Abstract
The nematicidal activity of two cassia, Cinnamomum cassia, oils (Especial and true), four cinnamon, Cinnamomum zey-lanicum, oils (technical, #500, bark and green leaf), and their compounds (e.g., trans-cinnamaldehyde and trans-cinnamic acid) toward adult Bursaphelenchus xylophilus was examined by a direct contact bioassay. Results were compared with those of 34 related compounds. As judged by 24-hour LC50 values, two cassia oils (0.084–0.085 mg/ml) and four cinnamon oils (0.064–0.113 mg/ml) were toxic toward adult B. xylophilus. Of 45 test compounds, trans-cinnamaldehyde (0.061 mg/ml) was the most active nematicide, followed by ethyl cinnamate, α-methyl-trans-cinnamaldehyde, methyl cinnamate and allyl cinnamate (0.114–0.195 mg/ml). Potent nematicidal activity was also observed with 4-methoxycinnamonitrile, trans-4-methoxycinnamaldehyde, trans-2-methoxy-cinnamaldehyde, ethyl α-cyanocinnamate, cinnamonitrile and cinnamyl bromide (0.224–0.502 mg/ml). Structure-activity relationships indicate that structural characteristics, such as types of functional groups, saturation and carbon skeleton, appear to play a role in determining the toxicities to adult B. xylophilus. Cassia and cinnamon oils and test compounds described merit further study as potential nematicides or leads for the control of pine wilt disease caused by B. xylophilus.
Keywords: Bursaphelenchus xylophilus, pine wood nematode, pine wilt disease, botanical nematicide, cassia oil, Cinnamomum cassia, cinnamon oil, Cinnamomum zeylanicum, cinnamaldehyde, structure-activity relationship
Pinewood nematode, Bursaphelenchus xylophilus (Sterner and Buhrer, 1934) Nickle, 1970, is associated with dead and dying conifers, particularly pines, and recently cut conifer logs throughout North America and Japan. The nematode is the causal agent of pine wilt disease (PWD) but is also associated with trees stressed or killed by other mortality factors, both biotic and abiotic (Linit, 1988). Among the cerambycid beetle species of the genus Monochamus, M. alternatus (Hope) and M. carolinensis (Olivier) are primary vectors of B. xylophilus in East Asia (Kobayashi et al., 1984) and the US (Linit, 1988), respectively. Control of PWD in East Asia has been principally provided by killing the vector beetles by aerial and ground applications of conventional insecticides, by fumigation of pine trees killed by the disease, or by eradicating B. xylophilus by trunk implantation of nematicides such as levamisol hydrochloride and emmamectin benzoate (Kishi, 1995; Kosaka et al., 2001; Lee et al., 2003a, 2003b). Certain trunk-implantation nematicides often caused phytotoxicity (Kishi, 1995). Growing public concern for the environmental effects of pesticides, human health effects and undesirable effects on nontarget organisms become more critical with repeated applications of pesticides (Kishi, 1995; Mun and Woo, 2001). Additionally, factors such as increased costs of nematicides and labor have made PWD control difficult in Korea. These problems substantiate the need for biorational B. xylophilus control alternatives.
Plant essential oils and their constituents have been suggested as alternative sources for nematode control products because some are selective, degrade to non-toxic products and have few harmful effects on nontarget organisms and the environment (Isman, 2000; Chit-wood, 2002). These potential new nematicides can be applied to pine trees in the same manner as the trunk-implanted nematicides currently used. Plant essential oils are widely available, and some are relatively inexpensive, compared with plant extracts (Isman, 2000). The US Environmental Protection Agency (EPA) lists only 17 different plant oils as being exempt from toxicity data requirements as minimum risk pesticides under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) (US EPA, 1996). Thus, much effort has been focused on these compounds and their constituents as potential sources of commercial nematode control agents. Previously the present authors reported that cinnamon, Cinnamomum zeylanicum Blume (Laura-ceae), bark oil had potent nematicidal activity toward adult B. xylophilus (Kong et al., 2006).
This paper describes a laboratory study aimed at isolating nematicidal constituents of oils of cassia, Cinnamomum cassia Blume, and cinnamon active toward adult B. xylophilus. Also, the structure-activity relationships of (E)-cinnamic acid and its related compounds toward adult B. xylophilus are discussed.
