Abstract
Few studies have addressed the role of the sexes in the emergence and dispersal of entomopathogenic nematodes from host insects. Individuals of two isolates of Steinernema feltiae, UK76 and SBIl, emerging from Galleria mellonella cadavers were classed as Non-Dispersed (remaining on the cadaver for up to nine days) and Dispersed (actively moving away from the cadaver). Sex ratios within both classes were examined in infective (individuals that successfully invaded bait G. mellonella) and entire (infective and noninfective individuals that matured in hanging drops of G. mellonella haemolymph) populations. Sex ratios differed significantly from 1:1 only in the SBIl Non-Dispersed entire population (female bias) and SBIl Non-Dispersed infective population (male bias). For each isolate, Dispersed individuals were significantly more infective than Non-Dispersed. However, only 11% of SBIl and 22% of UK76 Non-Dispersed individuals were found to be mature infective juveniles (IJ) compared with 78% of SBIl and 82% of UK76 Dispersed individuals (based on survival in SDS). Infective juveniles dispersing towards distant radial bait G. mellonella tended to migrate from the head region of the natal cadaver. For each isolate, a higher proportion of males than females arrived early at distant baits. SBIl males survived alone in G. mellonella cadavers for longer periods than did females, which supports the “male colonization” hypothesis.
Keywords: dispersal, infective juvenile, male colonisation, nematode, sex ratio
Insect-killing nematodes of the genera Steinernema and Heterorhabditis (Nematoda: Rhabditida) are ubiquitous soil organisms (Akhurst and Bedding, 1986; Hominick et al., 1996). The infective juvenile stage (termed IJ or dauer) seeks out and invades an insect host in soil. The searching strategies of different entomopathogenic nematodes (EPN) species have previously been classified into different behaviour types: ambusher, cruiser and intermediate (Lewis et al., 1992, 1995, 1997). Once inside the insect haemocoel, the nematode releases symbiotic bacteria (Xenorhabdus spp. for steinernematids and Photorhabdus spp. for heterorhabditids) that multiply and kill the host by septicaemia. The bacteria render the host suitable for nematode development and inhibit the development of other microorganisms (Poinar, 1979). Unlike the hermaphroditic Heterorhabditis spp., species of Steinernema are almost universally dioecious, requiring a minimum of one male and one female entering a host to reproduce. Reproduction occurs within the host for one to three generations, leading to the formation of new IJ as the nutrients within the host are depleted. The IJ emerge from the insect over a period ranging from several days to two weeks under moist conditions (Koppenhöfer et al., 1997). These free-living, nonfeeding juveniles disperse through the soil to find new insect hosts. Local dispersal from the natal patch may have important consequences for local populations of EPN.
Studies on the emergence and dispersal of EPN from the host cadaver in soil are surprisingly few considering that these traits are vital for the successful colonization of a new host insect. Only a small proportion of a population of IJ are successful invaders of insect larvae under apparently ideal conditions (e.g., Bedding and Akhurst, 1975; Mracek, 1982; Gaugler et al., 1989), leading Hominick and Reid (1990) to propose the “phased infectivity hypothesis.” This highly contentious hypothesis (see Campbell et al., 1999) states that infectivity among individual IJ varies with time. It is suggested that, at any time, infectious and noninfectious sub-populations of IJ exist. Changes in infectivity of an overall population may therefore be due to some noninfectious individuals becoming infectious, or vice versa. Campbell et al. (1999) dispute the existence of a noninfectious sub-population, having found that for three species of Steinernema most IJ could be recovered in one baiting round, provided sufficient suitable hosts were exposed. Hay and Fenlon (1995) suggested that there are three sub-populations of nematodes that may be distinguished by their infective behaviour: a first group of individuals that are capable of initiating infection in unparasitised insects; a second that only invade infected hosts, and a third group that fail to establish under either condition. This suggests that invasion by individuals of the first group renders hosts susceptible to invasion by the second group.
Studies on the dispersal and infectivity of EPN typically have been performed using IJ harvested into water from White traps. Shapiro and Glazer (1996) have shown that IJ allowed to emerge into sand directly from G. mellonella larvae differ from aqueously harvested nematodes. Very little attention has been paid to the status and behaviour of Steinernema juveniles as they emerge from the host into soil, although it is known that some species such as S. glaseri emerge from the cadaver as pre-IJ stages (Kaya and Stock, 1997).
The dispersal of many organisms is often pioneered by one sex. Greenwood (1980) suggests two reasons for this; firstly, dispersal by one sex only can act to prevent close inbreeding, and secondly, asymmetry between the sexes in resource competition may lead to differences in the costs and benefits of dispersal and philopatry for the two sexes. Grewal et al. (1993) reported that male IJ disperse, locate and establish in live hosts before females, the “male colonization” hypothesis. They suggest that this parasitism by male IJ renders infected hosts suitable for nematode development such that they are more attractive to female IJ. This “recruitment” may be a strategy to increase the chance of mate-finding, thereby enhancing reproductive success. Stuart et al. (1998), however, found no difference in the colonization of hosts by male and female S. glaseri, one of the five Steinernema species studied by Grewal et al. (1993). Of these five species, only S. feltiae did not conform to the “male colonization” hypothesis, a result supported by Bohan and Hominick (1997), Renn (1998) and Scheepmaker et al. (1998).
