Abstract
The oriental fruit moth (OFM), Grapholita molesta (Busck), which is among the most important insect pests of peaches and nectarines, has developed resistance to a wide range of insecticides. We investigated the ability of the entomopathogenic nematodes (EPN) Steinernema carpocapsae (Weiser), S. feltiae (Filipjev), S. riobrave (Cabanillas et al.), and Heterorhabditis marelatus (Liu and Berry) to control OFM under laboratory and fruit bin conditions. At a dosage of 10 infective juveniles (IJ)/cm2 in the laboratory, S. carpocapsae caused 63%, S. feltiae 87.8%, S. riobrave 75.6%, and H. marelatus 67.1% OFM mortality. All four nematode species caused significant OFM larval mortality in comparison to the nontreated controls. Steinernema feltiae was used for the bin assays due to the higher OFM mortality it caused than the other tested EPN species and to its ability to find OFM under cryptic environments. Diapausing cocooned OFM larvae in miniature fruit bins were susceptible to IJ of S. feltiae in infested corner supports and cardboard strips. Treatment of bins with suspensions of 10 or 25 S. feltiae IJ/ml water with wetting agent (Silwet L77) resulted in 33.3 to 59% and 77.7 to 81.6% OFM mortality in corner supports and cardboard strips, respectively. This paper presents new information on the use of EPN, specifically S. feltiae, as nonchemical means of OFM control.
Keywords: Biological control, cardboard strips, fruit bins, Grapholita molesta, entomopathogenic nematodes, Heterorhabditis marelatus, oriental fruit moth, Steinernema carpocapsae, S. feltiae, S. riobrave, wetting agent
Oriental fruit moth (OFM), Grapholita molesta, a native insect of China, has spread throughout the world and is considered the most important insect pest of peaches. Since its introduction into North America, OFM has become a serious pest of peaches, nectarines, apricots and apples (Rothschild and Vickers, 1991). It was first detected in the eastern United States in 1913, reached California by 1942, and is now found in all peach-growing areas of the United States and Canada (Rothschild and Vickers, 1991). The moth is difficult to control because it has several generations throughout the growing season and has developed resistance to organophosphate insecticides with cross-resistance to carbamates (Kanga et al., 1997, 1999). In addition, growers who have switched to pyrethroids are also concerned that OFM might develop resistance to this class of insecticides (Pree et al., 1998).
Incorporation of an effective biological control agent in the management of OFM might lead to reduction of insecticide use against this pest and alleviate the selective pressures that cause the insect to develop resistance. In addition, the use of biological control agents in lieu of insecticides could minimize human exposure to synthetic pesticides and eliminate the need for buffer zones in urban areas and sensitive habitats adjacent to orchards.
Entomopathogenic nematodes (EPN) have the potential to be effective biological control agents due to their broad host range, host-seeking abilities, high virulence, and safety for vertebrates and plants (Poinar, 1990). Efficacy of several EPN species has been demonstrated against a wide variety of insect pests (Kaya and Gaugler, 1993; Gaugler, 2002), including codling moths, Cydia pomonella (L.), in both orchards and fruit bins (Kaya et al., 1984; Unruh and Lacey, 2001; Lacey et al., 2005). Several species in the Tortricidae, in which genus G. molesta belongs, use cryptic habitats during some of their life stages. Full-grown OFM larvae over-winter in cocoons in bark crevices, orchard trash, weeds, ground cover, and fallen fruits. The OFM larvae pupate in early spring, and four to five generations can exist per year (Kanga et al., 1999).
Cryptic habitats used by insects are also good habitats for EPN applications (Kaya and Gaugler, 1993; Gaugler at al., 1997). Recently, Lacey et al. (2005) used EPN for control of overwintering codling moth in fruit bins by incorporating cardboard strips and wooden corners to mimic cryptic environment. In addition, they used a wetting agent, Silwet L77, to assist the penetration of EPN into codling moth hibernacula, and a humectant to prevent EPN desiccation.
OFM control could be effective if EPN were used to target the overwintering cocooned larvae found in cryptic habitats. The purpose of this study was to determine the ability of entomopathogenic nematodes to infect and kill OFM in laboratory and miniature fruit bin assays. This paper presents new information on the use of EPN for control of cocooned OFM larvae.
Materials and Methods
Test insects: OFM larvae used in experiments were reared on soy-wheat germ-starch artificial diet (Toba and Howell, 1991) under diapausing conditions (photoperiod of 12:12 [L:D], 21°C, 41–45% RH) in a colony maintained at the Yakima Agricultural Research Laboratory (YARL) in Wapato, Washington.
