Abstract
We studied the pathogenicity and overwintering survival of the foliar nematode, Aphelenchoides fragariae, infecting Hosta spp. Nematodes applied to either lower or upper sides of noninjured and injured hosta leaves were able to infect and produce typical symptoms on nine cultivars. Leaves of only four cultivars (Borschi, Fragrant Blue, Patomic Pride, and Olive Bailey Langdon) showed no symptoms of nematode infection. The nematodes overwintered as juveniles and adults in soil, dry leaves, and dormant buds, but not in roots. Nematode winter survival was higher in dormant buds and soil from the polyhouse than in an open home garden. Of the nematodes found in the dormant buds, 35% to 79% were located between the first two outside layers of the buds. The nematodes tolerated 8 hr exposure to 40°C and −80°C in leaf tissues. Relative humidity influenced nematode migration from soil to leaves. The presence of nematodes only on the outer surface of foliage (leaves and petioles) confirmed the migration of A. fragariae on the surface of the plants. Of the total number of nematodes found on the foliage, 25% to 46% and 66% to 77% were alive at 90% and 100% relative humidity, respectively, suggesting that high moisture is required for the survival and upward movement of nematodes. We conclude that A. fragariae can overwinter in soil, infected dry leaves, and dormant buds and migrate in films of water on the outer surface of the plant during spring to leaves to initiate infection.
Keywords: Aphelenchoides fragariae, dormant buds, foliar nematode, Hosta spp., overwintering, pathogenicity, temperature tolerance
Foliar nematodes, Aphelenchoides fragariae (Aphelen-chida: Aphelenchidae), cause serious damage to alfalfa, strawberries, lamium, and many ornamentals, including hosta and ferns in the nursery and landscape settings throughout the world (Decker, 1972; Johnson and Gill, 1975; Heinlein, 1982; Richardson and Grewal, 1993; Southey, 1993; LaMondia, 1995, 1999; Grewal and Jagdale, 2001; Jagdale and Grewal, 2002, 2004). The nematodes infect young leaves, presumably through stomata, and feed on the mesophyll cells, causing large sections of the leaf to become chlorotic (Wallace, 1959). The chlorotic sections subsequently become necrotic lesions, which are usually bounded by large veins (Sanwal, 1959). Sometimes an entire leaf may be infected, which then eventually dries and falls off the plant prior to autumn. Symptoms of foliar nematode infection on hosta leaves appear in July and August in the central US (Grewal and Jagdale, 2001; Jagdale and Grewal, 2002, 2004). However, where A. fragariae overwinter and how and when they migrate to the leaves is unknown. It has been speculated that foliar nematodes may overwinter in dormant crowns of plants, become active in spring and migrate in films of water on petioles or stems to cause infection in leaves (Wallace, 1959; Buckley and Gould, 2003).
In commercial nurseries, thousands of ornamental plants are traded each year, and there is growing concern among growers about the movement of nematode-infected plants and cut foliage across state and national boundaries due to quarantine regulations and fear of nematode dissemination to noninfested areas. Although nurseries are losing millions of dollars of revenue because of returned shipments of nematode-infected plants, little research has been conducted on the infection behavior and overwintering survival of foliar nematodes or general susceptibility and resistance of hosta cultivars to these nematodes. Information on the infection behavior and overwintering strategy of the foliar nematodes is essential to develop effective management strategies. Therefore, the purpose of this study was to determine the infection behavior and overwintering strategies of A. fragariae infecting hosta (Hosta spp). Specifically, we studied: (i) pathogenicity of A. fragariae on 23 different cultivars, (ii) overwintering survival of A. fragariae, (iii) temperature tolerance of A. fragariae, and (iv) migration of A. fragariae under different relative humidities.
Materials and Methods
Sources of nematodes: Nematodes were extracted from leaves of hosta cultivar Venessa and identified (Hunt, 1993; Siddiqi, 2000). A laboratory culture of nematodes was established on Rhizoctonia solani. The fungus was grown on potato dextrose agar medium in 15-cm-diam. petri dishes at 25°C ± 1°C. For pathogenecity tests, nematodes were obtained both from cultures maintained on R. solani and the naturally infected leaves of hosta Venessa. For the studies on overwintering survival of nematodes in polyhouses and under polythene covers, migratory behavior, and temperature tolerance, A. fragariae-infected hosta plants were obtained from commercial nurseries in Belleville, Perry, or Mansfield, OH. For the studies on overwintering survival of nematodes in a home garden, permanently planted hostas 5 to 10 m apart in a home garden in Wooster, OH, were sampled for the presence of nematodes.