This study examines for two isolates of S. feltiae the emergence and dispersal behaviour of IJ from host insect larvae in soil and investigates the role of the sexes in these behaviours.
Materials and Methods
Isolates: Two isolates of the insect parasitic nematode Steinernema feltiae were used: UK76, a commercial isolate originally obtained from MicroBio Ltd., Cambridge, England, and SBI1, isolated by baiting soil from a dune system at Bull Island, Dublin Bay, Ireland, with larvae of the wax moth Galleria mellonella. Following the development to adult stage of IJ in G. mellonella haemolymph (Kaya and Stock, 1997), the population sex ratio of each isolate was previously found to be balanced (Rolston, unpubl. data). Unless stated otherwise, all experiments were performed at 20°C.
Dispersal of nematodes towards distant G. mellonella bait insects: A ‘Soil Island’ arena was prepared as follows: six, 7-mm–diam. holes were drilled equidistantly around the circumference of the lid of a circular plastic container (100-mm height, 240-mm-diam.), 95 mm from the center. Several 9-cm-diam. filter papers were attached underneath the container lid to create a skirt around the edge of the container. A single skirted container was placed inside one of 15 larger containers (350-mm-diam. top, 300-mm-diam. base, 280-mm height). Cages were constructed to retain live G. mellonella bait larvae: wire mesh (1.5 × 1.5-mm-pore size) was cut into 30 × 30-mm squares and rolled into approximately 8-mm-diam. cylinders. One end of each cylinder was closed. A cylinder was then placed into each of the six holes in the container lid, closed end first, and a single G. mellonella larva was placed into each. The cylinder was closed using a cotton wool plug. Five hundred grams of Maynooth garden soil (19% clay; 48% silt; 33% sand; 22.5% organic matter) (Hass, 1999) was placed onto the container lid and slightly compressed. Ten G. mellonella larvae were infected with UK76 and SBI1 (5 larvae/isolate). Cadavers were placed on 5% agar plates and inspected frequently to detect the first signs of IJ emergence. One infected cadaver from which IJ were just beginning to emerge was then placed onto the center of the soil, ventral side to the soil, head pointing in the direction of one of the six holes, which was labelled. Another 500 g soil (totalling 1 kg soil/test arena) was placed on top of the cadaver and slightly compacted. The side of each wire cylinder was in contact with the soil. This was repeated for five arenas per isolate and five control arenas that did not have any cadavers placed in the soil, for a total of 15 arenas. All test arenas were then stored at 20°C for 10 d.
Galleria mellonella were exposed to the IJ dispersing from the cadavers by placing them in the cages for two periods of 4 hr and one of 16 hr in each 24-hr period. All G. mellonella were replaced after each of these periods. The 4-hr periods (combined) provide the data of the experiment; the G. mellonella from the 16-hr time periods were termed ‘reservoir’ and were discarded for convenience. This allowed the presumption that for each 4-hr period all IJ entering the baits would be recent arrivals at the insect sites. After each of the 4-hr time periods, the G. mellonella were recovered and replaced. The recovered larvae were washed under flowing tap water, placed singly in 6-cm-diam. petri dishes lined with moist filter paper, and stored at 20°C. After 5 d storage, dead larvae were dissected in quarter-strength Ringer solution to record the number of nematodes and to score the sex ratio. This was repeated each day for 10 d.
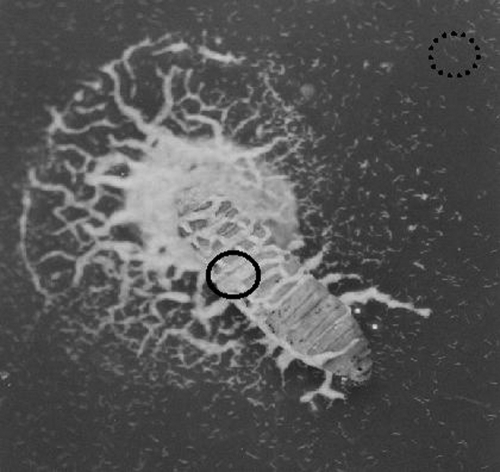
Emergence behaviour in soil dishes: Twenty late instar larvae of G. mellonella were infected with each of the two S. feltiae isolates (10 larvae/isolate): Ten larvae were placed in a 9-cm-diam. petri dish lined with filter paper. A water suspension of IJ (2,000 IJ/2 ml) was added to the dish. The dishes were stored at 20°C for 8 d, during which time the insects died. Twenty-eight grams of Maynooth garden soil of approximately 15% moisture content by weight (Sartorius Moisture Analyser MA 30) were placed in each of 20 9-cm-diam. petri dishes. Eight days after infection, one cadaver was placed on the soil surface in each dish (termed a soil dish), making 10 replicates per isolate. Patterns of emergence of nematodes from cadavers were observed over 10 d. Nematodes remaining on the cadaver for up to 9 d were classed as Non-Dispersed nematodes, whereas those that dispersed away from the cadaver were classed as Dispersed (at least 10 mm distant and actively moving away from the cadaver) (Fig. 1).