Materials and Methods
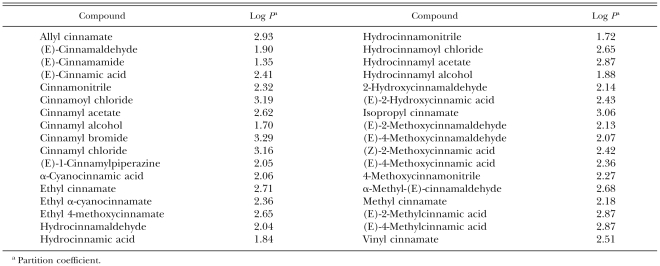
Materials: Chemicals used in this study were as follows: benzaldehyde, benzyl cinnamate, β-caryophyl-lene, (E)-cinnamaldehyde, (E)-cinnamic acid, (E)-cinnamamide, cinnamonitrile, cinnamoyl chloride, cinnamyl acetate, cinnamyl alcohol, cinnamyl bromide, cinnamoyl chloride, (E)-l-cinnamylpiperazine, p-cymene, ethyl cinnamate, hydrocinnamaldehyde, hydrocinnamoyl chloride, (E)-2-hydroxycinnamic acid, isopropyl cinnamate, linalool, (E)-2-methoxycinnamaldehyde, (Z)-2-methoxycinnamic acid, (E)-2-methoxycinnamic acid, (E)-4-methoxycinnamic acid, 4-methoxycinnamonitrile, α-methyl- (E) -cinnamaldehyde, methyl cinnamate, (E)-2-methylcinnamic acid, (E)-4-methylcinnamic acid and α-terpinene purchased from Aldrich (Milwaukee, WI); α-copaene and eugenol from Fluka (Bucks, Switzerland); allyl cinnamate, cinnamyl cinnamate, α-cyanocinnamic acid, ethyl α-cyanocinnamate, ethyl 4-methoxycinnamate, hydrocinnamic acid, hydrocinnamonitrile, hydrocinnamyl acetate, hydrocinnamyl alcohol, (E)-2-hydroxycinnamaldehyde, (E)-4-methoxycinnamaldehyde and vinyl cinnamate from Tokyo Kasei (Tokyo, Japan); and limonene and α-terpineol from Wako (Osaka, Japan). Hydrophobic parameter values for these compounds were calculated using ACD/Log P v8.02 (ACD/I-Lab, Montreal, Canada) (Table 1). The results of ACD/Log P v8.02 were obtained using the ACD/I-Lab service. Cassia oils Especial and true and cinnamon oils technical, #500 and green leaf oil were provided by Berjé (Bloomfield, NJ). Cinnamon bark oil was supplied by Jin-Ah Aromatics (Anyang, Gyeonggi, Korea). Polyoxyethylene hydrogenated castor oil (HCO-50), a solubilizer, was a gift from Nikko Chemical (Tokyo). Potato dextrose agar (PDA) was purchased from Becton, Dickinson and Company (Sparks, MD). All other chemicals were of reagent grade and available commercially.
Table 1.
Hydrophobic parameter values for test compounds.
Nematodes: Bursaphelenchus xylophilus isolated from chips of PWD-infected P. densiflora were cultured for 6 d on Botrytis cinerea Persoon cultured on PDA plates at 25 ± 1°C in darkness as previously described (Kong et al., 2006). The cultured nematodes were separated from the culture medium by the Baermann funnel technique (Viglierchio and Schmitt, 1983). The standard nematode suspension was prepared by appropriate dilution with distilled water, and the number of nematodes was counted under a binocular microscope (×20).
Gas chromatography (GC): A Shimadzu GC-2010 gas chromatograph (Tokyo, Japan), equipped with split injector, was used. Analytes were separated with a 0.32 mm i.d. × 60 m DB-1 MS bonded-phase fused-silica capillary column (J&W Scientific, Ringoes, NJ) with a film thickness of 0.25 μm. The oven temperature was programmed from 35°C (4 min isothermal) to 220°C at 2°C/min and then to 300°C (held for 5 min at final temperature) at 30°C/min. The linear velocity of the helium carrier gas was 25.4 cm/sec (30°C) at a split ratio of 1:10. Constituents were identified based on retention time and by co-injection with authentic compounds.
