Abstract
Field and laboratory research has repeatedly shown that free-living soil nematodes differ in their sensitivity to soil pollution. In this paper, we analyze whether nematode genera proved sensitive or tolerant toward heavy metals and organic pollutants in six long-term field experiments. We discuss overlaps between nematode physiological responses to heavy metals and to organic pollutants, which may explain why nematodes can exhibit co-tolerance toward several contaminants. We propose a simple method for separating direct effects of soil contamination on nematode populations from indirect effects mediated through the food chain. Finally, we analyze the extent to which nematodes exhibited consistent responses across the experiments analyzed. Our results show that (a) indirect effects of pollution were generally strong; (b) fewer nematode genera were tolerant than sensitive; (c) many genera, including practically all Adenophorea, exhibited a common response pattern to contaminants; and (d) several genera of the Secernentea exhibited differential tolerance toward particular pollutants. We conclude that bioindication of soil contamination should preferentially be based on tolerant, and less on sensitive, nematodes. We provide a list of nematode genera that may potentially serve as differential bioindicators for specific soil contaminants.
Keywords: Bioindicators, ecology, heavy metals, nematodes, organic toxicants, sentinels, soil pollution
Among soil organisms, nematodes are seen as the most promising candidates for bioindication of soil status (Cortet et al., 1999; Achazi, 2002). Using the well established classifications of nematode feeding types and cp-groups (Yeates et al., 1993; Bongers and Bongers, 1998), researchers have consistently exploited nematodes to investigate the propagation of broadly defined disturbance and fertilization effects through the soil ecosystem (Freckman and Ettema, 1993; Villenave et al., 2001). It has been shown repeatedly that nematodes respond differentially to xenobiotic substances (Bongers et al., 2001; De Nardo and Grewal, 2003; Jonker et al., 2004). However, a recognized concept enabling a nematode-based indication of more specific impacts than disturbance and fertilization, such as the indication of a specific heavy metal or a specific organic toxicant in the soil, is lacking.
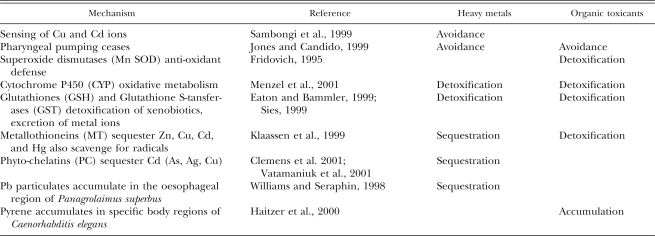
In fact, many of the physiological responses of nematodes to toxic substances are highly unspecific (Table 1). As the very first and immediate reaction on sensing a toxic substance, nematodes can cease pharyngeal pumping and thereby avoid intake of the toxicant (Jones and Candido, 1999). Nematodes have elaborate sensorial equipment, including receptors for cadmium and copper ions (Sambongi et al., 1999), which enables them to avoid intake of a broad spectrum of substances by means of the same behavioral response mechanism. Once a toxicant has passed into the body, a plethora of molecular decontamination mechanisms is induced (Downs et al., 2001). Superoxide dismutases accumulate in response to oxidative stress and are one of the main anti-oxidant defense pathways (Fridovich, 1995). Cytochrome P450 has both physiologically relevant oxidative and reductive reactions and catalyzes many xe-nobiotic-based substrates (Menzel et al., 2001). Glutathiones and glutathione S-transferases are involved in the detoxification of organic xenobiotics and in the discharge of metal ions from the cell (Eaton and Bammler, 1999; Sies, 1999). Metallothioneins can act as scavengers for radicals, and mainly they protect against metal toxicity by sequestering Zn, Cu, Cd, and Hg (Klaassen et al., 1999). Phyto-chelatins also sequester Cd, as well as As, Ag, and Cu (Clemens et al., 2001; Vatamaniuk et al., 2001). In this way, metal ions are neutralized within the nematode body if they cannot be excreted (Vijver et al., 2004). Storage of Pb can, for example, reach levels where visible lead particulates are formed in the oesophageal region of Panagrolaimus superbus (Williams and Seraphin, 1998). Similarly, organic pollutants can accumulate in the tissue if metabolization and excretion do not keep pace with intake rates. For example, Haitzer et al. (2000) observed accumulation of pyrene in specific, presumably lipid-rich body regions within Caenorhabditis elegans. The processes of avoidance, detoxification, and sequestration of pollutants are accompanied by a range of more general mechanisms for protein survey and repair (e.g., effected by ubiquitin or by heat shock proteins).