Identification of nematodes: Nematodes were extracted from leaves of Venessa using a technique described by Jagdale and Grewal (2002), heat-killed, fixed in an aqueous solution of 2% triethanolamine and 2.8% formaldehyde, and mounted in glycerol (Hooper, 1986). The morphological parameters were recorded using a light microscope (×400) for 10 females and 10 males isolated from hosta leaves (Hunt, 1993; Siddiqi, 2000).
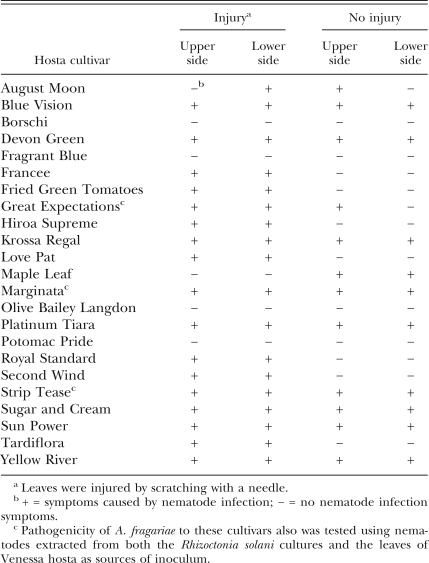
Pathogenicity: Two experiments were conducted to study pathogenicity of A. fragariae on different cultivars of hosta. The first experiment was conducted in the laboratory using A. fragariae extracted from either R. solani culture or naturally infected hosta leaves. The second experiment was conducted based on the results obtained from the first in a greenhouse using nema-tode-infected hosta leaves as the source of inoculum. The laboratory test was conducted on three hosta cultivars, Great Expectations, Marginata, and Strip Tease (Table 1), whereas the greenhouse experiment was conducted on 20 additional hosta cultivars (Table 1). For the laboratory trial, nematodes from R. solani and nematode- infected leaves of Venessa were usedasthe using a modified Baermann funnel technique (Whitehead and Hemming, 1965) and a technique described by Jagdale and Grewal (2002), respectively. The extracted nematodes were surface-sterilized with a 0.1% (v/v) sodium hypochlorite solution for 20 min. Five thousand mixed stages of nematodes suspended in 0.5 ml water were used for inoculation. The treatments consisted of nematodes inoculated on: (i) lower sides of noninjured leaves, (ii) upper-sides of noninjured leaves, (iii) lower sides of mechanically injured leaves, or (iv) upper-sides of mechanically injured leaves. In all the treatments, nematode inoculation was performed on four arbitrarily selected intact leaves on each of five plants of all three cultivars. Of the four inoculated leaves from each plant, two leaves were noninjured and two others were mechanically injured either on the upper or lower side by scratching with a needle. Inoculated leaves were immediately wrapped in a wet tissue paper (hereafter called ‘wet tissue-paper inoculation technique’) and covered with a plastic bag for 96 hr to minimize moisture loss. Observations on the infection status of plants were made 6 wk after inoculation.
Table 1.
Effect of the method of inoculation on the pathogenicity of Aphelenchoides fragariae to hosta cultivars.
In a shaded greenhouse, the pathogenicity of A. fragariae to 20 hosta cultivars was performed as described above except that it was conducted at 25°C ± 2°C and nematode-infected leaves of Venessa were used as the source of inoculum. In all treatments, one nematode-infected leaf of Venessa was placed on each of four arbitrarily selected intact leaves on each of five plants of all 20 cultivars using the wet-tissue paper inoculation technique. Observations on the infection status of plants were made 6 wk after inoculation.
Overwintering survival: Two tests, one during the winter of 2003–2004 and one during the winter of 2004–2005, were conducted in commercial nurseries (Perry and Mansfield, OH) and a home garden (Wooster, OH) to study the overwintering survival of A. fragariae-infected hosta plants (cultivar ‘Patriot’). Three treatments included overwintering plants held in: (i) a poly-house, (ii) under a polythene cover (hereafter termed as ‘polythene cover’), and (iii) planted in bare ground (unprotected method) in a home garden (hereafter termed as ‘home garden’). The polyhouse was a common Quonset-type of overwintering structure built from half-circle galvanized pipes and covered with a white polythene sheet during winter (Smith, 2005). The polythene cover is a structureless overwintering method in which containers are consolidated, covered with a white polythene sheet, and secured around the perimeter before winter's onset. In the unprotected overwintering method, plants were planted in soil in commercial landscapes/home gardens and left exposed to the environment. In the autumn of 2002 and 2004, 10 arbitrarily selected plastic pots (346 cm2 surface area) containing A. fragariae-infected hosta plants were transferred either to a polyhouse or put under polythene cover where they were maintained throughout the winter. Similarly, 10 nematode-infected plants were located in a home garden and labeled.
