Abstract
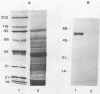
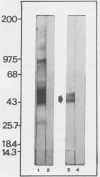
Trypanosoma cruzi antigenic specificities involved in human T-cell and antibody responses were compared in chronic chagasic patients affected with cardiomyopathy (C) or with the indeterminate form (I), the asymptomatic form of chronic Chagas' disease. T-cell Western blotting (immunoblotting) was performed to identify the most active antigens in epimastigote extracts (EPI-Ag). The patterns of peripheral blood mononuclear cell (PBMC) and T-cell proliferative responses induced by fractions blotted to nitrocellulose were heterogeneous, but the computation of their frequency distribution disclosed some important antigen specificities. Molecules ranging from 100 to 150 kilodaltons (kDa) were frequently stimulatory to PBMC from I patients (5 of 8 cases) and were less so when confronted with C patient (1 of 7 cases) lymphocytes. In contrast, both groups of patients actively responded to fractions ranging from 48 to 57 and 28 to 32 kilodaltons (kDa). The Western immunoblotting patterns of antibody reactivity displayed by 17 C and 15 I patients were also similar, yielding outstanding staining in the molecular mass ranges of 70 to 80 and 43 to 57 kDa. The latter antigen complex was recognized by 100% of the 32 chronic Chagas' disease serum specimens tested and closely corresponded to the migratory position recognized by T cells of most patients tested. The identification of the active molecules contained in the 43- to 57-kDa region was sought, with a focus on GP57/51, an antigen with well-established serodiagnostic properties. Immunoblotting analysis of EPI-Ag with a monoclonal antibody to GP57/51 confirmed its presence within the predicted molecular weight region. Highly purified GP51 was then used to demonstrate directly its capacity to promote specific PBMC proliferative responses in vitro. Data computed from a survey with 12 patients have shown a linear correlation (r = 0.93) between PBMC responses to EPI-Ag and to purified GP51, suggesting that the immune response to this particular glycoprotein may be an important component of human immune responses against T. cruzi.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acosta A. M., Santos-Buch C. A. Autoimmune myocarditis induced by Trypanosoma cruzi. Circulation. 1985 Jun;71(6):1255–1261. doi: 10.1161/01.cir.71.6.1255. [DOI] [PubMed] [Google Scholar]
- Andrade Z. A., Andrade S. G., Sadigursky M. Enhancement of chronic Trypanosoma cruzi myocarditis in dogs treated with low doses of cyclophosphamide. Am J Pathol. 1987 Jun;127(3):467–473. [PMC free article] [PubMed] [Google Scholar]
- Andrade Z. A. Mechanisms of myocardial damage in Trypanosoma cruzi infection. Ciba Found Symp. 1983;99:214–233. doi: 10.1002/9780470720806.ch12. [DOI] [PubMed] [Google Scholar]
- Bongertz V., Dvorak J. A. Trypanosoma cruzi: antigenic analysis of cloned stocks. Am J Trop Med Hyg. 1983 Jul;32(4):716–722. doi: 10.4269/ajtmh.1983.32.716. [DOI] [PubMed] [Google Scholar]
- CAMARGO E. P. GROWTH AND DIFFERENTIATION IN TRYPANOSOMA CRUZI. I. ORIGIN OF METACYCLIC TRYPANOSOMES IN LIQUID MEDIA. Rev Inst Med Trop Sao Paulo. 1964 May-Jun;6:93–100. [PubMed] [Google Scholar]
- Gattass C. R., Lima M. T., Nóbrega A. F., Barcinski M. A., Dos Reis G. A. Do self-heart-reactive T cells expand in Trypanosoma cruzi-immune hosts? Infect Immun. 1988 May;56(5):1402–1405. doi: 10.1128/iai.56.5.1402-1405.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli R. T., Morato M. J., Nunes R. M., Cançado J. R., Brener Z., Gazzinelli G. Idiotype stimulation of T lymphocytes from Trypanosoma cruzi-infected patients. J Immunol. 1988 May 1;140(9):3167–3172. [PubMed] [Google Scholar]
- Hontebeyrie-Joskowicz M., Said G., Milon G., Marchal G., Eisen H. L3T4+ T cells able to mediate parasite-specific delayed-type hypersensitivity play a role in the pathology of experimental Chagas' disease. Eur J Immunol. 1987 Jul;17(7):1027–1033. doi: 10.1002/eji.1830170720. [DOI] [PubMed] [Google Scholar]
- Israelski D. M., Sadler R., Araujo F. G. Antibody response and antigen recognition in human infection with Trypanosoma cruzi. Am J Trop Med Hyg. 1988 Nov;39(5):445–455. doi: 10.4269/ajtmh.1988.39.445. [DOI] [PubMed] [Google Scholar]
- Kirchhoff L. V., Engel J. C., Dvorak J. A., Sher A. Strains and clones of Trypanosoma cruzi differ in their expression of a surface antigen identified by a monoclonal antibody. Mol Biochem Parasitol. 1984 Apr;11:81–89. doi: 10.1016/0166-6851(84)90056-2. [DOI] [PubMed] [Google Scholar]
