Abstract
Parasitodiplogaster comprises a potentially large radiation of nematode species that appear to be parasitically bound to their Agaonid fig wasp hosts, which are mutualistically associated in the syconia (figs) of the diverse plant genus Ficus. Parasitodiplogaster laevigata n. sp. is described and illustrated as an associate of the fig wasp, Pegoscapus sp. from Ficus laevigata from southern Florida. It is the first species of Parasitodiplogaster reported from North America and is closest to P. trigonema from F. trigonata from Panama. Parasitodiplogaster laevigata n. sp. can be differentiated from all described species of Parasitodiplogaster based on stomatal morphology (presence of a large dorsal and a right subventral tooth) in the adults of both sexes, molecular comparisons of two expansion segments (D2,D3) of the large subunit (LSU) rRNAgene, and fig-fig wasp host affinities. The ultrastructure of P. laevigata n. sp. was elucidated using TEM and SEM for comparisons with other species of Parasitodiplogaster. The stoma of P. laevigata n. sp. possesses a nonsegmented cheilostomal ring that connects to the longitudinal body musculature per- and interradially, a claw-like dorsal tooth, a right subventral tooth, and telostegostomatal apodemes arising from the dorsal side of each subventral sector. The unification of the pro-, meso-, and metastegostom with the gymnostom in P. laevigata n. sp. and further simplification in other described species may be due to derived adaptations associated with the internal parasitism of fig wasps.
Keywords: Agaonidae; buccal capsule; Ficus, fig wasp; Hymenoptera; LSU rRNA; molecular phylogeny; morphology; nematode; parasitism; Diplogastridae; Parasitodiplogaster laevigata n. sp.; Pegoscapus sp.; stoma; taxonomy
Parasitodiplogaster laevigata n. sp. (Nematoda: Diplogastridae) was recovered as dauer (infective) juveniles from the hemocoel of newly emerged adult females of the fig wasp Pegoscapus sp. (Agaonidae) and lumen of phase D syconia (figs) of Ficus laevigata Vahl. (F. citrifolia Miller sensu DeWolf) from southern Florida (Giblin-Davis et al., 1992, 1995). It is carried by female fig wasps to a new phase B syconium, where a fig wasp host pollinates and oviposits into available female florets. The female fig wasp dies inside the syconium after oviposition. The nematodes greatly increase in size as they molt and develop into fourth-stage (J4) pre-adults inside the hemocoel of their host and emerge from the wasp cadaver to finish development to adults, mate, and lay eggs giving rise to dauer juveniles. These dauer juveniles are infective to the next generation of female fig wasps as they emerge from their floret galls (Giblin-Davis et al., 1995).
There are 11 described species of Parasitodiplogaster that parasitize fig wasps from figs in Panama and Africa (Poinar, 1979; Poinar and Herre, 1991). Parasitism is rare in the Diplogastridae, which are mostly omnivorous (predatory, bacterial, and/or fungal feeders) and free-living or phoretically associated with insects (Poinar, 1979; Luong et al., 1999; Fürst von Lieven and Sudhaus, 2000; Sudhaus and Fürst von Lieven, 2003). Thus, parasitism in the genus Parasitodiplogaster appears to be an innovation derived from free-living phoretic ancestors (Giblin-Davis et al., 2004). There is evidence from Dominican fossil amber (lower Miocene to Upper Eocene) that the association between Parasitodiplogaster and fig wasps is at least 19 to 25 million years old (Herre, 1995), suggesting an ancient radiation that may have developed early in the evolution of the pollination mutualism between fig wasps and figs (Giblin-Davis et al., 2004).
There are more than 700 species of Ficus in the world (Berg, 1989). Most species of fig wasps that are associated with species of Ficus from the Neotropics (140 species) and Africa (105 species) are hypothesized to harbor a unique species of Parasitodiplogaster (Giblin-Davis et al., 2004). Recent studies by Molbo et al. (2003) suggest that there maybe more than one cryptic species of fig wasp for each species of Ficus, leading to the possibility of more than one species of Parasitodiplogaster for some species of Ficus.
Stomatal features have been used in generating a phylogenetic tree with molecular data of some nematode groups (Baldwin et al., 1997a, b). However, relatively little work has been done on the stoma or the phylogeny of the Diplogastridae (Baldwin et al., 1997b; Fürst von Lieven and Sudhaus, 2000; Fürst von Lieven, 2002, 2003; Sudhaus and Fürst von Lieven, 2003). In the generic diagnosis of Parasitodiplogaster, the stoma is described as a variably shaped cylinder that is reduced to distinct in stature, without an apparent glottoid apparatus (Poinar and Herre, 1991; but see Fürst von Lieven and Sudhaus, 2000, for discussion on the presence of a glottoid apparatus vs. “bulges” in the Diplogastridae). The morphology of the stoma of nominal species of Parasitodiplogaster is poorly understood and has been described as “small,” “longer than wide,” or “distinct” (Poinar, 1979; Poinar and Herre, 1991). We have examined material that we collected from the type localities of several described species of Parasitodiplogaster for molecular analysis and concur that the stoma appears to be structurally simple as described by Poinar and Herre (1991). We know from other species of Diplogastridae that the buccal capsule/cavity is complicated to interpret (Baldwin et al., 1997b; Fürst von Lieven and Sudhaus, 2000; Fürst von Lieven, 2002, 2003) and that the parasitic habit of Parasitodiplogaster could have led to structural simplification or amalgamation that would make homology difficult to discover without ultrastructural observations.
An evolutionary framework is necessary to understand this genus and its relationship to other diplogastrids and for the designation and verification of new species. Although morphology, fig and fig wasp host associations and life history will all prove useful, gene sequences provide an independent and alternative data set for phylogenetic inferences. Thus, in addition to the ultrastructural (scanning electron microscopic [SEM] and transmission electron microscopic [TEM]) observations and taxonomic description of Parasitodiplogaster laevigata n. sp., this paper reports molecular phylogenetic comparisons of two expansion segments (D2,D3) of the large subunit (LSU) rRNA gene from Parasitodiplogaster laevigata n. sp. with several species of Parasitodiplogaster from Panama.
Materials and Methods
Phase B and early phase C syconia of Ficus laevigata were intermittently collected and dissected between 1991 and 2005 from 10 trees in a disused stretch of SE Palm Drive, Florida City, FL (25.44787° N, 80.45835° W and 25.44799° N, 80.45519° W). Parasitodiplogaster laevigata n. sp. adults that had exited their Pegoscapus sp. fig wasp host into the lumen of the sycone were hand-picked into water prior to processing for measurements, PCR amplification, fixation for type specimens, or electron microscopy.