Table 1.
Nematode physiological responses to toxic substances.
With respect to bioindication, the multitude of detoxification mechanisms available and the remarkable overlap between the mechanisms involved in the detoxification of heavy metals and organic pollutants may mean that nematodes frequently acquire multiple resistance against various pollutants simultaneously. The resulting nonspecific population response to pollutants will, in turn, largely blur an individual pollutant's signal in the nematode community. It seems, therefore, a relevant question whether nematodes exhibit sufficiently distinct responses to different toxic substances to enable a differential bioindication of these substances. We re-analyze published data from six large field investigations on long-term soil contamination as to the responses of nematodes to the pollutants applied. First, we illustrate the importance of indirect contamination effects on nematode populations and propose a simple method for isolating direct effects from food-chain effects. Then, we compare across experiments the consistency and the specificity in the numerical responses of nematode genera to selected toxicants.
Materials and Methods
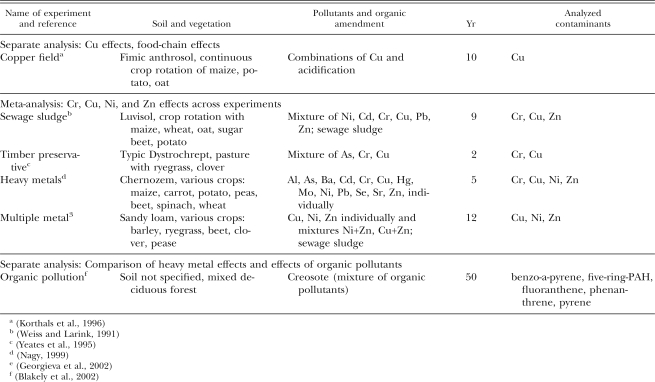
Data sources: Due to the destructive and risky nature of toxicological field experiments, few investigations are conducted in which soil contamination is deliberately introduced in a controlled way in the field. However, information can also be drawn from sites that have been contaminated by industrial waste or by accidental pollution. For our analysis, we selected six investigations from the literature that included at least 2-yr duration of contamination and provided quantitative documentation of soil pollution status and nematode community.
The copper field experiment (Korthals et al., 1996) was located near Wageningen, The Netherlands. The dominant soil type is a fimic anthrosol. A continuous crop rotation of maize, potato, and oats was maintained on the site. In 1982, 128 plots of 6 × 11 m were established in eight blocks. A full factorial design of four copper levels (0, 250, 500, and 750 kg Cu/ha) and four pH levels (4.0, 4.7, 5.4, and 6.1 pH-KCl) was implemented in eight replicates randomized in eight blocks. In 1992, 10 yr after the start of the experiment, 30 soil cores (17-mm-diam. × 10-cm-depth) were taken from each of the 128 plots, mixed, and analyzed for pH, total and available copper content, and nematodes.
The sewage sludge experiment (Weiss and Larink, 1991) was located near Braunschweig, Germany, on a luvisol. A rich rotation with maize, summer wheat, winter wheat, oat, sugar beet, and potato was grown on the site. From 1980 onward, native sewage sludge (12 t dry matter/ha/yr) was applied in one treatment and sewage sludge with added Ni, Pb, Cd, Cr, Cu, and Zn in a second treatment, while a third treatment was fertilized with 100 kg N/ha/yr. Soil concentrations of heavy metals were monitored regularly. In 1989, 9 yr after the start of the experiment, 10 soil cores (3.9-cm-diam. × 20-cm-depth) were taken in each of four replicate plots of the treatments, bulked, and analyzed for nematodes.
The timber preservative investigation (Yeates et al., 1995) took place near Levin, New Zealand, on stony silt loam (Typic Dystrochrept) under permanent pasture dominated by ryegrass and white clover. The site was contaminated by surface runoff of Cu-Cr-As-based timber preserving liquor from an adjacent timber-treatment plant. In 1989, the contamination was analyzed. In 1991, 50 soil cores (2.5-cm-diam. × 5-cm-depth) were taken from 11 plots on areas with low, medium, high, and extreme contamination. Soil concentrations of Cu, Cr, and As were measured and nematodes enumerated and identified.
