Abstract
Sleep is vital to cognitive performance, productivity, health and well-being. Earlier theories of sleep presumed that sleep occurred at the level of the whole organism and that sleep was governed by central control mechanisms. However, evidence now indicates that sleep might be regulated at a more local level within the brain: it seems to be a fundamental property of neuronal networks and is dependent on prior activity within each network. Such local network sleep might be initiated by metabolically driven changes in the production of sleep-regulatory substances. We discuss a mathematical model which illustrates that the sleep-like states of individual cortical columns can be synchronized through humoral and electrical connections, and that whole organism sleep occurs as an emergent property of local network interactions.
I. INTRODUCTION
The scientific community has yet to reach consensus as to exactly what it is, in body and brain, that sleeps and why we sleep. This lack of consensus confuses our understanding and confounds discussions of sleep. How can we explain the regulation of sleep if we do not know, nor have testable theories of, how the brain is organized to produce sleep and exactly what sleeps? Traditionally, sleep has been considered a property of the whole organism: an animal was either awake or drowsy or asleep. This idea was derived in part from the observation that sleep is homeostatic: prolonged wakefulness is followed by rebound sleep. Indeed, there are global ways to induce sleep-like states in animals via brain stimulation1 or induction of global metabolic2 or neurochemical3 changes in the body (Box 1). Within the past few years however, evidence has emerged that supports a new theory of sleep: sleep is local and sleep is use-dependent.4,5 According to this theory, the homeostatic regulation of sleep is not at the level of the whole organism but occurs in any brain region in response to its use.
In this Review, we present a comprehensive mechanistic view of the brain organization of sleep. First, we will discuss the recent evidence that directly demonstrates that a sleep-like state can exist in cortical columns,6 show that such local sleep-like states depend on the prior activity of the columns, and discuss the role of sleep-regulatory substances (SRS) in the regulation of local sleep. We then present a hypothesis that mechanistically links metabolism and brain activity to local sleep regulation and organization. We also discuss a quantitative model of how synchrony of cortical column states within cortical regions is achieved and how it enables the emergence of whole-organism sleep. Finally, the ramifications and manifestations of our theory for sleep function and consciousness are briefly presented.
II. SLEEP IS A LOCAL PROCESS
In this section, we present evidence that sleep is a spontaneous property of neuronal networks. Clinical evidence suggests that sleep can be a property of something less than the whole brain. Mahowald and Schenck7 describe patients with parasomnias, e.g. sleep walking, and propose that these individuals are awake (evidenced by their ability to negotiate around objects), and asleep (indicated by their lack of awareness of their actions) simultaneously. The pathology of strokes and other brain lesions indicates that if a human or animal survives acute brain damage for more than a few days, sleep reemerges – despite millions of cases, we know of no instance of complete insomnia following survivable acute brain lesions. This finding suggests that sleep is a robust fundamental self-organizing property of any surviving group of neurons.
Naturally occurring patterns of normal sleep also suggest that parts of the brain can be awake while other parts are asleep. For example, dolphins do not exhibit high-amplitude electroencephalogram (EEG) delta waves simultaneously in both cerebral hemispheres.8 High-amplitude EEG delta waves are a defining characteristic of non-rapid eye movement sleep (NREMS) (Box 1). Other species of whales, other marine mammals such as seals, and birds also exhibit unihemispheric sleep.9 In humans, as NREMS epochs unfold, there is more intense EEG delta-wave power in the frontal cortex earlier than in more posterior cortical areas, suggesting differential sleep intensities in different brain regions.10 Similarly, measurements of cerebral blood flow show regional differences during NREMS and rapid eye movement sleep (REMS).11 Collectively, these data suggest that sleep, or at least correlates of sleep or sleep intensity such as EEG delta-wave power and blood flow, are regional properties of brain tissue.
In cats, if cortical islands are prepared surgically such that they retain their blood flow but not their thalamic inputs, the local field potentials from those islands wax and wane over periods of 10–20 minutes through episodes with high-amplitude EEG delta waves.12 More recent data also indicate that there is an EEG slow-wave component (0.5-1.5 Hz) that originates locally within the cortex.13 Such findings indicate that high-amplitude EEG delta waves are a local property of cortical tissue. At the cellular level of analysis in monkeys that are at the transition between wakefulness and sleep, some neurons in the visual cortex display the characteristic sleep patterns of hyperpolarization followed by bursting action potentials even when the animal is performing a visual discrimination task.14 The coordination of such firing patterns contributes to the sleep phenotype of EEG delta-wave power.13 Although the animal is awake, the cellular processes characteristic of NREMS are evident, suggesting that there are local islands of sleep. Thus, we infer that these islands can integrate sleep, waking and vigilance transition states locally.
Cortical columns are anatomically well-defined examples of neuronal networks and are also called neuronal assemblies; they are thought to be a basic processing unit of the waking brain.15 Direct evidence indicates that cortical columns oscillate between functional states as defined by changing input-output relationships: if rat cortical columns are probed using afferent sensory stimulation and the subsequent amplitudes of the induced evoked response potentials are measured, wake-like and sleep-like states can be distinguished.6 The “sleep-like” state is characterized by evoked response potentials of greater magnitude than evoked response potentials that occur during waking. During whole-animal sleep, most of the cortical columns are in this sleep-like state. Conversely, when an animal is awake, most of the cortical columns are in an “awake-like” state, characterized by low-amplitude evoked response potentials. However, the findings that columns in a sleep-like state can occur during whole-animal wake episodes and conversely, that column wake-like states can occur during whole-animal sleep, suggest that sleep is a property of individual cortical columns.6
The cortical column sleep-like state, like whole-animal sleep, is predictable and homeostatically regulated: the probability of finding a column in the sleep-like state is dependent upon the length of time that the column has spent in the awake-like state. In other words, the longer a column is in an awake-like state, the higher the likelihood that it will make the transition to the sleep-like state.6 Such changes also manifest in behavioral consequences and thereby can be defined as functional state changes. Thus, rats that are subjected to a conditioned learning paradigm in which they are trained to lick in response to stimulation of a single facial whisker, show greater incidence of errors of commission (licking when the whisker is not stimulated) and omission (failure to lick in response to whisker stimulation) if the cortical column that receives the whisker input is in the sleep-like state.16 Thus, cortical columns might be the minimal unit of brain that can manifest a sleep-like state.4,6 It is possible that individual cells sleep independently of other cells, but this will be difficult to demonstrate because we currently cannot link electrical or metabolic activity of individual brain cells and sleep, despite the fact that there are numerous examples in the literature of correlations between single-neuron activity and sleep.13,14
In summary, the data reviewed in this section indicate that sleep can be a property of local neuronal assemblies. Even though there are central global coordinators of sleep-waking states, such as the clock mechanisms of the suprachiasmatic nuclei,17 we suggest that global coordination of NREMS is not due to a single sleep generator, but may largely reflect an emergent property of loosely coupled local processes. This may help to explain the common observation in human sleep laboratories that EEG indices of NREMS do not always correspond to the subjective experience of sleep quality. For instance, at times some patients report they have barely had any sleep, while EEG indicators suggest that is not true.
III. SLEEP DEPENDS ON PAST ACTIVITY
If sleep occurs locally in cortical columns, then what determines whether a cortical column is in a sleep-like or an awake-like state? Accumulating evidence suggests that brain areas enter a sleep-like state after a prolonged period of activity. For example, EEG delta-wave power is enhanced in the left somatosensory cortex compared to the right during the first NREMS episode after prolonged right hand stimulation prior to sleep onset in humans.18 Within the past 14 years many other studies have supported these findings. In mice, rats, chickens, pigeons, humans and cats, if a localized area is disproportionately stimulated during waking, EEG delta power is enhanced in the stimulated areas during subsequent NREMS.19–25 Conversely, if afferent activity to a localized area is reduced during waking, EEG delta power is reduced.26 There are also several findings showing that during sleep cerebral blood flow is targeted to, and enhanced in, areas that were disproportionately stimulated during prior waking.27,28 Finally, findings from the developmental plasticity literature29–31 indicate that changes in the sleep EEG are targeted to areas that were activated during prior waking. In summary, sleep intensity, a characteristic of sleep that is determined from EEG delta-wave power, is dependent upon prior use and is enhanced in areas that were disproportionately used during prior wakefulness.