Overwintering survival of nematodes was observed on 15 February 2003 and 20 February 2005 in dormant buds, roots, and soil collected from all three sites (treatments). Dry leaves were collected from plants grown in the home garden. In 2003 and 2005, three arbitrarily selected dormant buds from a crown of each plant were collected and washed for 2 to 3 min in a 50-ml beaker containing 10 ml tap water to remove soil particles or nematodes (if any) from their outer surfaces; 30 dormant buds were collected from each overwintering site. Each bud contained about seven small, unexpanded leaves (hereafter these leaves are called “layers”), which were peeled off individually starting from the outermost layer to the innermost layer, reaching the meristematic portion of the bud. These layers were then individually plunged in 2 ml water for 5 min to allow adhering nematodes to suspend in the water. The nematodes in the resulting suspension were counted and expressed as numbers per bud. After rinsing with water, each bud layer was stained with a red food color (Thies et al., 2002) to determine presence of nematodes inside the tissue.
For extraction of nematodes from dry leaves (collected only in 2003), a piece of chlorotic lesion was carefully separated from each leaf and its total area measured using a LI-3100 Area Meter (LI-COR, Inc., Lincoln, NE). Each piece was cut into approximately 1-cm2 pieces and transferred into a 3.5-cm-diam. petri dish containing 2 ml water. The samples were incubated for 24 hr to allow desiccated nematodes to revive and emerge in the water. After incubation, the nematodes were counted and expressed as nematodes per cm2 of lesion tissue. To assess the overwintering population of A. fragariae in the roots, roots from 10 plants were arbitrarily collected from each of three sites by cutting frozen soil with a knife. Frozen soil was immediately removed from all the roots by immersing them in tap water for 15 to 20 min. After removing soil, 2 g of roots from each plant was stained with red dye to determine presence of nematodes inside the root tissue. The proportions of nematode stages including eggs, juveniles, females, and males found in the buds, dry leaves, and soil (only in 2005) were recorded separately. To assess the overwintering population of A. fragariae in soil, a knife was used to cut frozen soil into 2 × 2-cm-wide × 8–10-cm-deep samples, which were collected from three different spots in each pot from all three sites (soil in the polyhouse was partially frozen). A composite soil sample for each pot was prepared and then held at 4°C overnight to thaw. After thawing, soil was thoroughly mixed, all pieces of roots were removed, and nematodes were extracted from a 10-g subsample using the Baermann funnel technique (Baermann, 1917).
Temperature tolerance: Temperature tolerance of A. fragariae in actively growing leaf tissues of hosta Patriot was studied at −80, −20, 35, and 40°C in 5-cm-diam. petri dishes. Based on visual observation, 2 to 3-cm2-size chlorotic lesions caused by nematode infection were cut from actively growing leaves and transferred into petri dishes. Twelve dishes were prepared for each temperature regime and then transferred to their respective temperatures for 2, 4, and 8 hr. As a control treatment, 12 dishes were maintained at 25 °C for 2, 4, and 8 hr. At each observation, four dishes (replicates) were removed from each temperature and incubated at room temperature for the emergence of the nematodes, after adding 10 ml water for 72 hr. The nematodes that emerged from leaves in water were counted and expressed as numbers survived per cm2 of infected leaf area.