Measurements and type specimens: Adults of Parasitodiplogaster laevigata n. sp. were collected and heat-killed for measurements in temporary water mounts, and some of these were fixed in formalin-glycerol for greater than 24 hr and processed slowly into glycerol before making permanent slides and measurements (Southey, 1970). Nematodes were drawn and measured with the aid of a camera lucida and a stage micrometer.
Scanning electron microscopy (SEM): We examined the external morphology of adults of both sexes of Parasitodiplogaster laevigata n. sp. with SEM. Specimens were fixed in 3% (v/v) glutaraldehyde, post-fixed in 2% OsO4 for 12 hr at 22 °C, rinsed in distilled H20, dehydrated in a graded ethanol series, critical point dried from liquid CO2, mounted on a stub with double sticky tape, sputter-coated with 20 nm of gold-palladium, and viewed with a Hitachi (Tokyo, Japan) S-4000 Field Emission SEM at 7 kV.
Transmission electron microscopy (TEM): Nematode specimens were collected live into 2% formaldehyde (prepared from paraformaldehyde) and 2.5% glutaraldehyde in 0.1 M cacodylate buffer at pH 7.2 and fixed overnight at 4°C. After repeated rinsing in buffer, specimens were post-fixed in 2% OsO4 in 0.1 M cacodylate buffer at pH 7.2 for 3.5 hr at 22 °C. Nematodes were rinsed in water, fixed with 1% aqueous uranyl acetate, dehydrated through 100% ethanol into 100% acetone, and infiltrated with Spurr's epoxy resin. Blocks were sectioned on an ultra-microtome, and nearly serial sections with silver refraction (75 nm) were picked up on copper grids with a 0.35% Formvar coating reinforced with a light carbon film. Sections were post-stained with 5% aqueous uranyl acetate and lead citrate before viewing with TEM at 80 kV. One adult male nematode was transverse-sectioned and another one was sagittally-sectioned through the stoma and anterior esophagus.
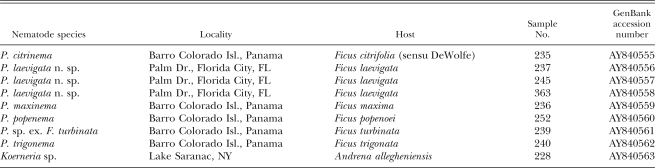
Molecular studies: Additional Parasitodiplogaster species were collected as described above from Ficus species from the Smithsonian Tropical Research Institute, Barro Colorado Island, Republic of Panama, with assistance from E. A. Herre (Table 1). Voucher specimens were collected from the same fig as samples for amplification and sequencing attempts. Two primers D2a (ACAAGTACCGTGAGGGAAAGT) and D3b (TGCGAAGGAACCAGCTACTA) (Nunn, 1992) were used for polymerase chain reaction (PCR) to amplify D2,D3 expansion segments (731 to 741 bp) of the large subunit of ribosomal DNA. The PCR conditions were 95°C for 5 min, 35 cycles (each cycle was 94°C, 30 sec, 55°C, 45 sec, 72°C, 2 min), then 72°C for 10 min. DNA sequencing was performed with the same primers on a CEQ 2000 DNA Analysis System (Beckman Coulter, Fullerton, CA) following manufacturer's protocols. The GenBank accession numbers for the sequences obtained are presented in Table 1. DNA sequence alignment and phylogenetic analyses were as described by Ye et al. (2006) with complete references. Briefly, DNA sequences were aligned by ClustalW (Clustal Reference) using Biology Workbench (http:// workbench.sdsc.edu/, Bioinformatics and Computational Biology group, Department of Bioengineering, University of California in San Diego, CA). Phylogenetic analyses were performed in PAUP* 4.0bl0. Sites with missing data or gaps were treated as missing characters for all analyses. The maximum parsimony (MP) method was performed using a heuristic search with stepwise-addition options and neighbor-joining (NJ) using Hasegawa-Kishino-Yano 85 DNA distances. Models of evolution for likelihood analysis, as well as parameter estimates, were determined via likelihood ratio tests as implemented in Modeltest 3.06 (Posada and Crandall, 1998). The robustness of the trees was tested using the bootstrap method with all bootstrap values being based on 1,000 replicates. The aligned DNA data matrices and phylogenetic trees are available at TreeBASE (http://www.treebase.org, submission number: SN2592).
Table 1.
Parasitodiplogaster species and outgroup (Koerneria sp.) samples used for DNA sequencing
Systematics
Parasitodiplogaster laevigata n. sp. (Figs. 1–14; Tables 1–2)
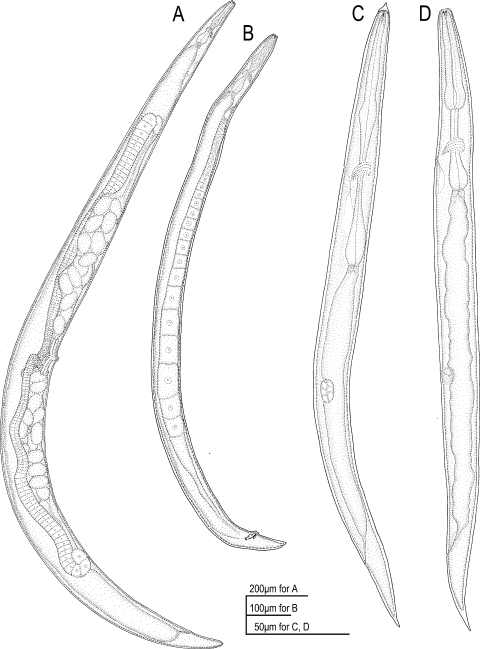
Fig. 1.
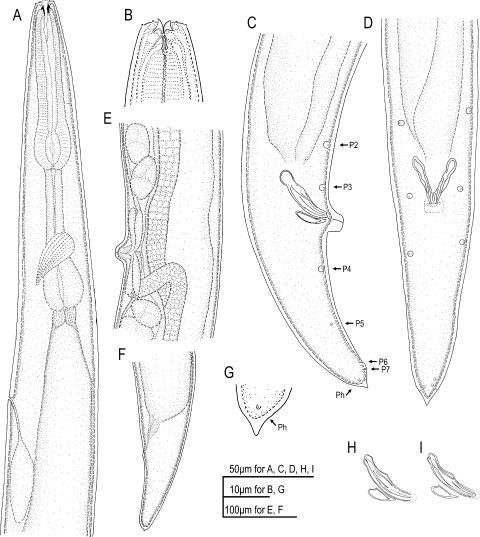
Drawings of adults and juveniles of Parasitodiplogaster laevigata n. sp. in lateral view. A) Female. B) Male. C) Dauer (infective) juvenile. D) Second-stage juvenile.