The heavy metals experiment (Nagy, 1999) was conducted at Nagyhorcsok, Hungary, on calcareous loamy chernozem with a medium-to-deep humus layer. Various crops, such as maize, carrot, potato, peas, beet root, spinach, and winter wheat, were planted during the experiment. In 1991, a split-plot design with two replicate blocks was established, in which 13 individual heavy metals and microelements were ploughed into the soil at three to four different contamination levels of up to 810 kg/ha. Soil element concentrations were annually monitored. In 1996 and 1997, a total of 56 soil cores (2-cm-diam. × 10-cm-depth) were taken from the controls and from the 13 treatments with the highest contaminations, and nematodes were extracted.
The multiple metal experiment (Georgieva et al., 2002) was conducted at North Nottinghamshire, United Kingdom, on sandy loam. Barley, Italian ryegrass, sugar beet, white clover, and peas were grown on the site. In 1982, a randomized plot design was established with the following treatments: Application of uncontaminated sludge (100 t dry solids/ha); sewage sludge contaminated artificially with four concentrations of either Ni, Cu, Zn, or combinations of Zn and Ni or Zn and Cu; and a control without any sewage sludge application. Four replicates were established for the uncontaminated sludge and no sludge treatments and two replicates for each heavy metal concentration. In 1986, further additions of naturally contaminated sludge were made to some of the plots. The soils were analyzed regularly for heavy metal content. In 1994, 12 yr after the first and 8 yr after the second treatment, each plot was sampled for nematodes with a bulk sample consisting of 20 cores (1.9-cm-diam. × 20-cm-depth).
The organic pollution investigation (Blakely et al., 2002) was performed in Toledo, OH, in the vicinity of a creosote reservoir. The reservoir was established at the site about 50 yr earlier and since then had been leaking into adjacent soil and groundwater. The soils analyzed were vegetated by mixed deciduous forest with a dense understory of perennial and annual herbs and vines. Thirty intact soil cores (5.1-cm-diam. × 7.6-cm-depth) were taken on each of three sampling occasions in 1998. Phenanthrene, fluoranthene, pyrene, and benzo-a-pyrene concentrations were measured, and nematode community composition was analyzed.
Data analysis: The copper field experiment was analyzed separately to serve as a validation control (see Table 2). To illustrate indirect Cu effects mediated via the food chain, we compared the responses of nematode feeding groups to the Cu concentration gradient of the copper field experiment. In a second analysis, we compared nematode responses of the four experiments with multiple heavy metal pollution. To make it possible to compare data across the different experiments, data were normalized as follows: Counts of nematode genera were expressed as relative proportions of their feeding type (DoFT = Dominance within Feeding Type), thereby correcting both for indirect toxicity effects through changes in feeding sources and for differences across investigations in sampling and extraction. We see this as a natural extension from the widely adopted habit of expressing genus importance as dominance, i.e., as relative proportions of the total community. To correct for differences between investigations in heavy metal bioavailability, the concentrations of heavy metals were given a different weight for each investigation and for each feeding type, to provide the best regression coefficient between heavy metals and nematode responses in the combined data of the meta-analysis. The Excel Solver tool was applied to optimize regression coefficients. The values of the relative weights were handled conservatively and were kept within 0.1 and 10. Spearman rank correlations were calculated for each nematode genus and each of the four heavy metals, Cu, Zn, Ni, and Cr, that were shared between at least two experiments. The truncated probability method (TPM; Zaykin et al., 2002; Neuhäuser, 2004) was applied to account for replicate testing and to keep cumulative error below 0.05. Finally, the regression coefficients obtained for heavy metals were compared to those reported from the organic pollution investigation. Regression coefficients were rearranged in a table by means of two-way joining to reflect similarities of the effects of toxicants and similarities of the responses of nematodes (Table 3). The Statistica software package (StatSoft Inc., Tulsa, OK) was used for all statistical analyses.
Table 2.
Data sources and analyses of the six studies used in this investigation, together with information on soil, vegetation, pollutants applied, organic amendments (if applied), years since contamination, and the contaminants considered. Data of the copper field experiment and of the organic pollution investigation were treated separately. Data of the four experiments with multiple heavy metal contamination were combined in a meta-analysis.
