Abstract
Changes in land use and the associated changes in land cover are recognized as the most important component of human-induced global change. Much attention has been focused on deforestation, but grasslands are among the most endangered ecosystems on Earth. The North American tallgrass prairie is a dramatic example, exhibiting a greater than 95% decline in historical area. Renewed interest in prairie conservation and restoration has highlighted the need for ecological indicators of disturbance and recovery in native systems, including the belowground component. The tallgrass prairie differs from the agricultural systems that have replaced it in having greater diversity and heterogeneity of resources, less physical soil disturbance (although other disturbances, such as fire and grazing, are prominent), and greater nitrogen limitation. Understanding the responses of nematode taxa to these characteristic differences is crucial to the development and improvement of community indices, but while knowledge of disturbance responses by individual taxa is accumulating, the level of necessary taxonomic resolution remains in question. Although nematode communities generally are better described for temperate grasslands than for other natural ecosystems, identification of sentinel taxa is further confounded by high levels of diversity, and both spatial and temporal heterogeneity.
Keywords: agroecosystem, community, disturbance, ecological indicator, land-use change, natural ecosystem, nematode, restoration, sentinel taxa, soil, tallgrass prairie
Land-use change and the associated changes in land cover are recognized as the leading component of global environmental change (Vitousek, 1994; Sala et al., 2000). Deforestation in the tropics may be the most dramatic contemporary example, but major land-use change has already occurred for grasslands, placing them among the most endangered ecosystems on Earth. Loss of tallgrass prairie, for example, represents the largest land-use conversion in North America (Samson and Knopf, 1994). Much of the former prairie has been replaced by agricultural ecosystems with disturbance regimes that fundamentally alter belowground communities (Wolters et al., 2000). Replacement of natural systems with managed ones is known to affect both the relative abundance and distribution of native species as well as introduce and disperse exotic nematode species (Yeates, 1991).
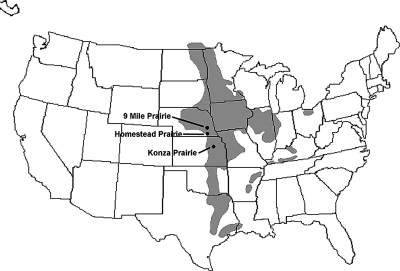
The tallgrass prairie once covered a vast area of North America, extending west from Indiana to Nebraska and north from Texas to Canada (Fig. 1). Soils rich in glacially derived loess and accumulated organic matter supported a diverse assemblage of warm-season grasses and deep-rooted herbaceous forbs. Today, less than 1% of the original tallgrass prairie remains (Ricketts et al., 1999). Remnant prairie exists mainly as small discontinuous patches, surrounded by agronomic fields or residential developments. Preservation and restoration of these species-rich prairies is a high-priority goal of conservation biology (Risser, 1996). Prairie restoration, however, is not a simple matter of establishing a prescribed mix of warm-season grasses and forbs. A functional prairie ecosystem includes innumerable soil microbes and soil invertebrates that comprise an elaborate food web that has evolved over thousands of years (Shirley, 1994). Among the most numerous and diverse organisms in this ecosystem are nematodes. We are only beginning to understand the functional roles and relationships among nematodes in prairie soils, and their application as indicators of ecosystem properties is in its nascence. In this paper, we interpret current and past research on nematodes of the tallgrass prairie in light of their potential use as indicators of ecosystem function.
Fig. 1.
Historical distribution of the tallgrass prairie in North America showing locations of Homestead National Monument, Konza Prairie, and Nine-mile Prairie.
Natural Ecosystems: The Tallgrass Prairie as a Model
The vegetation of the tallgrass prairie is characterized by a “core” of dominant C4 grasses (Andropogon gerardiiVitman, Schizachyrium scoparium (Michx.) Nash, Sorghastrum nutans (L.) Nash, and Panicum virgatum L.) that is spatially and temporally homogeneous relative to interspersed “satellite” species of subdominant grasses and forbs (Freeman, 1998; Hartnett and Fay, 1998). The tallgrass prairie is unique among natural grasslands in exhibiting high rates of primary productivity (most of it belowground) in the presence of low nutrient availability. In general, there is a poor correlation between plant productivity and soil nitrogen (N) availability at landscape scales (Turner et al., 1997), due largely to the high nitrogen-use efficiency exhibited by the dominant C4 grasses (Ojima et al., 1994). Still, N is a limiting nutrient in frequently burned tallgrass prairie, and large increases in productivity are possible following N fertilization (Owensby et al., 1970). Available phosphorus (P) levels are similarly low in tallgrass prairie soils, but mycorrhizal associations with the dominant grasses ensure that production limitations due to this nutrient are unlikely (Hetrick et al., 1988, 1989).