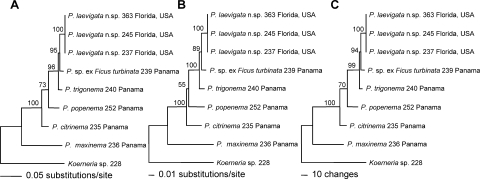
Fig. 14.
Phylogenetic trees were inferred from the analyses of molecular sequence data of Parasitodiplogaster laevigata n. sp. and other New World Tropical species of Parasitodiplogaster. Sequences from the D2,D3 expansion segments of LSU (731–741 bp) were aligned and used in A) maximum likelihood (ML), B) neighbor joining (NJ), and C) maximum parsimony (MP) analyses. All bootstrap values are based on 1000 replicates.
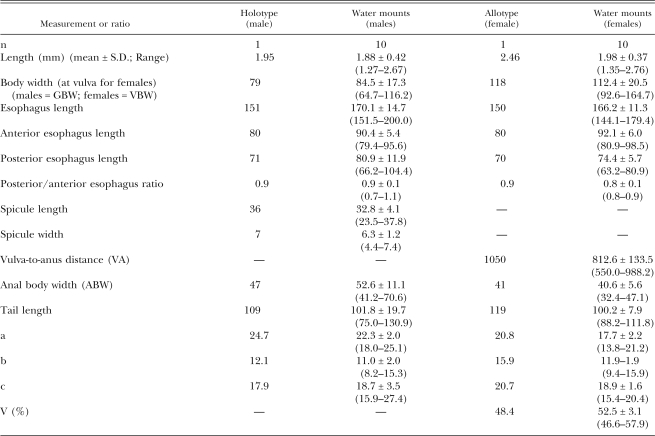
Table 2.
Morphometxics of male holotype and female allotype in glycerol and 10 male and female specimens each of Parasitodiplogaster laevigata n. sp. in temporary water mounts (measurements in micrometers [μm] unless otherwise stated).
Description
Measurements were made of specimens in temporary water mounts and are presented in Table 2.
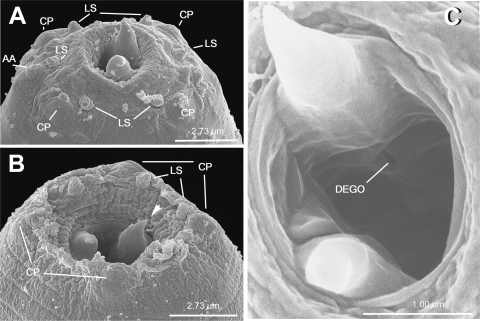
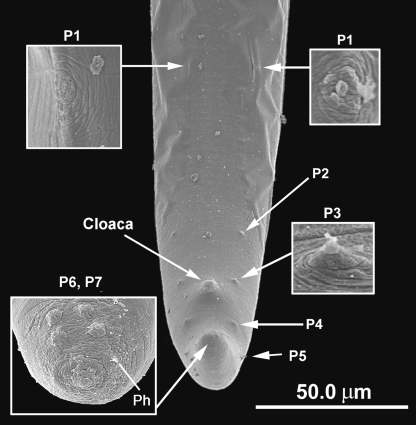
Male (n = 10): Body large and translucent white with faint transverse cuticular striations, ventrally arcuate when killed with heat (Fig. IB). Head without apparent lips, but with six adradial labial sensillae, one cephalic papilla dome present in each subdorsal and subventral sector (total of four), amphidial apertures circular and lateral (Fig. 3A). Lateral field not observed. Buccal capsule composed of an undivided cheilostomal ring that connects to longitudinal body musculature per- and in-terradially, a claw-like dorsal tooth, a right subventral tooth, and stegostomatal apodemes arising from the dorsal side of the each subventral sector that are difficult to see with light microscopy. The dorsal esophageal gland orifice exits through a stegostomatal plate below the dorsal tooth (Figs. 2B, 3C, 10B). Pro- and metacorpus muscular (Fig. 2A) central lumen of procorpus with small overlapping interradial spines that point inwardly (Figs. 2B, 8, 11B). Terminal bulb glandular (Fig. 2A). Nerve ring (circumesophageal commissure) circumscribes isthmus just anterior to terminal bulb (Fig. 2A). Cardia present. Excretory pore located ventrally about three cardia lengths posterior to the esophago-intestinal junction. Hemizonid not observed. Gonad outstretched, almost reaching excretory pore (Fig. IB). Tail ventrally curled; about 1.9 anal body widths long. No bursa present. Seven ventral, sub-ventral, or ventro-sublateral preanal (3) and postanal (4) pairs of papillae (according to terminology of Sudhaus and Fürst von Lieven [2003]; vl–3/v4, ad, v5–6, ph with the apparent loss of pd and one of the v5–7 triad). One pair of preanal subventral papillae (PI = vl) at about three anal body widths anterior to cloaca (visible only with SEM), one pair of preanal subventral papillae (P2 = v2) at about one anal body width anterior to cloaca, one pair of subventral preanal papillae (P3 = v3) just anterior to cloaca, one pair of subventral postanal papillae (P4 = v4) about 1/2 anal body width posterior to cloaca, one small pair of ventrosublateral postanal papillae (P5 = ad) posterior to P4 at about 55% of the tail length behind cloaca, two ventral pairs of papillae (P6–7 = v5–6), and pore-like phasmids located laterally just before tail tip (Figs. 2C-D, 4). Spicules separate; spicule length about 33 μm, gubernaculum about 40–50% of spicule length (Figs. 2C-D,H-I; Table 2). Tail with small mucron.
Fig. 3.
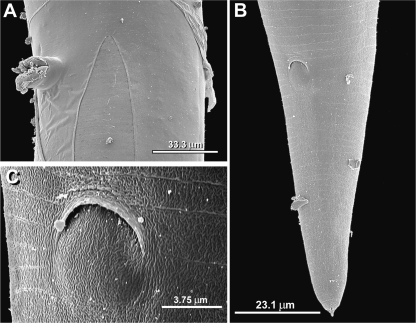
SEM photomicrographs of adult heads of Parasitodiplogaster laevigata n. sp. A) Male with partially protracted buccal capsule. B) Female with retracted stoma. C) Higher magnification of the buccal capsule, (arrow = dorsal tooth; AA = amphidial aperture; CP = cephalic papilla dome; DEGO = dorsal esophageal gland orifice; LS = labial sensilla).
Fig. 2.
Drawings of adults of Parasitodiplogaster laevigata n. sp. in lateral view (unless otherwise stated). A) Anterior region of female. B) Female stomatal region and anterior procorpus. C) Male tail (P2-P7 caudal papillae; Ph = phasmid). D) Male tail in ventral view. E) Female vulva and vaginal glands. F) Female tail. G) Female tail tip. H–I) Spicules and gubernaculum drawn in different focal planes.