Fig. 1.
Steinernema feltiae IJ emerging from a Galletia mellonella cadaver on 5% nutrient agar. Non-Dispersed (solid circle on the cadaver) and Dispersed (dotted circle dispersing from the cadaver) are evident. The typical “medusa” pattern of Non-Dispersed IJ on the agar, surrounding the head region of the cadaver, that persists for up to 9 d post emergence is also apparent.
Lethality and sex ratios of nematodes picked from soil surfaces or from natal cadavers: Infective juveniles were allowed to develop towards the adult stage in hanging drops (Kaya and Stock, 1997), enabling the combined infective and noninfective populations to be assessed for adult sex ratios. Haemolymph of G. mellonella larvae (20 μl) was pipetted onto the underside of a 5-cm-diam. petri dish lid to produce a hanging drop. Water was added to the petri dish base to prevent the hanging drop from drying out. Using a sharpened wooden stick (150 mm × 2 mm), for each isolate, 10 Non-Dispersed nematodes and up to 10 Dispersed nematodes (more than 10 mm from the cadaver, if present) arising from each of 10 cadavers were placed into separate hanging drops on each day for 9 d. The hanging drops were placed at 20°C for 5 d, after which the sex of each nematode was recorded.
One-on-one infections were performed to allow development to the adult stage within insect hosts; filter paper disks (55-mm-diam.) were folded into quarters, rolled into funnel shapes, and each placed into a 1.5-ml capped microcentrifuge tube (Greiner Labortechnik, Poitiers, France). A small hole was made in the lid of the tube. The filter paper was moistened with 25 μl water and one G. mellonella larva was placed in the tube. Ten Non-Dispersed nematodes occurring on or very close to the cadaver were removed from each of 10 soil dishes (100 Non-Dispersed nematodes/isolate). Each nematode was placed separately onto an insect larva, with no larva receiving more then one nematode. The procedure was repeated for the Dispersed nematodes, if present. Up to 10 Dispersed nematodes/soil plate were applied in this manner. This was repeated subsequently on each of 9 d. The microcentrifuge tubes were placed at 20°C for 5 d, and the insects were then dissected as previously described to ascertain the sex of each nematode. Any insect death that could not be confirmed to be an entomopathogenic nematode kill, i.e., was fungal, bacterial (other than the EPN symbiont Xenorhabdus: the cadaver was black and putrefied), or in which no IJ was found, was classed as a “non-nematode recovery” (NNR).
Are all emerging nematodes infective juveniles? In a separate experiment, 10 G. mellonella larvae were infected with each of the two Steinernema isolates (5 larvae/isolate) and placed on a soil dish as described above, making five replicates per isolate. Each d for up to 8 d, 10 Non-Dispersed and 10 Dispersed nematodes (if present) were removed from each cadaver and placed into 1% sodium dodecyl sulphate (SDS) solution for 1 h. Any nematodes that survived for this period were determined to be IJ (Cassada and Russell, 1975), rather than an earlier developmental stage.
Adult survivorship in cadavers: For 7 d, UK76 and SBI1 IJ were allowed to emerge into water from 5 cadavers/isolate. The IJ from the five cadavers were combined for each isolate. From the resulting two stocks, single IJ were removed to single G. mellonella late instar larvae in microcentrifuge tubes prepared as described above. Three hundred such transfers were made for each isolate. The tubes were stored at 20°C. After 2, 4 and 6 wk, 100 larvae were removed for each isolate. The dead larvae were dissected and the numbers of live and dead males and females were recorded.
Statistical Analysis: Statistical analyses were performed using Minitab and Statistix software packages.
Results
Emergence behaviour: Isolates UK76 and SBI1 had almost identical periods of development within the cadaver up to first emergence (means ± S.E. of 10.9 ± 1.71 and 10.8 ± 0.98 d, respectively). Dispersed nematodes of UK76 were first found on the soil surface at least 10 mm distant from the cadaver on average of 4.6 ± 0.67 d after emergence, compared with 5.8 ± 0.83 d for the SBI1 isolate.
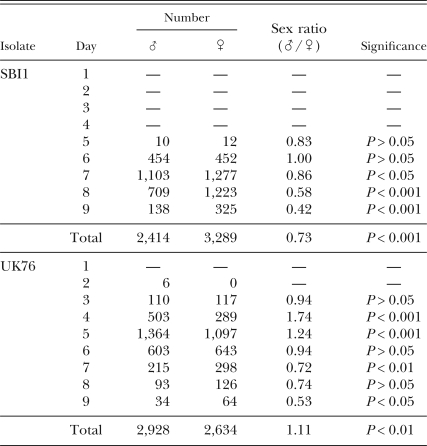
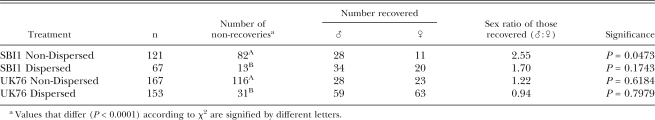
Dispersal of nematodes towards distant bait insects: No control G. mellonella were killed by EPN. No G. mellonella were killed the day the infected cadavers were placed in the soil (d 0). Data for the two 4-hr measurement periods were combined for each day and are the bases for the results reported here. Over the 10 d of the experiment, a total of 5,562 and 5,703 nematodes were found in these G. mellonella for UK76 and SBI1, respectively. No SBI1 nematodes were found in G. mellonella until d 5, 3 d after UK76 nematodes were first found there (Table 1).