Natural grassland and forest systems typically exhibit fungal-dominated decompositional pathways driven by high C:N substrate ratios (Ferris et al., 2001). In tall-grass prairie soil, fungi account for as much as three-fourths of the total microbial respiration (Rice et al., 1998), and this is reflected in a similar ratio of fungal-to bacterial-feeding nematodes (Todd, 1996). In contrast to natural systems, the agricultural systems that replaced much of the tallgrass prairie are characterized by nutrient enrichment and physical soil disturbance, both of which have important consequences for soil biotic communities and ecosystem processes (Neher, 1999). While these disturbance types can differentially affect nematode communities (Fiscus and Neher, 2002), they appear to have similar impacts on decompositional pathways, with both N enrichment and tillage favoring bacterial-dominated pathways and increased relative abundance of bacterial-feeding nematodes (Wardle, 1995; Todd, 1996; Bardgett and McAlister, 1999; Lenz and Eisenbeis, 2000; Liphadzi et al., 2005).
Natural ecosystems are best described by nonequilibrium models (Wu and Loucks, 1995), and this is a defining characteristic of the tallgrass prairie, where multiple limiting resources (light, water, and N) result in spatial and temporal heterogeneity in productivity patterns of above- and belowground plant and consumer communities (Seastedt and Knapp, 1993). This observation is relevant to any discussion of bioindicators of soil health because the structure and composition of indicator (e.g., nematode) communities is likely to be transient and variable across the landscape. In tallgrass prairie, the relative importance of multiple limiting resources is determined by the interaction of fire frequency, grazing intensity, and climate variability. A mechanistic understanding of nematode community responses to these characteristic disturbances should advance the broader use of nematodes as indicators of disturbance and recovery in both natural and managed ecosystems.
The Tallgrass Prairie Nematode Community: Conceptual Challenges
A fundamental assumption is that a tallgrass prairie nematode community exists. This assumption needs to be examined before we can ask what distinguishes a prairie nematode community from those in other ecosystems. First, we need to determine the appropriate scale for characterization of the reference community used in restoration efforts. The tallgrass prairie itself, as defined by a specific assemblage of plant species adapted for life on the Central Plains of North America, can exist on different soil types across a broad latitudinal gradient. For example, Konza Prairie, located in the Flint Hills of eastern Kansas (Fig. 1), exists on rolling hills comprised of shale and cherty limestone with a thin covering of loess. Plowing these rocky soils is extremely difficult, which explains why the Flint Hills region is the largest remaining intact remnant of tallgrass prairie. Eighty miles north is Homestead National Monument, which includes the second-oldest restored prairie in North America. Here, the flatter loess-covered hills have deeper silty clay loam soils typical of eastern Nebraska and much of the Western Corn Belt Plains. Based on native vegetation, both areas are classified as classic tallgrass prairies. These areas are in distinctly different ecoregions based on biotic and abiotic factors such as geology, physiography, vegetation, climate, land use, wildlife, hydrology, and soils (Chapman et al., 2001). A nematode community defined solely on the basis of tallgrass prairie plants may obscure patterns due to the coarse scale of analysis. A more appropriate level of analysis for the establishment and characterization of reference nematode communities may be at the ecoregional or subecoregional scale.
A second consideration when searching for patterns or associations indicative of nematode communities is the temporal scale. Dramatic climatic changes have occurred during the 1.8 million years of Pleistocene Ice Ages, influencing plant and animal communities through successive advances and retreats of glaciers. The retreat of the Wisconsin glaciations 18,000 years ago was followed by advancing coniferous and hardwood forests before drying conditions favored a grassland biota. The “true” prairie assemblage of plants and animals probably did not form until 8,000 to 10,000 years ago, derived from glacial refugia south, west, and northwest of the glacial front (Pielou, 1991). From an evolutionary perspective, this short time period is hardly sufficient for the evolution of many endemic species. Therefore, the distinctiveness of the prairie ecosystem may reside in the unique assemblage of organisms and not the existence of unique forms.
Early surveys of tallgrass prairie soils described nematode communities as exceptionally rich in species diversity (Orr and Dickerson, 1967; Norton and Ponchillia, 1968; Thorne and Malek, 1968; Schmitt and Norton, 1972; Thorne, 1974; Norton and Schmitt, 1978). More recent studies tend to substantiate this high diversity (Mullin et al., 2004). Comparisons among studies, however, are difficult because species concepts and sampling strategies cannot be standardized for area (Scheiner et al., 2000). At best, studies of nematode diversity on North American temperate grasslands allow us to say grasslands sustain high species richness relative to other biomes when standardized for sampling intensity (Boag and Yeates, 1998).