Fig. 10.
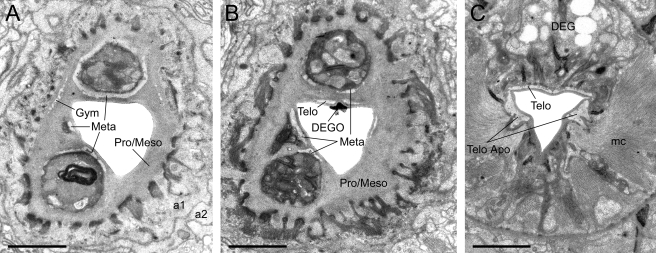
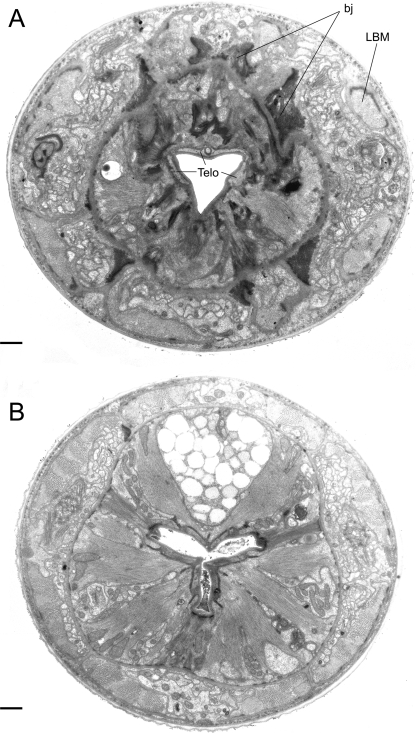
TEM micrographs of the stoma of an adult male of Parasitodiplogaster laevigata n. sp. A) Almost transverse section through the combined stegostomatal unit (al, a2 = arcade synctial rings; Gym = gymnostom; Meta = metastegostom; Pro/Meso = Pro/Mesostegostom). B) Almost transverse section through the combined stegostomatal unit posterior to section A (DEGO = dorsal esophageal gland orifice; Telo = telostegostom). C) Almost transverse section through the telostegostom posterior to the section in Figure 11A (DEG = dorsal esophageal gland; mc = second set of adradial muscles; Telo Apo = telostegostomatal apodemes) (Scale bar = 1 μm).
Fig. 8.
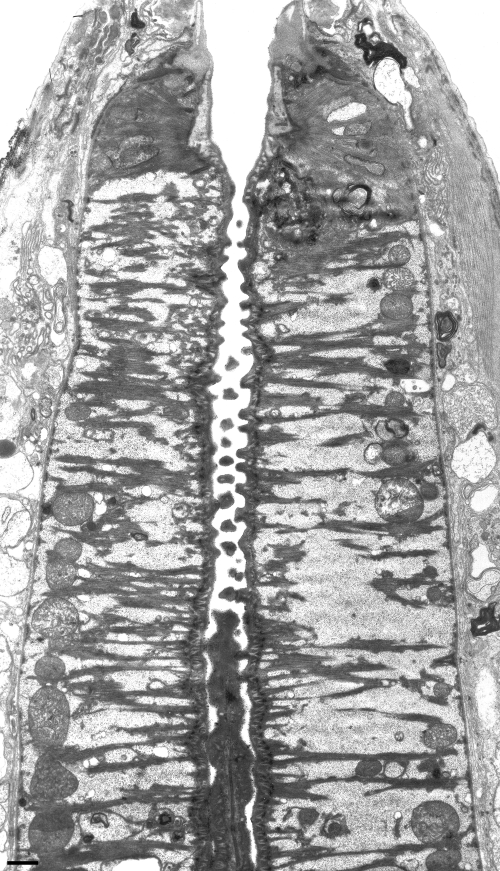
TEM micrograph of a sagittal mediolateral section through the central region of the stoma and anterior procorpus of an adult male of Parasitodiphgaster laevigata n. sp. showing orientation and morphology of muscles, mitochondria, and overlapping sclerotized spines in the lumen of the procorpus (Scale bar = 1 μm).
Fig. 11.
TEM micrographs of the stoma of an adult male of Parasitodiplogaster laevigata n. sp. A) Almost transverse section through the telostegostom (= Telo) posterior to section in Fig. 10B and anterior to section in Fig. IOC showing belt junctions (=bj) that extend from the base of the pro/mesostegostomatal matrix (see Fig. 10B) and anchor the stegostomatal unit to longitudinal body muscles (LBM) and cuticle. B) Almost transverse section through the anterior portion of the procorpus revealing the triradiate lumen of the esophagus and interdigitating spines that point inwardly in the central portion of the lumen. (Scale bar = 1 μm).
Fig. 4.
SEM photomicrographs of adult male tail of Parasitodiplogaster laevigata n. sp. PI, P2, P3 = precloacal pairs of papillae; P4–7 = post cloacal pairs of papillae; Ph = phasmid.
Female (n = 10): Adult female cuticular features and cephalic regions (exterior and interior) similar to males (Figs. 1A, 2A-B, 3B). Ventrally arcuate when killed with heat (Fig. 1A). Ovaries didelphic and reflexed, eggs usually present, in single cell through gastrula stages (Fig. 1 A). Vulval position at about 50% of body length (Fig. 1A; Table 2), vulva a protuberant oval pore (Figs. 1A, 2E, 5A), four vaginal glands present (Fig. 2E). Anus dome-shaped (Figs. 5B-C). Tail uniformly conoid with small mucron present (Figs. 2F-G, 5B). Phasmids located near tail tip (Fig. 2G).
Fig. 5.
SEM photomicrographs of adult female of Parasitodiplogaster laevigata n. sp. A) Newly molted adult ("J4-exuvia" partially attached) with protuberant vulva (lateral view). B) Tail (subventral view). C) Anus, close-up.
