Abstract
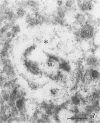
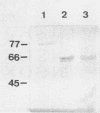
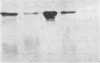
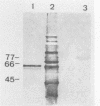
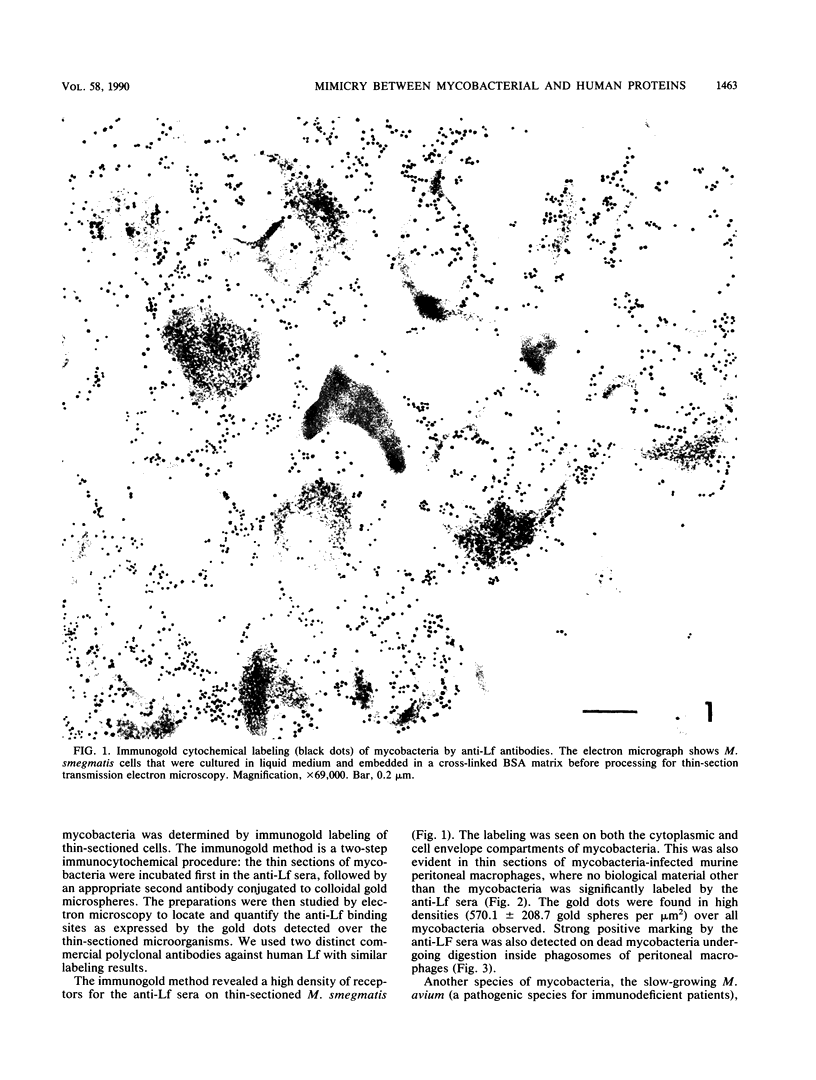
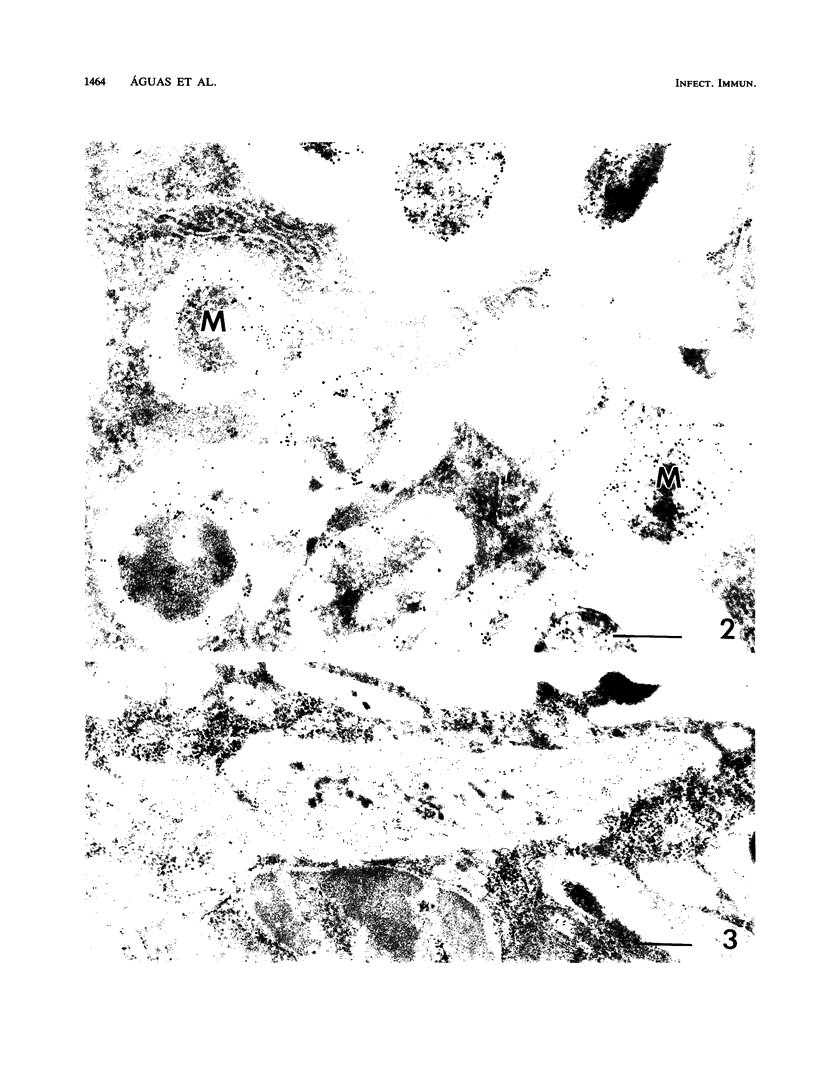
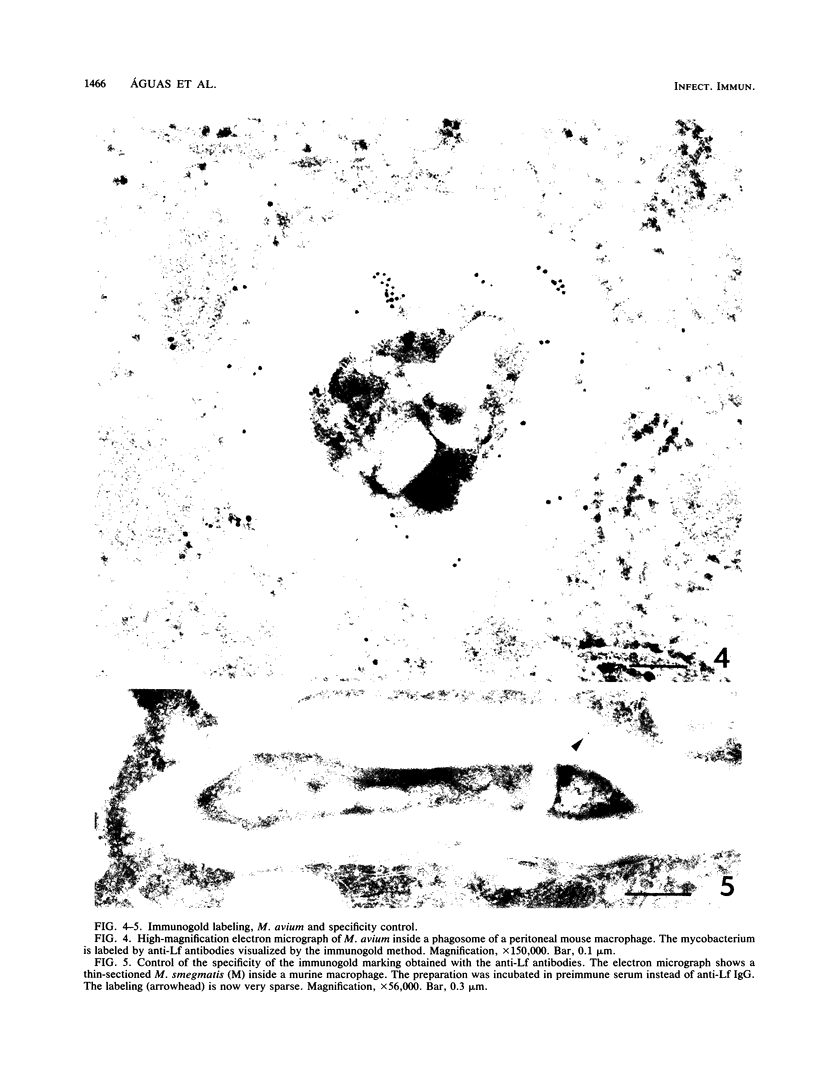
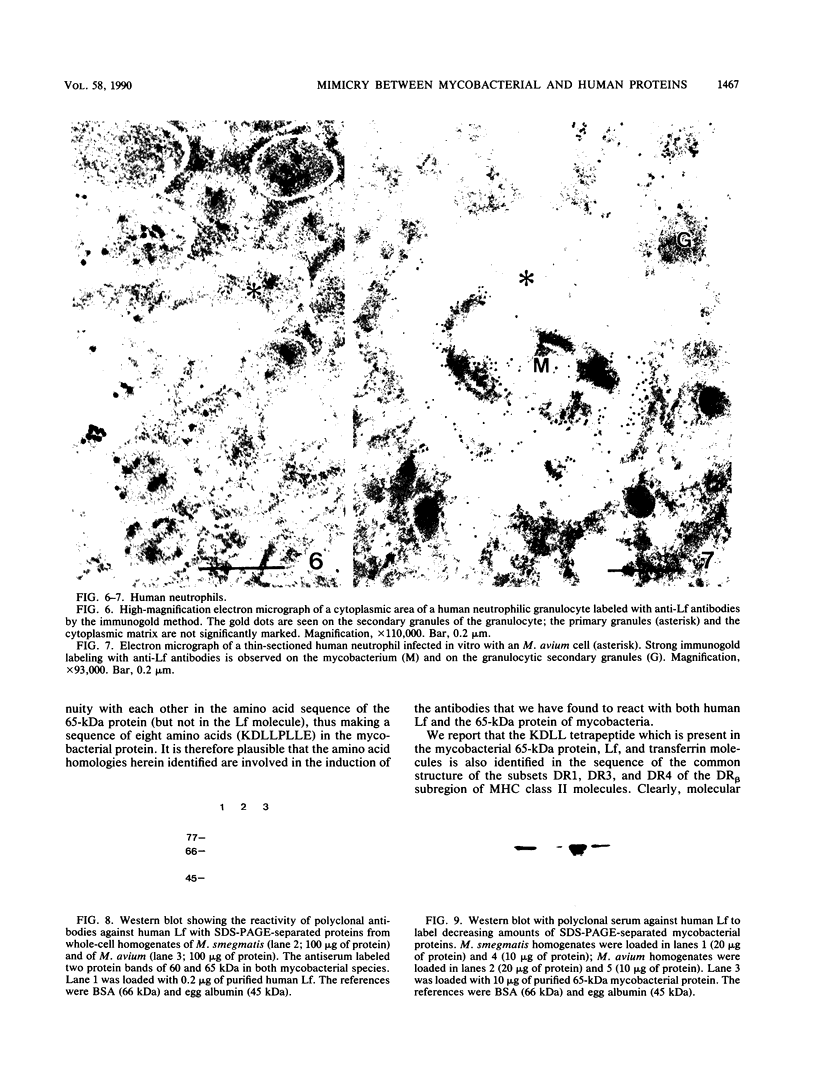
Immunogold ultracytochemistry and Western immunoblotting showed that polyclonal antibodies against human lactoferrin bind to the highly immunogenic 65-kilodalton (kDa) heat shock protein of mycobacteria. The fast-growing mycobacterial species Mycobacterium smegmatis showed a higher density of these receptors for antilactoferrin sera than the slow-growing M. avium. Polyclonal antibodies against mycobacteria (M. bovis BCG) recognized human lactoferrin. Comparison of the amino acid sequence of lactoferrin with that of the 65-kDa protein of M. tuberculosis revealed seven instances of four amino acid sequence homology between the microbial and the human iron-binding protein. Four of these tetrapeptide sequences were also shared with the human transferrin molecule. The shared amino acid sequence KDLL was also present in the DR1, DR3, and DR4 subsets of the DR beta subregion of major histocompatibility complex (MHC) class II molecules. The molecular mimicry between the 65-kDa mycobacterial protein and the human proteins (lactoferrin, transferrin, and MHC class II molecules) offers a molecular setting for mycobacteria-associated, T-cell-dependent autoimmune disease, namely, for rheumatoid arthritis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguas A. P., Pinto da Silva P. Regionalization of transmembrane glycoproteins in the plasma membrane of boar sperm head is revealed by fracture-label. J Cell Biol. 1983 Nov;97(5 Pt 1):1356–1364. doi: 10.1083/jcb.97.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. C., Barry M. E., Buchanan T. M. Exact definition of species-specific and cross-reactive epitopes of the 65-kilodalton protein of Mycobacterium leprae using synthetic peptides. J Immunol. 1988 Jul 15;141(2):607–613. [PubMed] [Google Scholar]