Thorne and Malek (1968) speculated that the nematode communities of the Northern Great Plains were homogeneous due to soil adhering to bison following their habit of rolling in muddy “wallows.” The nematodes would then be deposited across the prairie during the bison's seasonal migrations. A different view of nematode communities has been presented for tall-grass prairies in Iowa (Norton and Ponchillia, 1968; Schmitt and Norton, 1972; Norton and Schmitt, 1978). In these studies, plant-parasitic nematodes appear to be structured according to topography, with hillsides, ridges, and moist lowlands displaying distinctly different plant-parasitic nematode associations. Moist lowland habitats were populated typically by a nematode community that included Helicotylenchus hydrophilus, Hoplolaimus galeatus, Xiphinema chambersi, and an undescribed Tylenchorhynchus species. These nematodes were seldom a component of the drier ridges, where the characteristic nematode community included Helicotylenchus pseudorobustus, Tylenchorhynchus nudus, and Xiphinema americanum. It is notable that a generic-level analysis would combine these species, consequently overlooking them as potential indicators. This local variation among habitats is overlaid on a regional scale that exhibits different species occupying the role of predominant plant parasites. For example, seven Helicotylenchus species are distributed unevenly among four remnant prairies in Iowa (Norton and Ponchillia, 1968). We do not know if this distribution is due to subtle differences in plant species composition, soil factors, initial events in nematode community assemblage, and/or sampling intensity. Patterns of nematode distribution may be particularly difficult to infer from species lists when sampling intensity varies among studies. A sampling of 8,400 specimens along a single ridge on Konza Prairie revealed that, of the 375 morphologically identified species, most were present as rare species, with less than 25 taxa recovered at a frequency higher than 1% (Mullin et al., 2004). Rarity is a common feature in ecological communities and should be considered in sampling designs (Magurran and Henderson, 2003).
Nematodes as Indicators of Prairie Restoration
The definition and goals of ecological restoration remain somewhat contentious (Davis and Slobodkin, 2004; Winterhalder et al., 2004). Regardless of philosophy or objectives, however, good baseline data are required to assess the restoration process. The selection and use of extant reference sites is basic to restoration ecology (White and Walker, 1997). Before a local tall-grass prairie nematode community can be adequately characterized to serve as a reference site, nematode response to disturbance needs to be understood. Ironically, this task is especially complicated on the prairie because the prairie itself is a disturbance-adapted ecosystem (Knapp and Seastedt, 1998). Periodic fire and intense grazing, together with the physical disturbance and nutrient inputs associated with grazing, constitute normal ecosystem events that affect nematode communities directly and indirectly. The death of a large ungulate provides a good example of a complex natural disturbance. When a bison or cow dies, it contributes hundreds of pounds of nutrient-rich body contents to the soil (Towne, 2000). Within a few days, the nematode community beneath the carcass changes dramatically. The soil nematode community goes from a plant parasite/fungal feeder-dominated system to a community comprised solely of one to two species of bacterial feeders (Powers, unpubl.). The pre-disturbance community virtually disappears or is overwhelmed by the sheer abundance of the bacterial-feeding nematodes. It is not known if the specific bacterial feeders are transported phoretically by insects or if they normally reside in the soil as rare species. On Konza Prairie, bison death sites are associated with a drop in pH from 7.3 to 5.7 and a significant elevation of inorganic N and P levels that persist for several years after the death (Towne, 2000). Five years after bison death, the vegetation still resembles that of a disturbed community. Preliminary sampling of death site soils suggests that the changes also persist in the soil nematode community (pers. observ.). Clearly, changes of this nature must be considered when evaluating reference sites for prairie restoration.
Anthropogenic disturbances can cause similar dramatic disruptions of tallgrass prairie soil communities. Periodic pipeline spills of crude oil at active oil production sites on native prairie in the southern Flint Hills provide a salient example. The nematode communities at such hydrocarbon-impacted sites are sensitive indicators of soil food web recovery during the remediation and restoration process (Mehta, 2004). As observed for sites of bison death, severely impacted areas at one study site remained dominated by bacterial-feeding nematodes 5 yr after contamination, although there was a steady increase in abundance of cephalobid relative to rhabditid taxa during this period. Taxa showing the least recovery after 5 yr included Helicotylenchus, Criconematidae, Tylenchidae, Plectidae, and Prismatolaimus (Todd, unpubl.). It is yet to be determined if these anthropogenic disturbances follow a long-term trajectory similar to those associated with bison carcass decomposition.
Agricultural fields, of course, represent the more common and less extreme context for restoration of tallgrass prairie but, unfortunately, there has been limited characterization of nematode communities from areas restored after cultivation. A recent survey across a chronosequence of restoration at Homestead National Monument revealed that of the 94 genera and 213 species observed, 29% and 31%, respectively, occurred in native and restored prairie but not in adjacent cropland (Mullin and Powers, unpubl.). Most of these taxa, represented prominently by the Tylenchidae, Belondiridae, and Criconematoidea, were associated with restoration areas older than 30 yr or were found only in undisturbed prairie.
Yeates and Bongers (1999) have argued that established grasslands are the best benchmark land use for many agricultural regions, and this is certainly valid for much of temperate North America. We echo their concerns that scale, as well as variability in biotic and abiotic factors, must be considered in establishing such a reference. Grasslands are patchy. In such heterogeneous systems, soil texture, plant community, and management practice have marked effects on nematode community composition. Similarity among nematode assemblages is determined largely by habitat similarity, with both varying at the landscape scale (Johnson et al., 1972; Schmitt and Norton, 1972; Johnson et al., 1974).
