Abstract
BACKGROUND AND OBJECTIVE
Previous studies have found that obesity is associated with congestive heart failure. The goal of the present study was to evaluate the association between obesity and parameters of left ventricular (LV) diastolic dysfunction using a large echocardiographic database.
METHOD
Data from 13,382 echocardiograms were analyzed for associations between obesity and abnormal LV diastolic parameters. Body mass index (BMI) was categorized into two groups for univariate analysis (nonobese group: BMI less than 30 kg/m2; obese group: BMI 30 kg/m2 or greater). Obesity was correlated with left atrial (LA) enlargement (LA diameter greater than 40 mm), LV hypertrophy (posterior or anterior wall thickness greater than 11 mm), early versus late diastolic mitral flow reversal, abnormal LV mass (greater than 215 g) and abnormal relative wall thickness (greater than 0.43). Multivariate analysis was used to adjust for age and sex.
RESULTS
All diastolic parameters of heart failure were associated with obesity using univariate and multivariate analyses. The ORs for patients with a BMI of 30 kg/m2 or greater were 2.53 (95% CI 2.30 to 2.75; P<0.0001) for LA diameter greater than 40 mm, 1.61 (95% CI 1.45 to 1.80; P<0.0001) for LV hypertrophy, 1.14 (95% CI 1.02 to 1.25; P<0.0001) for early versus late diastolic mitral flow reversal, 2.33 (95% CI 2.10 to 2.58; P<0.0001) for LV mass greater than 215 g, and 1.14 (95% CI 1.02 to 1.26; P=0.01) for relative wall thickness greater than 0.43.
CONCLUSION
The present study suggests that obesity is associated with abnormal parameters of diastolic function.
Keywords: Body mass index, Congestive heart failure, Diastolic function, EF, Left ventricular dysfunction, Obesity
Obesity is a well-known risk factor for congestive heart failure (CHF) (1,2). Many studies in the literature have found body mass index (BMI) to be a risk factor for left ventricular (LV) remodelling, such as LV hypertrophy and dilation, potentially causing CHF (3–6). However, the majority of these studies are small in scale. In the present study, we used a very large database consisting of patients who had undergone echocardiograms for various clinical reasons. We investigated whether there was any association between BMI and parameters of LV diastolic dysfunction using univariate and multivariate analyses.
METHODS
A retrospective analysis of 24,265 echocardiograms performed at the University of California (California, USA) between 1984 and 1998 was conducted. Patients (age range, 18 to 90 years) consisted of inpatients and outpatients who were referred by clinicians for echocardiographic examination for various clinical reasons. The database included all echocardiograms performed during the study period. Data for weight and height were available for 13,382 patients, which were used to calculate BMI at the time of echocardiography. LV dimensions and wall thickness were measured using M-mode recording in parasternal view at or just below the tip of the mitral leaflets. Left atrial (LA) size was measured at the level of the left atrium in parasternal long-axis view. BMI categories were correlated with the following suspected echocardiographic parameters of diastolic dysfunction: LA enlargement (LA diameter greater than 40 mm), LV hypertrophy (LVH) (posterior or anterior wall thickness greater than 11 mm), early versus late diastolic mitral flow reversal (E/A reversal), abnormal LV mass (LVM) (greater than 215 g) and abnormal relative wall thickness (RWT) (greater than 0.43). LVM was measured using the Penn convention formula (7):
where IVS is the interventricular septum thickness, LVID is the LV internal diameter and PW is the posterior wall thickness. RWT was measured as follows (8):
Pearson’s χ2 test for univariate analysis was used. Multiple logistic regressions were used for multivariate analysis, adjusting for age and sex. SPSS version 13.0 statistical software (SPSS Inc, USA) was used for data analysis.
RESULTS
Of 24,265 patients, 13,382 had enough data available to calculate BMI. Ages ranged from 18 to 90 years, and mean (± SD) age of the study population was 51.6±18 years. Sex data were available in 23,337 echocardiograms (12,431 female [53.4%] and 10,906 male [46.6%] patients). BMI of less than 18.5 kg/m2 (underweight) was present in 5.7%, BMI of 18.5 kg/m2 or greater and less than 25 kg/m2 (normal weight) in 49.5%, BMI of 25 kg/m2 or greater and less than 30 kg/m2 (overweight) in 27.8 %, and BMI of 30 kg/m2 or greater (obese) in 17.1% of the patients. Furthermore, BMI was categorized into four groups, removing the underweight subgroup (BMI less than 25 kg/m2, BMI 25 kg/m2 or greater and less than 30 kg/m2, BMI 30 kg/m2 or greater and less than 35 kg/m2, BMI 35 kg/m2 or greater). BMI subgroups were found to be associated with LA enlargement (LA diameter greater than 40 mm), LVH (posterior or anterior wall thickness greater than 11 mm), E/A reversal, abnormal LVM (greater than 215 g) and abnormal RWT (greater than 0.43).