Dauer juveniles from newly emerged female wasps (n = 12): Measurements of the dauer juveniles follow (Mean ± SD; [range]): total body length = 260.0 ± 40.4 (198.7–344.1) um; esophagus length = 100.9 ± 14.9 (80.8–130.0) um; greatest body width = 18.7 ± 2.5 (16.2–25.0) μm; gonad primordium width = 9.5 ± 1.9 (7.5–13.3) μm; gonad primordium width = 5.9 ± 0.9 (4.6–7.5 μm); tail length = 29.5 ± 4.8 (21.7–35.8 μm); anal body width = 10.4 ±0.7 (9.2–11.7 μm); a = 13.9 ± 1.6 (11.4–16.6); b = 2.6 ± 0.2 (2.4–2.9); c = 7.7 ± 1.4 (5.8-9.9); and c’ ratio = 25.0 ± 3.8 (19.1–31.2). Body cylindrical, tapered at both ends (Fig. 1C). Small dorsal-ventral ridge on the head appears tooth-like in dorsal or ventral view (Fig. 6), lateral tooth-like protrusion anterior to lateral labial sensilla above amphid (Fig. 6D). Circular amphids visible (Fig. 6B-D), stoma and esophagus degenerate, excretory pore at level of nerve ring, anus visible, phasmids visible about one third of the tail length from terminus, tail uniformly conoid, slightly attenuated. The second-stage instar (Fig. ID) hatches from eggs in the fig sycone and precedes the dauer in development (Fürst von Lieven, 2005). The third instar appears to be an obligatory dauer because no further development occurs in the fig before infection of adult fig wasps. This second-stage instar has an apparently functional toothed stoma and esophagus (with an “a” ratio of about 3.4 vs. 2.4 in the dauer) (Figs. 1C-D).
Fig. 6.
Light and SEM photomicrographs of dauer juveniles of Parasitodiphgaster laevigata n. sp. A) Light micrograph of anterior head region showing head protuberance. B) Light micrograph of anterior head region showing amphid. C, D) SEMs of two dauer juveniles showing amphidial apertures and labial sensillae and protuberances.
Diagnosis: Parasitodiplogaster laevigata n. sp. is distinguished from all other described species of Parasitodi-plogasterby the unique morphology of the stoma in both sexes with the possession of large dorsal and right sub-ventral teeth, biogeography, and Agaonid and Ficus host range (associated with Pegoscapus sp. in Ficus laevigata in southern Florida).
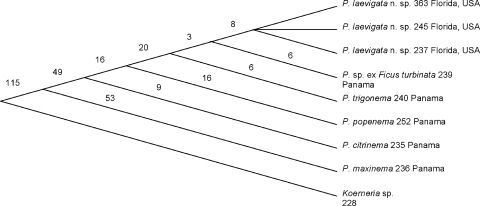
Relationships: Based upon D2,D3 LSU sequence data of collected Parasitodiplogaster, P. laevigata n. sp. is closest to an undescribed species of Parasitodiplogaster ex Ficus turbinata and to P. trigonema from F. trigonata from Panama (Figs. 14–Figs. 15). The number of nucleotide autapomorphies between P. laevigata n. sp. and P. trigonema (= 17) and P. sp. ex Ficus turbinata (= 14) is indicative of a large number of fixed novel differences in the concerted evolution of the D2,D3 LSU region, suggestive of separate but closely related species (Fig. 15). There is strong evidence for a clade of Parasitodiplogaster (P. laevigata n. sp., P. sp. ex Ficus turbinata, P. trigonema, P. popenema, and P. citrinema), all collected from the genus Ficus, subgenus Urostigma, section Americana (100% bootstrap support with MP, NJ, and ML analyses) (Fig. 14). Parasitodiplogaster maxinema, which was isolated from Ficus maxima, a member of the Ficus subgenus Pharmacosycea, section Pharmacosycea, appears outside this clade, suggesting that this lineage resulted from cospeciation with its fig and fig wasp hosts, as suggested by Giblin-Davis et al. (2004). Koerneria sp. was chosen as an outgroup for this study because of its apparent “nearest-relative” status in preliminary D2,D3 LSU trees within the Diplogastridae (Ye et al., unpubl. data), and this was corroborated by trees from wider surveys done of the Diplogastridae and clade V representatives using near full-length sequences of LSU and SSU rRNA genes (Kiontke and Fitch, pers. comm.).
Fig. 15.
Cladogram based upon the alignment of 746 characters (including gaps and insertions) from D2,D3 expansion segments of LSU. Each number represents the number of apomorphies.
Parasitodiplogaster laevigata n. sp. appears morphologically closest to P. trigonema from F. trigonata from Panama. In both species, the testes is outstretched, spicules are straight with blunt tips and a distinct manubrium, the gubernaculum is straight and wedge-shaped, often with a swollen proximal end, there are three (in Parasitodiplogaster laevigata n. sp.) and four (in P. trigonema) subventral preanal papillae in series up the body and four similarly placed pairs of postanal papillae, distinct phasmids at tail tip beyond last papilla, and the tail tip is usually mucronated. In addition, both sexes of both species have similar excretory pore positioning, posterior to the esophago-intestinal junction. Besides the difference in the number of preanal papillae in males, there are a number of significant morphological differences between these two species, including a muscular procorpus with a well-developed cardia in both sexes of P. laevigata n. sp. as opposed to a glandular-appearing procorpus and a weak cardia in P. trigonema. The structuring of the sclerotized lining of the procorpus into “tongue and groove” ridges and presence of small overlapping interradial spines that point inwardly appear to represent autapomorphies in P. laevigata n. sp. In addition, the stoma of P. laevigata n. sp., which possesses a distinct dorsal and right subventral tooth, is not only distinct from P. trigonema, which has a small 2-μm x 2-μm stoma with no teeth, but from all other described species that have variably described cylinders without major teeth.
Type host and locality: Holotype male and allotype female of P. laevigata n. sp., which had recently exited their Pegoscapus sp. fig wasp host into the lumen of the sycone in an early phase C syconia of F. laevigata, were collected from Florida City, FL.
Type designations: Holotype male, allotype female and additional material deposited at the University of California-Riverside Nematode Collection. Paratypes (males and females same data as holotype) deposited at the University of California, Davis; USDA Nematode Collection, Beltsville, MD; and the Nematology Department, Rothamsted Experiment Station, Harpenden, Herts., United Kingdom.
Etymology: This species name is derived from the species name of the Ficus host with which the nematode is associated in southern Florida, i.e., Ficus laevigata.
Ultrastructural observations and discussion: These are the first ultrastructural observations of the stoma of Parasitodiplogaster, which should prove useful for understanding changes in feeding structures that have occurred in the evolution of parasitism in the Diplogastridae. Previously used nomenclature in the ultrastructural work of the stoma of Acrostichus halicti by Baldwin et al. (1997b) was reconciled with the stomatal terminology of De Ley et al. (1995) for Rhabditidae and Panagrolaimidae, and Cephalobidae by Fürst von Lieven and Sudhaus (2000). In addition, they discussed their hypotheses concerning the homologization of the buccal regions of this nematode with other clade IV and V nematodes and provided some excellent light microscopic observations and drawings of a variety of stomatal structures in the Diplogastridae that will serve as a basis for the discussion herein.