Table 1.
Male and female nematodes of two Steinernema feltiae isolates recovered from six bait insects placed radially 95 mm from an infected cadaver, on each of 9 d. P-values were obtained following χ2 tests for equality of sex ratios.
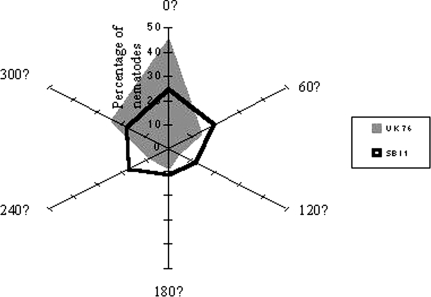
More IJ of each isolate were trapped by those G. mellonella toward which the cadaver head pointed (Figs. 2, 3). This was particularly striking in the case of UK76 and was observed for both male and female nematodes.
Fig. 2.
Percentage of total nematode recovery per isolate found in bait insects placed at six equidistant points, 95 mm from the centre of a circular soil island at which a cadaver with emerging IJ was placed. The head of the cadaver pointed towards 0°. There were 5 replicate islands/isolate.
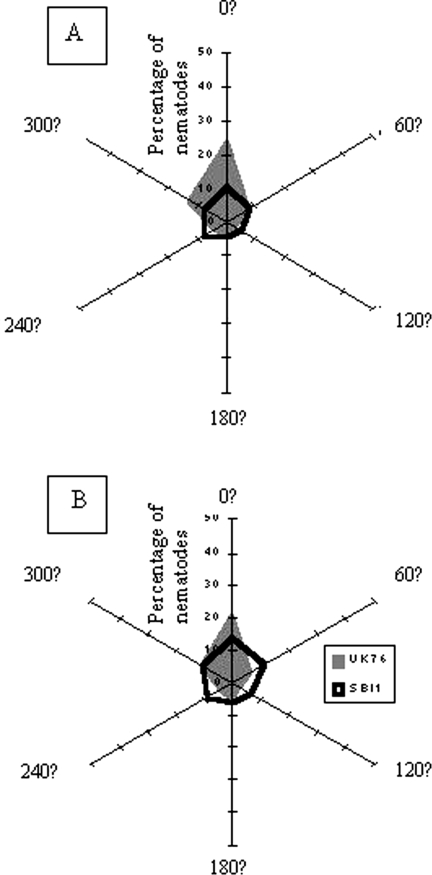
Fig. 3.
Percentage of total nematode recovery per isolate that was A) male and B) female, found in bait insects placed at six equidistant points, 95 mm from the centre of a circular soil island at which a cadaver with emerging IJ was placed. The head of the cadaver pointed towards 0°. There were 5 replicate islands/isolate.
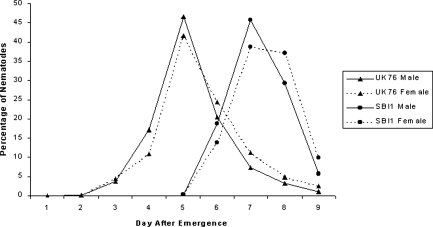
The numbers of SBI1 males and females trapped over time were equal at first, becoming significantly female biased (χ2 = 6.37, df = 1, P < 0.05; χ2 = 69.61, df = 1, P < 0.001; χ2 = 39.41, df = 1, P < 0.001 for 7, 8 and 9 d, respectively) (Table 1), with the total catch of the experiment being female biased also (χ2 = 67.63, df = 1, P < 0.001). Numbers of trapped UK76 nematodes were significantly male biased at first (χ2 = 29.45, df = 1, P < 0.001; χ2 = 14.53, df = 1, P < 0.001 for 4 and 5 d, respectively), becoming female biased with time (χ2 = 6.77, df = 1, P < 0.01; χ2 = 4.70, df = 1, P < 0.05 for 7 and 8 d, respectively), as for SBI1 (Table 1). In contrast to SBI1, the total UK76 catch was significantly male biased (χ2 = 7.78, df = 1, P < 0.01). These patterns were clarified and supported when the count values of the catches were expressed as percentages of the total catch for each isolate. It was then clear that whether the total catch was female biased (SBI1) or male biased (UK76), higher proportions of the male populations were trapped early in the experiment and higher proportions of the female populations later in the experiment (Fig. 4).
Fig. 4.
Percentage of nematodes of two isolates of Steinernema feltiae dispersing from a central host cadaver into six equidistant radial bait Galleria mellonella. For significant differences between the sexes for each isolate on each day, see Table 1.