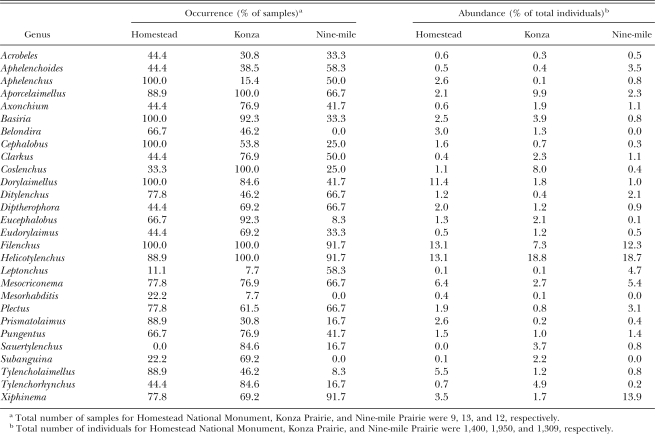
Still, available data suggest that general patterns in tallgrass prairie nematode assemblages are discernible. Frequency data from Konza Praire, Nine-mile Prairie, and the Homestead National Monument (Fig. 1) suggest a characteristic nematode assemblage consisting of (in order of importance): Filenchus, Helicotylenchus, Xiphinema, Aporcelaimellus, Dorylaimellus, Mesocriconema, Basiria, Plectus, Coslenchus, and Pungentus (Table 1). In some cases, species resolution is informative. For example, three co-occurring species of Helicotylenchus, H. digonicus, H. platyurus, and H. pseudorobustus are encountered across tallgrass prairie sites (Orr and Dickerson, 1967; Norton and Schmitt, 1978) but are not consistently associated with agricultural fields in the region (Powers et al., 1997). In other cases, such as with Aporcelaimellus obscurus, species with a high frequency of occurrence in tallgrass prairie are widespread across the region and offer less potential as indicator taxa (Thorne, 1974).
Table 1.
Common nematodes of the tallgrass prairie.
The relative abundances of the common taxa in tall-grass prairie soils are particularly informative. The dominant family in terms of both abundance and diversity is the Tylenchidae, which typically accounts for more than 30% of total abundance and approximately 20% of species richness (Todd, 1996; Todd et al., 1999; Mullin and Powers, unpubl. data). In contrast, this family comprises 10% to 20% of total nematode numbers in forest and agricultural systems (Freckman and Ettema, 1993; Lenz and Eisenbeis, 2000; Liphadzi et al., 2005; Neher et al., 2005). Unfortunately, feeding habits and functional contributions to soil processes remain poorly described for the group as a whole, limiting the potential of these taxa as ecological indicators. Both plant feeding and fungal feeding have been observed within the family (Wood, 1973), but evidence for a fungal-feeding habit for the major taxa found in prairie soils (e.g., Filenchus) is accumulating (Todd, 1996; Okada and Kadota, 2003). Even with the limited life-history information available, we suggest that Tylenchidae be given greater consideration when making inferences on the ecological condition of soils in general and that this group is particularly important when grasslands are the benchmark.
The most striking characteristic of prairie nematode assemblages is the low relative abundance of bacterial-feeding taxa, which averages no more than 10% to 20% (Todd, 1996; Todd et al., 1999), compared to 30% to 45% for forest and agricultural sites (Freckman and Ettema, 1993; Lenz and Eisenbeis, 2000; Yeates, 2003; Liphadzi et al., 2005; Neher et al., 2005). This feature is a predictable outcome of the nutrient limitation of the prairie system and the resultant soil food web structure discussed previously. In contrast to the suggestion by Wardle and Yeates (1993) that food resources are more likely to limit fungivores than bacterivores, it is the bac-terivorous nematode densities in tallgrass prairie that are N-limited (Todd et al., 1992; Todd, 1996). Cephalobidae is the dominant family, as is representative of many natural and managed ecosystems (Yeates, 2003). Unlike the majority of these systems, however, Rhabditidae typically represents < 1% of the total nematode abundance in tallgrass prairie soil. Resource pulses due to disturbance or changing land management practices often lead to increases in the relative abundance of this group of opportunistic taxa (Ferris et al., 2001; Yeates, 2003). The near absence of Rhabditidae from native, undisturbed prairie soils suggests that their increase and decline should be reliable indicators of disturbance and recovery, respectively, in the prairie system.
Dorylaimid taxa are well represented in prairie soil, comprising > 40% of species richness (Mullin and Powers, unpubl. data). Their usefulness as ecological indicators, however, is limited by low abundance (typically < 15–20% of total abundance for all taxa combined) and by inadequate information on trophic habits. One group that likely does deserve indicator status is the Belondiridae. Axonchium, Belondira, and Dorylaimellus all are frequently encountered in prairie soils (Orr and Dickerson, 1967; Mullin and Powers, unpubl.), but are relatively rare in agricultural soils (Freckman and Ettema, 1993; Neher et al., 1998, 1999). The use of assemblages of dorylaimid species to identify spatial distribution patterns and, potentially, soil disturbance and recovery offers another promising approach (Liebanas et al., 2002).