Migration of nematodes: A growth chamber test was conducted in 2003 to evaluate the effects of 90% and 100% RH on the migration of A. fragariae to hosta leaves at 22°C ± 1°C and 8-hr photoperiod. On 15 April 2003, 10 arbitrarily selected pots with nematode-infected dormant crowns of hosta Patriot were collected from a commercial nursery in Perry, OH, and transferred into a growth chamber at 90% RH where crowns were allowed to sprout and grow for 21 d. After 21 d, migration of A. fragariae was monitored on the outer surface of all leaves and petioles from four plants. The foliage (leaves plus petioles) from four plants was cut and plunged in 5 liters tap water in four separate plastic tubs for 15 to 20 min to allow adhering nematodes to float freely in the water. The remaining six plants were held in the same growth chamber to study the effect of 100% RH on the migration of A. fragariae for another 12 d by raising the RH from 90% to 100% while keeping temperature and photoperiod constant. The migration of nematodes was monitored as described above by cutting half of the leaves of all six plants after 6 d and the remaining half of the leaves of the same plants after 12 d at 100% RH. The resulting nematode suspensions (5 liters each) from both 90% and 100% RH trials were concentrated by passing through a 25-μm-pore sieve, and the live and dead nematodes were counted using a stereoscopic microscope. At each observation, 10 already washed leaves along with their petioles from each plant were stained with red dye to determine the presence of nematodes in the tissues. The growth chamber trial was repeated in 2004 as described above except that an additional treatment of 60% RH was included as a control and four replications were used instead of six. All 12 plants were allowed to sprout for 7 d at 90% RH; then four of them were maintained at 90% RH, four transferred to 60% RH, and four to 100% RH for 12 d to monitor the migration of nematodes on hosta foliage and in tissue, as described above.
Statistical analyses: Data on bud, leaf, and soil nematode populations from greenhouse, growth chamber, and overwintering sites and arcsine-transformed values of percentage mean number of nematodes per stages from overwintering, migration, and temperature tolerance studies were subjected to analysis of variance (ANOVA) using General Linear Models Procedure (SAS Institute, 1988). Significant differences between treatments were determined using the LSD test at P < 0.05. Changes in survival of nematodes in leaves exposed to different temperatures were compared using Tukey's mixed repeated measures analysis at P < 0.05 (SAS Institute, 1998).
Results
Identification of the nematodes: Based on morphometrics, 10 female [average body length = 0.68 mm (0.60–0.74 mm), a = 54.3 (46.2–60.0), b = 6.9 (6.00–8.2), c = 17.7 (10.0–13.6), V= 67.2 (64.9–68.8)] and 10 male nematodes [average body length = 0.63 mm (0.58–0.67 mm), a = 55.8 (44.7–65.0), b = 6.6 (5.8–7.0), c = 21.1 (18.5–23.6), length of dorsal limb of spicule = 0.01 mm (0.01–0.02)] from hosta Venessa were identified as A. fragariae.