Measurements of cortical-column activity also support the conclusion that sleep-like states depend on prior activity of the cortical column. In rats, afferent activity induced by twitching a facial whisker promotes the cortical column sleep-like state determined from the amplitudes of evoked response potentials as described in the previous section32. If one column is stimulated at twice the rate as another there is a higher probability that it will enter the sleep-like state.32 In summary, these data provide support for the idea that state oscillations in cortical columns are dependent on the amount of afferent activity. Sleep at this level of organization is auto-regulatory in the sense that activity within the network determines the probability of the network entering the sleep-like state.
IV. ROLE OF SLEEP-REGULATORY SUBSTANCES
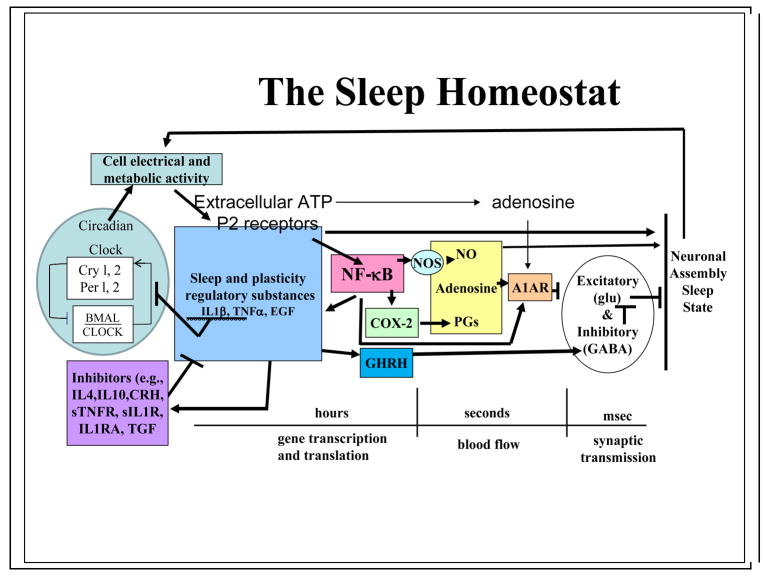
We have known for almost 100 years that cerebrospinal fluid contains substances that accumulate during wakefulness and, when transferred to the cerebrospinal fluid of recipient animals, elicit sleep.33 Many substances have now been implicated in sleep regulation. However, because all physiological variables vary concurrently with sleep it is not possible to isolate sleep as the independent variable. For that reason, investigators have developed lists of criteria that a substance should fulfill before it can be proposed that such a substance is a SRS.34–37 Briefly, a SRS should enhance sleep; if inhibited, it should reduce spontaneous sleep and sleep rebound after sleep loss; its brain levels should vary with sleep/wake history; it should act on sleep-regulatory circuits such as the anterior hypothalamus; and its levels should be altered in pathological states that are associated with enhanced sleepiness.33 There is consensus that adenosine, nitric oxide (NO), prostaglandin D2, tumor necrosis factor (TNF), interleukin-1 (IL1), and growth hormone releasing hormone (GHRH) are involved in the regulation of NREMS duration and intensity; each has met all or most of these criteria.33 These substances work in the biochemical cascades that form the NREMS homeostat (Figure 1). For instance, IL1 and TNF induce each other’s production and activate nuclear factor kappa B (NFkB), which in turn induces the production of NO and adenosine. There are separate substances that regulate rapid eye movement sleep (REMS) and waking, although if you affect duration of one state, other sleep-waking states are necessarily also affected. REMS-promoting substances (such as vasoactive intestinal polypeptide, prolactin) and wake-promoting substances (such as orexin, ghrelin, adrenocorticotropin hormone, and corticotropin releasing hormone) are not discussed in this article because the evidence from SRS studies that has bearing on sleep as a use-dependent local process is limited to NREMS-promoting SRSs.
Figure 1. Molecular networks of sleep-regulatory substances make up the NREMS homeostat.
The sleep-regulatory substances (SRSs) illustrated are produced in response to cellular activity and metabolism. These SRSs, as well as others not illustrated, are influenced by positive- and negative-feedback signals including transcription-enhancer substances such as nuclear factor kappa B (NFkB).33 The fruit fly homolog of NFkB, relish, is implicated in state control of those insects.71 The transcription and translation events that are needed to produce the proteins illustrated in this figure occur over periods of hours or longer. As such they offer a mechanism by which the brain can track its sleep/wake history. SRSs with direct sleep-promoting actions are labile substances with short half lives such as nitric oxide (NO) and adenosine. In the two-process model of sleep34 it is the output of this homeostat, as estimated from electroencephalogram (EEG) delta-wave power, that is modeled as process S. Several of the SRSs enhance EEG delta-wave power, e.g. interleukin-1 (IL1), tumor necrosis factor (TNF), and adenosine. A variety of experimental manipulations affects the homeostat and sleep in predictable ways. For instance, acute mild increases in ambient temperature enhance sleep duration and TNF levels.97 Infection enhances sleep duration and increases the concentration in the brain of almost every SRS.98 Excessive food intake enhances brain IL1 levels and sleep duration. Sleep loss increases levels of IL1, TNF, and adenosine33, whereas administration of antagonists of these substances before sleep deprivation attenuates the expected non-rapid eye movement sleep (NREMS) rebound.33 We propose that this biochemical network operates within cortical columns and other neuronal assemblies to affect state. Many of these substances also promote whole-organism sleep if they are applied directly to sleep-regulatory networks in subcortical networks33, suggesting that the homeostat operates at every level of the neural axis. Such findings also suggest that there is a high degree of evolutionary conservation of this fundamental mechanism.
SRSs act on neuronal networks
Several SRSs act on sub-cortical sleep-regulatory circuits.33 Thus, adenosine acts on basal forebrain neurons to promote sleep.38GHRH, TNF and IL1 act directly on hypothalamic preoptic neurons to promote NREMS.33 In fact, inhibition of either GHRH or TNF by microinjection of GHRH or TNF inhibitors into the preoptic hypothalamus inhibits NREMS.33 TNF and IL1 also act on the locus coeruleus39 and IL1 acts on raphe serotonergic neurons to promote sleep.40 IL1 acts in the hypothalamus on GHRH-receptive GABAergic neurons.41 SRSs also act locally in the cortex to enhance sleep phenotypes. If either IL142 or TNF43 or GHRH44 is applied to the cortex unilaterally, EEG delta-wave power during NREMS, but not during waking or REMS, is enhanced ipsilaterally for several hours. If inhibitors of either IL1 or TNF are applied unilaterally to the cortex the enhanced EEG delta-wave power that occurs during NREMS after sleep deprivation is attenuated ipsilaterally. Similarly, if the production of TNF is inhibited unilaterally by the microinjection of a TNF small-inhibitory RNA onto the surface of the cortex, EEG delta-wave power is inhibited ipsilaterally for days.45 At the cortical-column level of analysis, similar microinjections of TNF onto the cortex increase the probability of a cortical column being in the sleep-like state for 1–2 hours.46 These results are consistent with the view that SRSs act both on sleep-regulatory circuits and locally within the cortex and brainstem to promote sleep.
It is clear that SRSs act locally to change the electrical property of neurons and thereby alter their input-output relationships. The direct evidence for this statement was already mentioned; the application of TNF to the surface of the cortex enhances the probability of cortical-column sleep.46 Moreover, evidence from several independent literatures shows that SRSs such as cytokines and neurotrophins, alter network properties.47 For example, IL1 enhances presynaptic GABA inhibition of hypothalamic glutamatergic transmission.48 IL1 also enhances hypothalamic sleep-active neurons and inhibits wake-active neurons.49 From the fever literature, we know that IL1 and TNF alter hypothalamic neuron sensitivity to temperature.50 From another literature we know that IL1 receptors co-localize with GHRH receptors on GABAergic hypothalamic neurons.41 For other SRSs such as the neurotrophins, it is well known that they alter electrical properties of neurons.51 There is also a separate literature demonstrating that SRSs such as TNF alter K+ channels in neurons,52,53 glia54,55 and other cell types.56 Whether such actions are related directly to sleep remains unknown. The studies cited suggest multiple routes by which SRSs can alter network responsiveness in both the short- and long-term.