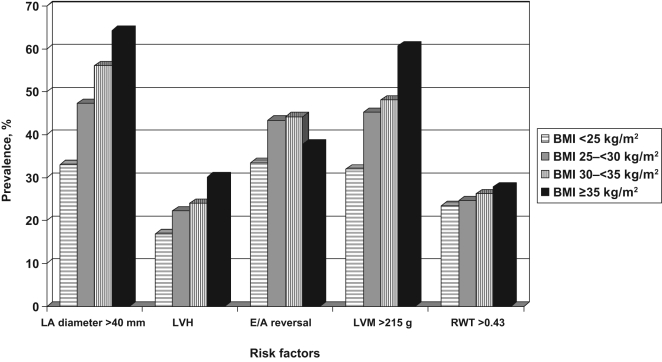
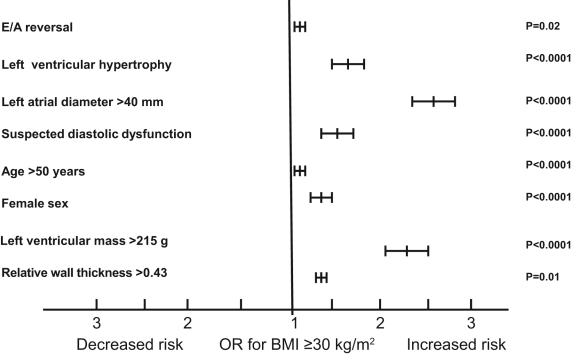
The parameters suggestive of diastolic dysfunction (LVH, LA enlargement, E/A reversal, LVM and RWT) were more common in patients with increasing BMI (Figures 1 and 2). Using multivariate analysis, adjusting for age and sex, increasing BMI and obesity were independently associated with abnormal diastolic parameters. All of the abnormal diastolic parameters were associated with increasing BMI values using univariate and multivariate analysis. The ORs for patients with a BMI of 30 kg/m2 or greater was 2.53 (95% CI 2.30 to 2.75; P<0.0001) for LA diameter greater than 40 mm, 1.61 (95% CI 1.45 to 1.80; P<0.0001) for LVH, 1.14 (95% CI 1.02 to 1.25; P<0.0001) for E/A reversal, 2.33 (95% CI 2.10 to 2.58; P<0.0001) for LVM greater than 215 g, and 1.14 (95% CI 1.02 to 1.26; P=0.01) for RWT greater than 0.43 (Figure 3). Furthermore, patients older than 50 years of age or of female sex were independently associated with increasing BMI (Figure 3).
Figure 1.
Prevalence of risk factors of congestive heart failure according to body mass index (BMI). Significant differences in the prevalence of increasing BMI categories with left atrial (LA) enlargement (P<0.0001), left ventricular hypertrophy (LVH) (P<0.0001), early versus late diastolic mitral flow reversal (E/A reversal) (P<0.0001) and combination of all abnormal diastolic parameters (P<0.0001) are observed
Figure 2.
Prevalence of risk factors of congestive heart failure according to body mass index (BMI). Significant differences in the prevalence of increasing BMI categories – using a lower cut-off value of 25 kg/m2 –with left atrial (LA) enlargement (P<0.0001), left ventricular hypertrophy (LVH) (P<0.0001), early versus late diastolic mitral flow reversal (E/A reversal) (P<0.0001), abnormal LV mass (LVM) (greater than 215 g) (P<0.0001) and abnormal relative wall thickness (RWT) (greater than 0.43) (P=0.009) are observed
Figure 3.
ORs for the association of BMI (body mass index) of 30 kg/m2 or greater (versus less than 30 kg/m2) with abnormal diastolic parameters. The graph shows significant independent associations with abnormal diastolic parameters. Suspected diastolic dysfunction is defined as the occurrence of all abnormal parameters in the same patient. E/A reversal Early versus late diastolic mitral flow reversal
DISCUSSION
It is well known that obesity is a risk factor for CHF. The Framingham Heart Study (1) showed that heart failure had developed in 8.4% of their study population, and that the risk of developing heart failure increased approximately twofold in people with obesity (BMI 30 kg/m2 or greater) when compared with the nonobese population (1). Based on their findings, they estimated that approximately 11% of CHF cases in men and 14% of CHF cases in women could be secondary to obesity alone. Furthermore, He et al (2) followed a cohort of 13,643 patients for an average of 19 years. They found that being overweight was an independent risk factor for heart failure. However, the types or causes of heart failure in the obese patients were not studied. In our study, we found a strong independent association between obesity and echocardiographic parameters of diastolic dysfunction, such as LA enlargement, LVH, E/A reversal, increased LVM and RWT. Furthermore, our study is the largest study evaluating the occurrence of abnormal diastolic parameters in relation to BMI. A large recent study of 1806 patients (9) found no association between obesity and LV systolic dysfunction, despite the known fact that obesity increases the risk of CHF (1,2). Therefore, other causes of CHF such as diastolic dysfunction should be considered in patients with obesity. Our results suggest that diastolic dysfunction may, in part, explain the increased incidence of heart failure in the present study population. Obesity is associated with several changes in cardiovascular function related to increases in cardiac work parameters such as LV dimension, stroke volume, cardiac output and eccentric hypertrophy. All of these parameters can cause diastolic heart failure consistent with our findings (5,6,10).