Pathogenicity: Aphelenchoides fragariae extracted from both R solani cultures and naturally infected leaves of Venessa were pathogenic to all three cultivars of hosta (Table 1). In addition, when naturally infected leaves of hosta were used as a source of inoculum, of the 20, only four cultivars (including Borschi, Fragrant Blue, Patomic Pride, and Olive Bailey Langdon) showed no typical symptoms of nematode infection on their leaves (Table 1). Nematodes applied to either lower or upper sides of the noninjured and mechanically injured leaves on hosta plants were able to infect leaves and produce typical symptoms on nine cultivars. In addition, leaf injury played an important role in successful penetration and production of typical symptoms of nematode infection on leaves of seven cultivars (Francee, Fried Green Tomatoes, Hiroa Supreme, Love Pat, Royal Standard, Second Wind, and Tardiflora) (Table 1).
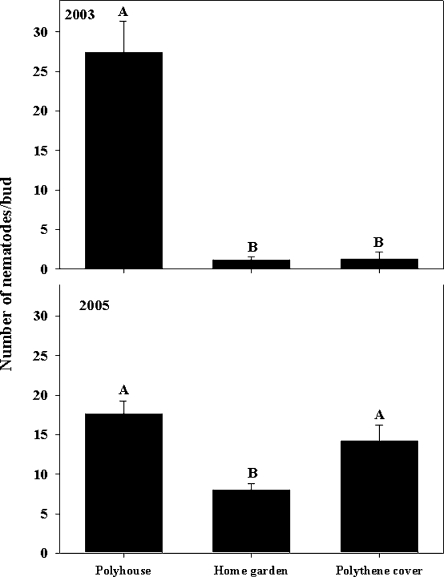
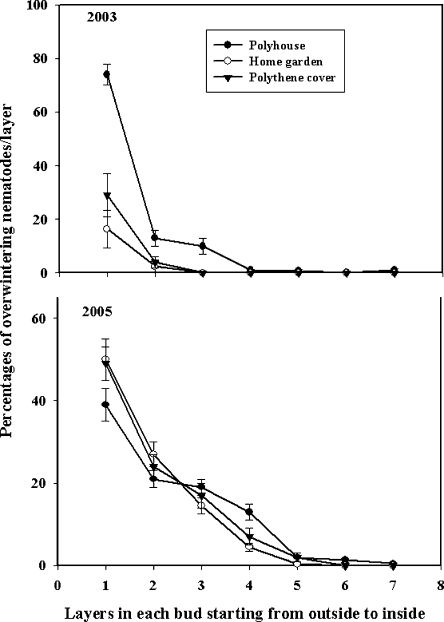
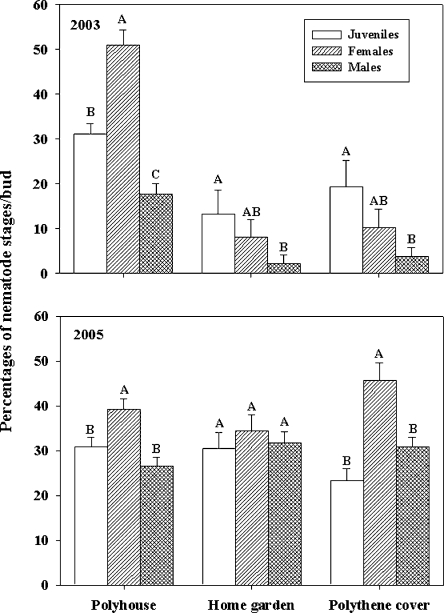
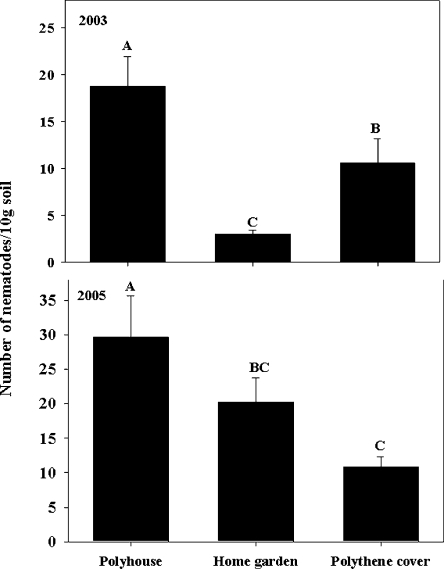
Overwintering survival: Aphelenchoides fragariae overwintered in the soil, dry leaves, and dormant buds but not in the roots of hosta. In 2003, nematode survival in the dormant buds was high (P < 0.05) only in the polyhouse; but in 2005, survival was higher (P < 0.05) in the polyhouse and under polythene cover than in the home garden (Fig. 1). Of the total number of nematodes found in each bud from all three overwintering sites, maximum numbers of nematodes (74%) were found between the two outermost layers of the bud (Fig. 2). Although nematodes were found as deep as the fourth and fifth layers of the buds, nematode numbers decreased in the deeper layers (Fig. 2). In the bud, nematodes survived as juveniles and adults (males and females) but not as eggs. In 2003, buds from the plants grown in the home garden harbored a higher (P < 0.05) percentage of juveniles (13%) and females (8%) compared to the males (2%), but in 2005, all stages were equally present (30–34%) in buds (Fig. 3). In 2003, the percentage of juveniles harbored in buds under polythene cover was higher (19%) than males (4%), but in 2005, the percentage of females was higher (46%) than both juveniles (23%) and males (31%) (P < 0.05). In both years, the proportion of females harbored in buds in the polyhouse was higher (39–51%) than juveniles (30–31%) and males (17–26%) (P < 0.05) (Fig. 3). Overall nematode survival in soil was higher (P < 0.05) in the polyhouse than under polythene cover or bare home garden in both years (Fig. 4). In soil, nematodes also survived as juveniles and adults (males and females) but not as eggs. In all overwintering sites, the proportion of females in soil was higher (P < 0.05) than the percentages of both juveniles and males, with the exception of the home garden, where equal numbers of juveniles and females were found (Fig. 5). Infected dry leaves from a home garden harbored approximately 39% juvenile, 39% female, and 22% male nematodes (data not shown).
Fig. 1.
Overwintering survival of Aphelenchoides fragariae during the winters of 2003–2004 and 2004–2005 in dormant buds of cultivar Patriot collected from three sites. Bars are means of 30 buds from each site. Bars (mean ± SE) with same letter(s) are not significantly (P < 0.05) different according to the LSD test.
Fig. 2.
Percent overwintering Aphelenchoides fragariae in each layer of dormant buds collected from three sites during 2003 and 2005. Each data point is a mean of 30 buds (± SE) from each site. Differences between layers were determined using the LSD test at P < 0.05.
Fig. 3.