Responses of Prairie Nematode Taxa to Disturbance
As emphasized throughout this discussion, the tall-grass prairie is a disturbance-adapted ecosystem, with its biotic communities determined by direct and indirect responses to fire, ungulate grazing, and a variable climate (Knapp and Seastedt, 1998). The role of these disturbances in defining ecosystem processes has been the focus of research at Konza Prairie, one of six original Long-Term Ecological Research (LTER) sites funded by the National Science Foundation (NSF), since 1980. Of the factors influencing tallgrass prairie, fire and its effects on nutrient dynamics provide a valuable tool for studying the mechanisms associated with soil ecosystem responses to an important component of land-use change, nutrient limitation/enrichment, in a natural setting. These relationships are emphasized in a long-term Belowground Plot experiment established in 1986 as part of the Konza Prairie LTER Program. The experimental design and a synthesis of biotic responses are available in Rice et al. (1998). Here, we discuss some of the nematode community responses to fire and N enrichment within the context of the major land-use change in the region, conventional agriculture.
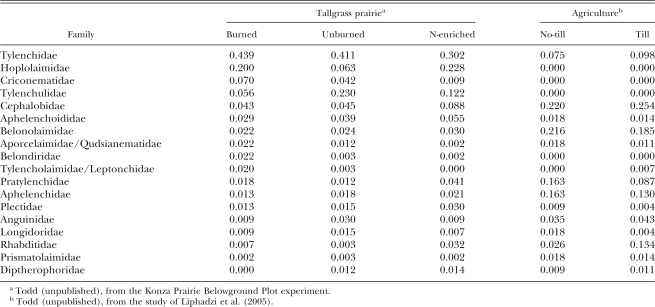
A comparison of the relative abundances of the major nematode families in Kansas prairie and agricultural soils under varying management practices reveals distinct patterns related to increasing levels of N enrichment (Table 2). Several important prairie families, including Tylenchidae and Belondiridae, decline under N enrichment and/or tillage. Similar declines in relative abundances of both groups occurred with increasing human intervention in a study at the Kellogg Biological Station LTER site in Michigan (Freckman and Ettema, 1993). In a comparison of pasture and cultivated soils in North Carolina, abundance of Tylenchidae and Belondiridae increased and decreased, respectively, with disturbance (Neher et al., 2005).
Table 2.
Relative abundance of the dominant nematode families under varying management practices in natural and agricultural prairie soils in Kansas.
Nematode families increasing with N enrichment include the bacterivorous Cephalobidae and Rhabditidae and the fungivorous Aphelenchidae (Table 2). The opposite relationship with disturbance also has been observed for cephalobid taxa (Freckman and Ettema, 1993; Neher et al., 2005). Substantial evidence remains, however, for using the relative abundance of cephalobid compared to rhabditid taxa as an indicator of disturbance and recovery, particularly where nutrient enrichment is involved (Yeates, 2003).
Dorylaimid and mononchid taxa are perennially favored for indicator status because they are reported to be broadly sensitive to soil disturbance (Johnson et al., 1974; Bongers, 1999; Ferris et al. 2001). These taxa traditionally are classified as indicators of community structure and stability along a colonizer-persister (CP) continuum (Bongers, 1990, 1999). The sensitivities of a number of taxa, however, are inconsistent with their CP value (Fiscus and Neher, 2002) and, with the exception of Belondiridae, this generalization of susceptibility to disturbance is not supported for the major prairie dorylaimid families (Table 2). Conflicting evidence of disturbance sensitivity exists for several major prairie genera, including Aporcelaimellus and Eudorylaimus (Freckman and Ettema, 1993; Fiscus and Neher, 2002).
The level of taxonomic resolution required to accurately assess and characterize nematode responses to disturbance remains a significant issue. A growing body of evidence suggests that genera within a family can vary markedly in their sensitivity to a given disturbance (Fiscus and Neher, 2002), and some have argued that species-level discrimination is necessary (Yeates, 2003). Bacterivorous taxa in the Belowground Plot experiment display both widely divergent within-family responses as well as similar across-family responses to burning and N enrichment (Jones et al., 2006). For example, although Cephalobidae, Plectidae, and Rhabditidae are thought to be indicators of resource limitation, degraded or stressed environments, and nutrient enrichment, respectively (Ferris et al., 2001), specific taxa within all three families respond similarly to N-enrichment. To date, consistent variation in species-level responses has not been detected. Clearly, this is a greater concern with herbivorous taxa, where the plant community is more likely to directly supersede or mask any disturbance effects.
