Abstract
Breast cancer (BC) is the most commonly diagnosed cancer among American women; however, the development of postmenopausal BC is significantly lower in African Americans as compared to Caucasians. Hormonal stimulation is important in BC development and differences in the conversion of dehydroepiandrosterone (DHEA) into estrogens may be involved in the lower incidence of post-menopausal BC in African American women. DHEA sulfation by SULT2B1b is important in regulating the conversion of DHEA into estrogens in tissues. SULT2B1b is localized in both cytosol and nuclei of some tissues including cancerous and associated-normal breast tissue. Immunohistochemical staining was used to evaluate the total expression and subcellular localization of SULT2B1b in African American and Caucasian breast tissues. Cell fractionation, immunoblot analysis and sulfation assays were used to characterize the subcellular expression and activity of SULT2B1b in BC tissues and T-47D breast adenocarcinoma cells. Immunohistochemical analysis of SULT2B1b showed that African Americans had a significantly greater amount of SULT2B1b in epithelial cells of associated-normal breast tissue as compared to Caucasians. Also, more SULT2B1b in African American associated-normal breast epithelial cells was localized in the nuclei than in Caucasians. Equivalent levels of SULT2B1b were detected in breast adenocarcinoma tissues from both African American and Caucasian women. Nuclei isolation and immunoblot analysis of both BC tissue and human T-47D breast adenocarcinoma cells demonstrated that SULT2B1b is present in nuclei and cytoplasm.
Keywords: Breast cancer, African American, Caucasian, sulfation, sulfotransferase, SULT2B1b, dehydroepiandrosterone, β-estradiol
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer among American women with approximately 200,000 cases diagnosed in 2005. Breast cancer is the second leading cause of cancer death in women with approximately 40,000 deaths in 2005 [1]. There is a slightly higher incidence of breast cancer in premenopausal African American women as compared to Caucasians. In contrast, the incidence of breast cancer in postmenopausal African American women is significantly lower than that of Caucasian women [1]. The reasons for the lower incidence in the postmenopausal African American women are not well understood.
Estrogenic stimulation is recognized as an important factor in the development of breast cancer [2]. Prior to menopause the majority of estrogens are synthesized and secreted from the ovaries whereas in postmenopausal women almost all estrogens are derived from the adrenal androgen dehydroepiandrosterone (DHEA) [3]. The enzymes necessary for the conversion of DHEA to β-estradiol (E2) are present in breast tissues [3]. Additionally, DHEA has been reported as being capable of binding and activating estrogen and androgen receptors directly [4, 5]. Therefore, mechanisms that inhibit the metabolism of DHEA may be important in regulating its estrogenic activity in postmenopausal breast tissues. One of the important mechanisms regulating DHEA activity and metabolism and therefore its hormonal activity is sulfation [6].
Sulfation involves the transfer of the sulfonate group from 3’-phosphoadenosine 5’-phosphosulfate (PAPS) to an acceptor compound [7]. The primary sites of sulfation are hydroxyl groups resulting in the formation of sulfate esters. The enzyme family responsible for the sulfation of drugs, xenobiotics and many small endobiotics is the cytosolic sulfotransferase (SULT) family. The SULTs are Phase II or conjugation enzymes involved in drug and xenobiotic metabolism. The conjugation of most small compounds with a sulfonate group inhibits their biological activity and increases their hydrophilicity and excretion [7].
The major SULT isoform in human breast responsible for DHEA sulfation is SULT2B1b [8]. The SULT2B1 gene encodes two different isoforms resulting from the use of different start sites of transcription resulting in the inclusion of different first exons [9]. SULT2B1b is slightly longer than SULT2B1a allowing for their resolution and detection by immunoblot analysis. SULT2B1b protein has been detected by immunoblot analysis in several human tissues including placenta, breast, prostate, lung and skin [8, 10–12]. In contrast, although message for SULT2B1a has been found in human tissues [9, 13], immunoreactive protein has not yet been detected in a human tissue [8].
SULT2B1b is selective for the conjugation of 3β-hydroxysteroids and does not sulfate most 3α-hydroxysteroids, estrogens or the D-ring hydroxyl groups of steroids [14]. SULT2B1b is the only SULT isoform that is localized in the nuclei of some human tissues. SULT2B1b has been localized to the nuclei of placental and breast cells but not lung or prostate cells [8, 10, 11]. In human BeWo choriocarcinoma cells, nuclear localization of SULT2B1b is associated with serine phosphorylation of a unique carboxyl-terminal proline and serine-rich sequence [15]. The functional role for nuclear localization of SULT2B1b has not been determined although its expression in estrogen responsive tissues would be expected to limit the conversion of DHEA to active estrogens.
In this study, the biochemical characterization of SULT2B1b in both T-47D breast cancer cells and human breast tissue was analyzed. Subcellular localization was evaluated by immunoblot and histochemical analyses, and SULT2B1b enzymatic activity was monitored with DHEA as substrate. Additionally, associated-normal and cancerous breast tissues from African American and Caucasian women were evaluated by histochemical staining to determine SULT2B1b levels as well as to investigate its subcellular localization.
MATERIALS AND METHODS
Cell culture
T-47D breast cancer cells were obtained from the ATCC and were maintained in RPMI 1640 supplemented with 10% FBS (Atlanta Biologicals) and 4 mg/ml insulin (Sigma). Cells were grown at 37° C in a humidified atmosphere with 5% CO2. Medium was replaced every three days and cells were passed upon reaching 80% confluency.
Biochemical characterization of SULT2B1b in T-47D cells and breast cancer tissue
For biochemical characterization of SULT2B1b, samples were prepared from T-47D cells and fresh breast cancer tissues from patients as follows. T-47D cells were plated in 100 mm plates and upon confluency, the cells were washed with PBS and scraped from the plates. Cytosolic and nuclear fractions were then prepared from the cells using the Nuclei Isolation Kit (Sigma). For the analysis of the biochemical localization o fSULT2B1b in breast tissue, cytosol and purified nuclear fractions were prepared from fresh human breast cancer tissue using Optiprep gradient density centrifugation (Sigma) as per the manufacturer’s instruction. Optiprep density medium is a 60% iodixanol solution used to create a self-forming iodixanol gradient during centrifugation to isolate nuclei by isopycnic banding [16, 17].