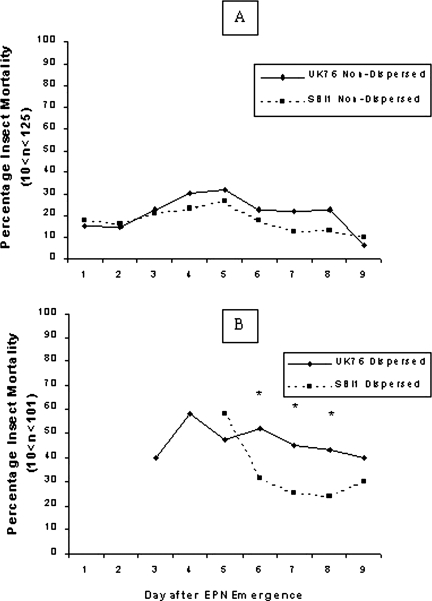
Lethality and sex ratios of nematodes picked blindly from soil surfaces or from natal cadavers: When the lethality of the two isolates emerging and dispersing from G. mellonella cadavers on the soil surface was investigated, UK76 was generally more lethal at successive assessment dates than SBI1, whether Dispersed or Non-Dispersed populations were compared between isolates (Fig. 5). The Dispersed populations showed differences between isolates at three successive dates (χ2 = 4.28, 5.50, and 5.54, P < 0.05, for 6, 7, and 8 d, respectively) and, while the Non-Dispersed populations did not differ significantly on any one occasion, those of UK76 were consistently more lethal on six successive test dates.
Fig. 5.
The mortality of Galleria mellonella larvae exposed to A) single Steinernema feltiae Non-Dispersed nematodes; B) single S. feltiae Dispersed IJ nematodes. Values of n varied each day depending on the presence of Non-Dispersed or Dispersed nematodes in each of the 10 replicates/isolate. Asterisks indicate significance difference between isolates on that day (χ2 Tests at P = 0.05 or better).
Within isolates, the Dispersed populations were always more lethal than Non-Dispersed (Fig. 5). However, large proportions (78%–89%) of Non-Dispersed nematodes were killed following immersion in 1% SDS (Table 2), indicating that fewer of them were fully developed IJ and so capable of infecting host insects. Small proportions (18%–22%) of Dispersed nematodes were also killed by SDS, more of SBI1 than of UK76.
Table 2.
Percentage of emerged nematodes surviving in 1% SDS (sodium dodecyl sulphate) solution for 1 hr. Entries bearing the same letters did not differ at P = 0.05 by χ2.
Some nematodes chosen to represent entire Dispersed and entire Non-Dispersed populations of the two isolates failed to develop to adulthood in hanging drops. This failure was much greater for the Non-Dispersed populations of each isolate (88%–93%) than for the Dispersed (35%–38%). These differences were significant for each isolate (χ2 = 7.50, df = 1, P < 0.01; χ2 = 442.14, df = 1, P < 0.001 for SBI1 and UK76, respectively) (Table 3). Of those nematodes that did develop into adults, the sex ratios only differed from 1:1 in the case of SBI1 Non-Dispersed, which was female biased (χ2 = 5.90, df = 1, P < 0.05). The sex ratios of Dispersed nematodes were less female biased than those of the Non-Dispersed for both isolates, significantly so in the case of SBI1 (Z = –3.77, P < 0.001) (Table 3). That difference was still highly significant when the data for the two isolates were combined (Z = –2.8, P < 0.01).
Table 3.
Recovery and sex ratios of entire Non-Dispersed and entire Dispersed populations of two Steinernema feltiae isolates developed in hanging drops of haemolymph. P-values were obtained following χ2 tests for equality of sex ratios.
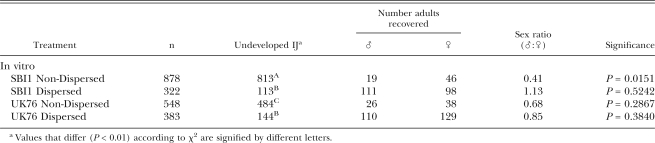
Despite the evidence of some female bias in the overall populations as measured in hanging drops, the sex ratios of those IJ that entered and developed in insect larvae were male biased in three out of the four cases (Table 4), though significantly so in the case of SBI1 Non-Dispersed only (χ2 = 3.93, df = 1, P < 0.05). Indeed, the proportion of insect-invaders that were male (Table 4) was higher in every case than the proportion of males that developed in hanging drops (Table 3), significantly so in the case of SBI1 Non-Dispersed (Z = 4.02, P < 0.0001).
Table 4.
Recovery and sex ratios of Dispersed and Non-Dispersed nematodes of SBIl and UK76 isolates infecting G. mellonella larvae. P-values were obtained following χ2 tests for equality of sex ratios.
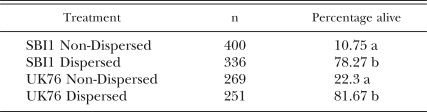
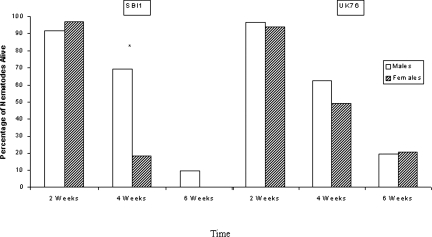
Adult survivorship in cadavers: Evidence for the longer survival of males within the cadaver was clear in the case of SBI1, where the difference between the sexes became significant at 4 wk (χ2 = 15.00, df = 1, P < 0.001) and 10% of males were still alive at 6 wk when all females were dead (Fig. 6). In the case of UK76 also there was higher, but not significantly so, survival of males at 4 wk. This was not evident at 6 wk when the experiment ended with approximately 20% of each sex still alive.