Research suggests that the indirect effects of disturbance (i.e., consequent changes in the soil environment) on nematode communities are greater than the direct effects (Blair et al., 2000; Fiscus and Neher, 2002). This necessarily introduces greater unpredictability into the measurement and interpretation of nematode responses across environmental and ecosystem gradients, as indirect effects are more likely to be context-dependent. The designation of sentinel status to key nematode taxa at regional or larger scales remains conditional on a better understanding (at the appropriate level of resolution) of the mechanisms driving their responses (Neher, 2001). Application of the sentinel concept to nematode communities in natural systems presents an obvious challenge: the assignment of environment-dependent disturbance responses at a fine level of taxonomic resolution across spatially and temporally heterogeneous, nonequilibrium systems.
Footnotes
This paper was edited by G. W. Yeates.
Symposium paper presented at the 43rd Annual Meeting of The Society of Nematologists, 7-11 August 2004, Estes Park, CO. Contribution no. 06-58-J, Kansas Agricultural Experiment Station, Manhattan; Journal Series no. 14667, Agricultural Research Division, University of Nebraska-Lincoln.
Literature Cited
- Bardgett RD, McAlister E. The measurement of soil fungal:bacterial biomass ratios as an indicator of ecosystem self regulation in temperate meadow grasslands. Biology and Fertility of Soils. 1999;29:282–290. [Google Scholar]
- Blair JM, Todd TC, Callaham MA., Jr . Responses of grassland soil invertebrates to natural and anthropogenic disturbances. In: Coleman DC, Hendrix PF, editors. Invertebrates as webmasters in ecosystems. New York: CABI Publishing; 2000. pp. 43–71. [Google Scholar]
- Boag B, Yeates GW. Soil nematode biodiversity in terrestrial ecosystems. Biodiversity and Conservation. 1998;7:617–630. [Google Scholar]
- Bongers T. The maturity index, an ecological measure of environmental disturbance based on nematode species composition. Oecologia. 1990;83:14–19. doi: 10.1007/BF00324627. [DOI] [PubMed] [Google Scholar]
- Bongers T. The maturity index, the evolution of nematode life history traits, adaptive radiation, and CP-scaling. Plant and Soil. 1999;212:13–22. [Google Scholar]
- Chapman SS, Omernik JM, Freeouf JA, Huggins DG, McCauley JR, Freeman CC, Steinauer G, Angelo RT, Schlepp RL. Ecoregions of Nebraska and Kansas. (Map poster) Reston, VA: US Geological Survey; 2001. [Google Scholar]
- Davis MA, Slobodkin LB. The science and values of restoration ecology. Restoration Ecology. 2004;12:1–3. [Google Scholar]
- Ferris H, Bongers T, de Goede RGM. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Fiscus DA, Neher DA. Distinguishing sensitivity of free-living soil nematode genera to physical and chemical disturbances. Ecological Applications. 2002;12:565–575. [Google Scholar]
- Freckman DW, Ettema CH. Assessing nematode communities in agroecosystems of varying human intervention. Agriculture, Ecosystems and Environment. 1993;45:239–261. [Google Scholar]
- Freeman CC. The flora of Konza Prairie: A historical review and contemporary patterns. In: Knapp AK, Briggs JM, Hartnett DC, Collins SL, editors. Grassland dynamics: Long-term ecological research in tallgrass prairie. New York: Oxford University processes; 1998. pp. 69–80. [Google Scholar]
- Hartnett DC, Fay PA. Plant populations: Patterns and processes. In: Knapp AK, Briggs JM, Hartnett DC, Collins SL, editors. Grassland dynamics: Long-term ecological research in tallgrass prairie. New York: Oxford University Press; 1998. pp. 81–100. [Google Scholar]
- Hetrick BAD, Kitt DG, Wilson GWT. Mycorrhizal dependence and growth habit of warm-season and cold-season tallgrass prairie plants. Canadian Journal of Botany. 1988;66:1376–1380. [Google Scholar]
- Hetrick BAD, Kitt DG, Wilson GWT. Relationship between mycorrhizal dependence and competitive ability of two tallgrass prairie grasses. Canadian Journal of Botany. 1989;67:2608–2615. [Google Scholar]
- Johnson SR, Ferris JM, Ferris VR. Nematode community structure of forest woodlots. III. Ordinations of taxonomic groups and biomass. Journal of Nematology. 1974;6:118–126. [PMC free article] [PubMed] [Google Scholar]
- Johnson SR, Ferris VR, Ferris JM. Nematode community structure of forest woodlots. I. Relationships based on similarity coefficients of nematode species. Journal of Nematology. 1972;4:175–183. [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Todd TC, Wall-Beam JL, Coolon JD, Blair JM, Herman MA. Molecular approach for assessing responses of microbial-feeding nematodes to burning and chronic nitrogen enrichment in a native grassland. Molecular Ecology. 2006 doi: 10.1111/j.l365-294X.2006.02971.x. [DOI] [PubMed] [Google Scholar]