For immunoblot analysis, nuclear and nuclei-free cytosolic fractions were prepared as described above. Protein concentrations were determined using the Bradford protein assay with γ-goblin as the protein standard. Nuclear and cytosolic fractions (100 µg) of the T-47D cells and breast cancer tissue were resolved by 10% SDS-PAGE and transferred to nitrocellulose membranes. The immunoblot protocol involved using 5% non-fat milk for blocking, primary antibody at a 1:1000 dilution and the appropriate secondary antibody, either goat anti-rabbit or goat anti-mouse IgG HRP conjugate (1:60,000). The following primary antibodies were used: polyclonal rabbit anti-SULT2B1b antiserum generated in our laboratory [15], mouse monoclonal anti-β-tubulin IgG (Sigma), and mouse monoclonal anti-histone IgG (Chemicon International). Bound antibodies were visualized using the SuperSignal West Pico chemiluminescent substrate system (Pierce).
To determine the enzymatic activity in intact T-47D cells, cells were plated in 6 well plates. When cells reached 80% confluence, 3 µM [3H]-DHEA in 1% stripped FBS phenol red-free RPMI 1640 media (approximately 500,000 cpm/well) was added to the cells. Reactions were incubated for various times after which aliquots of the medium were removed and [3H]-DHEA-sulfate quantified by scintillation counting of an aliquot of the aqueous phase after the removal of unsulfated [3H]-DHEA by chloroform extraction. SULT2B1b activity was expressed as pmol/mg protein.
Sulfation activity was evaluated using the same samples described above. T-47D and breast cancer nuclear and cytoplasmic fractions (100 µg) were incubated with 3 µM [3H]-DHEA (approximately 30,000 cpm/reaction), 50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, and 25 µM PAPS at 37°C for 20 min to detect SULT2B1b activity. DHEA-sulfate formation was quantified by scintillation counting of an aliquot of the aqueous phase after removal of unsulfated steroids by chloroform extraction [18]. SULT2B1b activity was expressed as pmol/min/mg protein.
Immunohistochemical staining of SULT2B1b in breast tissue and T-47D cells
Paraffin embedded normal and cancerous breast tissues were obtained from the Tissue Procurement Service of the Comprehensive Cancer Center at the University of Alabama at Birmingham after pathological diagnosis in accordance with Institutional Review Board approval. The breast tissues were from individuals that were self-identified as being African American or Caucasian. Following oven-drying, 5 micron breast tissue sections mounted on glass slides were deparaffinized and rehydrated by a series of xylene and graded ethanol washes. Low temperature antigen retrieval was used to optimize staining conditions [19]. Following endogenous peroxidase quenching with 3% H2O2 and non-specific biotin/avidin blocking with 1% goat serum (Sigma) for 1 h, sections were incubated with polyclonal rabbit anti-SULT2B1b primary antibodies (1:100) for 1 h [14]. Bound antibodies were then visualized by treating samples with biotin-SP-conjugated AffiniPure goat anti-rabbit IgG (H+L) followed by peroxidase-conjugated streptavidin (Jackson ImmunoResearch). The resulting antigen-antibody complex was then visualized using the diaminobenzidine tetrachloride Super Sensitive Substrate Kit (BioGenex). Sections were then counterstained with hematoxylin, rinsed with tap water, dehydrated with graded ethanol and washed in xylene followed by addition of a cover slip. Negative controls were processed along with stained sections except for the addition of primary antibodies. Images were acquired on an Axioskop 2 plus microscope equipped with an AxioCam digital camera and AxioVision Software (Zeiss, version 4.4). Sections were evaluated by two individual judges, a board certified histopathologist and experienced research technician. The sections were assigned a score of 0 to 4 based on intensity of staining in the nuclei and cytoplasm as described by Talley [20].
For immunohistochemical evaluation of T-47D cells, cells were plated on coverslips in a 6-well plate and allowed to grow to confluency. The cells attached to the coverslips were fixed in 70% ethanol for 2 h, permeabilized with acetone and quenched with 3% H2O2. Cells were then treated as described above except for blocking with 3% goat serum.
Statistical analysis
NCSS97 statistical and power analysis software was used to perform statistical analysis. Student’s t-test analysis was applied; however, because of the variability of the data Wilcoxon, non-parametric analysis was also utilized for confirmation. A p-value of 0.05 was set for significance. Means, medians, and standard deviations were also determined.
RESULTS
Subcellular distribution of SULT2B1b in T-47D cells and breast tissue
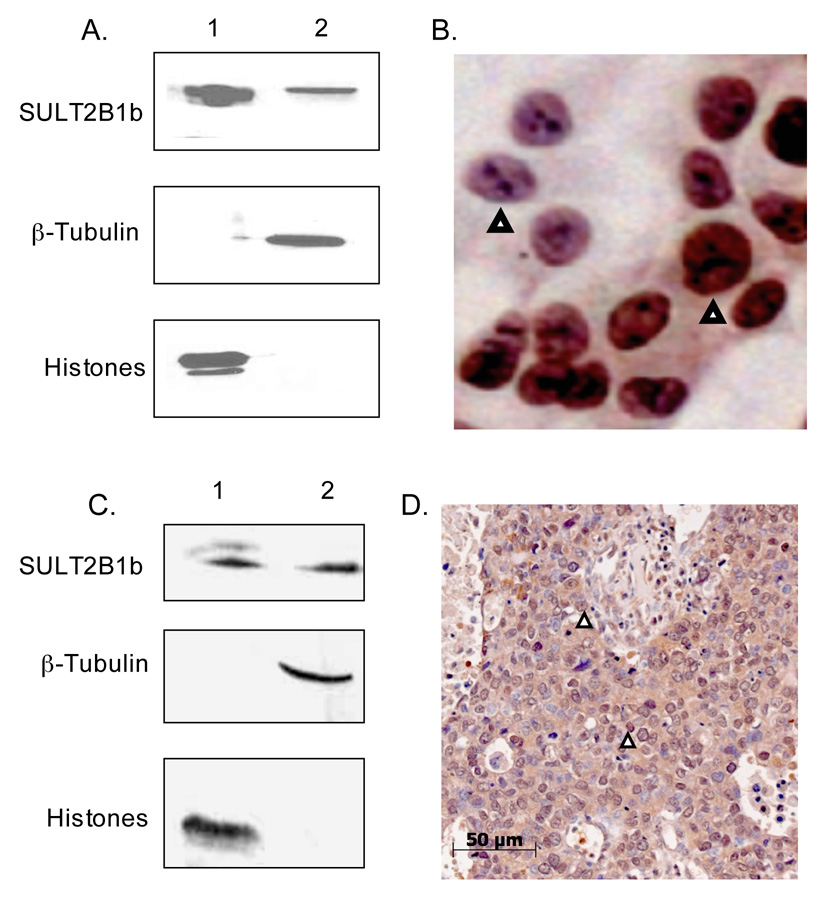
T-47D cells are an estrogen receptor (ER) positive human breast adenocarcinoma cell line [21]. Figure 1A shows that SULT2B1b is expressed in the cytoplasm as well as in the nuclei of T-47D cells following isolation of the subcellular fractions and immunoblot analysis. The nuclear fraction contained no apparent cytoplasmic contamination since β-tubulin, a cytoplasmic protein, could not be detected. The cytoplasmic fraction contained no apparent nuclear contamination as determined by the lack of immunodetection of nuclear histones (Fig. 1A). Figure 1B shows nuclear and cytosolic expression of SULT2B1b in intact T-47D cells plated on coverslips. Although not all cells express SULT2B1b, both nuclear and cytoplasmic staining was observed in those cells with positive staining. As reported previously [21], the T-47D cells did not possess detectable SULT2A1 immunoreactive protein.