NREMS is also influenced by activity of sub-cortical sleep-regulatory circuits.57 These sleep-regulatory circuits are kept informed of the status of cortical-column states. For example, application of either TNF or IL1 onto the cerebral cortex activates reticular thalamic neurons as determined by Fos expression.58,59 IL1 applied in this manner also activates prefrontal cortical neurons, ventral lateral preoptic neurons, and medial preoptic neurons.58 It seems likely that these sleep-regulatory circuits integrate information that is related to locally-induced cortical-column state with information that is important for the determination of whole animal sleep-waking states, such as time of day, sensory input, mental activity, emotion and current pathology.
Neuronal activity increases SRS levels
The production of every well-characterized NREMS SRS is enhanced by neuronal activity. For example, if activity is experimentally enhanced by epileptic episodes, levels of IL1 and TNF in the brain increase33. Neuronal expression of TNF and IL1 are enhanced in response to afferent activity. For instance, excessive stimulation of rat facial whiskers for 2 hours enhances IL1 and TNF immunoreactivity in cortical layers II-IV of columns in the somatosensory cortex that receive the enhanced afferent input.46,60 Glutamate, an excitatory neurotransmitter, enhances TNF production in cultured neurons.61 Whisker stimulation also enhances cortical expression of GHRH receptors.62 Adenosine and NO are produced locally in response to neuronal activity; both seem to be involved in local-sleep intensity.63 Caffeine, which is an adenosine-receptor antagonist, and inhibition of nitric-oxide synthase, the enzyme involved in the production of NO, reduces EEG delta-wave power.64,65 Collectively, such data demonstrate that in the brain several SRSs, that can operate locally, respond to cellular activity.
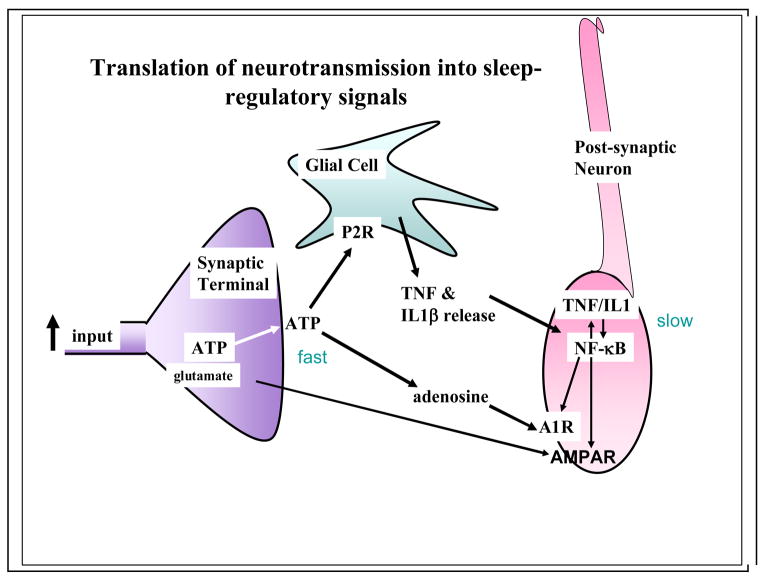
What is it about wakefulness and neuronal activity that causes the enhanced SRS activity? Neuronal activity manifests in pre- and post-synaptic events that act in both the short and long term. Neuronal activity in the pre-synaptic neuron results in the release of transmitters and ATP.66,67 ATP in turn does two things (Figure 2). Some of it is converted to adenosine and some ATP acts on purine P2X7 receptors on glia to release TNF and IL168–70. After conversion from ATP, adenosine interacts with neurons via the adenosine A1 receptor (A1AR); this action results in hyperpolarization via K+ channels.33,38 The ATP-released TNF also interacts with the post-synaptic and pre-synaptic neurons to activate NFkB.33,71 NFkB enhances the A1AR thereby making the cell more sensitive to adenosine.72 NFkB also enhances gluR1 mRNA production, a subunit of the AMPA receptor.73 The increased expression of AMPA receptors renders the post-synaptic neuron more sensitive to glutamate (up-scaling).74 The increases in receptor mRNA have a much longer time course than the direct actions of adenosine or TNF; the subsequent production of receptor protein offers a way for the brain to keep track of past neuronal network activity and translate that activity into a greater propensity for sleep or wakefulness. The differences in the time courses of action of the neurotransmitters (milliseconds), the conversions of ATP to adenosine and its actions (seconds) and the actions of ATP-induced release of cytokines and their subsequent actions on gene transcription and translation (minutes to hours) likely form a mechanism for activity-dependent oscillations of neuronal assembly sleep (Figure 1).
Figure 2. Translation of neurotransmission into sleep-regulatory substances.
The mechanism for the translation of synaptic activity into signals that act as sleep regulatory substances such as tumor necrosis factor alpha (TNF) and interleukin-1 beta (IL1), may involve ATP-enhanced release of these cytokines from glia. Thus, within the brain68–70 and the immune system99 ATP induces the release of cytokines via purine P2 receptors. During neurotransmission, ATP is released from presynaptic neurons.67 This extracellular ATP acts rapidly via its breakdown into adenosine on postsynaptic purine P1 receptors (the adenosine A1 receptor-A1R). The ATP-P2R-released TNF and IL1 act more slowly on postsynaptic neurons via activation of nuclear factor kappa B (NFkB) and its promotion of receptors such as the A1R and the glutamate AMPA receptor to change sensitivity of the postsynaptic neuron over longer periods (see Figure 3 and Box 3).
Adenosine and NO are cerebral vasodilators as well as SRSs. Their half lives are very short (seconds) and they probably are the immediate effector molecules for longer-lived vasodilators/sleep regulatory substances such as TNF and IL1.33,50 The dynamics of cerebral metabolism and blood flow are related to changes in the concentration of multiple SRSs. As electrical activity increases within a cortical column there is the potential for a parallel rise in metabolism and blood flow. However, blood flow and metabolism may reach their maxima before electrical activity does.75 As a result, intracellular ATP levels could be reduced while intracellular adenosine levels increase (although there is much more ATP than adenosine). In parallel with electrical activity, ATP in the synaptic cleft increases because it is released with neurotransmission (Figure 2). The released ATP causes the release of gliotransmitters, including cytokines, via binding to and activation of purine type 2 receptors68–70, so that levels of extracellular cytokines also follow neuronal electrical activity. Intracellular cytokine levels in post-synaptic neurons also increase with network activity46 (Figure 3). The enhanced intracellular cytokine levels persist beyond the changes in electrical activity, blood flow, ATP and adenosine, although to date the only experimentally determined point is 2 hours after afferent stimulation of a cortical column.46 These persistent increases in intracellular cytokine levels cause longer-term changes in gene expression76 and subsequent cell/network sensitivity via changes in the production of receptors that are responsive to short-lived molecules such as glutamate, NO and adenosine (Figure 1). It is thus the changes in cytokines that offer a plausible mechanism for keeping track of past wake (activity) levels of a cortical column and, ultimately, for the organism.
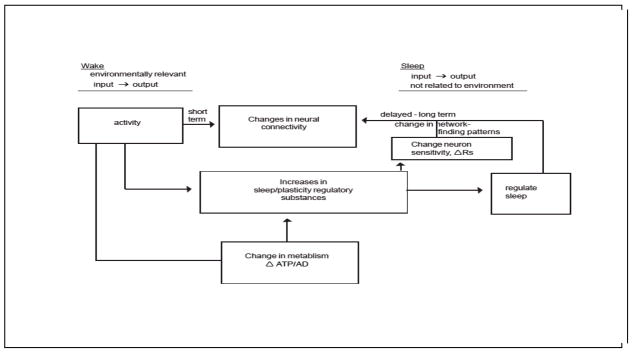
Figure 3.
Illustration of activity-driven changes in sleep regulatory substances regulate sleep at the local level. The same substances are involved in other cellular processes such as neuronal connectivity. These substances are induced by changes in ATP and adenosine and they affect sleep via their immediate actions on receptors and by longer-term actions on the synthesis of receptors. During waking environmental input to a neuronal assembly induces environmental relevant outputs. During sleep, due to the SRS-induced changes in receptor activation, the same environmental inputs result in a different output, one not directly relevant to the environment. This establishes the need for unconsciousness.