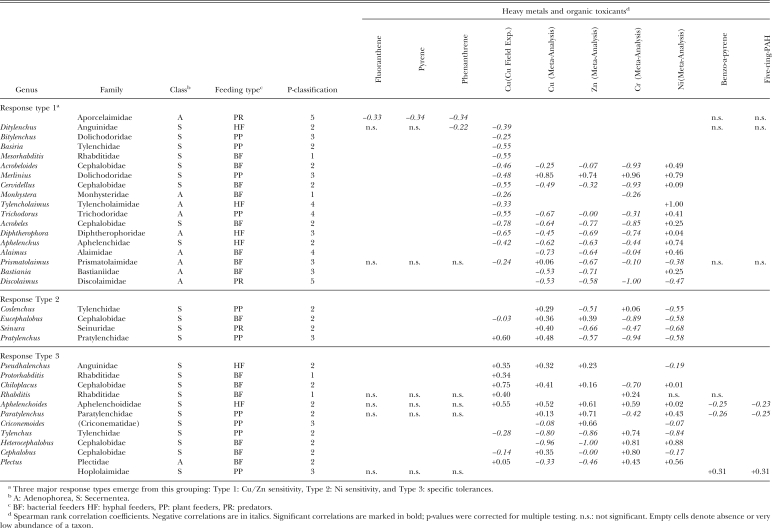
Table 3.
Overview of nematode responses to selected heavy metals and organic toxicants comparing results from the copper field experiment, the joint analysis of experiments with multiple heavy metal contamination, and the organic pollution investigation. Nematode genera and toxic substances are arranged to group similar nematode responses in adjacent cells of the table (two-way joining).
Results
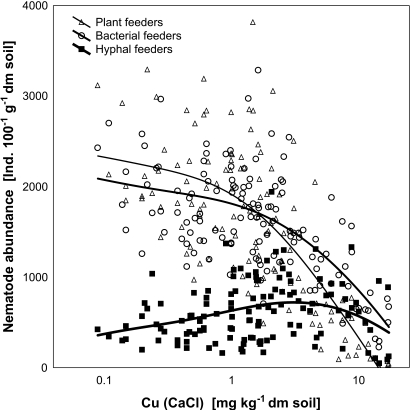
Differences between trophic channels: In the copper field experiment, there was a general and strong decrease of the nematode population density at higher Cu concentrations (Fig. 1). However, there were marked differences between feeding groups. Plant feeders proved the most strongly affected group, exhibiting a marked decline at Cu concentrations higher than 1 ppm. Bacterial feeders remained comparatively less affected up to a Cu concentration of around 2 ppm, while rapidly degrading at higher concentrations. In contrast, fungal feeders increased in abundance with increasing Cu concentrations up to 4 ppm and declined moderately at higher concentrations. We consider it unlikely that nematodes with a hyphal-feeding habit are generally resistant to Cu while nematodes with a plant-feeding habit are generally susceptible to Cu. Instead, we assume that the abundances of nematode trophic groups followed the responses of their feeding sources to the contamination. Specifically, plants showed a dramatic inhibition of growth at higher Cu concentrations, while fungal growth may have been partly fostered by the soil acidification brought about by the combined pH and Cu treatment in the experiment.
Fig. 1.
Effects of Cu on nematode feeding types. Nematode feeding types differed strongly in their response to soil Cu contamination. Plant-feeders and bacterial-feeders declined with higher Cu concentrations. Hyphal-feeders increased in abundance up to Cu concentrations of 4 ppm. (Data from the copper field experiment; Korthals et al., 1996).