- Anderson D. C., van Schooten W. C., Barry M. E., Janson A. A., Buchanan T. M., de Vries R. R. A Mycobacterium leprae-specific human T cell epitope cross-reactive with an HLA-DR2 peptide. Science. 1988 Oct 14;242(4876):259–261. doi: 10.1126/science.2459778. [DOI] [PubMed] [Google Scholar]
- Bainton D. F., Miller L. J., Kishimoto T. K., Springer T. A. Leukocyte adhesion receptors are stored in peroxidase-negative granules of human neutrophils. J Exp Med. 1987 Dec 1;166(6):1641–1653. doi: 10.1084/jem.166.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar S. M., Fischer B., Diamond B., Scharff M. D. Variable region gene utilization and environmental antigens in autoimmunity. Monogr Allergy. 1988;24:297–306. [PubMed] [Google Scholar]
- Bell J. I., Denney D., Jr, Foster L., Belt T., Todd J. A., McDevitt H. O. Allelic variation in the DR subregion of the human major histocompatibility complex. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6234–6238. doi: 10.1073/pnas.84.17.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Björkstén B., Björk J., Yang H. H., Allen J. M., Baehner R. L. Neutropenia induced by systemic infusion of lactoferrin. J Lab Clin Med. 1982 Jun;99(6):866–872. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brattig N. W., Medina-De la Garza C. E., Tischendorf F. W. Comparative study of eosinophil purification on Nycodenz, Metrizamide and Percoll density gradients. Eur J Haematol. 1987 Aug;39(2):148–153. doi: 10.1111/j.1600-0609.1987.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Smithyman A., Eger R. R., Meyers P. A., de Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J Exp Med. 1978 Oct 1;148(4):1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs O., Harboe M., Axelsen N. H., Bunch-Christensen K., Magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980;12(3):249–263. doi: 10.1111/j.1365-3083.1980.