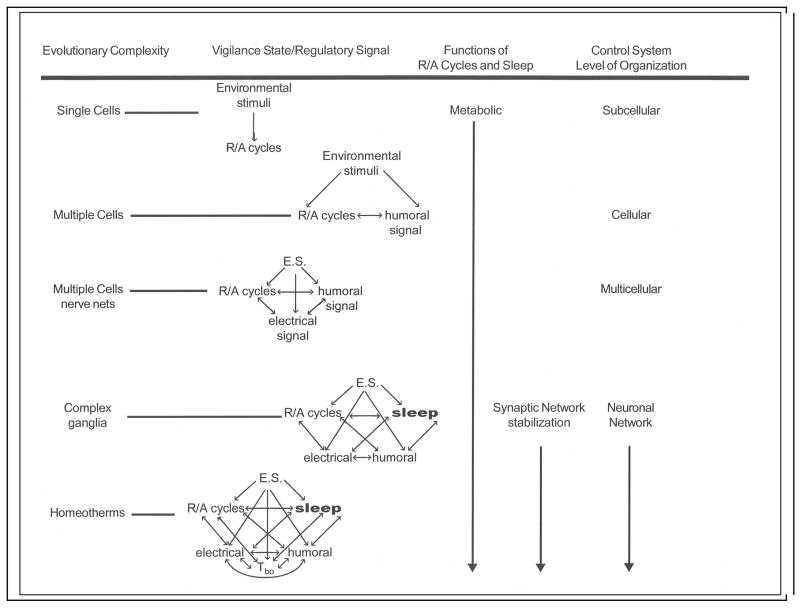
Cytokines are evolutionarily very old and likely were recruited to regulate rest-activity cycles and evolutionarily early versions of sleep-wake and metabolic cycles (Box 2). The hypothalamus and other subcortical circuits were probably undergoing activity-dependent oscillations of state even before there was a neocortex. Viewed in this way it is easy to envision that the biochemical mechanisms of SRS-regulated sub-cortical oscillations were extended to the new cortical assemblies as they appeared during evolution. Further, the new cortical assemblies were innervated by the sub-cortical networks that were already oscillating under activity-dependent auto-control. It is also possible to see how one could extend the application of this mechanism to animals such as octopi and fruit flies, whose brain organization is very different, but which have all the activity-dependent molecules in place. Indeed, the regulation of Drosophila sleep/wake cycles shares many biochemical mechanisms with those of mammals.71
Box 2 Figure.
When the cortical column goes into a sleep-like state the processes discussed in the previous paragraphs are reversed. Thus, electrical activity, blood flow, extracellular ATP and adenosine levels and intracellular adenosine levels all decrease while intracellular ATP levels increase. Some of the consequences of these changes are shown in Figures 1–3. The molecules that are responsible for changes in blood flow, adenosine, NO, TNF and IL1 are also involved in cortical-column state changes; thus, metabolism, blood flow and state are normally integrated with each other.
V. MODELING LOCAL-GLOBAL SLEEP TRANSITIONS
Thus far we have reviewed the evidence that different brain regions can exhibit different intensities of sleep simultaneously and that individual cortical columns oscillate between sleep and waking states. We also described the experimental finding that during sleep or wakefulness, most cortical columns are in the functional state that matches that of the whole animal.6 How is this achieved? In this section we briefly discuss a model that helps to explain how whole-animal sleep emerges from the interaction of many neuronal assemblies (for instance, cortical columns); this model is described in full in reference 77. We propose that synchrony of cortical-column states is a consequence of the electrical and humoral interactions that occur between columns. Naturally occurring networks that have weakly interacting components, such as cortical columns, have long been known to display emergent properties – collective behaviors that are not readily apparent from the features of the individual cortical columns - including in some cases achieving synchronization among the components and robustness to variations through the network interactions.78,79 We have introduced a plausible albeit abstract nonlinear mathematical model for interactions among cortical columns, and have shown that this particular model’s emergent properties capture well-known characteristics of sleep.
The local dynamics (the individual behavior of each neuronal assembly) of our model have some commonality with that of a standard model for a single neuron or neuronal assembly known as the Wilson-Cowan model,80 whereas the network interactions are similar in some respects to those of other network-regulation models. In our model77, the state variables change over time owing to integration of local activity, interactions with other assemblies, and threshold-based transitions. In addition to these internal variables for each assembly, the model comprises a network that describes the strengths of interactions between neuronal assemblies. Neuronal assemblies are more quickly driven towards the sleep-like state when connected neuronal assemblies have recently entered the sleep-like state. The strength of this interactive response scales with the strengths of the connections between the neuronal assemblies. Finally, if the circadian clock indicates that the organism should be asleep, the activity variable effectively increases more quickly and the state of the neuronal assembly moves more rapidly toward sleep.
With this model, in silico experiments as well as mathematical analysis demonstrate well-known characteristics of sleep as emergent properties of the network interactions; stability of the sleep/wake cycle under nominal conditions, emergence of a synchronous sleep state, and the regional activity dependence of sleep. In silico results and analysis also indicate that the over-stimulated neuronal assemblies (for instance, the impact on a rat’s sleep response of repeatedly stimulating a single whisker6 as described earlier in this Review) will enter the sleep-like state rapidly, and then (due to the coupling between assemblies) induce nearby neuronal assemblies to enter the sleep-like state, eventually leading to whole-animal sleep. We have verified that the model does indeed capture the evolution of a global sleep state, as theorized herein. As a further step, we note that the model permits us to tie emergent characteristics (for instance, the amount of asynchronicity between the columns during over-stimulation) to network properties.
VI. THEORETICAL PERSPECTIVES ON SLEEP FUNCTIONS
Sleep, as found in mammals, likely has multiple functions, although when it first evolved it probably did so for one crucial, primordial function. At the whole-animal behavioral level, sleep functions seem clear; calories are saved, performance is restored, and (in humans) affect becomes more positive as a result of sleep. Such findings have led to the universal acknowledgement that sleep restores brain function. However, at more mechanistic levels no one has yet identified what is being restored, and whether any restoring takes place at the cellular or molecular level. This evidential deficit has led many theorists to posit that optimizing neuronal connectivity is the primordial function of sleep.4,5,81–84
The functional importance of sleep is illustrated by the fact that during sleep one gives up opportunities to reproduce, eat, drink or socialize and one is subject to predation. Sleep could only have evolved despite these high evolutionary costs if it serves a crucial, primordial function. Maintaining adaptable flexible neural connectivity may be sufficiently important to the brain to allow the persistence of such a periodic disadvantaged state.4,5 The central idea in connectivity theories of sleep is the recognition that use-dependent changes in synaptic efficacy and connectivity would lead to dysfunction unless there were processes to stabilize, and thus preserve, functionally optimized synaptic networks (which are prima facie adaptive because the organism is alive) that are constantly being modified by the use-dependent-driven changes in connectivity.4,81,84 One of the processes that has been posited to be responsible for this stabilization is called ‘synaptic scaling’ in the more recent literature (Box 3).85 Synaptic scaling is a use-dependent, slow homeostatic process that occurs over hours to days.74,86 A proposed mechanism for synaptic network stabilization4 involves SRS-induced changes in local electrical properties as described above. Indeed, there is now direct evidence that TNF is involved in synaptic scaling (Box 3).74,86 Kavanau81 and Tononi and Cirelli83 proposed that intrinsic spontaneous electrical activity serves this scaling function. The various proposed scaling mechanisms, e.g. SRS-induced changes in receptor populations, transmitter release, ion channel populations, etc., are not mutually exclusive.
If we consider that sensory and internal afferent input during waking to induce an environmentally relevant neuronal network output (i.e. patterns of electrical and chemical signals from the network projecting to other brain areas and contributing to whole-animal cognitive and motor events), then after prolonged activation of neurons within a neuronal assembly, the consequent SRS release would induce changes in the network’s electrical and chemical outputs and responsiveness to inputs (Figure 2). These changes would presumably summate, perhaps with positive feedback, and result in a state shift within the local network. Sleep is thus initiated within neuronal assemblies and dependent upon prior use. The new output, because it is qualitatively and quantitatively different from the original output, may not be relevant to the environmentally driven input. Thus, if the initial environmentally-driven inputs induce adaptive outputs (waking), then a shift in input-output relationships could result in outputs (in this case sleep) that lack the necessary network activity patterns to induce environmentally relevant cognitive or motor outputs (Figure 3). This creates an adaptive need to prevent the organism from behaving and it is likely a role of the traditional sleep/wake regulatory circuits to ensure the absence of behavior at such times. Thus, not only are the mechanisms for local sleeping inseparable from the connectivity/metabolic function of sleep; they also cause the network outputs that lead to the necessity for the altered consciousness that typically pervades sleep. Previously, it was proposed by us5 and Kavanau81 that such outputs during sleep serve to stabilize the networks and thereby preserve them.
