusion and reperfusion
| Heart rate (beats/min)
|
|||||||
|---|---|---|---|---|---|---|---|
| Treatment groups | BO | MO | IAR | 1 h AR | 2 h AR | 3 h AR | 4 h AR |
| Normal sham control | 410.7±30.9 | 392.2±19.2 | 342.2±19.2 | 361.1±24.1 | 361.1±24.1 | 377.2±24.1 | 383.3±0.0 |
| Normal control | 434.5±62.7 | 366.1±37.5 | 326.1±37.5 | 338.9±47.7 | 338.9±47.7 | 344.9±12.8 | 358.9±10.4 |
| Normal + 10 mg/kg sulindac | 434.5±22.7 | 377.2±24.0 | 370.2±24.0 | 378.9±47.7 | 381.1±24.1 | 387.2±24.1 | 393.9±66.7 |
| Normal + 20 mg/kg sulindac | 422.4±11.2 | 389.2±14.5 | 377.2±14.0 | 392.8±8.9 | 392.8±10.9 | 399.1±24.1 | 403.1±24.1 |
| Diabetic sham control | 392.8±30.9 | 381.1±19.2 | 381.1±19.2 | 385.9±37.6 | 380.7±12.6 | 386.1±7.6 | 375.0±23.3 |
| Diabetic control | 374.9±12.5 | 332.2±9.2 | 315.1±12.2 | 311.1±14.1 | 319.5±13.8 | 328.0±13.3 | 333.9±16.7 |
| Diabetic + 10 mg/kg sulindac | 410.7±10.8 | 362.2±19.2 | 344.2±19.2 | 351.1±24.1 | 368.7±24.1 | 376.0±17.6 | 382.1±11.9 |
| Diabetic + 20 mg/kg sulindac | 418.4±9.65 | 382.1±8.7 | 356.6±13.3 | 362.5±9.2 | 369.8±6.2 | 385.0±11.5 | 386.6±13.5 |
Data are presented as mean ± SD. 1 h AR One hour after reperfusion; 2 h AR Two hours after reperfusion; 3 h AR Three hours after reperfusion; 4 h AR Four hours after reperfusion; BO Before occlusion; IAR Immediately after reperfusion; MO Middle of occlusion
DISCUSSION
Diabetes-induced changes within the heart appear to be important contributing factors to injury during and following acute myocardial infarction (24). Despite clinical data indicating that the diabetic heart is more sensitive to ischemic injury, animal studies (24) have produced inconsistent results regarding the sensitivity of diabetic hearts to ischemic injury.
Oxidative stress is believed to play a role in the pathogenesis of ischemic-reperfusion injury. During ischemic-reperfusion injury, a number of events that predispose the heart to the formation of reactive oxygen species may occur. These oxygen free radicals may result in depression of contractile function, arrhythmias, depletion of endogenous antioxidant enzymes, and membrane permeability changes resulting in an increase in myocardial lipid peroxidation. In addition, activation of phospholipases during reperfusion releases membrane fatty acids including arachidonic acid, thereby modulating the inflammatory response leading to free radical-mediated tissue damage.
In the present study, lipid peroxide levels in serum and heart tissue in the diabetic sham control group were significantly higher than those in the normal sham control group. This finding clearly indicates that diabetic hearts are more susceptible to oxidative stress. Increased lipid peroxide levels in serum and heart tissues of the diabetic control group, in comparison with the normal control group, further confirm oxidative-mediated damage during ischemia-reperfusion in diabetic hearts. Diabetic and nondiabetic groups treated with sulindac at 10 mg/kg and 20 mg/kg had decreased lipid peroxide levels in serum and heart tissues in a dose-dependent manner. In accordance with earlier studies (16), anti-inflammatory and antioxidant activities of sulindac may be responsible for the decreased lipid peroxide levels in nondiabetic and diabetic rats.
In the normal control group, a continuous decrease in heart rate was observed during 30 min coronary artery ligation and throughout the reperfusion period compared with the sham control group (Table 1). Similarly, in the diabetic control group, a continuous decrease in heart rate was observed during 30 min coronary artery ligation and throughout the reperfusion period compared with the diabetic sham control group. The groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg produced slight decrease in heart rate during 30 min coronary artery ligation. Thereafter, heart rate gradually increased throughout the reperfusion period and restored to near normal value at the end of the 4 h in both diabetic and nondiabetic rats.
