Abstract
Abamectin is nematicidal to Meloidogyne incognita and Rotylenchulus reniformis, but the duration and length of cotton taproot protection from nematode infection by abamectin-treated seed is unknown. Based on the position of initial root-gall formation along the developing taproot from 21 to 35 d after planting, infection by M. incognita was reduced by abamectin seed treatment. Penetration of developing taproots by both nematode species was suppressed at taproot length of 5 cm by abamectin-treated seed, but root penetration increased rapidly with taproot development. Based on an assay of nematode mobility to measure abamectin toxicity, the mortality of M. incognita associated with a 2-d-old emerging cotton radicle was lower than mortality associated with the seed coat, indicating that more abamectin was on the seed coat than on the radicle. Thus, the limited protection of early stage root development suggested that only a small portion of abamectin applied to the seed was transferred to the developing root system.
Keywords: abamectin, avermectin, cotton, Gossypium hirsutum, Meloidogyne incognita, nematicide, reniform nematode, root-knot nematode, Rotylenchulus reniformis, seed treatment
The root-knot nematode, Meloidogyne incognita race 3, and the reniform nematode, Rotylenchulus reniformis, are important plant-parasitic nematodes affecting cotton (Gossypium hirsutum) production (Koenning et al., 2004). Second-stage juveniles (J2) of M. incognita penetrate just behind the root tips and migrate toward the developing vascular tissue where they establish a feeding site, resulting in the characteristic galling of root tissue. Root-knot nematodes are found in coarse-textured soils in nearly all areas of cotton production (Koenning et al., 1996). Vermiform females of R. reniformis penetrate the root perpendicular to the root axis and establish a feeding site in the endodermis and pericycle. The reniform nematode is found primarily in fine-textured soils in the southeastern US, but commonly it is undetected because infection does not result in galling of root tissue (Koenning et al., 2004).
Management strategies for plant-parasitic nematodes continue to rely on nematicides because of limited availability of resistant cultivars with high yield potential (Koenning et al., 2004). Abamectin is nematicidal to both M. incognita and R. reniformis, and exposure for 1 hr to concentrations greater than 0.39 μg/ml for M. incognita and 8.22 μg/ml for R. reniformis inhibits tomato root infection (Faske and Starr, 2006). Applying abamectin in close proximity to plant-parasitic nematodes has been effective in suppressing infection (Cayrol et al., 1993; Jansson and Rabatin, 1998). However, commercialization of abamectin as a nematicide has been delayed because of limited foliar translocation, high affinity to bind to soil particles, and rapid decomposition in soil and sun light (Putter et al., 1981; Wislocki et al., 1989). In 2005, an abamectin formulation (Avicta 500 FS) was marketed by Syngenta Crop Protection as a seed treatment for early season control of nematode damage to cotton seedlings. However, abamectin seed treatment has been variable in suppressing M. incognita and R. reniformis and increasing cotton yields in nematode-infested fields (Lawrence et al., 2006a, 2006b; Monfort et al., 2006; Phipps et al., 2006). Further characterization of abamectin as a seed treatment is needed to better understand the variability in nematode suppression.
The objectives of this study were to determine (i) suppression of nematode penetration of young cotton roots in response to abamectin seed treatment, and (ii) mortality of M. incognita exposed to the excised radicle tip or seed coat of an abamectin-treated seed.
Materials and Methods
Nematode cultures: Meloidogyne incognita and R. reniformis were isolated from cotton and maintained in the greenhouse on Solanum lycopersicum L. cv. ‘Rutgers'. Eggs were collected from 8- to 10-wk-old M. incognita cultures with NaOCl (Hussey and Barker, 1973), from which J2 were collected in hatching chambers with a 20-μm-pore screen (Vrain, 1977). Only 24-hr-old J2 were evaluated in this study. Rotylenchulus reniformis was collected from infested soil using Baermann funnels (Chapman, 1958). Mixed-life-stages of R. reniformis were collected with a 25-μm-pore sieve after 48 hr and used immediately.
Seed treatments: Cotton (G. hirsutum) cv. Deltapine DP 444 BG/RR was used throughout this study. All seed treatments were applied by the manufacturer. Treated seed received 150 μg abamectin/seed (Avicta 500 FS, Syngenta Crop Protection, Greensboro, NC), the insecticide thiamethoxam (Cruiser 5FS, Syngenta Crop Protection) applied at 340 μg/seed, and a blend of the fungicides azoxystrobin, fludioxonil, and mefenoxam (Dynasty CST, Syngenta Crop Protection) applied at 30 ug/seed. Control treatments were seeds treated with thiamethoxam and azoxystrobin + fludioxonil + mefenoxam at the same rates.