Comparison across investigations: Of the 70 nematode taxa registered in total, 34 exhibited significant correlations with at least one of the toxic substances analyzed (Table 3). Correlations of nematode genera with Cu were coherent between the copper field experiment and the other heavy metal experiments. None of the significant correlations with Cu was contradictory between experiments; however, the ensemble of multiple heavy metal experiments showed less significant correlations, as is to be expected by the higher variation of data encompassed. The overall comparison of results from the copper field experiment, the four experiments with multiple heavy metals combined, and the organic pollution experiment reveals three major response types of nematodes: (i) Cu/Zn sensitivity, (ii) Ni sensitivity, and (iii) specific tolerances. Type 1 genera exhibited negative correlations with Cu and in many cases also with Zn. Possibly, genera of this response type also tend to be susceptible to organic pollutants, as indicated to some extent by Ditylenchus. The Aporcelaimidae were allocated to response type 1 due to their susceptibility to fluoranthene, pyrene, and phenanthrene. At the same time, type 1 nematodes were less affected by Cr and tended to be correlated positively with Ni, albeit not significantly in most cases. Practically all Adenophorea belonged to this response type. In direct contrast, response type 2 encompassed Secernen-tea showing negative correlations with Ni. The genera of response type 3 were characterized by positive correlations with pollutants. They showed a stronger differentiation of responses. Four genera were particularly tolerant to Cu, three to Zn, and three to Cr; Plectus was tolerant to Ni and the Hoplolaimidae were tolerant to benzo-a-pyrene and the five-ring-PAH. This response type was represented almost exclusively by Secernentea, the only exception being Plectus. The error probability for the positive correlation between Plectus and Ni is at the margin of the cut-off criterion for pairwise correlations (P pairwise < 0.005), which is required to keep the cumulative error below the significance threshold. The result for Plectus is therefore subject to the cumulative error probability, which amounted to 0.045.
Discussion
Our results suggest that a broad spectrum of nematode genera show a common response pattern to the toxicants analyzed, while some genera acquired specific and differential tolerances toward particular substances. It must be emphasized that dominance within feeding type (DoFT), used to quantify nematode importance in this investigation, does not offer an absolute measure of a nematode's potential to cope with a given impact. Instead, DoFT provides a relative measure of sensitivity as compared to the responses of the other nematodes from the same feeding type. We interpret the abundances of nematode feeding groups in the copper field experiment to be strongly influenced by responses of feeding sources to the copper contamination. Such food-chain effects distort the proportions of the individual genera within the nematode community, depending on the feeding type of each genus. Therefore, both nematode abundances and dominances reflect the superimposition of direct effects of toxicants on nematodes and indirect effects through the responses of feeding sources to the toxicants. In contrast, DoFT isolates the direct effects from the superimposition by treating the food chains separately. Analyses that we performed using nematode abundances and dominances did not yield consistent results, and we found DoFT by far the better choice.
Interpretation of the results is partially impeded by the fact that a number of nematode genera were present in only one of the datasets, which prevents a reliable generalization of their behavior. The sparse data available on long-term effects of organic toxicants means that only the most reserved of conclusions can be drawn. Generally, pollution can induce tolerance in nematodes through selection of tolerant strains within the population (Millward and Grant, 2000). Thus, our long-term analysis reflects the potential of nematode genera to adapt themselves to pollution in the long term, and this potential may not be representative of other investigations performed shortly after a contamination event. Given these limitations, our analysis revealed several consistent patterns. Consistency was explicitly visible by the lack of contradiction between the two separate analyses for Cu. Consistency was implicitly ensured by the meta-analysis of the four datasets with multiple heavy metals. If a nematode genus responded inconsistently between datasets, then no significant correlation would emerge across datasets.
Contrary to our expectation derived from the overlap of molecular mechanisms involved in the detoxification of heavy metals and organic toxicants, nematodes generally did not exhibit multiple resistance against various pollutants simultaneously. Instead, the majority of nematode taxa exhibited multiple sensitivity. Overall, tolerance of nematodes toward toxicants (16 significant positive correlations) was half as frequent as sensitivity (35 significant negative correlations), which illustrates that tolerance, not sensitivity, represented the special case. As a consequence, bioindication of specific toxicants often will be more powerful if based on tolerant
nematodes rather than on sensitive nematodes. The relative persistence of tolerant nematodes, in turn, qualifies them better as sentinels (Beeby, 2001). Tolerance to heavy metals and to organic toxicants was found almost exclusively in Secernentea. However, not all Secernentea exhibited such a behavior. Some Sercenentea were particularly sensitive to Ni. Many other Secernentea resembled the largely unspecific Adenophorea. This differentiation within the Secernentea will certainly obscure any relationship between the Secernentea/Adenophorea ratio and a toxicant. No coherent response pattern was visible on the family level. The Cephalobidae presented an extreme example where all kinds of different behaviors were shown by its genera. It is known that, even within the same genus, different species can exhibit contrasting responses to heavy metals (Sturhan, 1986). Therefore, it seems possible that tolerance to toxic substances, or the potential for selective adaptation, evolved repeatedly, independently, and differentially within the Secernentea based on a general pre-adaptation of this group. This implies that relatively little information can be drawn from the taxonomic relationships between nematodes about their potential as bioindicators of toxicants. Empirical knowledge of their responses to toxic substances is indispensable. From our analysis, we conclude that Chiloplacus and Pratylenchus are good candidates for substance-specific bioindication of Cu, Paratylenchus and Criconemoides of Cr, and Tylenchus and Cephalobus of Zn, as seen from their exclusive and positive correlations with these metals. Good candidates for bioindication of organic toxicants possibly can be found in the family Hoplolaimidae.