In concordance with our study, LV diastolic dysfunction correlated with BMI in a study of 48 obese and 25 normal weight women (11). Additionally, Krishnan et al (12) studied 122 patients and found no correlation between BMI and fractional shortening, nor between BMI and pulmonary artery systolic pressure. However, they found that obese persons had an increased LV end-diastolic cavity dimension, increased LVM to LV height ratio, and increased LA diameter consistent with our findings.
It has been recently reported that increased BMI is protective against mortality in patients with CHF (the so-called reverse epidemiology) (13–15). The cause of this apparent paradox is not known. It is suspected that lower body weight is associated with increased catabolic state, resulting in increased production of tumour necrosis factor and other cytokines (16,17). A higher prevalence of diastolic heart failure in obese patients may suggest better prognosis as a possible explanation for reverse epidemiology. However, recent studies (18,19) suggest that the prognosis of heart failure is similar between diastolic and systolic heart failure.
The present study was a retrospective study, and as such we did not have data of baseline clinical characteristics to adjust for comorbid conditions in the multivariate analysis. Therefore, we cannot prove that obesity is an independent risk factor of abnormal diastolic parameters. Furthermore, the diagnosis of diastolic dysfunction cannot be certain based on echocardiographic parameters, as previously mentioned in our discussion. LA volume is a more accurate estimate of LA dimension, which was not available in our database. Moreover, E/A ratio is pre- and afterload dependent. Many patients with normal E/A ratio could have psuedonormalization of their mitral inflow with grade 2 diastolic dysfunction. This could not be assessed in our database because we did not have any tissue Doppler studies available.
A prospective study adjusting for comorbid conditions using clinical data, tissue Doppler imaging, laboratory assessment of heart failure (eg, brain natriuretic hormones) and echocardiography is needed to accurately evaluate any association between obesity and LV diastolic dysfunction as an independent cause of heart failure. Our patients were referred for echocardiographic examination for various clinical reasons. Thus, our results cannot be extrapolated to the general population.
ACKNOWLEDGEMENTS
We would like to thank Ms Good Gale and Dr Mehrnoosh Hashemzadeh for their support in the editing of this manuscript.
REFERENCES
- 1.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 2.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 3.Messerli FH, Sundgaard-Riise K, Reisin ED, et al. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med. 1983;99:757–61. doi: 10.7326/0003-4819-99-6-757. [DOI] [PubMed] [Google Scholar]
- 4.Hammond IW, Devereux RB, Alderman MH, Laragh JH. Relation of blood pressure and body build to left ventricular mass in normotensive and hypertensive employed adults. J Am Coll Cardiol. 1988;12:996–1004. doi: 10.1016/0735-1097(88)90467-6. [DOI] [PubMed] [Google Scholar]
- 5.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA. 1991;266:231–6. [PubMed] [Google Scholar]
- 6.Alpert MA, Lambert CR, Terry BE, et al. Influence of left ventricular mass on left ventricular diastolic filling in normotensive morbid obesity. Am Heart J. 1995;130:1068–73. doi: 10.1016/0002-8703(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 8.Milani RV, Lavie CJ, Mehra MR, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97:959–63. doi: 10.1016/j.amjcard.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Dorbala S, Crugnale S, Yang D, Di Carli MF. Effect of body mass index on left ventricular cavity size and ejection fraction. Am J Cardiol. 2006;97:725–9. doi: 10.1016/j.amjcard.2005.09.122. [DOI] [PubMed] [Google Scholar]
- 10.Alpert MA, Hashimi MW. Obesity and the heart. Am J Med Sci. 1993;306:117–23. doi: 10.1097/00000441-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Pascual M, Pascual DA, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–6. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krishnan R, Becker RJ, Beighley LM, López-Candales A. Impact of body mass index on markers of left ventricular thickness and mass calculation: Results of a pilot analysis. Echocardiography. 2005;22:203–10. doi: 10.1111/j.0742-2822.2005.03138.x. [DOI] [PubMed] [Google Scholar]
- 13.Peterson LR, Waggoner AD, Schechtman KB, et al. Alterations in left ventricular structure and function in young healthy obese women: Assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 14.Davos CH, Doehner W, Rauchhaus M, et al. Body mass and survival in patients with chronic heart failure without cachexia: The importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–44. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: Possible relation to muscle wasting. J Am Coll Cardiol. 1997;30:997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 17.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–3. doi: 10.1016/S0140-6736(96)07015-8. (Erratum in 1997;349:1258) [DOI] [PubMed] [Google Scholar]
- 18.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 19.Badano LP, Albanese MC, De Biaggio P, et al. Prevalence, clinical characteristics, quality of life, and prognosis of patients with congestive heart failure and isolated left ventricular diastolic dysfunction. J Am Soc Echocardiogr. 2004;17:253–61. doi: 10.1016/j.echo.2003.11.002. [DOI] [PubMed] [Google Scholar]