Gas chromatography-mass spectroscopy (GC-MS): GC-MS analysis was performed using a gas chromatograph (Shimadzu GC-2010)-mass spectrometer (Shimadzu GC-MS QP-2010). The capillary column and temperature conditions for the GC-MS analyses were the same as described above for GC analysis. Helium carrier gas was used at a column head pressure of 26.9 kPa. The ion source temperature was 200°C, and the injector temperature was 170°C. The interface was kept at 280°C, and mass spectra were obtained at 70 eV. The effluent of the capillary column was introduced directly into the ion source of the mass spectrometer. The sector mass analyzer was set to scan from 50 to 500 amu every 0.5 sec. Components of cassia and cinnamon oils were identified by comparison of mass spectra of each peak with those of authentic samples in a mass spectra library (Anonymous, 2000).
Bioassays: A direct contact bioassay (Kong et al., 2006) was used to evaluate the toxicity to adult B. xylophilus of cassia and cinnamon oils and compounds tested. An aqueous nematode suspension (approx. 3,000 nematodes/ml) was prepared by diluting the standard nematode suspension. The nematode suspension (99 μl) was added separately to each well of a 96-well plate prior to toxicity tests. Six to eight concentrations of each test material in 1 μl of polyoxyethylene hydrogenated castor oil-ethanol solution (1 mg/ml) (Takai et al., 2000) were added to the wells. The castor oil-ethanol solution did not affect adversely adult B. xylophilus. Each well plate was then covered with a solid lid. Controls received 1 μl of the castor oil-ethanol solution.
Treated and castor oil-ethanol-treated control nematodes were held at the same conditions used for nematode maintenance. Nematicidal activity was evaluated 24 hr post-treatment under a binocular microscope. Nematodes were considered to be dead if their bodies showed straight shapes without movement when they were prodded with fine wooden dowels (Kong et al., 2006). The straight-shaped nematodes never recovered, even when transferred to untreated distilled water. All treatments were replicated four to six times.
Data analysis: Data were corrected for control mortality using Abbott's formula (Abbott, 1925). The LC50 values were calculated by probit analysis (SAS Online-Doc®, Version 8.01, Statistical Analysis System Institute, Cary, NC). Nematicidal activity was considered to be significantly different among treatments when 95% confidence limits of the LC50 values failed to overlap.
Results
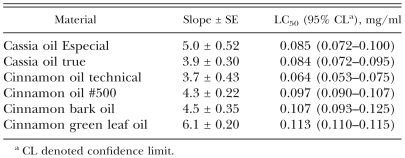
Nematicidal activity of cassia and cinnamon oils: The nematicidal activities of two cassia oils and four cinnamon oils were evaluated by comparing the LC50 values estimated from the bioassay toward adult B. xylophilus (Table 2). As judged by 24-hr LC50 values, cinnamon oil technical (0.064 mg/ml) was the most toxic oil, followed by cassia oil true and cassia oil Especial (approx. 0.084 mg/ml). Potent nematicidal activity was also observed with cinnamon oil #500, cinnamon bark oil and cinnamon green leaf oil (0.097–0.113 mg/ml). Mortality in the castor oil-ethanol-treated controls was less than 3%.
Table 2.
Nematicidal activity of cassia and cinnamon oils toward Bursaphelenchus xylophilus using the direct contact bioassay during a 24 hr exposure.
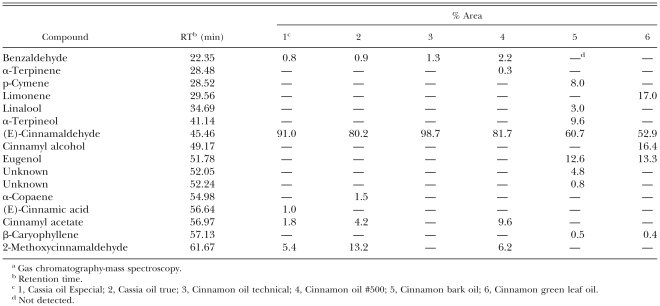
Chemical constituents of cassia and cinnamon oils: Cassia and cinnamon oils used were mainly composed of (E)-cinnamaldehyde (Table 3). The content of this compound was more pronounced in cinnamon oil technical (99%) and cassia oil Especial (91%) than in cinnamon oil #500 (82%), cassia oil true (80%), cinnamon bark oil (61%) or cinnamon green leaf oil (53%). The other major constituents, eugenol, α-terpineol and pcymene, comprised 13, 10 and 8% of cinnamon bark oil, respectively. The other major constituents, limonene, cinnamyl alcohol and eugenol, comprised 17, 16 and 13% of cinnamon green leaf oil, respectively.