VII. CONCLUSIONS AND FUTURE DIRECTIONS
Our mechanistic proposal for the generation of sleep at the local level involves afferent input-induced SRSs and their actions on neuronal assemblies to change local network input-output relationships. This mechanism is likely a property of neuronal assemblies throughout the brain, including the evolutionary older midbrain and lower brainstem. State changes of neuronal assembly at the lower brain levels would manifest themselves differently from those at higher levels because the various levels of the brain are involved in different functions.
The viewpoint presented in this Review allows one to frame important observations in sleep research in a different context than the current dominant paradigm of sleep regulation allows. Currently, sleep is typically viewed as being imposed upon the brain by sleep regulatory circuits (Box 1). However, that paradigm does not address many well-known phenomena such as sleep inertia, restoration of peak performance during sleep, recovery and reorganization of sleep after brain lesions, the nature of sleep homeostatic mechanisms, clinical conditions such as insomnia, and sleepiness and fatigue associated with multiple bodily ailments such as rheumatoid arthritis and most autoimmune disorders and with various chemotherapies. To the extent that our newer view posits that parts of the brain can be asleep while other parts are awake, it is easier to envision explanations for these phenomena. For example, sleep inertia may be a manifestation of some neuronal assemblies remaining in the sleep-like state after sufficient numbers of them have transitioned to the wake-like state to cause behavioral awakening. By extension, the degree of sleepiness, or the speed and accuracy of performance, may be dependent upon the fraction of neuronal assemblies that are in the sleep-like state compared to the wake-like state. There is brain imaging evidence that suggests that in insomnia patients some areas of brain show activation that is characteristic of waking while simultaneously other areas show the reduced activity that is characteristic of sleep.87
The theory presented here that sleep is an emergent property of cortical columns is not yet able to address the questions of how many assemblies need to be in the sleep-like state before consciousness changes. Of note, a similar issue is present in the more traditional paradigm of sleep regulation, which proposes a top-down imposition of sleep on the brain by regulatory circuits: it does not specify the areas (and how many of them) need to acted upon by the regulatory circuits to produce sleep. Experimentally it will remain difficult to investigate the ratio of awake-like to sleep-like assemblies that defines global sleep, as one would have to record from thousands of individual assemblies. However, we anticipate that our mathematical model will, with future developments, give some indication of system behaviour, e.g. fraction of assemblies that is needed for a rapid phase transition. Thus, although we are far from any comprehensive molecular or genetic understanding of sleep or the mechanistic details of local control of sleep, the present view provides a new evolutionary conceptual structure for continued discoveries in the field.
BOX 1: SLEEP
Sleep sustains physical and cognitive performance, productivity, health and well-being; even mild sleep restriction degrades performance over a few days. However, it is difficult to define sleep as there are no direct measures of sleep. Instead, sleep is inferred from a variety of measures including electroencephalograms, electromyograms, brain temperature, behaviour, posture and heart rate. Using those measures one characterizes epochs (usually less than one minute) as being in a particular state and then determines parameters, such as EEG power, that correlate with the state.
In almost all mammals there are two forms of sleep, non-rapid eye movement sleep (NREMS) and rapid eye movement sleep (REMS). In humans, NREMS occupies about 80% and REMS about 20% of sleep time. These two stages alternate with each other with about a 90–100 minute periodicity, with more NREMS occurring during the first half and more REMS occurring during the second half of the night. Despite this complex architecture, sustaining performance during waking is dependent on total sleep time. Central sleep-regulatory circuits appear to consolidate sleep into 1–2 daily bouts in humans. Circuits that regulate NREMS include the preoptic anterior hypothalamus, within which are found the ventrolateral preopic and the median preoptic areas, and the basal forebrain.57 REMS and the alternation between NREMS and REMS are also under central control. The areas responsible for these functions include the pedunculopontine tegmental and laterodorsal tegmental nuclei. There are also multiple wake-promoting networks, including the hypocretin/orexin system in the lateral hypothalamus, whose degeneration is responsible for the clinical condition of narcolepsy.
The currently dominant paradigm in sleep research views whole organism sleep as being initiated and regulated centrally by the interactions of specialized sleep-and wake-promoting neuronal networks.1,13,17,57 Notwithstanding this central regulatory control, it is the position of this Review that sleep is initiated locally as a consequence of use, and is only then consolidated by central mechanisms.
BOX 2: THE EVOLUTION OF SLEEP IS LINKED TO METABOLISM
There is no evidence that sleep serves distinctly different functions in different species. Indeed, studies in fruit flies have shown that at a biochemical level the substances that regulate fruit-fly sleep are the same as those regulating mammalian sleep, e.g. adenosine, NFkB and epidermal growth factor. The most parsimonious treatment, at least for now, is to consider sleep as a universal process. How might sleep have evolved? Temperature and light rhythms associated with the Earth’s rotation likely induced early cells to time the production of metabolic enzymes to the availability and energetic ease of processing nutrients (Box figure). With the sleep/wake cycle, the brain anticipates the earth’s orbital mechanics. This would have required chemical signaling within cells that later in evolution were likely used to signal other cells of their metabolic states. As multi-cellular organisms evolved, humoral signaling became more complex and was used to coordinate whole-animal physiology. The humoral signals themselves began to be influenced by rest-activity cycles as well as by environmental cues. Electrical signaling resulting from humoral signals occurred in single cells, although their use in higher-order information processing only truly came of age with the development of ganglia and nervous systems. As biochemical and neural complexity evolved to include specialization of neurons and epigenetic plasticity, the levels of organization at which the control systems operated also expanded. The evolutionary advantage of complex information processing is obvious. The flexible connectivity between neurons is experience-dependent.4,81,88 Such developments therefore required a mechanism, i.e. sleep, to insure the stability of synaptic networks that encode instinctual and learned memories.4,81,89 It seems probable that sleep developed from the rest portion of the behavioral rest-activity cycle, because during rest, niche-appropriate inactivity already existed. The regulatory mechanisms for sleep also likely developed, in part, from those that that already regulated rest. Over the past few years ample evidence has been reported that supports the notion that the genes involved in generating the circadian rhythm directly affect sleep.17 Sleep itself directly adds to the regulatory complexity by affecting rest/activity cycles, humoral signals, cellular electrical potentials, responses to environmental stimuli and every physiological system including metabolic regulation.90
BOX 3: SLEEP REGULATORY SUBSTANCES ARE INVOLVED IN SYNAPTIC SCALING
Sleep-regulatory substances seem to be involved in synaptic scaling (the up- or down-regulation of the strength of synapses within a neuron). Synaptic scaling is a slow homeostatic process that occurs over hours to days; it serves in part to balance, or scale, Hebbian plasticity changes that occur rapidly as a consequence of neuronal activity. Both Hebbian changes in synaptic weights and synaptic scaling can increase or decrease synaptic efficacy; the direction depends upon the nature of prior activity. The sleep-regulatory substance tumor necrosis factor alpha (TNF) promotes AMPA-receptor expression and enhances cytosolic Ca++ levels.73,91 An inhibitor of TNF inhibits AMPA-induced postsynaptic potentials92 and AMPA-induced changes in cytosolic Ca++91, suggesting that this action is physiological. If a TNF small interfering (si)RNA is applied to the cortex, levels of gluR1 mRNA are inhibited;45 gluR1 is a subunit of the AMPA receptor, and AMPA receptors influence EEG synchronization93 and synaptic plasticity.94 There is direct evidence for the involvement of TNF in synaptic up-scaling in cortical layers II-IV.74,86 IL1, another sleep-regulatory substance, might also affect AMPA-receptor expression.95 In cortical layer V, AMPA receptors are involved in downscaling during NREMS.96 The direction of scaling during sleep is probably specific to local brain areas and dependent on the nature of prior activity. Regardless, such data suggest a sleep-regulatory-substance-dependent mechanism for the reconfiguration of synaptic weights during NREMS. This may represent the first experimentally supported reductionist function of sleep.30
AT-A-GLANCE SUMMARY
Sleep is a fundamental property of neuronal assemblies such as coticla columns
Sleep is use-dependent
The mechanisms of neural assembly sleep include enhanced activity of sleep-regulatory substances, induced by neuronal use-enhanced metabolism
A mathematical model of loosely connected neuronal assemblies shows that they sybnshronyze their sleep-like/awake-like states
Sleep likely evolved from a metabolically quiescent rest state
Sleep seems to have a function of stabilization of instinctual and learned memories
Acknowledgments
This work was supported by the W.M. Keck Foundation, the National Institutes of Health (Grant No’s NS 25378, NS 31453, MH 71830), the U.S. Army Research Development and Material Command, the National Science Foundation, and the National Aeronautics and Space Administration.