Figure 1. Immunoblot analysis and immunohistochemistry of SULT2B1b in T-47D cells and breast cancer.
A. Immunoblot analysis of SULT2B1b in nuclear and cytosolic fractions isolated from T-47D cells. Nuclear and cytosolic proteins were resolved in 10% gels by SDS-PAGE and electrotransferred to nitrocellulose membranes. SULT2B1b, β-tubulin, and histones were detected by immunoblotting using antibodies as described in Methods. β-tubulin, and histones were used as cytosolic and nuclear standards, respectively. The lanes contain 100 µg: 1) T-47D cell nuclei; 2) T-47D cell nuclei-free lysate. B. Immunostaining of T-47D cells for SULT2B1b. Rabbit anti-SULT2B1b antibodies were used to stain T-47D cells plated on coverslips. Arrows depict nuclear staining. The panel is enlarged to demonstrate the differences in SULT2B1b staining in the nuclei of T47D cells. C. Immunoblot analysis of SULT2B1b in nuclear and cytosolic fractions isolated from a fresh breast cancer specimen. Nuclear and cytosolic proteins were isolated from a fresh breast adenocarcinoma specimen obtained from a 50 yo African American woman. The proteins were resolved by SDS-PAGE in 10% gels and electro-transferred to nitrocellulose membrane. The membranes were immunoblotted with a rabbit anti-SULT2B1 antibody as described in Methods. The lanes contain 100µg protein each from: 1) breast cancer nuclei; 2) breast cancer cytosol. E. Rabbit anti-SULT2B1 antibodies were used to stain the paraffin embedded breast tissue from the same patient. Arrowheads depict nuclear staining.
Isolated nuclei and cytosol were prepared from freshly isolated breast adenocarcinoma tissue obtained from a 56 year old African American woman using the Optiprep centrifugation procedure and analyzed for SULT2B1b expression by immunoblot and immunohistochemical methods (Figures 1C and 1D). As seen with T-47D cells, immunoblot analysis demonstrated that SULT2B1b is expressed in the cytoplasm as well as in nuclei of the breast cancer tissue specimen. Immunoblot analysis for marker proteins confirms that the nuclear fraction has no apparent cytoplasmic contamination and that the cytosolic fraction has no apparent nuclear contamination (Fig. 1C). Immunohistochemical analysis of a paraffin embedded breast cancer section obtained from the same 56 year old woman also shows nuclear and cytoplasmic expression of SULT2B1b (Fig. 1D).
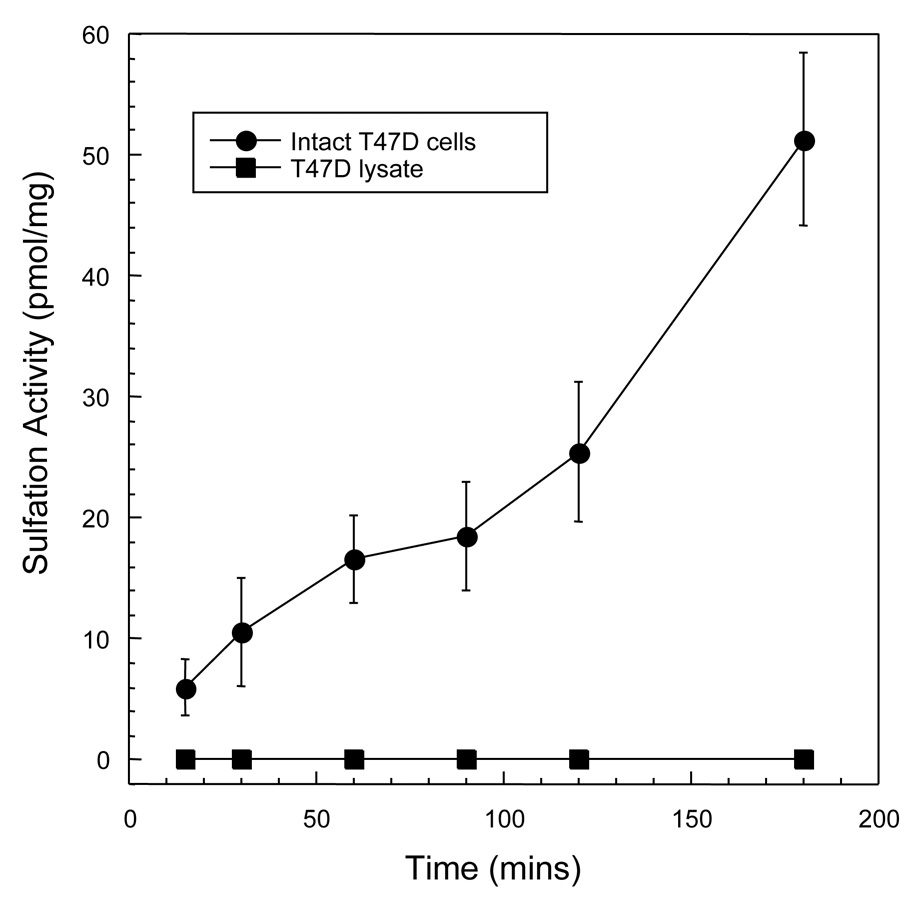
Figure 2 shows that DHEA sulfation by SULT2B1b was observed in intact T-47D cells at a rate of 3.3 pmol/mg/10 min. The sulfation activity is attributable to SULT2B1b because our laboratory has reported previously that SULT2A1, the other SULT capable of DHEA sulfation, is not present in T-47D cells [21]. Upon the preparation of T-47D cytosolic and crude nuclear fractions SULT2B1b enzymatic activity was no longer detectable consistent with the loss of activity found with cell disruption in other cell lines and tissues [8]. However, isolated nuclei from the fresh breast cancer sample possessed DHEA sulfation activity similar to that reported previously in isolated human placental nuclei [10]. Lysis of the isolated intact nuclei also resulted in the loss of DHEA sulfation activity although SULT2B1b protein could be detected by immunoblot analysis.