Test nematodes: Steinernema feltiae (Umea strain), S. carpocapsae (Sal strain), S. riobrave (originally obtained from H. Kaya, University of California, Davis) and Heterorhabditis marelatus (originally obtained from R. Berry, Oregon State University, Corvallis, OR) were used in this study. Infective juveniles (IJ) used were produced in wax moth, Galleria mellonella (L.), according to procedures described by Kaya and Stock (1997), stored at 10°C in moist sponges, and used within 2 wk of production. Quality control of test nematode infectivity was conducted for each experiment against diapausing cocooned OFM larvae in 15.2-cm2 perforated cardboard strips (double-faced, B flute, Weyerhaeuser, Tacoma, WA) using 152 IJ in 1 ml water (10 IJ/cm2, 4 strips per treatment and control) and methods prescribed by Lacey and Unruh (1998). The treated strips were placed in filter-paper-lined petri dishes (9-cm-diam.), incubated for 6 d at 25°C ± 1.7°C, and then assessed for mortality. Quality control was performed directly on OFM as the mortality observed in OFM strips was very close to that of codling moth larvae reported in studies conducted by Lacey et al. (2005). We used 25°C as the optimal temperature for testing EPN, as this temperature can be obtained in fruit-packing houses where bins would be stored for the first 24 hr following treatment (Lacey et al., 2005).
Susceptibility of OFM to EPN: Steinernema carpocapsae, S. feltiae, S. riobrave, and H. marelatus IJ were used to treat cocooned larvae or pupae in 15.2-cm2 perforated cardboard strips using the procedures described by Lacey and Unruh (1998). Five strips were used for each EPN species and control. The strips were treated with 1 ml of water or 1 ml of water containing 152 IJ (10 IJ/cm2) placed in filter-paper-lined-petri dishes, incubated for 6 d at 25°C ± 1.7°C, and then assessed for mortality. Although we aimed for 20 OFM per cardboard strip, the number was variable. In the control strips, the average number of OFM larvae per strip was 13.2 (ranged from 11–16), in S. carpocapsae it was 13.2 (ranged from 9–17), in S. feltiae it was 16.4 (ranged from 13–19), in S. riobrave it was 13.4 (ranged from 9–17), and in H. marelatus it was 15 (ranged from 14–17). The experiments were replicated on three separate dates.
Nematode applications against OFM in miniature fruit bins: The miniature fruit bins used in our tests were described by Lacey and Chauvin (1999) and Lacey et al. (2005) and are one eighth the size of regular wooden fruit bins. The same materials used to construct commercial fruit bins (1.6-cm-thick CDX plywood and fir corner supports) were used to construct the miniature fruit bins. Each bin was modified to include one corner support that had been grooved (13 horizontal grooves per side, 2–3-mm wide, 3–5 mm-deep) on the sides facing the bin wall to facilitate infestation with OFM diapausing larvae. Four round head screws were embedded into the surface of the corner supports that faced the sides of the bins to provide a 2- to 3-mm gap between the corner support and side of the bin. Corner supports that had been removed from bins were placed on rearing trays that contained diapause-destined maturing OFM larvae. Full-grown larvae were allowed to spin cocoons in the supports several days prior to testing. After formation of cocoons, the corner supports were stored in a 12°C ± 0.5°C incubator until they were used for testing. On the day of the test, they were inserted into the corners of the fruit bins with wood screws and an electric drill. The number of OFM larvae per corner support was variable. The average number of OFM larvae in controls was 33.4 (ranged from 7–45), in tests with S. feltiae (10 IJ/ml) was 104 (ranged from 62–139), and in tests with S. feltiae (25 IJ/ml) was 87 (ranged from 51–169).
Similarly, perforated cardboard strips (41.0-cm long by 1.9-cm wide, double-faced, C flute) were infested with diapausing OFM. The number of OFM larvae per strip was variable. In control strips the average number of OFM larvae was 32.6 (ranged from 7–62), in tests with S. feltiae (10 IJ/ml) the average was 30 (ranged from 7–64), and in tests with S. feltiae (25 IJ/ml) the average was 37 (ranged from 6–86). The infested cardboard strips were placed in spaces in one corner of each of the bins. The space was made by placing a strip of Plexiglas (24.-m long × 2.5-cm wide × 6.4-mm thick) between one of the corner supports and adjacent walls 2 cm from the corner.
The miniature bins were treated by immersing them in a deep, straight-sided wheelbarrow (Rubbermaid farm cart, Fairlawn, OH) filled with 170.3 liters of water to within 2.8 cm of the top. Two concentrations (10 and 25) of S. feltiae IJ/ml of water were used. In all tests, immersion time in the tank was 1 min. Water used in the experiments was nonchlorinated well water (20.6°C ± 0.5°C; pH 7.5). In all experiments, 0.063% of the adjuvant Silwet L77® (Silicone-polyether copolymer, Loveland Industries, Inc., Greeley, CO) was added to the water as a wetting agent. Silwet L77 was shown in the studies of Lacey et al. (2005) to enhance EPN activity by permitting penetration of hibernacula and bin crevices. After treatments, the bins were stacked in the greenhouse at 20°C to 25°C for 24 h and covered with a tarp to maintain moisture. After the initial 24 h, the corner supports and cardboard strips were removed and stored in a walk-in incubator at 25°C ± 1.7°C. Mortality of larvae in the supports was determined after 6 d of incubation. Bins were stored outdoors between tests and subjected to natural weathering. Analysis of variance was used to determine the effect of EPN on OFM mortality in the laboratory and in bins at P < 0.05, respectively. Statistical differences of means among EPN were tested using Tukey's multiple comparison method (Devore, 1987). Five bins were used per treatment and per control. The bin assay was repeated on three separate dates.