Fig. 6.
Percentage of lone adult nematodes alive in Galleria mellonella cadavers after 2, 4 and 6 wk. Asterisk indicates significant difference between the sexes (χ2 Test, P < 0.01).
Discussion
Clobert et al. (2001) stated that the emergence and dispersal of an organism may be some of the most important life history traits involved in both species persistence and evolution. Kin interaction, inbreeding avoidance, local mate and resource competition, and temporal and spatial heterogeneity are all forces that guide the evolution of dispersal. Levels of sexual selection acting on male and female S. feltiae can be important with regard to dispersal, host invasion, and mating dynamics.
The soil island experiment demonstrates what might be expected to happen in the vicinity of a large insect infected with EPN. Most of the trap insects 95 mm from the cadaver were each invaded with hundreds of nematodes, despite being left in situ for only 4 hr. In nature, S. feltiae IJ probably frequently find themselves invading a host together with many other nematodes from the same natal host.
Infective juveniles of Steinernema invade the host insect through its natural openings, the mouth, anus and spiracles. Nematodes of both UK76 and SBIl have been observed emerging from the anus and spiracles of the natal cadaver, but the head region, in particular the mouth, is commonly where the majority of emergence occurs for these isolates (Rolston, pers. obs.). It is reasonable to expect the nematodes to disperse in the direction from which they have emerged as shown by UK76, yet SBIl nematodes showed a more directionally balanced radial dispersal. Such a strategy may increase the chance that the emerging population exploits all available hosts and may also help to avoid competition for these hosts.
In this paper, we have classified the emerging nematodes of S. feltiae into two distinct groups, Non-Dispersed and Dispersed. The majority of Non-Dispersed individuals were deemed not to be IJ, based on their failure to survive immersion in 1% SDS. This presumably also explains the failure of the majority of Non-Dispersed individuals to develop in hanging drops. Nematode dauer or IJ differ in several respects from other juvenile stages, both morphologically and behaviourally; they do not feed, have a unique morphology and resistance to stress, are altered in energy metabolism, and are developmentally arrested (Poinar and Leutenegger, 1968; Riddle, 1988; Fodor et al., 1990). While it is commonly stated that it is the IJ of EPN that emerge from insect cadavers, our results indicate that most S. feltiae juveniles emerge before completing their development to the resistant IJ. Non-infective juveniles may emerge from the cadaver before development is complete because conditions within the cadaver, such as food and crowding levels, have become intolerable (Fodor et al., 1990). However, emerging early from a cadaver may leave the nematodes susceptible to attack from fungi and other predators, as well as desiccation, since IJ are more resistant than other developmental stages. Most of these non-IJ presumably continue their development to the IJ stage whilst remaining on the cadaver and then disperse, although approximately 20% of the Dispersed populations were also killed by SDS.
The proportion of juveniles developing to adult in hanging drops was lower than the proportion that survived SDS treatment. The juveniles that failed to develop may have had inadequate population densities of Xenorhabdus with which to infect the haemolymph, although this is yet to be investigated. Martens et al. (2003) identified a category of immature IJ in S. carpocapsae developing on bacterial lawns; these were resistant to SDS, but had not yet migrated away from the lawn and were usually colonized by only a few bacteria. According to Martens et al. (2003), IJ are initially colonized by just one or two bacterial clones that proliferate in the mature IJ.
Not all of the infective nematodes continued their development in the killed insects. This may again represent an inadequate population density of symbiont bacteria with which to infect the host. This is supported by the finding that more of the Non-Dispersed than of the Dispersed failed to develop after infection. Only approximately 30% of Non-Dispersed nematodes developed after infecting compared to approximately 80% of Dispersed, and Martens et al. (2003) demonstrated that premigratory IJ are colonized by few bacteria. Although axenic S. feltiae can kill G. mellonella (Ehlers et al., 1997), they may be unable to continue developing adequately. The absence of symbiotic bacteria in S. carpocapsae IJ did not affect their infectivity of Spodoptera exigua, but did inhibit nematodes producing IJ progeny within the host cadavers (Mitani et al., 2004).
While approximately 80% of the Dispersed populations of both isolates were determined to be IJ stages by the SDS test, only 30% to 47% of these populations were infective. It is possible that immersion in SDS for 1 hr is too harsh a test for the present purpose and that some IJ were killed by this treatment. Alternatively, the results may be explained by the existence of a noninfectious population of individuals, as suggested by Hominick and Reid (1990), or by a population of secondary invaders, IJ that will invade an already occupied host, but not an unoccupied one (Hay and Fenlon, 1995). As we used a one-on-one test for infectivity, secondary invaders would not be scored as infective.