Figure 2. DHEA sulfation by intact T-47D cells.
The T-47D cells were washed twice with PBS then placed in serum-free RPMI1640 without phenol-red in 6 well plates. [3H]-DHEA was added to the medium to a final concentration of 3 µM. The formation of [3H]-DHEA-sulfate was determined in aliquots of the medium by scintillation counting after chloroform extraction to remove unsulfated [3H]-DHEA. DHEA sulfation is expressed as pmol/mg protein. The standard derivation of the [3H]-DHEA-sulfate determinations are included within the points.
Immunohistochemical evaluation of SULT2B1b protein expression in breast tissues
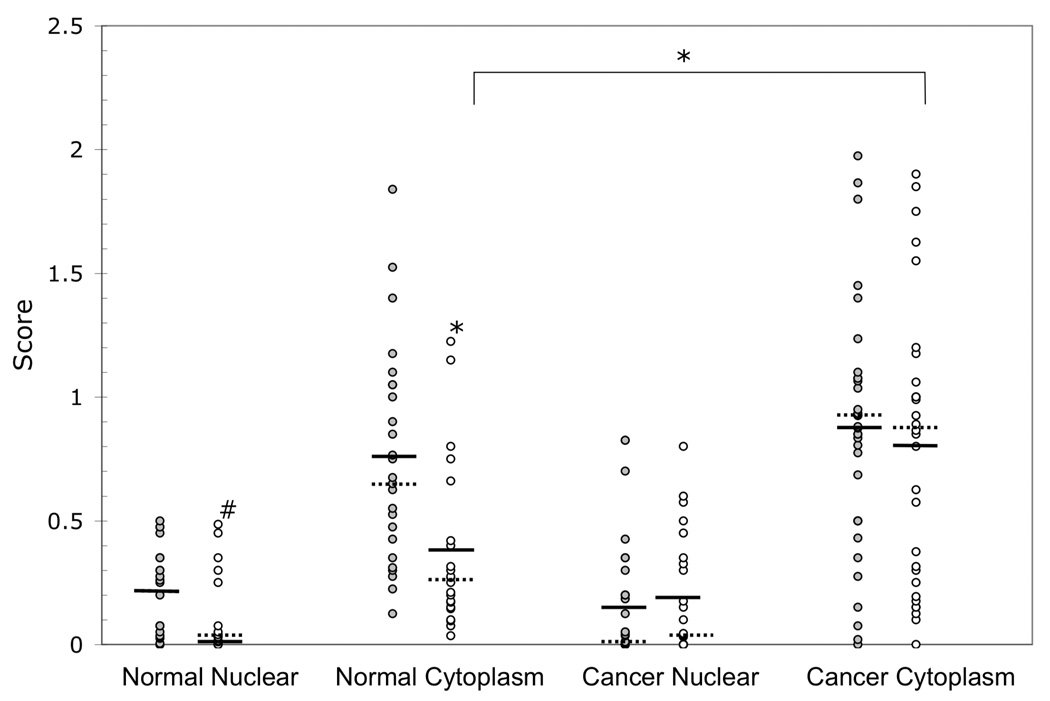
Since SULT2B1b immunoreactive protein was detected in the nuclear and cytoplasmic fractions of T-47D breast cancer cells (Fig. 1A and Fig. 1B), SULT2B1b expression was examined immunohistochemically in cancerous and associated normal breast tissues from African Americans and Caucasians. Sections of breast tissue containing adenocarcinoma and associated-normal tissue were immunostained with the rabbit anti-human SULT2B1b antibody. Staining was only observed in normal epithelial cells or adenocarcinoma cells. The level of immunostaining in the nuclei and cytoplasm was estimated using the standardized protocol described by Talley et al. [20]. Each section was assigned a nuclear and cytoplasmic score where applicable. Table 1 summarizes the statistical analysis of SULT2B1b expression in African American and Caucasian normal and cancerous breast tissues. Figure 3 shows the individual normal and cancerous immunoscores for SULT2B1b expression in the nuclei and cytoplasm of the 68 African American and Caucasian breast tissues examined. Statistical analysis reveals that African Americans have significantly more SULT2B1b expression in associated-normal tissue, but not in cancerous tissue as compared to Caucasians. African Americans express higher levels of nuclear and cytoplasmic SULT2B1b in associated-normal tissue than Caucasians with p-values of 0.02 and 0.001, respectively. There was no significant difference in SULT2B1b expression between African Americans and Caucasians in the cancerous breast tissue. Caucasians express significantly higher amounts of SULT2B1b in the cytoplasm of cancerous tissues as compared to normal tissues with a p-value of 0.001. African American normal nuclei, African American normal cytoplasm, and Caucasian normal nuclei show no significant difference in SULT2B1b expression for the corresponding cancerous tissues.
Table 1.