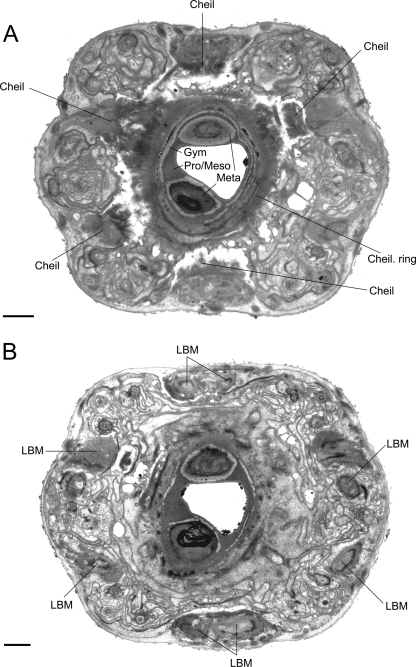
The stoma of P. laevigata n. sp. is composed of a short telescoping unit involving the cheilostom and a combined unit of the gymnostom and stegostom. The undivided cheilostomal ring attaches to longitudinal body musculature in the head (Figs. 9A, 12) at the six perradial and interradial sectors (Fig. 9A). At the dorsal interradial and the ventral perradial (marginal) sectors, the cheilostomal ring connects to two longitudinal body muscles as opposed to the right and left subdorsal perradial and the right and left subventral interradial sectors, which each connect to a single longitudinal body muscle (Fig. 9B). Interestingly, J4 juveniles of Koerneria sp. (Strain RG20 = RGD228 in this study) have an undivided cheilostomal ring with vertical striations vs. adults, in which the cheilostom is divided into six per- and interradial plates (Fürst von Lieven and Sudhaus, 2000). This contrasts with A. halicti, where the cheilostom is divided into six adradial plates (Baldwin et al., 1997b; Fürst von Lieven and Sudhaus, 2000).
Fig. 9.
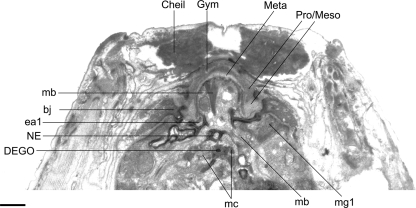
TEM micrographs of the stoma of an adult male of Parasitodiplogaster laevigata n. sp. A) Almost transverse section through the cheilostomatal ring (Cheil. ring), per- and interradial cheilostomatal connections (Cheil) to the longitudinal body musculature (LBM), and combined stegostomatal unit with gymnostom and dorsal tooth and right subventral tooth embedded in pro/mesostegostomatal matrix that is partially telescoped through the cheilostomatal ring (Gym = gymnostom; Meta = metastegostom; Pro/Meso = Pro/ Mesostegostom). B) Almost transverse section posterior to section A through combined stegostomatal unit showing longitudinal body muscles (LBM) (Scale bar = 1 μm).
Fig. 12.
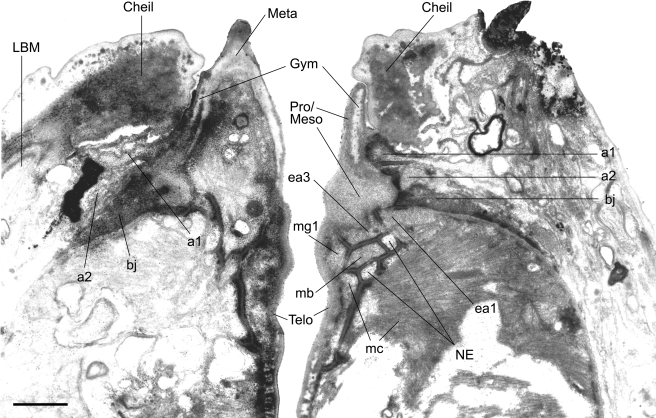
TEM micrograph of an almost sagittal section through the right subventral tooth (left side of photograph) and the ventrosublateral aspect of the left subventral sector (right side of the photograph) of the stoma of an adult male of Parasitodiplogaster laevigata n. sp. (al, a2 = arcade synctial rings; bj = belt junction; Cheil = cheilostom; eal = first set of interradial epithelial cells; ea3 = second set of interradial epithelial cells; Gym = gymnostom; LBM = longitudinal body muscle; mb = first set of adradial muscles; me = second set of adradial muscles; Meso = mesostegostom; Meta = metastegostom; mgl = first set of marginal cells; NE = nerve ending; Pro = Prostegostom; Telo = Telostegostom). (Scale bar = 1 μm).
The gymnostom of P. laevigata n. sp. is an electron lucent ring in transverse section, associated with two rings of arcade synctia, which is apparently fused with most of the stegostomatal components into a cohesive, non-telescoping unit (Figs. 7, 9, 12). The gymnostom of P. laevigata n. sp. appears embedded in what we are interpreting to be a matrix produced by the first and second sets of interradial epithelial cells (eal, ea3) (Figs. 12, 13). According to Fürst von Lieven and Sudhaus (2000), this cuticular matrix, which is similar to that observed in A. halicti, should be homologized with a fused pro/mesostegostom which, in C. elegans, appears as unresolved linear stomatal components that are associated with each interradial epithelial cell (iel, ie2). In some sagittal sections from P. laevigata n. sp., a zone of demarcation between the relatively darker-staining prostegostom and the lighter-staining mesostegostom could be discerned (Figs. 12, 13). However, in other sections it was not possible to separate the two zones.
Fig. 7.
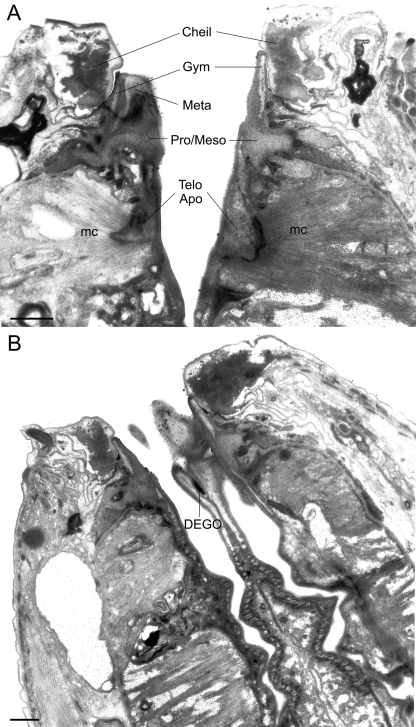
TEM micrographs of the stoma of an adult male of Parasitodiphgaster laevigata n. sp. A) Sagittal section through the ventrosublateral aspect of the right subventral tooth (left side of photograph) and the mediolateral aspect of the left ventrosublateral sector (right side of the photograph) (Cheil = cheilostom; Gym = gymnostom; Meta = metastegostom; mc = second set of adradial muscles; Pro/Meso = Pro/Mesostegostom; Telo apo = Telostegostomatal apodeme). B) Sagittal section through the mediodorsal aspect of the dorsal tooth and the anterior triradiate branches of the procorpus showing “grinding” plates (DEGO = dorsal esophageal gland orifice) (Scale bar = 1 μm).