GLOSSARY TERMS
- Clock mechanisms of the suprachiasmatic nuclei
In mammals the suprachiasmatic nuclei of the hypothalamus house the cellular and molecular mechanisms responsible for circadian rhythms. If the suprachiasmatic nuclei are lesioned circadian rhythms entrained by light are lost. In the absence of light/dark cues, animals wil exhibit rhythms that approximate 24 hours based on the free-running molecular oscillators within the suprachiasmatic cells
- Cortical columns
Cortical columns are collections of highly interconnected neurons that often focus on particular tasks, e.g. in rats somatosensory cortical columns receive input from individual facial whiskers. Columns are layered structures with individual layers concerned with different functions such as receipt of afferent input and column output. Cells within a column are much more likely to be connected to other cells in the same column, rather than to cells of another column. This creates a functional unit. In humans, each column contains 1000 to 10,000 cells and there are about 100,000 cortical columns
- Delta wave power
This term refers to the calculated power (uV2) obtained from Fast Fourier Transformations of the electroencephalogram. Delta waves are from ½–3.5 Hz. Delta wave power increases during sleep after sleep loss and is considered an indicator of NREMS intensity. Delta waves are generated by the synchronous and cyclical alteration of cortical cells between a hyperpolarized and depolarized state. While this rhythm is present in isolated cortical slices, similar rhythms occur in the thalamus that may drive the cortical cells into alternating hyperpolarized and depolarized states
- Evoked response potentials
These are the field potentials measured from electrodes on the surface of the cerebral cortex or scalp that occur in response to sensory afferent stimulation. Synchronous synaptic activation of many cells within a cortical column generate an electrical potential gradient that can be detected by the electrodes. By using multiple electrodes and/or discrete sensory stimuli, these potentials can be localized to individual cortical columns
- Hebbian plasticity
This term refers to the type of rapid (min-hours) changes in neuronal connectivity associated with use. It is derived from the work of Donald Hebb, a Canadian psychologist who first posited in 1949 that neurons that fire together tend to wire together
- Non-rapid eye movement sleep
This type of sleep is characterized by high amplitude (up to 500 uV) electroencephalographic slow waves (delta waves). In humans, it is often divided into 4 stages, the first two characterized less by delta waves than by a transition from wake fast activity to slower electroencephalographic activity. In intermediate stages of NREMS, thalamic cells produce 8 to 12 Hz bursts known as spindles which also characterize lighter stages of NREMS. (See Box 1)
- Parasomnias
These are states in which behavioral or physical phenomena characteristic of waking occur mostly during sleep. Sleep-walking is a well-known example of a parasomnia
- Rapid eye movement sleep
This state is characterized by a relatively fast wave form electroencephalogram and a flat electromyogram in postural muscles. This stage is also well known for the vivid dreams that can be remembered
- Sleep active neurons
These are neurons whose activity (action potentials) increases during sleep or just before sleep onset
- Wake-active neurons
These are neurons whose activity increases during wakefulness or just before wakefulness onset
Biographies
James M. Krueger, PhD, is a Regents Professor of Neurobiology at Washington State University. His laboratory is focused on the biochemical regulation of sleep, the role sleep plays in the acute phase response to inflammatory challenge and the brain organization of sleep. His accomplishments include studies that: a) implicate cytokines and the somatotropic axis in sleep regulation; b) demonstrate that brain cytokine levels change with physiology; c) show that sleep changes over the course of an infection; d) provide a testable theory of brain organization of sleep and sleep function.
David M. Rector, PhD, is an Associate Professor of Neurobiology at Washington State University. His interests focus on using light in biological measurements. He developed an implantable video system for imaging scattered light changes in neural tissue from freely behaving animals. He also developed high speed electronic equipment for imaging scattered light changes from neural tissue and is currently developing high speed imaging devices to use scattered light in neural imaging. He also studies sensory processing during sleep using both optical and electrical mapping techniques.
Sandip Roy, PhD, is an Assistant Professor in the School of Electrical Engineering and Computer Science at Washington State University. His research is focused on modeling and control of network dynamics, and has application in such domains as autonomous-vehicle control, air traffic management, and virus-spreading control.
Hans P.A. Van Dongen, PhD, is Associate Research Professor in the Sleep and Performance Research Center at Washington State University. His research foci include the effects of sleep loss on neurobehavioral function and sleep architecture, and particularly individual differences therein, as well as new approaches to biomathematical modeling of fatigue and performance.
Gregory Belenky, MD, is Research Professor and Director, Sleep and Performance Research Center, Washington State University. Previously he was Director of the Division of Neuroscience at the Walter Reed Army Institute of Research and Professor in the Department of Psychiatry of the Uniformed Services University of the Health Sciences. Dr. Belenky’s research includes sleep, sleep deprivation, sleep restriction and their effects on human performance and informs the development of the emerging science of fatigue risk management.
Jaak Panksepp, PhD, is the Baily Endowed Chair of Animal Well-Being Science at Washington State University. His work is devoted to the analysis of the neuroanatomical and neurochemical mechanisms of instinctual emotional and motivational behaviors in the emerging fields of affective neuroscience with a focus on understanding how various affective processes are evolutionarily organized in the brain. His early work was devoted to analyzing energy balance and brain mechanisms of sleep.