Summary of statistical analysis of immunohistochemical analysis of SULT2B1b expression in African American (AA) and Caucasian (Cau) breast tissues. Results from statistical analysis of tissue scoring. Bold print indicates significant difference between races and the parentheses indicates significant difference within a race between normal and cancerous tissues.
| Race | Stats | Normal Nuclear | Normal Cyto | Cancer Nuclear | Cancer Cyto |
|---|---|---|---|---|---|
| AA | Mean | 0.2 | 0.76 | 0.14 | 0.86 |
| AA | Median | 0.2 | 0.68 | 0.01 | 0.88 |
| AA | Std Dev | 0.18 | 0.43 | 0.24 | 0.49 |
| AA | p-value | 0.02 | 0.001 | 0.46 | 0.32 |
| Cau | Mean | 0.01 | 0.37 | 0.15 | 0.79 |
| Cau | Median | 0.02 | 0.26 | 0.03 | 0.86 |
| Cau | Std Dev | 0.16 | 0.34 | 0.23 | 0.56 |
| Cau | p-value | 0.02 | 0.001 (0.001) | 0.46 | 0.32 (0.001) |
Figure 3. Individual immunohistochemical analysis of SULT2B1b expression in African American and Caucasian breast tissues.
Paraffin sections of Caucasian and African American women diagnosed with breast adenocarcinoma were obtained from the Tissue Procurement Service of the UAB Comprehensive Cancer Center. The paraffin sections were stained with rabbit anti-SULT2B1b antibodies. Each tissue section was scored based on intensity of nuclear and cytosolic SULT2B1b staining and that score is represented by a dot. The gray dots, ( ) represent the African American scores and the white dots ( ○ ) represent the Caucasian scores. A pound sign (#) indicates a significant difference at p<0.05 and an asterisk (*) indicates a significant difference at p<0.001 between African Americans and Caucasians or between normal and cancerous tissues. A solid bar, (
) represent the African American scores and the white dots ( ○ ) represent the Caucasian scores. A pound sign (#) indicates a significant difference at p<0.05 and an asterisk (*) indicates a significant difference at p<0.001 between African Americans and Caucasians or between normal and cancerous tissues. A solid bar, ( ) depicts the mean and the dashed bar, (
) depicts the mean and the dashed bar, ( ) depicts the median. The numbers of specimens were: AA normal n=25; Cau normal n=22; AA cancer n=34; and Cau cancer n=30.
) depicts the median. The numbers of specimens were: AA normal n=25; Cau normal n=22; AA cancer n=34; and Cau cancer n=30.
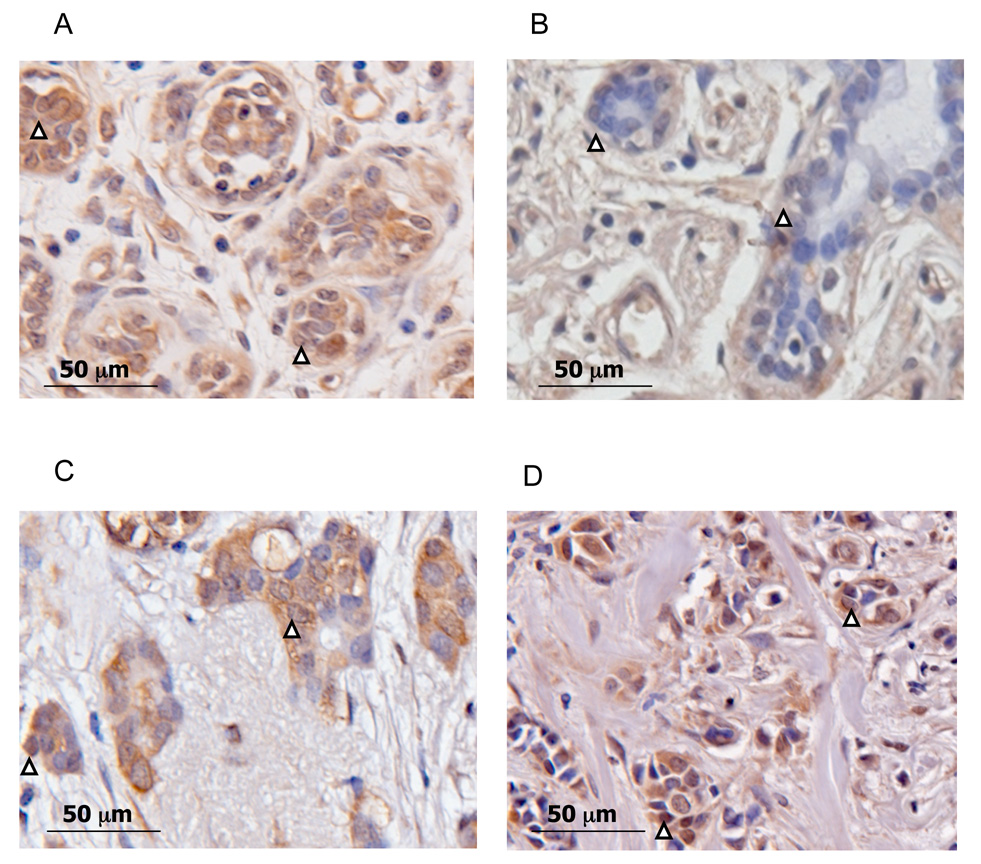
Figure 4 shows SULT2B1b immunohistochemical staining in both associated-normal and cancerous African American and Caucasian breast tissues and illustrates the significant differences seen in the tissues analyzed. It is apparent that African American normal breast tissues show a significantly higher expression of nuclear and cytoplasmic SULT2B1b in epithelial cells as compared to Caucasians (Figures 4A and 4B). Additionally, Caucasian cancerous breast tissues express significantly higher amounts of SULT2B1b in cytoplasm as compared to Caucasian normal breast tissues (Figures 4D and 4B, respectively). Although SULT2B1b expression increases in breast adenocarcinoma in Caucasians, there is no significant difference between normal-associated and adenocarcinoma in African Americans.
Figure 4. Immunohistochemical staining of SULT2B1b in African American and Caucasian breast tissues.
Representative sections of African American and Caucasian normal and cancerous breast tissues were stained with the rabbit anti-SULT2B1b antibodies. Shown here is a representation of the significant differences seen in the tissues analyzed. A) African American (45 yo) normal breast tissue. B) Caucasian (48) normal breast tissue. C) African American (69 yo) cancerous breast tissue. D) Caucasian (66 yo)cancerous breast tissue. Arrows depict nuclear staining.