Footnotes
Symposium paper presented at the 43rd Annual Meeting of The Society of Nematologists, 7–11 August 2004, Estes Park, CO.
The authors acknowledge D. Neher and T. Bongers for motivating this cooperation and work.
This paper was edited by G. W. Yeates.
Literature Cited
- Achazi RK. Invertebrates in risk assessment. Journal of Soils and Sediments. 2002;2:174–178. [Google Scholar]
- Beeby A. What do sentinels stand for? Environmental Pollution. 2001;112:285–298. doi: 10.1016/s0269-7491(00)00038-5. [DOI] [PubMed] [Google Scholar]
- Blakely JK, Neher DA, Spongberg AL. Soil invertebrate and microbial communities, and decomposition as indicators of polycyclic aromatic hydrocarbon contamination. Applied Soil Ecology. 2002;21:71–88. [Google Scholar]
- Bongers T, Bongers M. Functional diversity of nematodes. Applied Soil Ecology. 1998;10:239–251. [Google Scholar]
- Bongers T, Ilieva-Makulec K, Ekschmitt K. Acute sensitivity of nematode taxa to CuSO4 and relationships with feeding-type and life-history classification. Environmental Toxicology and Chemistry. 2001;20:1511–1516. doi: 10.1897/1551-5028(2001)020<1511:asontt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Clemens S, Schroeder JI, Degenkolb T. Caenorhabditis elegans expresses a functional phytochelatin synthase. European Journal of Biochemistry. 2001;268:3640–3643. doi: 10.1046/j.1432-1327.2001.02293.x. [DOI] [PubMed] [Google Scholar]
- Cortet J, Gomot-De Vauflery A, Poinsot-Balaguer N, Gomot L, Texier C, Cluzeau D. The use of invertebrate soil fauna in monitoring pollutant effects. European Journal of Soil Biology. 1999;35:115–134. [Google Scholar]
- De Nardo EAB, Grewal PS. Compatibility of Steinernema feltiae (Nematoda: Steinernematidae) with pesticides and plant-growth regulators used in glasshouse plant production. Biocon-trol Science and Technology. 2003;13:441–448. [Google Scholar]
- Downs CA, Dillon RT, Fauth JE, Woodley CM. A molecular biomarker system for assessing the health of gastropods (Ilyanassa obsoleta) exposed to natural and anthropogenic stressors. Journal of Experimental Marine Biology and Ecology. 2001;259:189–214. doi: 10.1016/s0022-0981(01)00233-7. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicological Sciences. 1999;49:156–164. doi: 10.1093/toxsci/49.2.156. [DOI] [PubMed] [Google Scholar]
- Freckman DW, Ettema CH. Assessing nematode communities in agroecosystems of varying human intervention. Agriculture. Ecosystems and Environment. 1993;45:239–261. [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annual Review of Biochemistry. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Georgieva SS, McGrath SP, Hooper DJ, Chambers BS. Nematode communities under stress: The long-term effects of heavy metals in soil treated with sewage sludge. Applied Soil Ecology. 2002;20:27–42. [Google Scholar]
- Haitzer M, Lohmannsroben HG, Steinberg CEW, Zimmermann U. In vivo laser-induced fluorescence detection of pyrene in nematodes and determination of pyrene binding constants for humic substances by fluorescence quenching and bioconcentration experiments. Journal of Environmental Monitoring. 2000;2:145–149. doi: 10.1039/a907341h. [DOI] [PubMed] [Google Scholar]