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaguy N., Aguas A. P., Silva M. T. High-resolution localization of lactoferrin in human neutrophils: labeling of secondary granules and cell heterogeneity. J Leukoc Biol. 1989 Jul;46(1):51–62. doi: 10.1002/jlb.46.1.51. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Heitzmann J. G. Autoantibodies: terms and concepts. Clin Immunol Immunopathol. 1988 Jun;47(3):245–252. doi: 10.1016/s0090-1229(88)80002-3. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Barteling S. J., Meloen R. H. Small peptides induce antibodies with a sequence and structural requirement for binding antigen comparable to antibodies raised against the native protein. Proc Natl Acad Sci U S A. 1985 Jan;82(1):178–182. doi: 10.1073/pnas.82.1.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Sritharan M., Messenger A. J., Ratledge C. Iron transport in Mycobacterium smegmatis: occurrence of iron-regulated envelope proteins as potential receptors for iron uptake. J Gen Microbiol. 1987 Aug;133(8):2107–2114. doi: 10.1099/00221287-133-8-2107. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Koga T., Wand-Württenberger A., DeBruyn J., Munk M. E., Schoel B., Kaufmann S. H. T cells against a bacterial heat shock protein recognize stressed macrophages. Science. 1989 Sep 8;245(4922):1112–1115. doi: 10.1126/science.2788923. [DOI] [PubMed] [Google Scholar]
- Kraus W., Ohyama K., Snyder D. S., Beachey E. H. Autoimmune sequence of streptococcal M protein shared with the intermediate filament protein, vimentin. J Exp Med. 1989 Feb 1;169(2):481–492. doi: 10.1084/jem.169.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Lactoferrin effects on phagocytic cell function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985 Jun;134(6):4176–4183. [PubMed] [Google Scholar]