DISCUSSION
SULT2B1b is expressed in several hormone responsive human tissues including breast, placenta and prostate. Falany et al. [8] have reported the nuclear and cytoplasmic localization of SULT2B1b in MCF-7 breast cancer cells. SULT2B1b expression is also observed in the nuclei and cytoplasm of T-47D breast cancer cells although every cell does not show expression, possibly due to cycle dependent expression of SULT2B1b (Fig. 1B). Although T-47D cell lysates show no DHEA sulfation activity, intact T47D cells are capable of sulfating DHEA (Fig. 2). This agrees with studies in other tissues demonstrating that SULT2B1b activity is lost upon cell lysis or lysis of intact nuclei [10, 14]. Ji et al. [22] report that SULT2B1b has a paranuclear localization in MCF-7 breast cancer cells using confocal microscopy; however, Fuda et al. [23] observed nuclear localization of SULT2B1b in THP-1 macrophages with confocal microscopy. In addition to its expression in breast cancer cell lines, SULT2B1b is detected in the nuclei and cytoplasm of breast cancer sections and in freshly isolated cytosol and intact nuclei from a breast cancer patient (Fig. 1C and D). The specific function of SULT2B1b in the nuclei remains unknown; however, SULT2B1b in the cell has a role in the sulfation of DHEA, cholesterol and oxysterols [23–25]. SULT2B1b expression potentially regulates steroid hormone levels via DHEA metabolism in peripheral tissues where SULT2B1b is expressed.
In human breast cancer T-47D cells, SULT2B1b is responsible for the sulfation of DHEA, which is the major steroid precursor of estrogen and androgen synthesis after menopause [26]. As mentioned previously, SULT2B1b is enzymatically inactive in preparations of cell cytosol or nuclear extracts although SULT2B1b protein is detectable by immunoblot analysis. DHEA sulfation activity is present in intact cells as well as in isolated nuclei suggesting that membrane and/or heterologous protein interactions are required for stability of SULT2B1b [10]. SULT2B1b is also active when expressed with a histidine-tag that apparently allows for proper folding and remains active after the enzymatic removal of the histidine-tag [24]. Results from the present study show that intact human breast cancer T-47D cells have DHEA sulfation activity but that T-47D lysate, cytosol, and crude nucleiar extracts have no detectable activity towards DHEA. As shown in Table 2, Optiprep-isolated nuclei from fresh breast tissue have 3 pmol/mg/min activity towards DHEA whereas cytosol and nuclear extract from the same tissue have no detectable sulfation activity. These results correlate with those of He et al. [10] reporting DHEA sulfation activity in isolated placenta nuclei. SULT2B1b is also the only human SULT isoform that has a proline rich carboxyl terminus that may be involved in both its nuclear localization and enzyme activity characteristics [15]. The mechanism of nuclear localization is not known, but involves phosphorylation of the unique 3’-tail of SULT2B1b in human BeWo choriocarcinoma cells [15].
Table 2.
DHEA sulfation in breast cancer tissues. Sulfation activity was evaluated as described in Methods in lysate of T47D breast cancer cells and in cytosol and intact nuclei isolated from fresh breast adenocarcinoma specimen. Samples were incubated with 3 µM [3H]-DHEA at 37°C for 20 min to detect SULT2B1b activity. DHEA-sulfate formation was quantified by scintillation counting of an aliquot of the aqueous phase after removal of unsulfated steroids by chloroform extraction. DHEA sulfation is expressed as pmol/mg/min.
| Tissues | DHEA Sulfation Activity (pmol/mg/min) |
|---|---|
| T-47D Cell Lysate | ND |
| Breast Cancer Cytosol | ND |
| Breast Cancer Intact Nuclei | 3.0 |
Pursuant to the studies in breast cancer cell lines, the subcellular localization and levels of expression of SULT2B1b in normal and cancerous breast tissues from pre- and post-menopausal women was studied. Breast cancer develops a variety of ways, one of which is in response to long-term exposure to estrogens and androgens [27]. In the breast DHEA can readily be converted into estrogens and androgens [3] and thus the level of available DHEA may contribute to the development of breast cancer. Low DHEA levels are associated with increased risk of pre-menopausal breast cancer while higher DHEA levels are associated with increased risk of postmenopausal breast cancer [28–32]. The level of estrogens and androgens within the breast are important factors in the development or progression of breast cancer in comparison to circulating hormone levels, particularly in the case of post-menopausal breast cancer where the plasma levels of estrogens are low. Because SULT2B1b sulfates and thereby inactivates DHEA, the level of estrogens and androgens should be significantly lower in normal tissue where SULT2B1b is expressed at significantly higher levels. Thus, elevated SULT2B1b expression in normal breast may result in a greater conversion of DHEA to DHEA-S and therefore a lower concentration of estrogens in the breast. Estrone, which is a metabolite of DHEA and androstenediol is associated with an increased risk for developing breast cancer [2, 3, 25].
As the study of SULT2B1b expression in breast tissues specimens was underway, it became apparent that there were differences in SULT2B1b levels between the normal breast tissues from African Americans and Caucasians. It is known that African Americans have a slightly greater incidence of pre-menopausal breast cancer but have a significantly lower incidence of postmenopausal breast cancer compared to their Caucasian counterparts [1]. African Americans have higher mortality possibly due to later detection of the cancer and poorer access to treatment [1] as well as a higher incidence of estrogen receptor negative, progesterone receptor negative and more advanced stage cancer at time of detection [33–34]. Pre-menopausal African American women diagnosed at stage I or II have the same mortality as the general population while African American women with advanced stage diagnosis have a worse prognosis [35]. This finding explains why African Americans have increased breast cancer mortality but does not explain the significantly lower incidence of post-menopausal breast cancer in African Americans. There are several studies designed to determine why African Americans have higher breast cancer mortality, but few designed to determine why African Americans have a significantly lower incidence of post-menopausal breast cancer [34, 36–38]. Our results demonstrate that African Americans express significantly higher levels of SULT2B1b in epithelial cells of normal cancer-associated post-menopausal breast tissue than Caucasians (Fig. 4) while there is no difference in SULT2B1b expression in cancerous tissues between the races (Fig. 4). Higher DHEA sulfation activity in the epithelial cells will inhibit both the activity of DHEA as well as its and its conversion to other estrogens. The intracrinology of DHEA metabolism in the post-menopausal breast epithelial cell may be more important in the transformation of the epithelial cell than the low circulating levels of estrogens. Thus, the lower levels of free DHEA in breast epithelial cells of African Americans presents a possible explanation as to why African Americans have a lower incidence of post-menopausal breast cancer than Caucasians.
