Abstract
A 49-year-old woman was admitted to hospital because of heart failure. She was diagnosed as having mitochondrial cardiomyopathy and diabetes mellitus. Echocardiography revealed a hypertrophic and poorly contracting left ventricle. A diagnosis of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes was established by muscle biopsy. She underwent technetium-99m-sestamibi (99mTc-MIBI) and beta-methyl-p-123I-iodophenyl-pentadecanoic acid (123I-BMIPP) scintigraphic examinations. 99mTc-MIBI single-photon emission computed tomography revealed reduced tracer uptake in the hypertrophic left ventricular inferior wall. In contrast, there was an increase in 123I-BMIPP uptake in the in the region of reduced 99mTc-MIBI uptake (99mTc-MIBI/123I-BMIPP mismatch). There was rapid washout of 99mTc-MIBI from the myocardium (washout rate increased by 30%). Decreased 99mTc-MIBI and increased 123I-BMIPP uptake (99mTc-MIBI/123I-BMIPP mismatch) were the characteristics of cardiac involvement in mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes.
Keywords: 99mTc-MIBI, 123I-BMIPP mismatch, 99mTc-MIBI washout, MELAS
Mitochondrial disorders are a heterogeneous group of diseases resulting from abnormalities in mitochondrial DNA and function. In mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS), cardiac involvement manifesting as hypertrophic (symmetrical or asymmetrical) or dilated cardiomyopathy is frequently observed (1–6). We describe cardiac scintigraphic findings in a patient with MELAS and reviewed previous reports in the literature.
CASE PRESENTATION
A 49-year-old woman was admitted to hospital with dyspnea accompanied by orthopnea. She had been treated for insulin-dependent diabetes mellitus for four years. Three years before admission, she had undergone coronary angiography, which revealed normal coronary arteries. One year before admission, she had had pacemaker implantation because of a third-degree atrioventricular block. She had symptoms of a common cold eight days before admission. One day before admission, she had diarrhea and vomiting, as well as dyspnea, leading to hospital admission. On admission, her blood pressure was 80/46 mmHg, and her heart rate was 120 beats/min when 6 μg/kg/min infusions of dopamine and dobutamine were administered. Physical examination revealed a third heart sound, moist rales in the bilateral lung fields and pretibial edema. She was 145 cm tall and her weight was 40 kg. Her two daughters were also of short stature. She had difficulty hearing and muscle weakness. Her mother had difficulty hearing and died of a stroke. Echocardiography revealed biphasic P waves on the right pre-cordial leads, left ventricular hypertrophy and decreased amplitude of R waves. Chest x-ray showed pulmonary congestion and cardiomegaly (cardiothoracic ratio was 62%). Laboratory tests found increased levels of brain natriuretic peptide (6.8 × 105 ng/L), glutamicpyruvic transaminase (13,102 U/L) and glutamate oxaloacetate transaminase (5167 U/L). Her fasting blood glucose level was 1.44 mmol/L and hemoglobin A1c was 6.7%. Echocardiography (Figure 1) showed a markedly dilated, as well as diffusely hypertrophic and hypocontractile, left ventricle (end-diastolic septal thickness was 11 mm, posterior end-diastolic dimension was 77 mm, end-systolic dimension was 67 mm and ejection fraction was 16%). In addition, there was a small amount of pericardial effusion. She underwent plasma exchange, continuous hemodiafiltration, volume expansion and infusion of inotropic agents for heart failure. Arterial blood gas data on the third day showed worsening of metabolic acidosis. Incubation and arterial ventilation were performed. She was given a large dose of coenzyme Q10 (210 mg/day).
Figure 1.