Percentage of overwintering juveniles, females, and males of Aphelenchoides fragariae found in the dormant buds collected from three sites during 2003 and 2005. Bars are means of 30 buds from each site. Bars (mean ± SE) in the same overwintering site with same letter(s) are not significantly (P < 0.05) different according to the LSD test.
Fig. 4.
Overwintering survival of Aphelenchoides fragariae during 2003 and 2005 in the soil collected from three sites. Bars are means of 10 replications from each site. Bars (mean ± SE) with same letter) are not different according to the LSD test (P < 0.05).
Fig. 5.
Percentage of overwintering juveniles, females, and males of Aphelenchoides fragariae found in the soil collected from three sites during 2005. Bars are means of 10 replications from each site. Bars (mean ± SE) in the same overwintering site with same letter(s) are not significantly (P < 0.05) different according to the LSD test.
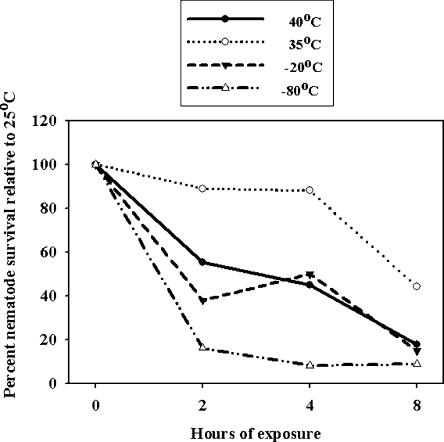
Temperature tolerance of A. fragariae: The nematodes tolerated exposure to extreme hot (40°C) and cold (−80°C) temperatures for 8 hr (Fig. 6). Percent survival of nematodes in leaves that were exposed to 40°C for 4 and 8 hr and to 35°C for 2 and 8 hr was lower (P < 0.05) than the control (25°C). In addition, the numbers of surviving nematodes in leaves were reduced (P < 0.05) within 2 hr of exposure at both −20°C and −80°C relative to the control (25°C) (Fig. 6).
Fig. 6.
Temperature tolerance of Aphelenchoides fragariae in infected hosta leaves exposed to −80°C, −20°C, 35°C, or 40°C for 2, 4, and 8 hr. Data are mean percent survival (four replicates) of nematodes relative to the control temperature (25°C) at which 100% nematode survival (0-hr exposure) was considered.
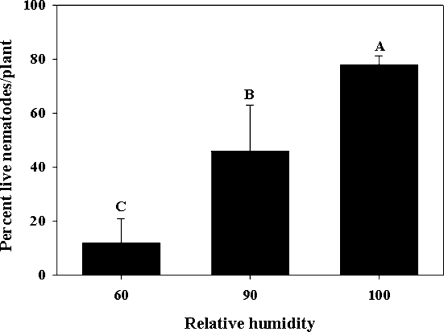
Migration of nematodes: In both years, relative humidity influenced the migration of A. fragariae from soil/crowns to leaves. Although many nematodes were found on the outer surface of both leaves and petioles (foliage) of plants when they were exposed to 90% or 100% RH, the percentage of live nematodes was higher (P < 0.05) at 100% RH than at 90% (51–66% vs. 25% survival) in 2003 (data not shown). At 100% RH, the percentage of live nematodes on the surface of plants was 51% and 66% at 6 and 12 d, respectively. Similar results were obtained in 2004 except that the percentage of live nematodes was much higher in both 90% RH (46% live nematodes) and 100% RH (77% live nematodes). Also, lower (P < 0.05) percentages of live nematodes were observed on leaves at 60% RH than at 90% and 100% RH (Fig. 7). In both trials, no nematodes were found inside the leaf or petiole tissues.
Fig. 7.
Percentage of live Aphelenchoides fragariae found on hosta foliage (leaves and petiole) after 12 d at different relative humidities during 2004. Data are means of four replications. Bars (mean ± SE) with same letter(s) are not significantly (P < 0.05) different according to the LSD test.
Discussion
Aphelenchoides fragariae isolated from naturally infected hosta plants and from fungal cultures were pathogenic to several cultivars of hosta. Previously, it had been reported that foliar nematodes enter leaf tissue only through stomata, which are located on the lower sides of leaves (Wallace, 1959). However, in the present study, we found that the nematodes applied on the upper side of leaves were also successful in entering leaf tissue and causing typical symptoms, suggesting that stomata are not the only portal of entry for the nematodes. In addition, we observed that when nema-tode-infected leaf tissue was used as a source of inoculum, nematodes were able to emerge and successfully infect healthy leaves. This suggests that nematodes can spread directly from infected plants to healthy plants via contact between healthy and infected leaves. We also found that in 13 cultivars of hosta it did not matter whether or not leaves were injured for successful nematode infection but in seven cultivars mechanical injury was required for infection. Decker (1972) also reported that A. fragariae enter the leaves through wounds. This suggests that the spread of foliar nematodes can be reduced if contact between healthy and infected plants can be avoided.