Nematode penetration: Two experiments were conducted to determine the suppression of penetration by M. incognita and R. reniformis on developing roots in response to abamectin seed treatment. In the first experiment, conducted in a greenhouse, abamectin-treated and control seeds were planted into 656 cm3 Deepots (Stuewe and Sons, Inc, Corvallis, OR) containing pasteurized sand:sandy loam (2:1 v/v; <1% organic matter; pH 8.0) soil mix infested with 1,000 M. incognita J2/500 cm3. The root systems from these plants were harvested at 7, 14, 21, 28, and 35 d after planting (DAP). Nematode penetration of roots was determined at 7 and 14 DAP by staining J2 with acid fuchsin (Byrd et al., 1983). Infection was estimated at 21, 28, and 35 DAP based on total number of galls per root system. Reproduction was determined at 28 and 35 DAP based on numbers of eggs per gram of root. The experiment was arranged in a randomized complete block with each treatment replicated five times, and the experiment was conducted twice.
In a second experiment, taproots of 5-cm, 10-cm and 20-cm length of plants grown from abamectin-treated seed were inoculated with M. incognita or R reniformis to determine suppression of nematode penetration at different taproot lengths. Abamectin-treated and control seeds were germinated on germination paper (Anchor Paper Co., St. Paul, MN) at 26°C for 3, 5, and 7 d to obtain taproots lengths of 5 cm, 10 cm, and 20 cm, respectively. Cotton taproots were inserted inside a 7-cm-long plastic cylinder (5-mm diam.), then covered in pasteurized sand (<7l0 μm) through a gap (2 × 40-mm) cut from the plastic cylinder. Approximately 100 J2 in 100 μl distilled water were inoculated into the sand 1 cm below the root tips. Taproots were placed in a moisture chamber for 48 hr at 26°C. For R. reniformis, taproots were prepared as described above. Approximately 120 it reniformis in 100 μl distilled water were inoculated into the sand at target lengths. Taproots were placed in a moisture chamber for 6 d at 26°C. Nematode penetration of taproots was determined by staining nematodes with acid fuchsin (Byrd et al., 1983). Treatments were replicated six times per taproot length, and each experiment was conducted twice.
Toxicity associated with developing radicle: In this study, M. incognita J2 were exposed to a radicle tip or seed coat for 48 hr, and nematode mortality was measured. Abamectin-treated and control seeds were germinated in sand:sandy loam (2:1 v/v) soil mix for 48 hr at 26°C, resulting in a radicle length of 3 cm. The excised radicle tip (2 cm) and remaining seed coat were placed in separate 3-cm diam. glass petri dishes containing 30 to 40 J2 in 2 ml distilled water. Nematodes exposed to distilled water served as controls. Nematode mortality was estimated following a 48-hr exposure at 26°C. Nematodes were considered dead if they were not moving and did not respond to being touched by a small probe. Each treatment was replicated six times, and the experiment was conducted three times.
Statistical analysis: Data from repeated experiments were combined and subjected to general linear model analysis of variance using SPSS 11.5 (SPSS Inc., Chicago, 111). Mean separations were established by least significant difference.
Results
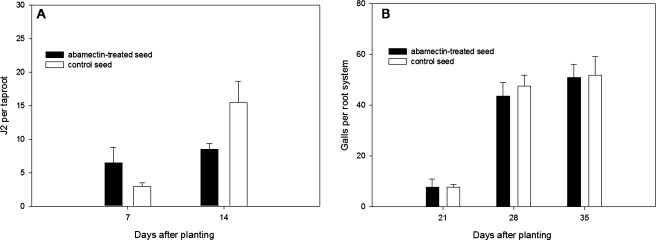
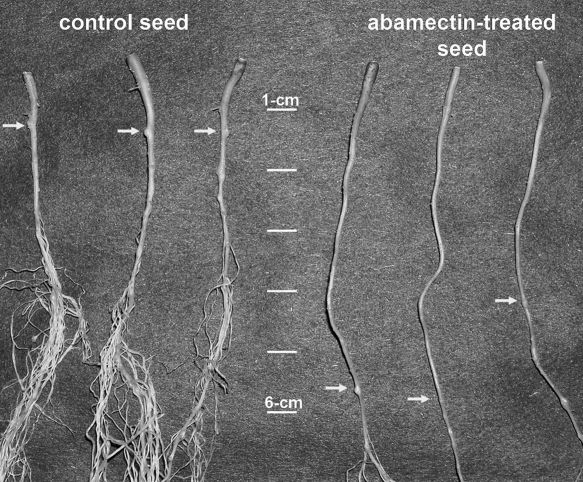
In the greenhouse experiment, suppression of M. incognita root infection by abamectin seed treatment varied depending on observation date (Fig. 1). The number of M. incognita in the taproot 7 DAP was higher for abamectin-treated than non-treated seed, but the reverse was observed at 14 DAP. Seedling heights, total galls per taproot, secondary roots, and eggs per gram of root from 21 to 35 DAP were similar between seed treatments (data not shown). Taproot length between the crown and position of the initial gall was longer (5.6 cm, P = 0.001) for abamectin-treated than non-treated seed (2.4 cm, Fig. 2).