Fig. 13.
TEM micrograph of an almost sagittal section through the back of the dorsal tooth in the stoma of an adult male of Parasitodiplogaster laevigata n. sp. (bj = belt junction; Cheil = cheilostom; DEGO = dorsal esophageal gland orifice; eal = first set of interradial epithelial cells; Gym = gymnostom; mb = first set of adradial muscles; mc = second set of adradial muscles; Meso = mesostegostom; Meta = metastegostom; mgl = first set of marginal cells; NE = nerve ending; Pro = Prostegostom). (Scale bar = 1 μm).
Another striking feature of P. laevigata n. sp. is the asymmetry of the metastegostomatal teeth, apparent fusion or suspension of the metastegostomatal teeth in the pro/mesostegostomatal matrix, and significant reduction in size of the associated first set of adradial muscle cell homologs (mb). As we interpret it, the contractile component of the first set of adradial muscle cell homologs (mb) is reduced and inside each tooth (Fig. 13). This contrasts with Koerneria sp., which possesses very large and longitudinally oriented adradial muscles that attach to the dorsal tooth and extend into the esophagus (Fürst von Lieven and Sudhaus, 2000). The dorsal and right subventral teeth are quite robust and claw-like in P. laevigata n. sp., similar to Koerneria sp. There is a small mediolateral projection in the right subventral sector, of apparent metastegostomatal origin, that appears to be associated with cellular components (Figs. 3C, 10A,B). However, its derivation and function are unknown. If we accept that Parasitodiplogaster is the sister group of Koerneria, as the molecular data suggest, then the loss or reduction of stegostomatal armatures in all of the currently described species of Parasitodiplogaster becomes problematic. The dorsal and right subventral claw-like teeth in P. laevigata n. sp. could have evolved de novo after being lost in the stem-ancestor of Parasitodiplogaster, or the teeth could have been lost independently several times (Figs. 14–15). We hypothesize that when Parasitodiplogaster is fully characterized, it will represent a large radiation of species from the Neotropics, Africa, and other parts of the world. Thus, interpretations of evolutionary trends must be postponed until we have characterized more species from a wider geographical sampling of species.
Fürst von Lieven and Sudhaus (2000) posit that homologization of the stoma in C. elegans and the Diplogastridae is aided by the presence or absence of a pair of putative proprioreceptive nerve endings at the anterior and posterior midline of each subventral metastegostomatal sector and a single nerve ending in the dorsal telostegostomatal plate just above the orifice of the dorsal esophageal gland. Two nerve endings each were clearly present in P. laevigata n. sp. in the left subventral sector in sagittal section (Fig. 12) and the right subventral sector (not shown), and a single one was confirmed in the dorsal sector (Fig. 13), but the presence of foramina (innervated indentations in the meta- or telostegostomatal plates) were not confirmed for any of these nerve endings.
The following is our hypothesis for functionality of the stoma in P. laevigata n. sp. given our understanding of the ultrastructure. When the longitudinal body muscles are contracted, the oral opening is dilated and the cheilostomal ring is pulled back, allowing for the protraction of the large, claw-like metastegostomatal dorsal and right subventral teeth slightly beyond the entrance of the mouth (Fig. 3A). When the body muscles are relaxed, the cheilostomal ring returns to its normal shape, and the belt junctions (or tendons?) connecting the pro/mesostegostom at its base (Figs. 10A,B) to larger belt junctions (or tendons?) (Fig. 11 A) that connect to a framework of bands that attach to the longitudinal body muscles and cuticle hold the fused gymnostom and pro/meso and metastegostom in place, allowing for retraction of the teeth. Each tooth appears to lack the musculature that would allow independent movement.
The dorsal esophageal gland orifice exits the dorsal telostegostomatal plate into the buccal cavity. The anterior-most region of this plate is embedded in the pro/ mesostegostom matrix without direct fusion to the dorsal tooth (Fig. 7B, 10B) as apparently occurs in other Diplogastridae (Baldwin et al., 1997b). There is a small pair of adradial muscles that attach to this plate. In contrast, strong adradial muscles (mc) attach to the telostegostomatal apodemes that arise from the dorsal side of each subventral sector (Figs. 7A, 10C) that, when contracted, may dilate the lumen of the stoma from a triangle in transverse section to a more pentagonal shape. This action could create suction or help to mix food. Fürst von Lieven and Sudhaus (2000) consider the presence of stegostomatal apodemes to be an apomorphy that helps to justify the Koerneria clade.
Each marginal branch of the triradiate lining of the procorpus has sclerotized, integrating “tongue and groove-like” ridges through its entire length that are visible only in sagittal view (Fig. 7B) and suggest a grinding or crushing function for the entire procorpus. Interestingly, the radial muscles of the procorpus are relatively thin and evenly distributed among large glycogen-looking reserves with mitochondria oriented mostly at the periphery and perpendicular to the lumen (Fig. 8). In addition, the dorsal and subventral interradial aspects of the central lumen of the procorpus are equipped with small, overlapping sclerotized spines that point inwardly (Fig. 8, 11B), perhaps for further piercing or crushing of food.
Thus, although it is difficult to discern feeding habits from morphology alone, we hypothesize that the unique stomatal morphology of P. laevigata n. sp. may be adapted for tearing and/or hooking internal organs of its fig wasp host when its large teeth are protracted through the action of longitudinal body muscle contractions and that host tissue is pulled into the buccal cavity when longitudinal body muscles are relaxed. In the telostegostom, secretions from the dorsal esophageal gland may be added for extra-corporeal digestion, while contraction and relaxation of adradial muscles (mc) attached to the dorsal subventral telostegostomatal sectors may begin to help move tissue into the anterior procorpus for crushing and piercing by the unique sclerotized lining.
Poinar and Herre (1991) suggested that the Panama Parasitodiplogaster species represent a monophyletic radiation from a single stem species with a variety of stomatal and esophageal manifestations to match their ecology. Herre (1995) has argued that the expression of virulence by Parasitodiplogaster is correlated with its chances for horizontal or vertical transmission by the fig wasp, with virulence being expressed most in associations where the number of female foundresses per fig is high and least in associations where the number is low. Giblin-Davis et al. (1995) reported a level of about 1.5 pollinator wasp foundresses per fig for Pegoscapus. sp. in Ficus laevigata, but observed that ovipositing foundresses were infested with P. laevigata n. sp. dauers that had not yet developed or were very small J4s, suggesting that the nematodes may delay development to reduce parasitic effects on their host until oviposition and pollination have occurred. Thus, even if the morphology of the stoma or esophagus suggests tissue destruction and potential virulence such as in P. laevigata n. sp., much more work is needed to confirm the expression of virulence. Poinar and Herre (1991) suggested that Parasitodiplogaster with small stomas, glandular esophagi, and thin-walled intestines (e.g., P. trigonema) may be adapted for a liquid diet as opposed to those with large stomas, muscular esophagi, and small intestinal cells with a border that may be adapted for solid food (e.g., P. laevigata n. sp.). These two Parasitodiplogaster species may be good candidates for further study because they have divergent feeding morphologies but are closely related according to male primary and secondary sexual characters and molecular phylogenetic analysis (Fig. 14).