It is evident from our results that the nematodes could overwinter in soil, dry infected leaves, and dormant crowns, but not in the roots. We also discovered that A. fragariae nematodes overwinter as juveniles and adults, but not as eggs. Our results are similar to those of Lewis and Mai (1960), which were that the stem and bulb nematode, Ditylenchus dipsaci, overwintered in soil as pre-adults and adults but not as eggs. However, Mor and Spiegel (1993) reported that Aphelenchoides sub-tenuis, which is considered a root-feeding nematode, overwintered as eggs in the roots of Narcissus tazetta papyraceus. It has been suggested that D. dipsaci adults leave onion bulbs in the autumn to overwinter in soil and re-infect plants in the spring (Lewis and Mai, 1960). Our study indicates that A. fragariae can overwinter in soil, dry leaves, and the outermost layers of the dormant buds of hosta.
Survival of A. fragariae in soil and dormant buds was influenced by the location of plants. We found that higher numbers of nematodes survived in soil collected from the polyhouse than that collected from plants held under polythene cover or plants in the bare ground in the home garden. This suggests that the polyhouse may provide a more favorable microclimate, protection from extreme temperatures, and higher humidity compared to the other two overwintering sites. At the time of sampling, we recorded higher soil temperature in the polyhouse (−0.5°C to 0°C) than under polythene cover (−2°C) or bare home garden (−4°C). According to French and Barraclough (1962), a closely related species, A. ritzemabos, survived in frozen soil at −5°C for 15 mon. Ladigina (1956) also reported the tolerance of mixed stages of both D. destructor and D. allii and cysts of Heterodera schachtu to freezing temperatures below −28°C and −20°C, respectively. In the present study, we also found that A. fragariae could tolerate temperatures as low as −80°C.
As foliar nematodes feed and cause damage to leaves, the migration of overwintering nematodes from soil and dormant buds to leaves is an important step in their life cycle. In the present study, RH influenced nematode migration from soil to leaves. In addition, nematodes were present only on the outer surface of both leaves and petioles and not inside plant tissues. We found higher numbers of live nematodes on leaves and petioles at 100% than at 90% RH, suggesting that free moisture is required for the survival and movement of nematodes. These results are in agreement with the findings of Wallace (1959), who reported that wet-weather conditions or thin films of water were required for the movement and migration of A. ritzemabosi to leaves of chrysanthemum. Thus, the presence of both live and dead nematodes on the upper- and lower sides of leaves and petioles of hosta in the May confirmed that A. fragariae had migrated from soil to leaves. This suggests that the overwintering A. fragariae could become active in early spring and migrate to leaves in films of water on the leaf petioles or stems to cause infection during spring by infecting leaf tissue. This provides impetus for developing effective management practices as demonstrated by Jagdale and Grewal (2004). These researchers showed that drench application of 90°C water in the autumn suppressed the population of foliar nematodes in the soil and reduced the numbers of nematode-infected leaves per plant in summer.
Footnotes
This research was supported by the American Hosta Society, Great Lakes Hosta Society, Midwest Hosta Society, Central Ohio Hosta Society, Ohio Agricultural Research and Development Center, and Horticulture Research Institute. The authors thank C. W. Hoy for advice on the use of image analysis system, J. Smith for helping to set up LI-3100 Area Meter, and Klyn Nurseries Inc., Perry, OH, Blueberry Patch Nursery, Mansfield, OH, and Wade and Gaton Nurseries, Belleville, OH, for providing their greenhouse facilities, plants, and assistance for the experiments.
This paper was edited by Jim LaMondia
Literature Cited
- Baermann G. Eine einfache methode zur auffindung von anchylostomum- (Nematoden)-larven in erdproben. Geneeskundig Tijdschrift Nederlands Indië. 1917;57:131–137. [Google Scholar]
- Buckley, R. J., and Gould, A. B. 2003. Foliar nematodes in ornamental plants. Plant Disease Control fact sheet. Rutgers Co-operative Extension, The State University of New Jersey. https://www.rce.rutgers.edu/pubs/pdfs/fs878.pdf.