- Martínez-Maza O., Fehniger T. E., Ashman R. F. Antibody-secreting cell precursor frequencies among the sheep-erythrocyte-binding cells after immunization. Scand J Immunol. 1983 Apr;17(4):345–354. doi: 10.1111/j.1365-3083.1983.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz-Boutigue M. H., Jollès J., Mazurier J., Schoentgen F., Legrand D., Spik G., Montreuil J., Jollès P. Human lactotransferrin: amino acid sequence and structural comparisons with other transferrins. Eur J Biochem. 1984 Dec 17;145(3):659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]
- Nishiya K., Horwitz D. A. Contrasting effects of lactoferrin on human lymphocyte and monocyte natural killer activity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1982 Dec;129(6):2519–2523. [PubMed] [Google Scholar]
- Nunes J. F., Soares J. O., Alves de Matos A. P. Micro-buffy coats of whole blood: a method for the electron microscopic study of mononuclear cells. Stain Technol. 1979 Sep;54(5):257–260. doi: 10.3109/10520297909110681. [DOI] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Shinnick T. M., Houghten R. A., Kvalheim G., Degre M., Lundin K. E., Godal T. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J Immunol. 1988 Oct 15;141(8):2749–2754. [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A., Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988 Apr;9(4):117–120. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- Sher R., Anderson R., Glover A., Wadee A. A. Polymorphonuclear cell function in the various polar types of leprosy and erythema nodosum leprosum. Infect Immun. 1978 Sep;21(3):959–965. doi: 10.1128/iai.21.3.959-965.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Sweetser D., Thole J., van Embden J., Young R. A. The etiologic agents of leprosy and tuberculosis share an immunoreactive protein antigen with the vaccine strain Mycobacterium bovis BCG. Infect Immun. 1987 Aug;55(8):1932–1935. doi: 10.1128/iai.55.8.1932-1935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Appelberg R., Silva M. N., Macedo P. M. In vivo killing and degradation of Mycobacterium aurum within mouse peritoneal macrophages. Infect Immun. 