Although DHEA can be converted to estrogens, DHEA has been reported to alter the expression of estrogen receptors in hormone-responsive cells that have lower levels of SULT2B1b activity. He et al. [25] reported that the siRNA-mediated inhibition of SULT2B1b was important in the androgen and possibly estrogen regulation of human prostate cancer LNCaP cells. After inhibition of SULT2B1b activity in human LNCaP prostate cancer cells with siRNA, exposure of these cells to nanomolar concentrations of DHEA or androstenediol was associated with a decrease in levels of ER-β expression and an increase in the growth rate of the LNCaP cells. Thus, the concept that the presence of SULT2B1b in estrogen-responsive tissues may play a role in their responsiveness to DHEA and androstenediol is supported by the results in prostate cancer cells.
The present study demonstrates that SULT2B1b protein is expressed in both the cytoplasm and nucleus of human breast tissues, although the specific function of SULT2B1b in the nucleus is unknown. Significantly, the presence of higher levels of SULT2B1b in both the nucleus and cytoplasm of epithelial cells in post-menopausal African American normal breast tissues may play a contributing role in the lower incidence of breast cancer in these women as compared to that of Caucasians. The metabolism of DHEA in epithelial cells in normal breast tissue may influence the occurrence of post-menopausal breast cancer. However, the levels of SULT2B1b expression in the transformed breast cancer tissue are apparently secondary to the loss of growth regulation. The high levels of SULT2B1b in the breast adenocarcinoma tissues may be related to a loss of growth control or to an attempt to decrease the conversion of DHEA to estrogens and slow estrogen-stimulated cell growth.
ACKNOWLEDGEMENTS
The authors would like to thank Josie Falany for her helpful comments and careful reading of the manuscript, and Dr. Michael Ruppert for the β-tubulin antibody and use of the AxioCam HRc digital camera. This research was supported by NIH grant GM38953 to CNF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.ACS. Breast Cancer Facts and Figures 2005–2006. Atlanta: American Cancer Society, Inc; 2006. [Google Scholar]
- 2.Adly L, Hill D, Sherman ME, Sturgeon SR, Fears T, Mies C, Ziegler RG, Hoover RN, Schairer C. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int J Cancer. 2006;119:2402–2407. doi: 10.1002/ijc.22203. [DOI] [PubMed] [Google Scholar]
- 3.Labrie F. Dehydroepiandrosterone, androgens and the mammary gland. Gynecol Endocrinol. 2006;22:118–130. doi: 10.1080/09513590600624440. [DOI] [PubMed] [Google Scholar]
- 4.Mo Q, Lu SF, Simon NG. Dehydroepiandrosterone and its metabolites: differential effects on androgen receptor trafficking and transcriptional activity. J Steroid Biochem Mol Biol. 2006;99:50–58. doi: 10.1016/j.jsbmb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Chen F, Knecht K, Birzin E, Fisher J, Wilkinson H, Mojena M, Moreno CT, Schmidt A, Harada S, Freedman LP, Reszka AA. Direct agonist/antagonist functions of dehydroepiandrosterone. Endocrinology. 2005;146:4568–4576. doi: 10.1210/en.2005-0368. [DOI] [PubMed] [Google Scholar]
- 6.Falany CN, Wheeler J, Oh TS, Falany JL. Steroid sulfation by expressed human cytosolic sulfotransferases. J Steroid Biochem Mol Biol. 1994;48:369–375. doi: 10.1016/0960-0760(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 7.Falany CN. Enzymology of human cytosolic sulfotransferases. Faseb J. 1997;11:206–216. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- 8.Falany CN, He D, Dumas N, Frost AR, Falany JL. Human cytosolic sulfotransferase 2B1: isoform expression, tissue specificity and subcellular localization. J Steroid Biochem Mol Biol. 2006;102:214–221. doi: 10.1016/j.jsbmb.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Her C, Wood TC, Eichler EE, Mohrenweiser HW, Ramagli LS, Siciliano MJ, Weinshilboum RM. Human hydroxysteroid sulfotransferase SULT2B1: two enzymes encoded by a single chromosome 19 gene. Genomics. 1998;53:284–295. doi: 10.1006/geno.1998.5518. [DOI] [PubMed] [Google Scholar]
- 10.He D, Meloche CA, Dumas NA, Frost AR, Falany CN. Different subcellular localization of sulfotransferase 2B1b (SULT2B1b) in human placenta and prostate. Biochem J. 2004;379:533–540. doi: 10.1042/BJ20031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He D, Frost AR, Falany CN. Identification and immunohistochemical localization of Sulfotransferase 2B1b (SULT2B1b) in human lung. Biochim Biophys Acta. 2005;1724:119–126. doi: 10.1016/j.bbagen.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Higashi Y, Fuda H, Yanai H, Lee Y, Fukushige T, Kanzaki T, Strott CA. Expression of cholesterol sulfotransferase (SULT2B1b) in human skin and primary cultures of human epidermal keratinocytes. J Invest Dermatol. 2004;122:1207–1213. doi: 10.1111/j.0022-202X.2004.22416.x. [DOI] [PubMed] [Google Scholar]
- 13.Geese WJ, Raftogianis RB. Biochemical characterization and tissue distribution of human SULT2B1. Biochem Biophys Res Commun. 2001;288:280–289. doi: 10.1006/bbrc.2001.5746. [DOI] [PubMed] [Google Scholar]
- 14.Meloche CA, Falany CN. Expression and characterization of the human 3 beta-hydroxysteroid sulfotransferases (SULT2B1a and SULT2B1b) J Steroid Biochem Mol Biol. 2001;77:261–269. doi: 10.1016/s0960-0760(01)00064-4. [DOI] [PubMed] [Google Scholar]