References
- 1.Koella WP. The organization and regulation of sleep. A review of the experimental evidence and a novel integrated model of the organizing and regulating apparatus. Experientia. 1984;40:309–38. doi: 10.1007/BF01952538. [DOI] [PubMed] [Google Scholar]
- 2.Panksepp J, Jalowiec JE, Zolovick AJ, Stern WC, Morgane PJ. Inhibition of glycolytic metabolism and sleep-waking states in cats. Pharm Biochem Behav. 1973;1:41–46. doi: 10.1016/0091-3057(73)90065-8. [DOI] [PubMed] [Google Scholar]
- 3.Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- 4.Krueger JM, Obal JrF. A neuronal group theory of sleep function. J Sleep Res. 1993;2:63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. This was the first statement of the hypothesis that sleep was a local use-dependent process. [DOI] [PubMed] [Google Scholar]
- 5.Krueger JM, Obal F., Jr Sleep function. Frontiers in Biosci. 2003;8:511–519. doi: 10.2741/1031. [DOI] [PubMed] [Google Scholar]
- 6.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. This was the first demonstration that cortical columns oscillate between functional states, one of which had properties similar to whole organism sleep. [DOI] [PubMed] [Google Scholar]
- 7.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. These authors have recognized for over 20 years that human parasomnias suggest that the brain can be awake and asleep simultaneously. This is a comprehensive review of that work. [DOI] [PubMed] [Google Scholar]
- 8.Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 1977;134:581–584. doi: 10.1016/0006-8993(77)90835-6. This pioneering study demonstrated that dolphins have high amplitude delta sleep on only one half of the brain at a time and that they lack rapid-eye movement sleep. [DOI] [PubMed] [Google Scholar]
- 9.Rattenborg NC, Amlaner CJ, Lima SL. Unilateral eye closure and interhemispheric EEG asymmetry during sleep in the pigeon (Columba livia) Brain Behav Evol. 2001;58:323–332. doi: 10.1159/000057573. [DOI] [PubMed] [Google Scholar]
- 10.Werth E, Achermann P, Dijk DJ, Borbely AA. Spindle frequency activity in the sleep EEG: Individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol. 1997;103:535–542. doi: 10.1016/s0013-4694(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 11.Braun AR, Balkin TJ, Wesenten NJ, Carson RE, Varga M, Baldwin P, Selbie S, Belenky G, Herscovitch P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain. 1997;120:1173–1197. doi: 10.1093/brain/120.7.1173. [DOI] [PubMed] [Google Scholar]
- 12.Kristiansen K, Courtois G. Rhythmic activity from isolated cerebral cortex EEG. Clin Neurophysiol. 1949;1:265–272. [PubMed] [Google Scholar]
- 13.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–d899. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 14.Pigarev IN, Nothdurf HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. NeuroReport. 1997;8:2557–2560. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 15.Koch C. The quest for consciousness. Roberts and Company; Englewood, CO: 2004. [Google Scholar]
- 16.Walker AJ, Topchiy I, Kouptsov K, Rector DM. ERP differences during conditioned lick response in the rat. Sleep. 2005;28:A15. [Google Scholar]
- 17.Moore RY. Suprachiasmatic nucleus in sleep-wake regulation. Sleep Med. 2007;8(supp3):27–33. doi: 10.1016/j.sleep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Kattler H, Dijk DJ, Borbely AA. Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J Sleep Res. 1994;3:1599–1604. doi: 10.1111/j.1365-2869.1994.tb00123.x. The authors tested the hypothesis put forward in reference 4 that sleep was a local activity-dependent process. Much research has since extended and confirmed this pioneering study. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto H, Katagiri H, Hensch T. Experience-dependent slow-wave sleep development. Nat Neurosci. 2003;6:553–554. doi: 10.1038/nn1064. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki N, Karashima A, Tamakawa Y, Katayama N, Nakao M. Sleep EEG dynamics in rat barrel cortex associated with sensory deprivation. NeuroReport. 2004;15:2681–2684. doi: 10.1097/00001756-200412030-00026. [DOI] [PubMed] [Google Scholar]
- 21.Vyazovskiy V, Borbely AA, Tobler I. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J Sleep Res. 2000;9:367–371. doi: 10.1046/j.1365-2869.2000.00230.x. [DOI] [PubMed] [Google Scholar]
- 22.Cottone LA, Adamo D, Squires NK. The effect of unilateral somatosensory stimulation on hemispheric asymmetries during slow wave sleep. Sleep. 2004;27:63–68. doi: 10.1093/sleep/27.1.63. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara M, De Gennaro L, Curcio G, Cristiani R, Bertini M. Interhemispheric asymmetry of human sleep EEG in response to selective slow-wave sleep deprivation. Behav Neurosci. 2002;116:976–981. doi: 10.1037//0735-7044.116.6.976. [DOI] [PubMed] [Google Scholar]
- 24.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 25.Yasuda T, Yasuda K, Brown RA, Krueger JM. State-dependent effects of light-dark cycle on somatosensory and visual cortex EEG in rats. Am J Physiol. 2005;289:R1083–R1089. doi: 10.1152/ajpregu.00112.2005. [DOI] [PubMed] [Google Scholar]
- 26.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci. 2006;9:1169–1176. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- 27.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 28.Drummond SPA, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. NeuroReport. 1999;10:3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 29.Mascetti GG, Rugger M, Vallortigara G, Boddo D. Monocular-unihemispheric sleep and visual discrimination learning in the domestic chick. Exp Brain Res. 2007;176:70–84. doi: 10.1007/s00221-006-0595-3. [DOI] [PubMed] [Google Scholar]
- 30.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 31.Marks GA, Shaffery JP, Oksenberg A, Speciale SG, Roffwarg HP. A functional role for REM sleep in brain maturation. Behav Brain Res. 1995;69:1–11. doi: 10.1016/0166-4328(95)00018-o. [DOI] [PubMed] [Google Scholar]
- 32.Rector DM, Topchiy I, Rojas M. Local cortical column activity states and localized delta wave differences. Sleep. 2005;28:A26. [Google Scholar]
- 33.Obal F, Jr, Krueger JM. Biochemical regulation of sleep. Front Biosci. 2003;8:520–550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 34.Borbély AA, Tobler I. Endogenous sleep-promoting substances and sleep regulation. Physiol Rev. 1989;69:605–670. doi: 10.1152/physrev.1989.69.2.605. [DOI] [PubMed] [Google Scholar]
- 35.Jouvet M. Neuromediateurs et facteurs hypnogenes. Rev Neurol (Paris) 1984;140:389–400. [PubMed] [Google Scholar]
- 36.Krueger JM, Obál F., Jr . Sleep and Breathing. New York: Marcel Dekker, Inc.; 1994. Sleep Factors; pp. 79–112. [Google Scholar]
- 37.Inoué S. Biology of sleep substances. Boca Raton, Florida: CRC Press, Inc.; 1989. [Google Scholar]
- 38.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 39.De Sarro G, Gareri P, Sinopoli VA, David E, Rotiroti D. Comparative, behavioural and electrocortical effects of tumor necrosis factor-alpha and interleukin-1 microinjected into the locus coeruleus of rat. Life Sci. 1997;60:555–564. doi: 10.1016/s0024-3205(96)00692-3. [DOI] [PubMed] [Google Scholar]
- 40.Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1 beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18:1041–1049. doi: 10.1046/j.1460-9568.2003.02836.x. [DOI] [PubMed] [Google Scholar]
- 41.De A, Churchill L, Obal F, Jr, Simasko S, Krueger JM. GHRH and IL1beta increase cytoplasmic Ca2+ levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda T, Yoshida H, Garcia-Garcia F, Kay D, Krueger JM. Interleukin-1βhas a role in cerebral cortical state dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida H, Peterfi Z, Garcia-Garcia F, Garcia-Garcia F, Kirkpatrick R, Yasuda T, Krueger JM. State-specific asymmetries in EEG slow wave activity induced by local application of TNF alpha. Brain Res. 2004;1009:129–136. doi: 10.1016/j.brainres.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 44.Szentirmai E, Yasuda T, Taishi P, Wang W, Churchill L, Bohnet S, Mcgrath P, Kacsoh B, Jimenez L, Krueger JM. Growth hormone releasing hormone: Cerebral cortical EEG actions and expression. Am J Physiol. 2007;293:R922–R930. doi: 10.1152/ajpregu.00237.2007. [DOI] [PubMed] [Google Scholar]
- 45.Taishi P, Churchill L, Wang M, Kay D, Davis CJ, Guan X, De A, Yasuda T, Liao F, Krueger JM. TNFαsiRNA reduces brain TNF, EEG delta wave activity in rats. Brain Res. 2007;1156:125–132. doi: 10.1016/j.brainres.2007.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Churchill L, Rector DM, Yasuda K, Fix C, Rojas MJ, Yasuda T, Hall SJ, Guan X, Krueger JM. Tumor necrosis factorα: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. doi: 10.1016/j.neuroscience.2008.06.066. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Obal F, Jr, Krueger JM. Humoral mechanisms of sleep. In: Parmeggiani PL, Velluti R, editors. The Physiological Nature of Sleep. Imperial College Press; 2004. pp. 23–44. [Google Scholar]
- 48.Tabarean IV, Korn H, Bartfai T. Interleukin-1βinduces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Cell Neurosci. 2006;4:1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, Szymusiak R. Interleukin-1 beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. Eur J Neurosci. 2004;20:207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- 50.Shibata M. Hypothalamic neuronal responses to cytokines. Yale J Biol Med. 1990;63:147–156. [PMC free article] [PubMed] [Google Scholar]