Table 3.
Chemical constituents of cassia and cinnamon oils identified by GC and GC-MS.a
Nematicidal activity of cassia and cinnamon oil compounds: The toxicity of cassia and cinnamon oil compounds to adult B. xylophilus is given in Table 4. On the basis of 24-hr LC50 values, (E)-cinnamaldehyde was the most toxic compound toward adult B. xylophilus, followed by (E)-2-methoxycinnamaldehyde and (E)-cinnamic acid.
Table 4.
Toxicity of cassia and cinnamon oil compounds to adult Bursaphelenchus xylophilus using the direct contact bioassay during a 24 hr exposure.
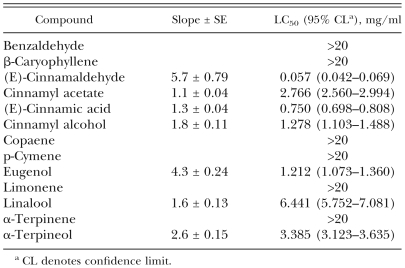
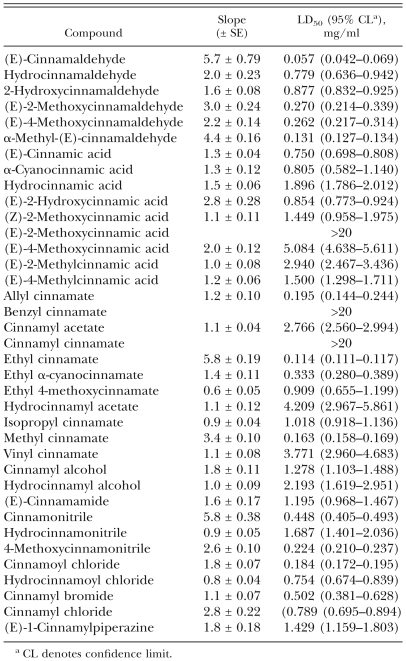
Nematicidal activity of cinnamic acid derivatives: Toxic effects on adult B. xylophilus in the direct contact bioassay of (E)-cinnamaldehyde, (E)-cinnamic acid and their related compounds are recorded in Table 5. Potencies varied according to the compound tested. (E)-Cinnamaldehyde was the most potent compound, followed by ethyl cinnamate, α-methyl-(E)-cinnamaldehyde, methyl cinnamate, cinnamoyl chloride and allyl cinnamate (LC50, 0.114–0.195 mg/ml). Potent nematicidal activity (0.224–0.502 mg/ml) was also observed with six compounds. Moderate toxicity (0.750–1.896 mg/ml) was obtained from 16 compounds, whereas weak nematicidal activity (2.193–5.084 mg/ml) was produced from six compounds. The body of a nematode treated with these compounds usually showed a straight shape without movement when prodded with a wooden dowel. The castor oil-ethanol-treated control nematodes moved actively without being prodded.
Table 5.
Toxicity of cinnamaldehyde, cinnamic acid and their related compounds to adult Bursapheknchus xylophilus using the direct contact bioassay during a 24 hr exposure.