- Decker H. Plant nematodes and their control (phytonematology) Moscow: Kolos Publishers; 1972. Leaf-parasitic nematodes; pp. 354–368. [Google Scholar]
- French N, Barraclough RM. Survival of Aphelenchoides ritzemabosi (Schwartz) in soil and dry leaves. Nematologica. 1962;7:309–316. [Google Scholar]
- Grewal PS, Jagdale GB. Biology and management of foliar nematodes. The Hosta Journal. 2001;32:64–66. [Google Scholar]
- Heinlein MA. Columbus: The Ohio State University; 1982. Symptomology and host range of Aphelenchoides fragariae (Ritzema Bos, 1890) Christie, 1932, on the gesneriaceae. MSc Thesis. [Google Scholar]
- Hooper DJ. Handling, fixing, staining, and mounting nematodes. In: Southey JF, editor. Laboratory methods for work with plant soil nematodes. London: Her Majesty's Stationery Office; 1986. pp. 59–80. [Google Scholar]
- Hunt DJ. Wallingford, UK: CAB International; 1993. Aphelenchida, Longidoridae, and Trichodoridae: Their systematics and bionomics. [Google Scholar]
- Jagdale GB, Grewal PS. Identification of alternatives for the management of foliar nematodes in floriculture. Pest Management Science. 2002;58:451–458. doi: 10.1002/ps.472. [DOI] [PubMed] [Google Scholar]
- Jagdale GB, Grewal PS. Effectiveness of a hot water drench for the control of foliar nematodes, Aphelenchoides fragariae, in floriculture. Journal of Nematology. 2004;36:49–53. [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Gill DL. Chemical control of foliar nematodes (Ahpelenchoidesfragariae) on ‘Fluffy Ruffles’ fern. Plant Disease Reporter. 1975;59:272–274. [Google Scholar]
- Ladigina NM. Problemi-parazitologii. Transactions of the Scientific Conference of Parasitologists of the Ukrainian SSR; 1956. pp. 298–299. [Google Scholar]
- LaMondia JA. First report of the foliar nematode Aphelenchoides fragariae infecting lamium. Plant Disease. 1995;79:642–642. [Google Scholar]
- LaMondia JA. Efficacy of insecticides for control of Aphel-enchoide fragariae and Ditylenchus dipsaci in flowering perennial ornamentals. Supplement to the Journal of Nematology. 1999;31:644–649. [PMC free article] [PubMed] [Google Scholar]
- Lewis GD, Mai WF. Overwintering and migration of Ditylenchus dipsaci in organic soils of New York. Phytopathology. 1960;50:341–343. [Google Scholar]
- Mor M, Spiegel Y. Infection of Narcissus roots by Aphelenchoides subtenuis . Journal of Nematology. 1993;25:476–479. [PMC free article] [PubMed] [Google Scholar]
- Richardson PN, Grewal PS. Nematode pests of glasshouse crops and mushrooms. In: Evans K, Trudgill DL, Webster JM, editors. Plant-parasitic nematodes in temperate agriculture. Wallingford, UK: CAB International; 1993. pp. 501–544. [Google Scholar]
- Sanwal KC. A simple method for rearing pure populations of the foliar nematode, Aphelenchoide ritzemabosi, in the laboratory. Canadian Journal of Zoology. 1959;37:707–711. [Google Scholar]
- Siddiqi MR. Wallingford, UK: CAB International; 2000. Tylenchida parasites of plants and insects. [Google Scholar]
- Smith, T. M. 2005. Greenhouse management. Extension fact sheet, University of Massachusetts. http://www.umass.edu/umext/floriculture/fact_sheets/greenhouse_management/overwint.html.
- Southey JF. Nematode pests of ornamental and bulb crops. In: Evans K, Trudgill DL, Webster JM, editors. Plant-parasitic nematodes in temperate agriculture. Wallingford, UK: CAB International; 1993. pp. 463–500. [Google Scholar]
- Thies JA, Merrill SB, Corley EL. Red food coloring stain: New, safer procedures for staining nematodes in roots and egg masses on root surfaces. Journal of Nematology. 2002;34:179–181. [PMC free article] [PubMed] [Google Scholar]
- Wallace HR. Movement of eelworms. V. observations on Aphelenchoides ritzemabosi (Schwartz, 1912) Steiner, 1932, on florists' chrysanthemums. Annals of Applied Biology. 1959;47:350–360. [Google Scholar]
- Whitehead AG, Hemming JR. Acomparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology. 1965;55:25–38. [Google Scholar]