In the present study, infarct size was larger in the diabetic control group than in the normal control group when subjected to ischemia-reperfusion injury. Enhanced polyol pathway activation might be responsible for the increased infarct size as evidenced by enhanced levels of sorbitol dehydrogenase in diabetic rats. Activation of the polyol pathway has long been implicated as one of the major underlying causes for the development of diabetic complications (25). Studies (11,12) of cardiac tissue from diabetic rats have also suggested the possible involvement of the polyol pathway in the development of cardiac-related diabetic complications. High levels of glucose in many tissues lead to accumulation of sorbitol via the polyol pathway. In this way, glucose is reduced to sorbitol by aldose reductase and sorbitol is then oxidized by sorbitol dehydrogenase to fructose. However, it is well reported that elevated levels of sorbitol have been considered to be pathogenic in diabetic complications. Tilton et al (26) have demonstrated that polyol pathway-linked vascular dysfunction induced by diabetes is more closely linked to increased oxidation of sorbitol to fructose than to either the osmotic effects of elevated sorbitol levels or metabolic imbalances associated with reduction of glucose to sorbitol by aldose reductase. Hwang et al (27) demonstrated that sorbitol dehydrogenase is a novel target for the protection of ischemic myocardium. Increased sorbitol dehydrogenase activity directly influences the redox ratio of NADH/NAD+ in diabetic hearts and profoundly affects energy use during ischemia-reperfusion (28). In the present study, sorbitol dehydrogenase activity in the diabetic control group was increased compared with the diabetic sham control group in accordance with previous findings, which revealed that cardiac sorbitol dehydrogenase activity is increased during ischemia. In groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, sorbitol dehydrogenase activity significantly decreased the in diabetic rats in a dose-dependent manner.
In groups treated with the sulindac, infarct size was reduced significantly in both diabetic and nondiabetic rats. In diabetic groups treated with sulindac at doses of 10 mg/kg and 20 mg/kg, infarct size decreased in a dose-dependent manner, whereas in normal groups treated with sulindac, the decrease in infarct size was not dose dependent. In addition, diabetic groups treated with sulindac exhibited a higher degree of cardioprotection than normal-treated groups. The enhanced cardioprotection offered by sulindac in diabetic rats might be responsible for aldose reductase inhibition thereby inhibiting the polyol pathway. This was evident by significant reductions in the sorbitol dehydrogenase levels.
CONCLUSION
The present study indicates that the observed cardioprotection provided by sulindac in terms of reducing infarct size in normal rats may be due to its combined antioxidant and anti-inflammatory activities. The inhibitory activity of sulindac on aldose reductase is probably responsible for the enhanced cardioprotection offered by sulindac in diabetic rats. Thus, the present study suggests a potential role of sulindac in inhibiting aldose reductase, thereby reducing infarct size in ischemia-reperfusion-induced myocardial infarction in both diabetic and nondiabetic rats.
REFERENCES
- 1.Stone GW, Grines CL, Browne KF, et al. Predictors of in-hospital and 6-month outcome after acute myocardial infarction in the reperfusion era: The Primary Angioplasty in Myocardial Infarction (PAMI) trial. J Am Coll Cardiol. 1995;25:370–7. doi: 10.1016/0735-1097(94)00367-y. [DOI] [PubMed] [Google Scholar]
- 2.Lehto S, Pyörälä K, Miettinen H, et al. Myocardial infarct size and mortality in patients with non-insulin-dependent diabetes mellitus. J Intern Med. 1994;236:291–7. doi: 10.1111/j.1365-2796.1994.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 3.Williamson JR, Chang K, Frangos M, et al. Hyperglycemic pseudohypoxia and diabetic complications. Diabetes. 1993;42:801–13. doi: 10.2337/diab.42.6.801. [DOI] [PubMed] [Google Scholar]