- Knapp AK, Seastedt TR. Introduction: Grasslands, Konza Prairie, and long-term ecological research. In: Knapp AK, Briggs JM, Hartnett DC, Collins SL, editors. Grassland dynamics: Long-term ecological research in tallgrass prairie. New York: Oxford University Press; 1998. pp. 3–15. [Google Scholar]
- Lenz R, Eisenbeis G. Short-term effects of different tillage in a sustainable farming system on nematode community structure. Biology and Fertility of Soils. 2000;31:237–244. [Google Scholar]
- Liebanas G, Pena-Santiago R, Real R, Marquez AL. Spatial distribution of dorylaimid and mononchid nematodes from southeast Iberian Peninsula: Chorological relationships among species. Journal of Nematology. 2002;34:390–395. [PMC free article] [PubMed] [Google Scholar]
- Liphadzi KB, Al-Khatib K, Bensch G, Stahlman PW, Dille JA, Todd T, Rice CW, Horak MJ. Soil microbial and nematode communities as affected by glyphosate and tillage practices in a glyphosate-resistant cropping system. Weed Science. 2005;53:536–545. [Google Scholar]
- Magurran AE, Henderson PA. Explaining the excess of rare species in natural species abundance distributions. Nature. 2003;422:714–716. doi: 10.1038/nature01547. [DOI] [PubMed] [Google Scholar]
- Mehta C. University of Tulsa; 2004. A study of the ecological indicators in the biore-mediation and restoration of crude oil contaminated soil. P.hD. dissertation. [Google Scholar]
- Mullin PG, Harris TS, Higgins RS, Powers TO. An 18S DNA barcode applied to nematodes from the Konza Tallgrass Prairie. Journal of Nematology. 2004;36:336. (abstr.). [Google Scholar]
- Neher DA. Soil community composition and ecosystem processes: Comparing agricultural ecosystems with natural ecosystems. Agroforestry Systems. 1999;45:159–185. [Google Scholar]
- Neher DA. Role of nematodes in soil health and their use as indicators. Journal of Nematology. 2001;33:161–168. [PMC free article] [PubMed] [Google Scholar]
- Neher DA, Easterling KN, Fiscus D, Campbell CL. Comparison of nematode communities in agricultural soils of North Carolina and Nebraska. Ecological Applications. 1998;8:213–223. [Google Scholar]
- Neher DA, Weicht TR, Savin M, Gorres JH, Amador JA. Grazing in a porous environment. 2. Nematode community structure. Plant and Soil. 1999;212:85–99. [Google Scholar]
- Neher DA, Wu J, Barbercheck ME, Anas O. Ecosystem type affects interpretation of soil nematode community measures. Applied Soil Ecology. 2005;30:47–64. [Google Scholar]
- Norton DC, Ponchillia PE. Stylet-bearing nematodes associated with plants in Iowa prairie. Proceedings of the Iowa Academy of Science. 1968;75:32–35. [Google Scholar]
- Norton DC, Schmitt DP. Community analyses of plant-parasitic nematodes in the Kalsow Prairie, Iowa. Journal of Nematology. 1978;10:171–176. [PMC free article] [PubMed] [Google Scholar]
- Ojima DS, Schimel DS, Parton WJ, Owensby CE. Long- and short-term effects of fire on nitrogen cycling in the tall-grass prairie. Biogeochemistry. 1994;24:67–84. [Google Scholar]
- Okada H, Kadota I. Host status of 10 fungal isolates for two nematode species, Filenchus misellus and Aphelenchus avenae . Soil Biology and Biochemistry. 2003;35:1601–1607. [Google Scholar]
- Orr CC, Dickerson OJ. Nematodes in true prairie soils of Kansas. Transactions of the Kansas Academy of Science. 1967;69:317–334. [Google Scholar]
- Owensby CE, Hyde RM, Anderson KL. Effects of clipping and supplemental nitrogen and water on loamy upland bluestem range. Journal of Range Management. 1970;23:341–346. [Google Scholar]
- Pielou EC. After the Ice Age: The return of life to glaciated North America. Chicago: University of Chicago Press; 1991. [Google Scholar]
- Powers TO, Todd TG, Burnell AM, Murray PCB, Fleming CC, Szalanski AL, Adams BA, Harris TS. The rDNA internal transcribed spacer region as a taxonomic marker for nematodes. Journal of Nematology. 1997;29:441–450. [PMC free article] [PubMed] [Google Scholar]
- Rice CW, Todd TC, Blair JM, Seastedt TR, Ramundo RA, Wilson GWT. Belowground biology and processes. In: Knapp AK, Briggs JM, Hartnett DC, Collins SL, editors. Grassland dynamics: Long-term ecological research in tallgrass prairie. New York: Oxford University Press; 1998. pp. 244–264. [Google Scholar]
- Ricketts TH, Dinerstein E, Olson DM, Loucks CJ, Eichbaum W, DellaSala D, Kavanagh K, Hedao P, Hurley PT, Carney KM, Abell R, Walters S. Terrestrial ecoregions of North America: A conservation assessment. Washington, DC: Island Press; 1999. [Google Scholar]