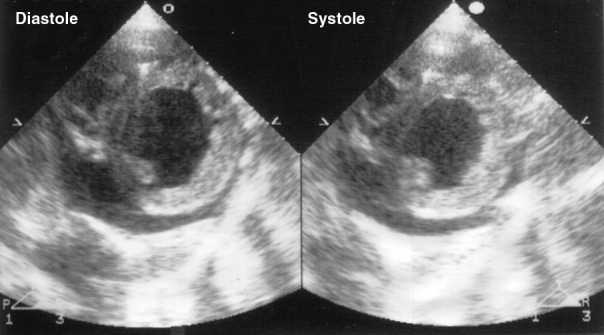
Echocardiogram in the short-axis view showing left ventricular hypertrophy and left ventricular dilation with decreased contraction. A small amount of pericardial effusion is also noted
On the ninth day in hospital, muscle biopsy (taken from musculus sternocleidomastoideus) was performed. Mitochondrial DNA sequence analysis by polymerase chain reaction revealed an A-to-G mutation in the mitochondrial transfer RNA(Leu[UUR]) gene at nucleotide position 3243 (Figure 2), a finding consistent with MELAS (7). Myocardial biopsy was not carried out because a pacemaker had been implanted.
Figure 2.
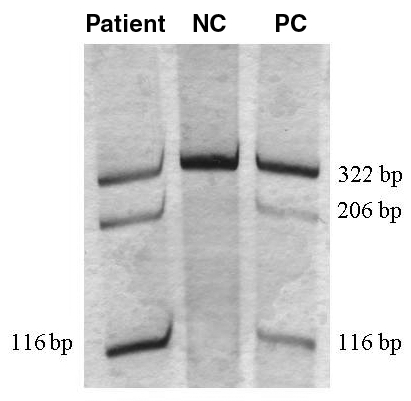
Southern blot analysis of mitochondrial DNA in the mitochondrial transfer RNA (Leu[UUR]) gene showing, in the presence of A3243G point mutation, the 322 bp polymerase chain reaction product is cut into a 206 bp and a 116 bp fragment. NC Negative control; PC Positive control
On the 55th day in hospital, the patient underwent rest technetium-99m-sestamibi (99mTc-MIBI) cardiac scintigraphy (740 MBq). The planar and single-photon emission computed tomography (SPECT) views were obtained approximately 30 min after injection as previously reported (8). Analysis by the electrocardiograph-gated quantitative gated SPECT software revealed decreased left ventricular systolic function with an end-diastolic volume of 83 mL, end-systolic volume of 60 mL and ejection fraction of 29%. Heart to mediastinum count ratio (H/M) of 99mTc-MIBI on the initial and delayed images were 2.4 and 2.5, respectively. There was an increase of 30% in the 99mTc-MIBI washout rate (normal washout rate is 15% or lower). SPECT images showed mild hypoperfusion in the inferior segments (Figure 3, upper panel).
Figure 3.
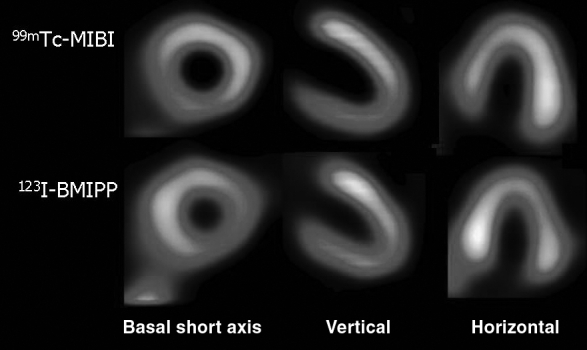
99mTc-sestamibi (99mTc-MIBI) (upper panel) and beta-methyl-p-123I-iodophenyl-pentadecanoic acid (123I-BMIPP) (lower panel) single-photon emission computed tomography images showing decreased 99mTc-MIBI uptake in the inferoseptal segments and increased 123I-BMIPP uptake in the inferoseptal and septal segments (99mTc-MIBI/123I-BMIPP mismatch)
On the 58th hospital day, beta-methyl-p-123I-iodophenyl-pentadecanoic acid (123I-BMIPP, Nihon Medi-Physics, Japan) scintigraphy was performed. Under fasting and resting conditions, 111 MBq of 123I-BMIPP was administered intravenously and immediately flushed with 10 mL of saline. 123I-BMIPP SPECT images were obtained as previously reported (9), and it showed increased uptake in the inferoseptal segment (Figure 3, lower panel).