- Jones D, Candido EPM. Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: Relationship to the cellular stress response. Journal of Experimental Zoology. 1999;284:147–157. doi: 10.1002/(sici)1097-010x(19990701)284:2<147::aid-jez4>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Jonker MJ, Piskiewicz AM, Castella NII, Kammenga JE. Toxicity of binary mixtures of cadmium-copper and carbendazim-copper to the nematode Caenorhabditis elegans . Environmental Toxicology and Chemistry. 2004;23:1529–1537. doi: 10.1897/03-49. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Liu J, Choudhrui S. Metallothionein: An intracellular protein to protect against cadmium toxicity. Annual Review of Pharmacology and Toxicology. 1999;39:267–294. doi: 10.1146/annurev.pharmtox.39.1.267. [DOI] [PubMed] [Google Scholar]
- Korthals GW, Alexiev AD, Lexmond TM, Kammenga JE, Bongers T. Long-term effects of copper and pH on the nematode community in an agroecosystem. Environmental Toxicology and Chemistry. 1996;15:979–985. [Google Scholar]
- Menzel R, Bogaert T, Achazi R. A systematic gene expression screen of Caenorhabditis elegans cytochrome P450 genes reveals CYP35 as strongly xenobiotic inducible. Archives of Biochemistry and Biophysics. 2001;395:158–168. doi: 10.1006/abbi.2001.2568. [DOI] [PubMed] [Google Scholar]
- Millward RN, Grant A. Pollution-induced tolerance to copper of nematode communities in the severely contaminated Restronguet Creek and adjacent estuaries, Cornwall, UK. Environmental Toxicology and Chemistry. 2000;19:454–461. [Google Scholar]
- Nagy P. Effect of an artificial metal pollution on nematode assemblage of a calcareous loamy chernozem soil. Plant and Soil. 1999;212:35–43. [Google Scholar]
- Neuhäuser M. Testing whether any of the significant tests within a table are indeed significant. Oikos. 2004;106:409–410. [Google Scholar]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans . Neuroreport. 1999;10:753–757. doi: 10.1097/00001756-199903170-00017. [DOI] [PubMed] [Google Scholar]
- Sies H. Glutathione and its role in cellular functions. Free Radical Biology and Medicine. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Sturhan D. Influence of heavy metals and other elements on soil nematodes. Revue de Nématologie. 1986;9:311. [Google Scholar]
- Vatamaniuk OK, Bucher EE, Ward JT, Rea PA. Worms take the ‘phyto’ out of “phytochelatins”. Trends in Biotechnology. 2001;20:61–64. doi: 10.1016/s0167-7799(01)01873-x. [DOI] [PubMed] [Google Scholar]
- Vijver MG, Van Gestel CAM, Lanno RP, Van Straalen NM, Peijnenburg WJGM. Internal metal sequestration and its ecotoxicological relevance: A review. Environmental Science and Technology. 2004;38:4705–4712. doi: 10.1021/es040354g. [DOI] [PubMed] [Google Scholar]
- Villenave C, Bongers T, Ekschmitt K, Djigal D, Chotte JL. Changes in nematode communities following cultivation of soils after fallow periods of different length. Applied Soil Ecology. 2001;17:43–52. [Google Scholar]
- Weiss B, Larink O. Influence of sewage sludge and heavy metals on nematodes in an arable soil. Biology and Fertility of Soils. 1991;12:5–9. [Google Scholar]
- Williams MSR, Seraphin S. Heavy metal biominer-alization in free-living nematodes, Panagrolaimus spp. Materials Science and Engineering C-Biomimetic Materials Sensors and Systems. 1998;6:47–51. [Google Scholar]
- Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS. Feeding habits in soil nematode families and genera—an outline for soil ecologists. Journal of Nematology. 1993;25:315–331. [PMC free article] [PubMed] [Google Scholar]
- Yeates GW, Orchard VA, Speir TW. Reduction in faunal populations and decomposition following pasture contamination by a Cu–Cr–As based timber preservative. Acta Zoologica Fennica. 1995;196:297–300. [Google Scholar]
- Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS. Truncated product method for combining P-values. Genetic Epidemiology. 2002;22:170–85. doi: 10.1002/gepi.0042. [DOI] [PubMed] [Google Scholar]