The shift from balanced or male biased to female biased sex ratio in the Soil Island experiment may indicate either that male IJ develop earlier than females, as found for S. glaseri (Lewis and Gaugler, 1994), or that they disperse first, in line with the Grewal et al. (1993) “male colonization” hypothesis. Males may have been trapped earlier than females either because they reached the baits sooner or because they invaded more quickly when they arrived there. Previous findings for UK76 and SBI1 agree with Bohan and Hominick (1997) that the underlying sex ratio of S. feltiae is balanced (Rolston, unpubl. data). Support for early dispersal and/or infection by males comes from the soil surface experiment also. When picked from the soil surface and reared in hanging drops, Dispersed populations were less female biased than the Non-Dispersed populations, supporting the proposition that, whatever the relative numbers of males and females present, more of the males migrate early. That the nematodes that entered insects were more male biased than those that developed in hanging drops suggests that when differences in the numbers of the two sexes present are allowed for, males are more infective than females. This too can be seen as conformity with the “male colonization” hypothesis.
If male colonization does occur, it is possible that the first invading male(s) of SBI1 and perhaps other S. feltia, may be alone in the cadaver for a long period before a female enters. Over time, natural selection should result in males being able to survive for longer periods alone in the host than later-entering females. This was true for SBI1, but not for UK76. After 6 wk, generally less than 20% of nematodes had survived inside cadavers. As specially adapted dauers, IJ are able to survive without feeding for many months, yet no previous study has investigated adult survivorship in cadavers. On the (possibly rare) occasions when S. feltiae colonizes an insect alone, it is important to be able to survive until potential mates arrive. Since lone nematodes survived up to 6 wk, this would allow more than adequate time for IJ to emerge from neighbouring cadavers invaded by multiple nematodes at the same time as the singletons invaded. How long these surviving adults remain fertile and whether the cadavers in which they are found are still attractive to other IJ are yet to be investigated. The fact that males are indeed capable of surviving longer than females in the cadaver is interesting, as males are known to be more vulnerable to environmental stress than females in many other species (Charnov, 1982).
While it is generally fair to summarize that IJ of EPN emerge from the depleted insect cadaver and disperse in search of new hosts, the results of our experiments show that the situation is more complex. The majority of S. feltiae emerge from the cadaver before completing their development to the IJ stage. Most of these non-IJ remain close to the cadaver, though about 20% of the nematodes found 10mm or more from the cadaver were still not IJ (as determined by resistance to SDS). Many SDS-resistant IJ failed to develop in vivo or in vitro, possibly due to inadequate Xenorhabdus inoculum. In nature, it is unlikely that such immature IJ would find themselves alone in an insect. Where large numbers of IJ emerge and locate nearby hosts, as in our soils island experiment, IJ with few bacteria would be supported by the mass invasion of many nematodes together. By the time they have migrated further away from the natal host and lost the support of co-emerging and co-migrating juveniles, their bacterial complement would have increased (Martens et al., 2003). Further diversity in the juvenile population is introduced by prospective males and females behaving differently; males either develop or disperse earlier and show a higher propensity to invade an unoccupied insect. One of the risks of this behaviour is of failing to find (or be found by) a mate, but being able to survive a long time in the cadaver reduces this risk.
Footnotes
A portion of a Ph.D. thesis by the first author, supported in part by the British-Irish Scholarship Exchange Program, the Environmental Protection Agency of Ireland and the EMBARK initiative of the Irish Research Council for Science, Engineering and Technology.
This paper was edited by S. Patricia Stock
Literature Cited
- Akhurst R, Bedding R. Natural occurrence of insect pathogenic nematodes (Steinernematidae and Heterorhabditidae) in soil in Australia. Journal of the Australian Entomological Society. 1986;25:241–244. [Google Scholar]
- Bedding R, Akhurst R. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- Bohan DA, Hominick WM. Sex and the dynamics of infection in the entomopathogenic nematode Steinernema feltiae (site 76 strain) Filipjev. Parasitology. 1997;114:301–308. [Google Scholar]
- Campbell JF, Koppenhöfer AM, Kaya H, Chinnasri B. Are there temporarily non-infectious dauer stages in entomopathogenic nematode populations: A test of the phased infectivity hypothesis. Parasitology. 1999;118:499–508. doi: 10.1017/s0031182099003984. [DOI] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans . Developmental Biology. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Charnov EL. Princeton, NJ: Princeton University Press; 1982. The theory of sex allocation. [PubMed] [Google Scholar]