DISCUSSION
Lactic acidosis is one of the common biochemical hallmarks of mitochondrial disorders. However, it also occurs in healthy subjects as nonspecific laboratory abnormalities after episodes of anoxic events, shock, hypoperfusion or CO intoxication. Because the brain and nervous system, in addition to muscle, are greatly dependent on energy production by mitochondrial oxidation, these tissues are more vulnerable to mitochondrial defects. The majority of patients with MELAS have malignant clinical outcomes, and it is frequently associated with heart failure (1–6) and arrhythmias, including advanced atrioventricular block (10), as observed in our patient.
Echocardiography demonstrated left ventricular hypertrophy, which is believed to be one of the morphological features of cardiac involvement in MELAS (4). A reduced uptake of 99mTc-MIBI was observed in the inferior myocardial segments. Also, there was a rapid washout of 99mTc-MIBI from the myocardium. 99mTc-MIBI circulates as a monovalent positive ion and distributes specifically in the myocardium after intravenous administration. Although 99mTc-MIBI was initially developed as a tracer for the evaluation of myocardial blood flow, recent studies (11–14) indicate that it is selectively incorporated (over 90%) into mitochondria in a membrane potential-dependent manner after passive diffusion into the myocardium. Thus, 99mTc-MIBI is a potentially useful agent for evaluating mitochondrial function. It has been suggested that mitochondrial DNA mutation in patients with dilated cardiomyopathy may also be associated with mitochondrial dysfunction. Mitochondrial dysfunction may be indicated by increased 99mTc-MIBI washout from the myocardium, presumably because of an inability of mitochondria to retain the tracer (15). Several studies (16,17) have reported mitochondrial DNA mutation along with mitochondrial abnormalities. Arbustini et al (16) identified a small subgroup of patients with pathological mutations in mitochondrial DNA in idiopathic dilated cardiomyopathy. The mutations may be a sign of increasing stress to the myocardium, promoting additional damage to mitochondrial DNA (17). The kinetics of 99mTc-MIBI in MELAS is not well documented, but a recent report by Ikawa et al (18) described increased 99mTc-MIBI washout in five patients with MELAS. In their study, patients had decreased 99mTc-MIBI uptake and an increased 99mTc-MIBI washout rate, which correlated inversely with left ventricular ejection fraction. In addition, they found increased uptake of 123I-BMIPP in the region of decreased 99mTc-MIBI uptake (99mTc-MIBI/123I-BMIPP mismatch) in two patients with severe left ventricular dysfunction, which was consistent with our patient. 123I-BMIPP is an analogue of free fatty acid, which enters the intracellular triglyceride pool. In mitochondrial respiratory chain failure, energy production shifts from the aerobic to the anaerobic pathway (glycolytic pathway), resulting in increased lactic acid formation and increased uptake of 123I-BMIPP (19). Thus, 99mTc-MIBI/123I-BMIPP mismatch, together with the increased 99mTc-MIBI washout rate, appears to reflect alterations in energy state due to mitochondrial respiratory failure, and this association may be useful to evaluate the severity of cardiac involvement in MELAS.