Fig. 1.
Suppression of root penetration and infection by Meloidogyne incognitaon developing seedling roots in response to abamectin-treated seed. Initial population density for M. incognita was1,000 J2/500 cm3soil. A. Second-stage juveniles. B. Galls per root system. Bars indicate standard error.
Fig 2.
Effect of abamectin seed treatment on the position of initial gall formation by Meloidogyne incognitaon cotton taproots. To better visualize galling, secondary roots were removed 2 cm past the initial gall for both control and abamectin-treated seed. Arrows indicate position of first gall from crown.
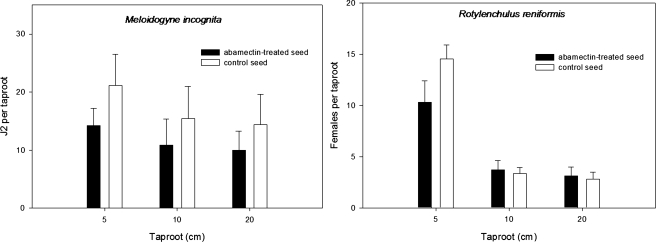
The length of taproot protection from M. incognita and R. reniformis infection by abamectin-treated seed varied at taproot lengths of 5 cm, 10 cm, and 20 cm (Fig. 3). Suppression of nematode penetration was greatest at taproot length of 5 cm for both nematode species. At taproot lengths of 10 cm and 20 cm, fewer M. incognita were observed on taproots from abamectin-treated seed whereas no suppression was observed for R reniformis.
Fig. 3.
Suppression of root penetration by plant-parasitic nematodes at different taproot lengths in response to abamectin seed treatment. Target taproot lengths were sleeved and centered inside a plastic cylinder then filled with sand. Taproot tips inoculated with 100 J2 of Meloidogyne incognita were harvested 48 hr after inoculation. Taproots inoculated with 120 vermiform-stages of Rotylenchulus reniformis were harvested 6 d after inoculation. Bars indicate standard error.
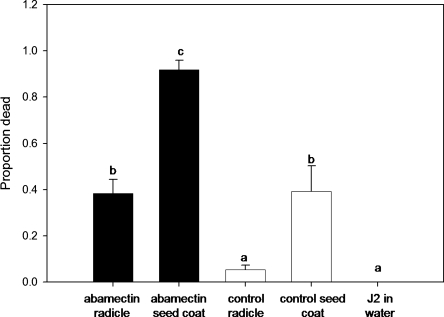
In preliminary experiments based on assays of nematode mobility, no mortality was observed in response to pesticide-free cotton seed or fungicide seed treatment, and extremely low toxicities were observed for the insecticide seed treatment. The proportion of dead J2 following 48 hr exposure to a radicle of an abamectin-treated seed was higher (0.39, P = 0.05) compared to non-treated seed (0.05), but lower (P = 0.05) than J2 exposed to abamectin-treated seed coats (0.87, Fig. 4).
Fig 4.
Mortality of Meloidogyne incognita associated with the emerging radicle and residual seed coat from abamectin-treated cotton seed. Bars indicate standard error. Different letters over bars indicate significant difference according to LSD at α = 0.05.
Discussion
Cotton yield response and suppression of M. incognita and R reniformis by abamectin-treated seed has been variable in replicated field trials (Lawrence et al., 2006a, 2006b; Monfort et al., 2006; Phipps et al., 2006). In a greenhouse trial, Monfort et al. (2006) reported lower root gall rating and nematode reproduction on cotton roots grown in pasteurized soil 35 DAP by seed treated with only abamectin. However, in our greenhouse trial, root gall rating and nematode reproduction were similar between abamectin and non-abamectin treated seed, which were also treated with three fungicides and an insecticide. Interactions among these seed treatments may have contributed to the small portion of abamectin transferred along the developing cotton root system.