- 4.Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987;316:599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- 5.Roy TM, Broadstone VL, Peterson HR, et al. The effect of an aldose reductase inhibitor on cardiovascular performance in patients with diabetes mellitus. Diabetes Res Clin Pract. 1990;10:91–7. doi: 10.1016/0168-8227(90)90086-9. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Xia P, Kramer RM, King GL. Identification of the mechanism for the inhibition of Na+,K(+)-adenosine triphosphatase by hyperglycemia involving activation of protein kinase C and cytosolic phospholipase A2. J Clin Invest. 1995;96:733–40. doi: 10.1172/JCI118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry GJ, Kozu H. Piroxicam may reduce the rate of progression of experimental diabetic neuropathy. Neurology. 1990;40:1446–9. doi: 10.1212/wnl.40.9.1446. [DOI] [PubMed] [Google Scholar]
- 9.Zochodne DW, Ho LT. The influence of indomethacin and guanethidine on experimental streptozotocin diabetic neuropathy. Can J Neurol Sci. 1992;19:433–41. [PubMed] [Google Scholar]
- 10.Krishna KM, Gopal GS, Chalam CR, et al. The influence of sulindac on diabetic cardiomyopathy: A non-invasive evaluation by Doppler echocardiography in streptozotocin-induced diabetic rats. Vascul Pharmacol. 2005;43:91–100. doi: 10.1016/j.vph.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Oates PJ, Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes. 1997;46:292–300. doi: 10.2337/diab.46.2.292. [DOI] [PubMed] [Google Scholar]
- 12.Ramasamy R, Trueblood N, Schaefer S. Metabolic effects of aldose reductase inhibition during low-flow ischemia and reperfusion. Am J Physiol. 1998;275:H195–203. doi: 10.1152/ajpheart.1998.275.1.H195. [DOI] [PubMed] [Google Scholar]
- 13.Simpson PJ, Lucchesi BR. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987;110:13–30. [PubMed] [Google Scholar]
- 14.Klein HH, Pich S, Bohle RM, et al. Antiinflammatory agent BW 755 C in ischemic reperfused porcine hearts. J Cardiovasc Pharmacol. 1988;12:338–44. doi: 10.1097/00005344-198809000-00012. [DOI] [PubMed] [Google Scholar]
- 15.O’Neill PG, Charlat ML, Kim HS, et al. Lipoxygenase inhibitor nafazatrom fails to attenuate postischaemic ventricular dysfunction. Cardiovasc Res. 1987;21:755–60. doi: 10.1093/cvr/21.10.755. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes E, Toste SA, Lima JL, Reis S. The metabolism of sulindac enhances its scavenging activity against reactive oxygen and nitrogen species. Free Radic Biol Med. 2003;35:1008–17. doi: 10.1016/s0891-5849(03)00437-4. [DOI] [PubMed] [Google Scholar]
- 17.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 18.Johnson G, 3rd, Tsao P, Lefer AM. Synergism between superoxide dismutase and sodium nitrite in cardioprotection following ischemia and reperfusion. Am Heart J. 1990;119:530–7. doi: 10.1016/s0002-8703(05)80275-3. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein MC, Meerabaum S, Rit J. Early phase acute myocardial infarct size quntification: Validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 20.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–6. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 21.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich HB. Methods of Enzymatic Analysis. 2. London: Verlag Chemie Weinheim, Academic Press Inc; 1974. pp. 569–73. [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 24.Feuvray D, Lopaschuk GD. Controversies on the sensitivity of the diabetic heart to ischemic injury: The sensitivity of the diabetic heart to ischemic injury is decreased. Cardiovasc Res. 1997;34:113–20. doi: 10.1016/s0008-6363(97)00037-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee SM, Schade SZ, Doughty CC. Aldose reductase, NADPH and NADP+ in normal, galactose-fed and diabetic rat lens. Biochim Biophys Acta. 1985;841:247–53. doi: 10.1016/0304-4165(85)90065-0. [DOI] [PubMed] [Google Scholar]
- 26.Tilton RG, Chang K, Nyengaard JR, Van den Enden M, Ido Y, Williamson JR. Inhibition of sorbitol dehydrogenase: Effects on vascular and neural dysfunction in streptozocin-induced diabetic rats. Diabetes. 1995;44:234–42. doi: 10.2337/diab.44.2.234. [DOI] [PubMed] [Google Scholar]
- 27.Hwang YC, Bakr S, Ellery CA, Oates PJ, Ramasamy R. Sorbitol dehydrogenase: A novel target for adjunctive protection of ischemic myocardium. FASEB J. 2003;17:2331–3. doi: 10.1096/fj.03-0128fje. [DOI] [PubMed] [Google Scholar]
- 28.Hwang YC, Kaneko M, Bakr S, et al. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J. 2004;18:1192–9. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]