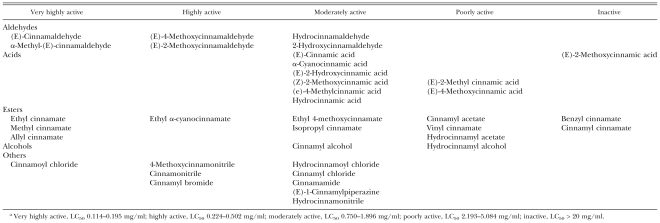
Structure-activity relationship: Comparisons were made to determine nematicidal activity differences involving the skeletal structure, saturation and functional groups of cinnamaldehyde, cinnamic acid and their related compounds using the toxicity data obtained (Table 6). Aldehydes were more effective nematicides than their corresponding acids (e.g., cinnamaldehyde vs. cinnamic l; hydrocinnamaldehyde vs. hydrocinnamylalc acid) and alcohols (cinnamaldehyde vs. cinnamyl alcohol; hydrocinnamaldehyde vs. hydrocinnamyl alcohol). Introduction of a functional group, such as hydroxy, methoxy or methyl, in cinnamaldehyde or cinnamic acid reduced its nematicidal activity. Allyl, ethyl and methyl cinnamates were significantly more toxic than isopropyl and vinyl cinnamates. Benzyl and cinnamyl cinnamates were ineffective. The toxicity of cinnamaldehyde, cinnamic acid, cinnamyl alcohol and cinnamonitrile was more pronounced than that of their corresponding saturated compounds.
Table 6.
Structure-activity relationships of test chemicals against adult Bursaphelenchus xylophilus.
Linear regression analysis of the toxicities of the test compounds toward adult B. xylophilus was performed using their LC50 values and the values of the hydrophobicity parameter for the test compounds. Compounds showing LC50 values >20 mg/ml were excluded for the analysis. Hydrophobicity parameter (r2 = 0.024, n = 34) was not significantly related to the observed toxicities of the test compounds in the contact toxicity bioassay.
Discussion
Very little work has been done to consider the potential of cassia and cinnamon oils to manage B. xylophilus, although extracts of some higher plants, particularly asteraceous and euphorbiaceous plants, have been found to possess good nematicidal activity against B. xylophilus (Kawazu et al., 1980a). In the present study, two cassia oils and four cinnamon oils exhibited good nematicidal activity toward adult B. xylophilus. Cassia and cinnamon oils might be good candidates for natural B. xylophilus control agents.
Mono- and sesquiterpenoids and related phenols exist in essential oils (Isman, 2000; Lawless, 2002), and jointly or independently they contribute to a variety of biological activities, including repelling or attracting nematodes, stimulating or inhibiting egg hatch, and nematicidal activity (Chitwood, 2002). Much effort has been focused on determining the distribution, nature and practical use of plant-derived chemicals that have nematicidal activity. Naturally occurring compounds nematicidal against B. xylophilus include the acetylenes (Z)-dehydromatricaria ester, 9,10-epoxyheptadec-16-en-4,6-diyn-8-ol, l-phenylhepta-l,3,5-triyne, 2-phenyl-5-(l-propynyl)-thiophene and tridec-l-en-3,5,7,9,11-pentayne (Kawazu et al., 1980b); the quinolizidine alkaloids, such as aloperine, cytisine, N-methylcytisine and matrine (Matsuda et al., 1989, 1991; Zhao, 1999); the lignans (−)-nortrachelogenin and (+)-pinoresinol, the phenylpropanoid methyl ferulate and the stilbene pinosylvin monomethyl ether (Suga et al., 1993); and the phenolics 3-undecylphenol and 3-(8Z-tridecenyl)phenol (Alen et al., 2000). In this study, the nematicidal principles of cassia and cinnamon oils were the phenylpropanoids (E)-cinnamaldehyde, cinnamyl acetate and eugenol, and the monoterpenoid α-terpineol. The nematicidal activity of these essential oils appeared to be associated with cinnamaldehyde content. Of the test compounds, (E)-cinnamaldehyde was the most active nematicide. Potent nematicidal activity was also observed with ethyl cinnamate, α-methyl-(E)-cinnamaldehyde, methyl cinnamate, cinnamoyl chloride and allyl cinnamate. Structural characteristics such as types of functional groups, saturation and carbon skeleton appear to play a role in determining the toxicity of these chemicals to adult B. xylophilus. The hydrophobicity parameter was not significantly related to the observed toxicities.