- Clobert EL, Danchin E, Dhondt AA, Nichols JD. Oxford, UK: Oxford University Press; 2001. Dispersal. [Google Scholar]
- Ehlers R, Wulff A, Peters A. Pathogenicity of axenic Steinernema feltiae, Xenorhabdus bovienii and the bacto-helminthic complex to larvae of Tipula oleracea (Diptera) and Galletia mellonella (Lepi-doptera) Journal of Invertebrate Pathology. 1997;69:212–217. doi: 10.1006/jipa.1996.4647. [DOI] [PubMed] [Google Scholar]
- Fodor A, Vesceri G, Farkas T. Caenorhabditis elegans as a model for study of entomopathogenic nematodes. In: Gaugler R, Kaya HK, editors. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC Press; 1990. pp. 249–265. [Google Scholar]
- Gaugler R, McGuire T, Campbell J. Genetic variability among strains of the entomopathogenic nematode Steinernema feltiae . Journal of Nematology. 1989;21:247–253. [PMC free article] [PubMed] [Google Scholar]
- Greenwood PJ. Mating systems, phylopatry, and dispersal in birds and mammals. Animal Behaviour. 1980;28:1140–1162. [Google Scholar]
- Grewal PS, Selvan S, Lewis EE, Gaugler R. Male insect-parasitic nematodes: A colonizing sex. Experientia. 1993;49:605–608. [Google Scholar]
- Hass B, Griffin CT, Downes MJ. Persistence of Heterorhabditis infective juveniles in soil: Comparison of extraction and infectivity methods. Journal of Nematology. 1999;31:508–516. [PMC free article] [PubMed] [Google Scholar]
- Hay DB, Fenlon JS. A modified binomial model that describes the infection dynamics of the entomopathogenic nematode Steinernema feltiae (Steinernematidae; Nematoda) Parasitology. 1995;111:627–633. [Google Scholar]
- Hominick WM, Reid AP. Perspectives on Entomopathogenic Nematology. In: Gaugler R, Kaya HK, editors. Entomopathogenic nematodes in biological control. Boca Raton, FL: CRC Press; 1990. pp. 327–345. [Google Scholar]
- Hominick WM, Reid AP, Bohan DA, Briscoe BR. Entomopathogenic nematodes: Biodiversity, geographical distribution and the convention on biological diversity. Biocontrol Science and Technology. 1996;6:317–331. [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. Manual of techniques in insect pathology. In: Lacey L, editor. San Diego, CA: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Koppenhofer AM, Baur ME, Stock SP, Choo HY, Chinnasri B, Kaya H. Survival of entomopathogenic nematodes within host cadavers in dry soil. Applied Soil Ecology. 1997;6:231–240. [Google Scholar]
- Lewis EE, Campbell JF, Gaugler R. The effects of aging on the foraging behaviour of Steinernema carpocapsae (Rhabdita: Steinernematidae) Nematologica. 1997;43:355–362. [Google Scholar]
- Lewis EE, Gaugler R. Entomopathogenic nematode (Rhabdita, Steinernematidae) sex-ratio relates to foraging strategy. Journal of Invertebrate Pathology. 1994;64:238–242. [Google Scholar]
- Lewis EE, Gaugler R, Harrison R. Entomopathogenic nematode host finding: Response to host contact cues by cruise and ambush foragers. Parasitology. 1992;105:103–107. [Google Scholar]
- Lewis EE, Selvan S, Campbell JF, Gaugler R. Changes in foraging behaviour during the infective stage of entomopathogenic nematodes. Parasitology. 1995;110:583–590. [Google Scholar]
- Martens EC, Heungens K, Goodrich-Blair H. Early colonization events in the mutualistic association between Steinernema carpocapsae nematodes and Xenorhabdus nematophila bacteria. Journal of Bacteriology. 2003;185:3147–3154. doi: 10.1128/JB.185.10.3147-3154.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani DK, Kaya HK, Goodrich-Blair H. Comparative study of the entomopathogenic nematode, Steinernema carpocapsae, reared on mutant and wild-type Xenorhabdus nematophila . Biological Control. 2004;29:382–391. [Google Scholar]
- Mracek Z. Horizontal distribution in soil, and seasonal dynamics of the nematode Steinernema kraussei, a parasite of Cephalcia abietis . Journal of Applied Entomology (zeitschrift fur Angewandte Entomologie) 1982;94:110–112. [Google Scholar]
- Poinar GO., Jr . Boca Raton, FL: CRC Press; 1979. Nematodes for the biological control of insects. [Google Scholar]
- Poinar GO, Jr, Leutenegger R. Anatomy of the infective and normal third-stage juveniles of Neoaplectana carpocapsae Weiser (Steinernematidae: Nematoda) Journal of Parasitology. 1968;54:340–350. [PubMed] [Google Scholar]
- Renn N. Routes of penetration of the entomopathogenic nematode Steinernema feltiae attacking larval and adult houseflies (Musca domestica) Journal of Invertebrate Pathology. 1998;72:281–287. doi: 10.1006/jipa.1998.4811. [DOI] [PubMed] [Google Scholar]
- Riddle DL. The dauer larva. The nematode Caenorhabditis elegans . In: Wood WB, editor. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. pp. 393–412. [Google Scholar]
- Scheepmaker JWA, Geels FP, van Griensven LJLD, Smits PH. Susceptibility of larvae of the mushroom fly Megaselia halterata to the entomopathogenic nematode Steienernema feltiae in bioassys. Biocontrol. 1998;43:201–214. [Google Scholar]
- Shapiro DI, Glazer I. Comparison of entomopathogenic nematode dispersal from infected hosts vs. aqueous suspension. Environmental Entomology. 1996;25:1455–1461. [Google Scholar]
- Stuart RJ, Abu Hatab M, Gaugler R. Sex ratio and the infection process in entomopathogenic nematodes: Are males the colonising sex? Journal of Invertebrate Pathology. 1998;72:288–295. doi: 10.1006/jipa.1998.4789. [DOI] [PubMed] [Google Scholar]