- 15.He D, Falany CN. Characterization of proline-serine-rich carboxyl terminus in human sulfotransferase 2B1b: immunogenicity, subcellular localization, kinetic properties, and phosphorylation. Drug Metab Dispos. 2006;34:1749–1755. doi: 10.1124/dmd.106.011114. [DOI] [PubMed] [Google Scholar]
- 16.Graham J, Ford T, Rickwood D. The preparation of subcellular organelles from mouse liver in self-generated gradients of iodixanol. Anal Biochem. 1994;220:367–373. doi: 10.1006/abio.1994.1351. [DOI] [PubMed] [Google Scholar]
- 17.Provost J, Fudge J, Israelit S, Siddiqi AR, Exton JH. Tissue-specific distribution and subcellular distribution of phospholipase D in rat: evidence for distinct RhoA- and ADP-ribosylation factor (ARF)-regulated isoenzymes. Biochem. J. 1996;319:285–291. doi: 10.1042/bj3190285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falany CN, Vazquez ME, Kalb JM. Purification and characterization of human liver dehydroepiandrosterone sulphotransferase. Biochem J. 1989;260:641–646. doi: 10.1042/bj2600641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost AR, Sparks D, Grizzle WE. Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Appl Immunohistochem Mol Morphol. 2000;8:236–243. doi: 10.1097/00129039-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Talley LI, Grizzle WE, Waterbor JW, Brown D, Weiss H, Frost AR. Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in preand postmenopausal women. Int J Cancer. 2002;98:118–127. doi: 10.1002/ijc.10171. [DOI] [PubMed] [Google Scholar]
- 21.Falany JL, Falany CN. Expression of cytosolic sulfotransferases in normal mammary epithelial cells and breast cancer cell lines. Cancer Res. 1996;56:1551–1555. [PubMed] [Google Scholar]
- 22.Ji Y, Moon I, Zlatkovic J, Salavaggione OE, Thomae BA, Eckloff BW, Wieben ED, Schaid DJ, Weinshilboum RM. Human hydroxysteroid sulfotransferase SULT2B1 pharmacogenomics: gene sequence variation and functional genomics. J Pharmacol Exp Ther. 2007;322:529–540. doi: 10.1124/jpet.107.122895. [DOI] [PubMed] [Google Scholar]
- 23.Fuda H, Javitt NB, Mitamura K, Ikegawa S, Strott CA. Oxysterols are substrates for cholesterol sulfotransferase. J Lipid Res. 2007;48:1343–1352. doi: 10.1194/jlr.M700018-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Meloche CA. Pharmacology. Birmingham: University of Alabama at Birmingham; 2003. The Activity and Expression of the Sulfotransferase 2B1 Isoforms in Human Prostate, Placenta, Breast and Skin. Editor^Editors. [Google Scholar]
- 25.He D, Falany CN. Inhibition of SULT2B1b expression alters effects of 3beta-hydroxysteroids on cell proliferation and steroid hormone receptor expression in human LNCaP prostate cancer cells. Prostate. 2007;67:1318–1329. doi: 10.1002/pros.20615. [DOI] [PubMed] [Google Scholar]
- 26.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 27.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 28.Helzlsouer KJ, Gordon GB, Alberg AJ, Bush TL, Comstock GW. Relationship of prediagnostic serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing premenopausal breast cancer. Cancer Res. 1992;52:1–4. [PubMed] [Google Scholar]
- 29.Pasqualini JR. Role of androgens in breast cancer. J Steroid Biochem Mol Biol. 1993;45:167–172. doi: 10.1016/0960-0760(93)90137-l. [DOI] [PubMed] [Google Scholar]
- 30.Zumoff B, Levin J, Rosenfeld RS, Markham M, Strain GW, Fukushima DK. Abnormal 24-hr mean plasma concentrations of dehydroisoandrosterone and dehydroisoandrosterone sulfate in women with primary operable breast cancer. Cancer Res. 1981;41:3360–3363. [PubMed] [Google Scholar]
- 31.Gordon GB, Bush TL, Helzlsouer KJ, Miller SR, Comstock GW. Relationship of serum levels of dehydroepiandrosterone and dehydroepiandrosterone sulfate to the risk of developing postmenopausal breast cancer. Cancer Res. 1990;50:3859–3862. [PubMed] [Google Scholar]
- 32.Johnson MD, Bebb RA, Sirrs SM. Uses of DHEA in aging and other disease states. Ageing Res Rev. 2002;1:29–41. doi: 10.1016/s0047-6374(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 33.Mehrotra J, Ganpat MM, Kanaan Y, Fackler MJ, McVeigh M, Lahti-Domenici J, Polyak K, Argani P, Naab T, Garrett E, Parmigiani G, Broome C, Sukumar S. Estrogen receptor/progesterone receptor-negative breast cancers of young African- American women have a higher frequency of methylation of multiple genes than those of Caucasian women. Clin Cancer Res. 2004;10:2052–2057. doi: 10.1158/1078-0432.ccr-03-0514. [DOI] [PubMed] [Google Scholar]
- 34.Ademuyiwa FO, Olopade OI. Racial differences in genetic factors associated with breast cancer. Cancer Metastasis Rev. 2003;22:47–53. doi: 10.1023/a:1022259901319. [DOI] [PubMed] [Google Scholar]
- 35.De Jesus MA, Fujita M, Kim KS, Goldson AL. Retrospective analysis of breast cancer among young African American females. Breast Cancer Res Treat. 2003;78:81–87. doi: 10.1023/a:1022161629156. [DOI] [PubMed] [Google Scholar]
- 36.Trock BJ. Breast cancer in African American women: epidemiology and tumor biology. Breast Cancer Res Treat. 1996;40:11–24. doi: 10.1007/BF01805999. [DOI] [PubMed] [Google Scholar]
- 37.Gray GE, Henderson BE, Pike MC. Changing ratio of breast cancer incidence rates with age of black females compared with white females in the United States. J Natl Cancer Inst. 1980;64:461–463. [PubMed] [Google Scholar]
- 38.Marbella AM, Layde PM. Racial trends in age-specific breast cancer mortality rates in US women. Am J Public Health. 2001;91:118–121. doi: 10.2105/ajph.91.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]