- 51.Schinder AF, Poo MM. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 52.Houzen H, Kikuchi S, Kanno M, Shinpo K, Tashiro K. Tumor necrosis factor enhancement of transient outward potassium currents in cultured rat cortical neurons. J Neurosci Res. 1997;50:990–999. doi: 10.1002/(SICI)1097-4547(19971215)50:6<990::AID-JNR9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 53.Diem R, Meyer R, Weishaupt JH, Bahr M. Reduction of potassium currents in phosphatidylinositol 3-kinase-dependent AKT phosphorylation by tumor necrosis factor-(alpha) rescues axotomized retinal ganglion cells from retrograde cell death in vivo. J Neurosci. 2001;21:2058–2066. doi: 10.1523/JNEUROSCI.21-06-02058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koller H, Allert N, Oel D, Stoll G, Siebler M. TNF alpha induces a protein kinase C-dependent reduction in astroglial K+ conductance. NeuroReport. 1998;9:1375–1378. doi: 10.1097/00001756-199805110-00023. [DOI] [PubMed] [Google Scholar]
- 55.McLarnon JG, Franciosi S, Wang X, Bae JH, Choi HB, Kim SU. Acute actions of tumor necrosis factor-alpha on intracellular Ca (2+) and K(+) currents in human microglia. Neuroscience. 2001;104:1175–1184. doi: 10.1016/s0306-4522(01)00119-1. [DOI] [PubMed] [Google Scholar]
- 56.Kawada H, Niwano S, Niwano H, Yumoto Y, Wakisaka Y, Yuge M, Kawahara K, Izumi T. Tumor necrosis factor-alpha downregulates the voltage gated outward K+ current in cultured neonatal rat cardiomyocytes: a possible cause of electrical remodeling in diseased hearts. Circ J. 2006;70:605–609. doi: 10.1253/circj.70.605. [DOI] [PubMed] [Google Scholar]
- 57.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 58.Yasuda K, Churchill L, Yasuda T, Blindheim K, Falter M, Krueger JM. Unilateral cortical application of interleukin-1β(IL1β) induces asymmetry in Fos- and IL1β-immunoreactivity: Implication for sleep regulation. Brain Res. 2007;1131:44–59. doi: 10.1016/j.brainres.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 59.Churchill L, Yasuda K, Yasuda T, Blindheim K, Falter M, Garcia-Garcia F, Krueger JM. Unilateral cortical application of tumor necrosis factor alpha induces asymmetry in Fos- and interleukin-1 beta-immunoreactive cells within the corticothalamic projection. Brain Res. 2005;1055:15–24. doi: 10.1016/j.brainres.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 60.Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Med Clinics. 2007;2:161–170. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De A, Krueger JM, Simasko SM. Glutamate induces expression and release of tumor necrosis factor alpha in cultured hypothalamic cells. Brain Res. 2005;1053:54–61. doi: 10.1016/j.brainres.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 62.De A, Fix C, Hall S, Churchill L, Krueger JM. Growth hormone releasing hormone receptive immuno-reactive cells increase in the barrel field in response to whisker deflection in rats. Sleep. 2006;29:A9. [Google Scholar]
- 63.Gerashchenko D, Wisor JP, Burns D, Reh RK, Shiromani PJ, Sakurai T, de la Iglesia HO, Kilduff TS. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci USA. 2008;105:10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kapas L, Fang J, Krueger JM. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994;664:189–196. doi: 10.1016/0006-8993(94)91969-0. [DOI] [PubMed] [Google Scholar]
- 65.Landolt HP, Dijk DJ, Gaus SE, Borbely AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995;12:229–238. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- 66.Farber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Arch – Eur J Physiol. 2006;452:615–621. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- 67.Burnstock G. Physiology and pathology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 68.Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki T, Hide I, Ido K, Kohsaka S, Inoue K, Nakata Y. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 71.Williams JA, Sathyanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in drosophila: A role for the NFkB Relish. Sleep. 2007;30:389–401. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jajoo S, Mukherjea D, Pingle S, Sekino Y, Ramkumar V. Induction of adenosine A1 receptor expression by pertussis toxin via an adenosine 5′-diphosphate ribosylation-independent pathway. J Pharmacol Exp Ther. 2006;317:1–10. doi: 10.1124/jpet.105.096255. [DOI] [PubMed] [Google Scholar]
- 73.Yu Z, Cheng G, Wen X, Wu GD, Lee WT, Pleasure D. Tumor necrosis factor alpha increases neuronal vulnerability to excitotoxic necrosis by inducing expression of the AMPA-glutamate receptor subunit GluR1 via an acid sphingomyelinase-NF-kappaB-dependent mechanism. Neurobiol Dis. 2002;11:199–213. doi: 10.1006/nbdi.2002.0530. [DOI] [PubMed] [Google Scholar]
- 74.Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schei JL, Foust AJ, Rojas MJ, Navas JA, Rector DM. Evoked optical responses differ between quiet sleep stages. Sleep. 2008;31:A7. [Google Scholar]
- 76.Taishi P, Churchill L, De A, Obal F, Krueger JM. Cytokine mRNA induction by interleukin-1 beta or tumor necrosis factor alpha in vitro and in vivo. Brain Res. 2008;1226:89–98. doi: 10.1016/j.brainres.2008.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy S, Krueger JM, Rector DM, Wan Y. Network models for activity-dependent sleep regulation. J Theor Biol. 2008;253:462–468. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mirollo RE, Strogatz SH. Synchronization of pulse-coupled biological oscillators. SIAM J Appl Math. 1990;50:1645–1662. [Google Scholar]
- 79.Asavathiratham C, Roy S, Lesieutre BC, Verghese GC. The influence model. IEEE Control Systems Magazine. 2001 Dec; [Google Scholar]
- 80.Wilson HR, Cowan JD. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1974;12:1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kavanau JL. Sleep and dynamic stabilization of neural circuitry: A review and synthesis. Behav Brain Res. 1994;63:111–126. doi: 10.1016/0166-4328(94)90082-5. This is a scholarly development of the idea that experience-driven changes in neural connectivity leads to dysfunction unless there is a mechanism in place to temper such activity. The author posits that intrinsic electrical activity of the brain stabilizes the activity-altered circuits and that this is a function of sleep. [DOI] [PubMed] [Google Scholar]
- 82.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Crick F, Mitchinson G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 85.Abbott LF, Nelson SB. Synaptic plasticity: Taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 86.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. Synaptic scaling is an activity-driven synaptic plasticity stabilization process. The authors show that TNF, a sleep regulatory substance, is involved in synaptic scaling. The implications for sleep are that sleep regulatory mechanisms can not be separated from a sleep-connectivity stabilization function. [DOI] [PubMed] [Google Scholar]
- 87.Nofzinger EA, Nissen C, Germain A, Moul D, Hall M, Price JC, Miewald JM, Buysse DJ. Regional cerebral metabolic correlates of WASO during NREMS sleep in insomnia. J Clin Sleep Med. 2006;2:316–322. [PubMed] [Google Scholar]
- 88.Edelman GH. Neural Darwinsim. Basic Books; New York: 1987. [Google Scholar]
- 89.Panksepp J. Affective Neuroscience. Oxford University Press; New York: 1998. [Google Scholar]
- 90.Brandt JA, Churchill L, Guan Z, Fang J, Chen L, Krueger JM. Sleep-deprivation but not a whisker trim increases nerve growth factor within barrel cortical neurons. Brain Res. 2001;898:105–112. doi: 10.1016/s0006-8993(01)02149-7. This report demonstrates that the interaction of afferent activity and sleep alters expression of a sleep regulatory substance in neurons. As such it provides evidence for a mechanism by which local sleep is tied to prior activity. [DOI] [PubMed] [Google Scholar]
- 91.De A, Krueger JM, Simasko SM. Tumor necrosis factor alpha increases cytosolic calcium response AMPA and KCl in primary cultures of rat hippocampal neurons. Brain Res. 2003;981:133–142. doi: 10.1016/s0006-8993(03)02997-4. [DOI] [PubMed] [Google Scholar]
- 92.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNF alpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 93.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Model of thalamocortical slow-wave sleep oscillations and transitions to activated states. J Neurosci. 2002;22:8691–704. doi: 10.1523/JNEUROSCI.22-19-08691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 95.Lai AY, Swayze RD, El-Husseini A, Song C. Interleukin-1 beta modulates AMPA receptor expression and phosphorylation in hippocampal neurons. J Neuroimmunol. 2006;175:97–106. doi: 10.1016/j.jneuroim.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 96.Czarnecki A, Birtoli B, Ulrich D. Cellular mechanisms of burst-firing mediated long-term depression in rat neocortical pyramidal cells. J Physiol. 2007;578:471–479. doi: 10.1113/jphysiol.2006.123588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takahashi S, Krueger JM. Inhibition of tumor necrosis factor prevents warming-induced sleep responses in rabbits. Am J Physiol. 1997;272:R1325–R1329. doi: 10.1152/ajpregu.1997.272.4.R1325. [DOI] [PubMed] [Google Scholar]
- 98.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: A key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]