In our studies, suppression of penetration and infection on cotton taproots by M. incognita and R reniformis was greatest at a taproot length of 5 cm and decreased as taproots elongated, suggesting that abamectin concentration decreased rapidly as root length increased. The concentration of abamectin associated with developing cotton roots was more effective against M. incognita, which was reported to be more sensitive than R. reniformis to abamectin (Faske and Starr, 2006).
Based on the known concentration response [y = 1.022/(l+exp(−(x-0.4l7)/0.203))] for M. incognita (Faske and Starr, 2006) and the mortality of J2 after a 24 hr exposure, the concentrations of abamectin released into solution from the radicle and seed coat were estimated to be 0.13 μg and 0.68 μg abamectin/ml, respectively. Thus, the majority of abamectin applied as a seed treatment remains on the seed coat. Exposing M. incognita to a concentration less than 0.15 μg abamectin/ml for 1 hr did not reduce root-galling of tomato (Faske and Starr, 2006).
Applying abamectin as a seed treatment delivers the nematicide in close proximity to the developing root system. Limited protection of early stage cotton root development was related to the small portion of abamectin transferred to the developing root system, which decreased rapidly as roots elongated. These observations contribute to the understanding of variability in suppression of plant-parasitic nematodes by abamectin seed treatment.
Footnotes
This paper was edited by Philip Roberts.
Literature Cited
- Byrd DW, Jr, Kirkpatrick T, Barker KR. An improved technique for clearing and staining tissues for detection of nematodes. Journal of Nematology. 1983;15:142–143. [PMC free article] [PubMed] [Google Scholar]
- Cayrol JC, Djian C, Frankowski JP. Efficacy of abamectin Bl for the control of Meloidogyne arenaria . Fundamental and Applied Nematology. 1993;16:239–246. [Google Scholar]
- Chapman RA. An evaluation of methods for determining the number of nematodes in soil. Plant Disease Reporter. 1958;42:1351–1356. [Google Scholar]
- Faske TR, Starr JL. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to abamectin. Journal of Nematology. 2006;38:240–244. [PMC free article] [PubMed] [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;59:1025–1028. [Google Scholar]
- Jansson RK, Rabatin S. Potential of foliar, dip, and injection applications of avermectins for control of plant-parasitic nematodes. Journal of Nematology. 1998;30:65–75. [PMC free article] [PubMed] [Google Scholar]
- Koenning SR, Walters SA, Barker KR. Impact of soil texture on the reproductive and damage potentials of Rotylenchulus reniformis and Meloidogyne incognita on cotton. Journal of Nematology. 1996;28:527–536. [PMC free article] [PubMed] [Google Scholar]
- Koenning SR, Wrather JA, Kirkpatrick TL, Walker NR, Starr JL, Mueller JD. Plant-parasitic nematodes attacking cotton in the United States: Old and emerging production challenges. Plant Disease. 2004;88:100–113. doi: 10.1094/PDIS.2004.88.2.100. [DOI] [PubMed] [Google Scholar]
- Lawrence KS, Burmester CH, Lawrence GW, Norris C. Evaluation of Avicta formulations as compared to Temik 15G for reniform nematode management in cotton in north Alabama, 2005. Fungicide and Nematicide Tests. 2006a;61:N015. [Google Scholar]
- Lawrence KS, Gazaway WS, Lawrence GW, Akridge R. Evaluation of Avicta, Vydate CLV, and Temik 15G combinations for reniform nematode management in cotton in south Alabama, 2005. Fungicide and Nematicide Tests. 2006b;61:N014. [Google Scholar]
- Monfort WS, Kirkpatrick TL, Long DL, Rideout S. Efficacy of a novel nematicidal seed treatment against Meloidogyne incognita on cotton. Tournal of Nematoloey. 2006;38:245–249. [PMC free article] [PubMed] [Google Scholar]
- Phipps PM, Partridge DE, Eisenback JD. Efficacy of abamectin (A16006) on seed and Temik 15G in-furrow for root-knot nematode control in cotton, 2005. Fungicide and Nematicide Tests. 2006;61:N003. [Google Scholar]
- Putter I, MacConnell JG, Prieser FA, Haidri AA, Ristich SS, Dybas RA. Avermectins: Novel insecticides, acaricides and nematicides from a soil microorganism. Experientia. 1981;37:963–964. [Google Scholar]
- Vrain TC. A technique for the collection of larvae of Meloidogyne spp. and a comparison of eggs and larvae as inocula. Journal of Nematology. 1977;9:249–251. [PMC free article] [PubMed] [Google Scholar]
- Wislocki PG, Grosso LS, Dybas RA. Environmental aspects of abamectin use in crop protection. In: Campbell CW, editor. Ivermectin and abamectin. New York: Springer-Verlag; 1989. pp. 182–200. [Google Scholar]