- Krettli A. U., Brener Z. Protective effects of specific antibodies in Trypanosoma cruzi infections. J Immunol. 1976 Mar;116(3):755–760. [PubMed] [Google Scholar]
- Köberle F. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBaer J., Tsien R. Y., Fahey K. A., DeFranco A. L. Stimulation of the antigen receptor on WEHI-231 B lymphoma cells results in a voltage-independent increase in cytoplasmic calcium. J Immunol. 1986 Sep 15;137(6):1836–1844. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987 Jan;60(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Llop E., Rothhammer F., Acuña M., Apt W. HLA antigens in cardiomyopathic Chilean chagasics. Am J Hum Genet. 1988 Nov;43(5):770–773. [PMC free article] [PubMed] [Google Scholar]
- Martins M. S., Hudson L., Krettli A. U., Cançado J. R., Brener Z. Human and mouse sera recognize the same polypeptide associated with immunological resistance to Trypanosoma cruzi infection. Clin Exp Immunol. 1985 Aug;61(2):343–350. [PMC free article] [PubMed] [Google Scholar]
- Melby P. C., Neva F. A., Sacks D. L. Profile of human T cell response to leishmanial antigens. Analysis by immunoblotting. J Clin Invest. 1989 Jun;83(6):1868–1875. doi: 10.1172/JCI114093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morato M. J., Brener Z., Cançado J. R., Nunes R. M., Chiari E., Gazzinelli G. Cellular immune responses of chagasic patients to antigens derived from different Trypanosoma cruzi strains and clones. Am J Trop Med Hyg. 1986 May;35(3):505–511. doi: 10.4269/ajtmh.1986.35.505. [DOI] [PubMed] [Google Scholar]
- Nickell S. P., Gebremichael A., Hoff R., Boyer M. H. Isolation and functional characterization of murine T cell lines and clones specific for the protozoan parasite Trypanosoma cruzi. J Immunol. 1987 Feb 1;138(3):914–921. [PubMed] [Google Scholar]
- Nogueira N., Ellis J., Chaplan S., Cohn Z. Trypanosoma cruzi: in vivo and in vitro correlation between T-cell activation and susceptibility in inbred strains of mice. Exp Parasitol. 1981 Jun;51(3):325–334. doi: 10.1016/0014-4894(81)90120-x. [DOI] [PubMed] [Google Scholar]
- Petry K., Eisen H. Chemical characterization of epitopes common to Trypanosoma cruzi and mammalian nervous cells. Mem Inst Oswaldo Cruz. 1988 Nov;83 (Suppl 1):498–501. doi: 10.1590/s0074-02761988000500057. [DOI] [PubMed] [Google Scholar]
- Postan M., McDaniel J. P., Dvorak J. A. Comparative studies of the infection of Lewis rats with four Trypanosoma cruzi clones. Trans R Soc Trop Med Hyg. 1987;81(3):415–419. doi: 10.1016/0035-9203(87)90155-6. [DOI] [PubMed] [Google Scholar]
- Rangel-Aldao R., Comach G., Allende O., Cayama E., Delgado V., Piras R., Piras M., Henriquez D., Negri S. Trypanosoma cruzi: polypeptide markers of epimastigotes and trypomastigotes. Mol Biochem Parasitol. 1986 Jul;20(1):25–32. doi: 10.1016/0166-6851(86)90139-8. [DOI] [PubMed] [Google Scholar]
- Scharfstein J., Luquetti A., Murta A. C., Senna M., Rezende J. M., Rassi A., Mendonça-Previato L. Chagas' disease: serodiagnosis with purified Gp25 antigen. Am J Trop Med Hyg. 1985 Nov;34(6):1153–1160. doi: 10.4269/ajtmh.1985.34.1153. [DOI] [PubMed] [Google Scholar]
- Scharfstein J., Rodrigues M. M., Alves C. A., de Souza W., Previato J. O., Mendonça-Previato L. Trypanosoma cruzi: description of a highly purified surface antigen defined by human antibodies. J Immunol. 1983 Aug;131(2):972–976. [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snary D., Flint J. E., Wood J. N., Scott M. T., Chapman M. D., Dodd J., Jessell T. M., Miles M. A. A monoclonal antibody with specificity for Trypanosoma cruzi, central and peripheral neurones and glia. Clin Exp Immunol. 1983 Dec;54(3):617–624. [PMC free article] [PubMed] [Google Scholar]
- Takehara H. A., Perini A., da Silva M. H., Mota I. Trypanosoma cruzi: role of different antibody classes in protection against infection in the mouse. Exp Parasitol. 1981 Aug;52(1):137–146. doi: 10.1016/0014-4894(81)90069-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunón P., Johansson K. E. Yet another improved silver staining method for the detection of proteins in polyacrylamide gels. J Biochem Biophys Methods. 1984 May;9(2):171–179. doi: 10.1016/0165-022x(84)90008-3. [DOI] [PubMed] [Google Scholar]
- Wood J. N., Hudson L., Jessell T. M., Yamamoto M. A monoclonal antibody defining antigenic determinants on subpopulations of mammalian neurones and Trypanosoma cruzi parasites. Nature. 1982 Mar 4;296(5852):34–38. doi: 10.1038/296034a0. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]