Footnotes
The authors thank Barbara J. Center, Nicole DeCrappeo, E. Allen Herre, Linda Frisse, James G. Baldwin, Henry Aldrich, and Claudia Vanderbilt for technical assistance and suggestions, and Alex Fürst von Lieven and an anonymous reviewer for helpful suggestions concerning the manuscript. This study was supported by the National Science Foundation (NSF) Tree of Life project (DEB 0228692) and a USDA Special Grant in Tropical and Subtropical Agriculture CRSR-99–34135–8478.
This paper was edited by Zafar A. Handoo.
Literature Cited
- Baldwin JG, Frisse LM, Vida JT, Eddleman CD, Thomas WK. An evolutionary framework for the study of developmental evolution in a set of nematodes related to Caenorhabditis elegans . Molecular Phylogenetics and Evolution. 1997a;8:249–259. doi: 10.1006/mpev.1997.0433. [DOI] [PubMed] [Google Scholar]
- Baldwin JG, Giblin-Davis RM, Eddleman CD, Williams DS, Vida JT, Thomas WK. Buccal capsule of Aduncospiculum halicti (Nemata: Diplogasterina): An ultrastructural and molecular study with implications for phylogeny. Canadian Journal of Zoology. 1997b;33:407–423. [Google Scholar]
- Berg CC. Classification and distribution of Ficus . Experientia. 1989;45:357–358. [Google Scholar]
- DeLey P, Van De Velde MC, Mounport D, Baujard P, Coomans A. Ultrastructure of the stoma in Cephalobidae, Panagrolaimidae, and Rhabditidae, with a proposal for a revised stoma terminology in Rhabditida (Nematoda) Nematologica. 1995;44:153–182. [Google Scholar]
- Fürst von Lieven A. Functional morphology, origin, and phylogenetic implications of the feeding mechanism of Tylopharynx foetida (Nematoda: Diplogastrina) Russian Journal of Nematology. 2002;10:11–23. [Google Scholar]
- Fürst von Lieven A. The genus Oigolaimella Paramonov, 1952 (Nematoda: Diplogastridae) and description of Oigolaimella kruegeh n. sp. and O. ninae n. sp. Nematology. 2003;5:583–600. [Google Scholar]
- Fürst von Lieven A. The embryonic moult in diplogastrids (Nematoda) - homology of developmental stages and heterochrony as a prerequisite for morphological diversity. Zoologischer Anzeiger. 2005;244:79–91. [Google Scholar]
- Fürst von Lieven A, Sudhaus W. Comparative and functional morphology of the buccal cavity of Diplogastrina (Nematoda) and a first outline of the phylogeny of this taxon. Journal of Zoological Systematics and Evolutionary Research. 2000;38:37–63. [Google Scholar]
- Giblin-Davis RM, Center BJ, Nadel H, Frank JH, Ramirez BW. Nematodes associated with Fig wasps, Pegoscapus spp. (Agaonidae), and the syconia of native Floridian figs (Finis spp. Journal of Nematology. 1995;27:1–14. [PMC free article] [PubMed] [Google Scholar]
- Giblin-Davis RM, Davies KA, Taylor GS, Thomas WK. Entomophilic nematode models for studying biodiversity and cospeciation. In: Chen ZX, Chen SY, Dickson DW, editors. Nematology, advances and perspectives, vol. 1. New York: Tsinghua University Press/CABI Publishing; 2004. pp. 492–538. [Google Scholar]
- Giblin-Davis RM, Nadel H, Frank JH. New species of Schistonchus and Parasitodiplogaster parasitizing the fig wasp, Pegoscapus assuetes, in the syconia of Ficus citrifolia . Journal of Nematology. 1992;24:592. [Google Scholar]
- Herre EA. Factors affecting the evolution of virulence: Nematode parasites of fig wasps as a case study. Parasitology. 1995;111:S179–S191. [Google Scholar]
- Luong LT, Platzer EG, De Ley P, Thomas WK. Morphological, molecular, and biological characterization of Mehdinema alii (Nematoda: Diplogasterida) from the decorated cricket (Gryllodes sigillatus) Journal of Parasitology. 1999;85:1053–1064. [PubMed] [Google Scholar]
- Molbo D, Machado CA, Sevenster JG, Keller L, Herre EA. Cryptic species of fig-pollinating wasps: Implications for the evolution of the fig-wasp mutualism, sex allocation, and precision of adaptation. Proceedings of the National Academy of Sciences. 2003;100:5867–5872. doi: 10.1073/pnas.0930903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn GB. Nematode molecular evolution. PhD dissertation. U.K: University of Nottingham; 1992. [Google Scholar]
- Poinar GO., Jr Parasitodiplogaster sycophilon gen. n., sp. n. (Diplogasteridae: Nematoda), a parasite of Elisabethiella stuckenbergi Grandi (Agaonidae: Hymenoptera) in Rhodesia. Proceedings of the Koninklijke Nederlandse Akademie van Wetenschappen, Series C. Biological and Medical Sciences. 1979;82:375–381. [Google Scholar]
- Poinar GO, Jr., Herre EA. Speciation and adaptive radiation in the fig wasp nematode Parasitodiplogaster (Diplogasteridae: Rhabditida) in Panama. Revue de Nematologie. 1991;14:361–374. [Google Scholar]
- Posada D, Crandall KA. Modeltest: Testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Southey JF, editor. Laboratory methods for work with plant and soil nematodes. London: Her Majesty's Stationery Office; 1970. [Google Scholar]
- Sudhaus W, Fürst von Lieven A. A phylogenetic classification and catalogue of the Diplogastridae (Secernetea, Nematoda) Journal of Nematode Morphology and Systematics. 2003;6:43–90. [Google Scholar]
- Ye W, Giblin-Davis RM, Braasch H, Morris K, Thomas WK. Molecular Phylogenetics and Evolution In Press. 2006. Phylogenetic relationships among Bursaphelenchus species (Nematoda: Parasitaphelenchidae) inferred from nuclear ribosomal and mitochondrial DNA sequence data. [DOI] [PubMed] [Google Scholar]