Results
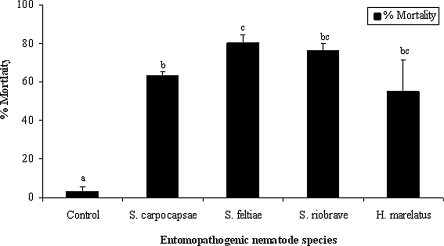
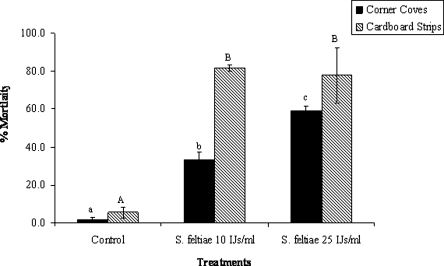
Under laboratory conditions, in perforated cardboard strips, all four EPN species tested against OFM larvae caused significantly higher mortality in comparison to the nontreated controls (F4,10 = 16.17, P = 0.0002) (Fig. 1). Steinernema feltiae caused significantly higher mortality than S. carpocapsae but not higher than H. marelatus or S. riobrave. In miniature fruit bins, S. feltiae caused significantly higher mortality to cocooned OFM larvae both in corner supports (F2,6 = 101, P = 0.0001) and cardboard strips at 10 and 25 IJ/ml in comparison to the nontreated controls (F2,6 = 25, P = 0.001) (Fig. 2). Significantly higher mortality was observed in cocooned larvae in cardboard strips treated with S. feltiae IJ than in the corner supports. The concentration of S. feltiae that was most effective against OFM in corner supports was 25 IJ/ml (F1,4 = 28.41, P = 0.006) (Fig. 2). There was no significant difference between the concentrations of nematodes in terms of larval mortality in the cardboard strips (F1,4 = 0, P = 0.9502) (Fig. 2).
Fig. 1.
Mortality in cocooned Grapholita molesta diapausing larvae treated with Steinernema carpocapsae, S. feltiae, S. riobrave, and Heterorhabditis marelatus in the laboratory at 10 IJ/cm2. Columns with the same letter are not significantly different at the 0.05 level.
Fig. 2.
Mortality in cocooned Grapholita molesta larvae treated with two concentrations of Steinernema feltiae (10 and 25 IJ/ml) and wetting agent (Silwet L77® ) in fruit bins using infested corner coves or cardboard strips. Columns with the same upper- or lower-case letter within each series are not significantly different at the 0.05 level.
Discussion
Steinernema feltiae caused mortality against OFM and was chosen as the EPN species with which to conduct further experiments in miniature fruit bins, a cryptic environment in which OFM can be found. Although S. feltiae did not cause significantly higher mortality than S. riobrave and H. marelatus, it was chosen as an OFM control agent due to its attributes that will enhance its effectiveness in cryptic habitats. For example, S. feltiae is more active at cooler temperatures, i.e., 8°C and lower (Grewal et al., 1996), than S. riobrave or S. carpocapsae. In addition, S. feltiae is considered an intermediate host search strategist exhibiting both ambusher and cruiser behavior (Lewis et al., 1995) and will seek out insect hosts, unlike S. carpocapsae, which is considered an ambusher. Miniature fruit bins containing cardboard strips and grooved corners were chosen for our studies as they provide a cryptic environment appropriate for Tortricidae and EPN studies. Treatments of miniature fruit bins in water suspensions of S. feltiae with wetting agent provided encouraging results, especially in infested strips where 77.7 to 81.6% mortality was observed.
The efficacy of EPN in cryptic environments was enhanced with the use of wetting agents. Lacey et al. (2005) reported that the use of adjuvants to increase penetration of hibernacula and retard desiccation of S. feltiae in fruit bins resulted in improved efficacy against cocooned codling moth larvae. In our study, S. feltiae was more successful against larvae in cardboard strips than the grooved corner supports within bins. It is possible that S. feltiae along with the wetting agent was more successful in reaching the OFM larvae in the cardboard strips than in the corner supports. The cardboard is rapidly wetted and retains water better than wood.