1987 Sep;55(9):2006–2016. doi: 10.1128/iai.55.9.2006-2016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. The interpretation of the ultrastructure of mycobacterial cells in transmission electron microscopy of ultrathin sections. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):225–234. [PubMed] [Google Scholar]
- Thole J. E., Hindersson P., de Bruyn J., Cremers F., van der Zee J., de Cock H., Tommassen J., van Eden W., van Embden J. D. Antigenic relatedness of a strongly immunogenic 65 kDA mycobacterial protein antigen with a similarly sized ubiquitous bacterial common antigen. Microb Pathog. 1988 Jan;4(1):71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J. E., van Schooten W. C., Keulen W. J., Hermans P. W., Janson A. A., de Vries R. R., Kolk A. H., van Embden J. D. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BCG. Infect Immun. 1988 Jun;56(6):1633–1640. doi: 10.1128/iai.56.6.1633-1640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan J. H., Kouri T., Petersen J., Roudier J., Rhodes G. H. On the etiology of rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;74:19–28. doi: 10.3109/03009748809102935. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Goralnick S., Bullock W. E. Defective leukotaxis in patients with lepromatous leprosy. J Lab Clin Med. 1976 Jun;87(6):1025–1032. [PubMed] [Google Scholar]
- Wood J. N., Hudson L., Jessell T. M., Yamamoto M. A monoclonal antibody defining antigenic determinants on subpopulations of mammalian neurones and Trypanosoma cruzi parasites. Nature. 1982 Mar 4;296(5852):34–38. doi: 10.1038/296034a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Cohen I. Antigenic mimicry between mycobacteria and cartilage proteoglycans: the model of adjuvant arthritis. Concepts Immunopathol. 1987;4:144–170. [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]