- Risser PG. A new framework for prairie conservation. In: Samson FB, Knopf FL, editors. Prairie conservation: Preserving North America's most endangered ecosystem. Washington, DC: Island Press; 1996. pp. 261–274. [Google Scholar]
- Sala OE, Chapin FS, III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Samson F, Knopf F. Prairie conservation in North America. Bioscience. 1994;44:418–421. [Google Scholar]
- Scheiner SM, Cox SB, Willig M, Mittelbach GG, Osenberg C, Kaspari M. Species richness, species-area curves, and Simpson's paradox. Evolutionary Ecology Research. 2000;2:791–802. [Google Scholar]
- Schmitt DP, Norton DC. Relationships of plant-parasitic nematodes to sites in native Iowa prairies. Journal of Nematology. 1972;4:200–206. [PMC free article] [PubMed] [Google Scholar]
- Seastedt TR, Knapp AK. Consequences of nonequilibrium resource availability across multiple time scales: The transient maxima hypothesis. American Naturalist. 1993;141:621–633. doi: 10.1086/285494. [DOI] [PubMed] [Google Scholar]
- Shirley S. Restoring the tallgrass prairie: An illustrated manual for Iowa and the upper midwest. Iowa City, IA: University of Iowa Press; 1994. [Google Scholar]
- Thorne G. Brookings, SD: Technical Bulletin 41, Agricultural Experiment Station, South Dakota State University; 1974. Nematodes of the northern great plains. Part II. Dorylaimoidea in part [Nemata: Adenophorea] [Google Scholar]
- Thorne G, Malek RB. Brookings, SD: Technical Bulletin 41, Agricultural Experiment Station, South Dakota State University; 1968. Nematodes of the northern great plains. Part I. Tylenchida [Nemata: Secernentea] [Google Scholar]
- Todd TC. Effects of management practices on nematode community structure in tallgrass prairie. Applied Soil Ecology. 1996;3:235–246. [Google Scholar]
- Todd TC, Blair JM, Milliken GA. Effects of altered soil-water availability on a tallgrass prairie nematode community. Applied Soil Ecology. 1999;13:45–55. [Google Scholar]
- Todd TC, James SW, Seastedt TR. Soil invertebrate and plant responses to mowing and carbofuran application in a North American tallgrass prairie. Plant and Soil. 1992;144:117–124. [Google Scholar]
- Towne EG. Prairie vegetation and soil nutrient responses to ungulate carcasses. Oecologia. 2000;122:232–239. doi: 10.1007/PL00008851. [DOI] [PubMed] [Google Scholar]
- Turner CL, Blair JM, Shartz RJ, Neel JC. Soil Nand plant responses to fire, topography, and supplemental N in tall-grass prairie. Ecology. 1997;75:1861–1876. [Google Scholar]
- Vitousek PM. Beyond global warming: Ecology and global change. Ecology. 1994;75:1861–1876. [Google Scholar]
- Wardle DA. Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Advances in Ecological Research. 1995;26:105–185. [Google Scholar]
- Wardle DA, Yeates GW. The dual importance of competition and predation as regulatory forces in terrestrial ecosystems, evidence from decomposer food webs. Oecologia. 1993;93:303–306. doi: 10.1007/BF00317685. [DOI] [PubMed] [Google Scholar]
- White PS, Walker JL. Approximating nature's variation: Selecting and using reference information in restoration ecology. Restoration Ecology. 1997;5:338–349. [Google Scholar]
- Winterhalder K, Clewell AF, Aronson J. Values and science in ecological restoration – a response to Davis and Slobodkin. Restoration Ecology. 2004;12:4–7. [Google Scholar]
- Wolters V, Silver WL, Bignell DE, Coleman DC, Lavelle P, Van Der Putten WH, De Ruiter P, Rusek J, Wall DH, Wardle DA, Brussaard L, Dangerfield JM, Brown VK, Giller KE, Hooper DU, Sala O, Tiedje J, Van Veen JA. Effects of global changes on above- and belowground biodiversity in terrestrial ecosystems: Implications for ecosystem functioning. Bioscience. 2000;50:1089–1098. [Google Scholar]
- Wood FH. Nematode feeding relationships: Feeding relationships of soil-dwelling nematodes. Soil Biology and Biochemistry. 1973;5:593–601. [Google Scholar]
- Wu J, Loucks OL. From balance of nature to hierarchical patch dynamics: A paradigm shift in ecology. Quarterly Review of Biology. 1995;70:439–466. [Google Scholar]
- Yeates GW. Impact of historical changes in land use on the soil fauna. New Zealand Journal of Ecology. 1991;15:99–106. [Google Scholar]
- Yeates GW. Nematodes as soil indicators: Functional and biodiversity aspects. Biology and Fertility of Soils. 2003;37:199–210. [Google Scholar]
- Yeates GW, Bongers T. Nematode diversity in agroecosystems. Agriculture. Ecosystems and Environment. 1999;74:113–135. [Google Scholar]