OFM has three to four generations each year, resulting in damage to both growing twigs and fruit. The late-stage OFM larvae overwinter under tree bark, in crevices, or in the soil (Dustan, 1961) and have also been reported in fruit bins (L. Gut and D. Epstein, pers. com.). The OFM overwintering larvae may prove to be appropriate stages to target in the early fall or spring using suspensions of S. feltiae IJ. It is possible that reducing the number of OFM adults that emerge at the beginning of the peach-growing season could substantially reduce subsequent OFM generations, thereby enabling a significant reduction in chemical insecticide use. Field trials are needed to determine the effect of EPN on OFM under various orchard conditions.
Footnotes
The authors thank Lorraine Seymour for assistance with assays and bin tests, Pauline Anderson for rearing OFM larvae, and Don Hostetter and Steve Arthurs for review of the manuscript.
This paper was edited by Patricia Stock.
Literature Cited
- Devore JL, editor. 2nd ed. Monterey, CA: Brooks/Cole Publishing Company; 1987. Probability and statistics for engineering and sciences. [Google Scholar]
- Dustan GG. The Oriental fruit moth, Grapholita molesta (Busck), in Ontario. Proceedings of the Entomological Society of Ontario. 1961;91:215–227. [Google Scholar]
- Gaugler R, editor. Wallingford, UK: CABI Publishing; 2002. Entomopathogenic nematodes. [Google Scholar]
- Gaugler R, Lewis E, Stuart RJ. Ecology in the service of biological control: The case of entomopathogenic nematodes. Oecologia. 1997;109:483–489. doi: 10.1007/s004420050108. [DOI] [PubMed] [Google Scholar]
- Grewal PS, Gaugler R, Wang Y. Enhanced cold tolerance of the entomopathogenic nematode Steinernema feltiae through genetic selection. Annals of Applied Biology. 1996;129:335–341. [Google Scholar]
- Kanga LHB, Pree DJ, van Lier JL. Monitoring for resistance to organophosphorus, carbamate, and pyrethroid insecticides in the Oriental fruit moth (Lepidoptera: Tortricidae) The Canadian Entomologist. 1999;131:441–450. [Google Scholar]
- Kanga LHB, Pree DJ, van Lier JL, Whitty KJ. Mechanisms of resistance to organophosphorous and carbamate insecticides in Oriental fruit moth populations, Grapholita molesta (Busck) Pesticide Biochemistry and Physiology. 1997;59:11–23. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Joos JL, Falcon LA, Berlowitz A. Suppression of the codling moth (Lepidoptera: Olethreutidae) with the entomogenous nematode, Steinernema feltiae (Rhabditida: Steinerne-matidae) Journal of Economic Entomology. 1984;77:1240–1244. [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. London, UK: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Lacey LA, Chauvin RL. Entomopathogenic nematodes for control of codling moth in fruit bins. Journal of Economic Entomology. 1999;92:104–109. doi: 10.1093/jee/92.1.104. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Neven LG, Headrick HL, Fritts R., Jr Factors affecting entomopathogenic nematodes (Steinernematidae) for the control of overwintering codling moth (Lepidoptera: Tortricidae) in fruit bins. Journal of Economic Entomology. 2005;98:1863–1869. doi: 10.1603/0022-0493-98.6.1863. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Unruh TR. Entomopathogenic nematodes for control of codling moth: Effect of nematode species, dosage, temperature, and humidity under laboratory and simulated field conditions. Biological Control. 1998;13:190–197. [Google Scholar]
- Lewis EE, Grewal PS, Gaugler R. Hierarchical order of host cues in parasite foraging strategies. Parasitology. 1995;110:207–213. [Google Scholar]
- Poinar GO., Jr . Taxonomy and biology of Steinernematidae and Heterorhabditadae. In: Gaugler R, Kaya HK, editors. Entomopathogenic nematodes in biological control. Boca Raton: CRC Press; 1990. pp. 23–61. [Google Scholar]
- Pree DJ, Whitty KJ, van Driel L. Resistance to insecticides in Oriental fruit moth populations (Grapholita molesta) from the Niagara Peninsula of Ontario. The Canadian Entomologist. 1998;130:245–256. [Google Scholar]
- Rothschild GHL, Vickers RA. Biology. Ecology and control of the Oriental fruit moth. In: van der Geest LPS, Evenhuis HH, editors. Totricid pests, their biology, natural enemies, and control. Amsterdam, The Netherlands: Elsevier Science Publishers; 1991. pp. 389–412. [Google Scholar]
- Toba HH, Howell JF. An improved system for mass-rearing codling moths. Journal of the Entomological Society of British Columbia. 1991;88:22–27. [Google Scholar]
- Unruh TR, Lacey LA. Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae), with Steinernema carpocapsae. Effects of supplemental wetting and pupation site on infection rate. Biological Control. 2001;20:48–56. [Google Scholar]