REFERENCES
- 1.Sato W, Tanaka M, Sugiyama S, et al. Cardiomyopathy and angiopathy in patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Am Heart J. 1994;128:733–41. doi: 10.1016/0002-8703(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 2.Hiruta Y, Chin K, Shitomi K, et al. Mitochondrial encephalomyopathy with A to G transition of mitochondrial transfer RNA(Leu(UUR)) 3,243 presenting hypertrophic cardiomyopathy. Intern Med. 1995;34:670–3. doi: 10.2169/internalmedicine.34.670. [DOI] [PubMed] [Google Scholar]
- 3.Shinomiya H, Fukuda N, Takeichi N, et al. Evaluation of cardiac function by various cardiac imaging techniques in mitochondrial cardiomyopathy: A case report. J Cardiol. 1998;31:109–14. [PubMed] [Google Scholar]
- 4.Okajima Y, Tanabe M, Takayanagi M, Aotsuka H. A follow up study of myocardial involvement in patients with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) Heart. 1998;80:292–95. doi: 10.1136/hrt.80.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ueno H, Shiotani H. Cardiac abnormalities in diabetic patients with mutation in the mitochondrial tRNA(Leu(UUR)) gene. Jpn Circ J. 1999;63:877–80. doi: 10.1253/jcj.63.877. [DOI] [PubMed] [Google Scholar]
- 6.Higashikata T, Koyama J, Shimada H, Yazaki M, Owa M, Ikeda S. An 80-year-old mitochondrial disease patient with A3243G tRNA(Leu(UUR)) gene presenting cardiac dysfunction as the main symptom. Intern Med. 2001;40:405–8. doi: 10.2169/internalmedicine.40.405. [DOI] [PubMed] [Google Scholar]
- 7.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–3. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo S, Nakae I, Matsumoto T, Horie M. Impact of endothelial dysfunction on left ventricular remodeling after successful primary coronary angioplasty for acute myocardial infarction – analysis by quantitative ECG-gated SPECT. Ann Nucl Med. 2006;20:57–62. doi: 10.1007/BF02985592. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo S, Nakamura Y, Takahashi M, Mitsunami K, Kinoshita M. Myocardial metabolic abnormalities in hypertrophic cardiomyopathy assessed by iodine-123-labeled beta-methyl-branched fatty acid myocardial scintigraphy and its relation to exercise-induced ischemia. Jpn Circ J. 1998;62:167–72. doi: 10.1253/jcj.62.167. [DOI] [PubMed] [Google Scholar]
- 10.Lev D, Nissenkorn A, Leshinsky-Silver E, et al. Clinical presentations of mitochondrial cardiomyopathies. Pediatr Cardiol. 2004;25:443–50. doi: 10.1007/s00246-003-0490-7. [DOI] [PubMed] [Google Scholar]
- 11.Okada RD, Glover D, Gaffney T, Williams S. Myocardial kinetics of technetium-99m-hexakis-2-methoxy-2-methylpropyl-isonitrile. Circulation. 1988;77:491–8. doi: 10.1161/01.cir.77.2.491. [DOI] [PubMed] [Google Scholar]
- 12.Piwnica-Worms D, Kronauge JF, Chiu ML. Uptake and retention of hexakis (2-methoxyisobutyl isonitrile) technetium (I) in cultured chick myocardial cells: mitochondrial and plasma membrane potential dependence. Circulation. 1990;82:1826–38. doi: 10.1161/01.cir.82.5.1826. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho PA, Chiu ML, Kronauge JF, et al. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med. 1992;33:1516–22. [PubMed] [Google Scholar]
- 14.Crane P, Laliberté R, Heminway S, Thoolen M, Orlandi C. Effect of mitochondrial viability and metabolism on technetium-99m-sestamibi myocardial retention. Eur J Nucl Med. 1993;20:20–5. doi: 10.1007/BF02261241. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo S, Nakae I, Tsutamoto T, Okamoto N, Horie M. A novel clinical indicator using Tc-99m sestamibi for evaluating cardiac mitochondrial function in patients with cardiomyopathies. J Nucl Cardiol. 2007;14:215–20. doi: 10.1016/j.nuclcard.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Arbustini E, Diegoli M, Fasani R, et al. Mitochondrial DNA mutations and mitochondrial abnormalities in dilated cardiomyopathy. Am J Pathol. 1998;153:1501–10. doi: 10.1016/S0002-9440(10)65738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruppert V, Maisch B. Mitochondrial DNA deletions in cardiomyopathies. Herz. 2000;25:161–7. doi: 10.1007/s000590050002. [DOI] [PubMed] [Google Scholar]
- 18.Ikawa M, Kawai Y, Arakawa K, et al. Evaluation of respiratory chain failure in mitochondrial cardiomyopathy by assessments of 99mTc-MIBI washout and 123I-BMIPP/99mTc-MIBI mismatch. Mitochondrion. 2007;7:164–70. doi: 10.1016/j.mito.2006.11.008. [DOI] [PubMed] [Google Scholar]