Available information is limited because immobile nematodes intoxicated by some plant-derived materials and chemicals were described as dead (Jourand et al., 2004). Jourand et al. (2004) reported that an aqueous extract of Crotalaria virgulata subsp. grantiana Harvey leaves caused paralysis of Meloidogyne incognita (Kofoid & White) Chitwood juveniles, but the effect was reversible, i.e., nematostatic. They suggested that pyrrolizidine alkaloids such as grantianine and grantaline of C. grantiana (Hartmann and Witte, 1995) might be responsible for the nematostatic effect on the basis that Fabaceae (formerly Leguminosae) plant alkaloids possess anthelmintic properties (Fassuliotis and Skucas, 1969; Zhao, 1999). It has been reported that nematode bodies treated with the muscle activity blockers levamisol hydrochloride and morantel tartrate usually exhibit semicircular and coiling shapes, respectively (Kong et al., 2006). In the current study with adult B. xylophilus, the active compounds tested had rapid nematicidal action. The bodies of nematodes killed by these compounds usually showed straight shapes. Our present and previous other studies suggest that the nematicide mode of action of the test phenylpropanoids might be different from that of either levamisol hydrochloride and morantel tartrate or plant alkaloids.
Results of this study indicate that the cassia and cinnamon oils and test compounds described could be useful as potential nematicides for B. xylophilus. Cinnamon oil is listed on the US Food and Drug Administration's GRAS (Generally Recognized as Safe) list and is exempt from toxicity data requirements by the US EPA (2004). For the practical use of the oils and test compounds as novel nematicides to proceed, further research is necessary on their systemic action, because the injected oils and compounds must be translocated and diffused to every part of the pine tree at an effective concentration. Other areas requiring attention are nematicide mode of action, phytotoxicity and formulations for improving nematicidal potency and stability and for reducing cost.
Footnotes
This paper was edited by Ed Lewis.
Literature Cited
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Alen Y, Nakajima S, Nitoda T, Baba N, Kanzaki H, Kawazu K. Two antinematodal phenolics from Knema hookeriana, a Sumatran rainforest plant. Zeitschrift fur Naturforschung. 2000;55C:300–303. doi: 10.1515/znc-2000-3-426. [DOI] [PubMed] [Google Scholar]
- Anonymous. 7th edition. New York: John Wiley & Sons, Inc; 2000. The Wiley registry of mass spectral data. [Google Scholar]
- Chitwood DJ. Phytochemical based strategies for nematode control. Annual Review of Phytopathology. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Fassuliotis G, Skucas GP. The effect of a pyrrolizidine alkaloid ester and plants containing pyrrolizidine on Meloidogyne incognita acrita . Journal of Nematology. 1969;1:287–288. [Google Scholar]
- Hartmann T, Witte L. Chemistry, biology and chemoecology of the pyrrolizidine alkaloids. In: Pelletier SW, editor. Alkaloids: Chemical & biological perspectives. vol. 9. Oxford, UK: Elsevier Science; 1995. pp. 155–233. [Google Scholar]
- Isman MB. Plant essential oils for pest and disease management. Crop Protection. 2000;19:603–608. [Google Scholar]
- Jourand P, Rapior S, Fargette M, Mateille T. Nematostatic effects of a leaf extract from Crotalaria virgulata subsp. grantiana on Meloidogyne incognita and its use to protect tomato roots. Nematology. 2004;6:79–84. [Google Scholar]
- Kawazu K, Nishii Y, Ishii K, Tada M. A convenient screening method for nematicidal activity. Agricultural and Biological Chemistry. 1980a;44:631–635. [Google Scholar]
- Kawazu K, Nishii Y, Nakajima S. Two nematicidal substances from roots of Cirsium japonicum . Agricultural and Biological Chemistry. 1980b;44:903–906. [Google Scholar]
- Kishi Y. Tokyo: Thomas Company; 1995. The pine wood nematode and the Japanese pine sawyer. [Google Scholar]
- Kobayashi F, Yamane A, Ikeda T. The Japanese pine sawyer beetle as a vector of pine wilt disease. Annual Review of Entomology. 1984;29:115–135. [Google Scholar]
- Kong JO, Lee SM, Moon YS, Lee SG, Ahn YJ. Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) Journal of Asia-Pacific Entomology. 2006;9:173–178. [Google Scholar]
- Kosaka H, Aikawa T, Ogura N, Tabata K, Kiyohara T. Pine wilt disease caused by the pine wood nematode: The induced resistance of pine trees by the avirulent isolate of nematode. European Journal of Plant Pathology. 2001;107:667–675. [Google Scholar]
- Lawless J. London, UK: Thorsons; 2002. The encyclopedia of essential oils. [Google Scholar]
- Lee SM, Chung YJ, Lee SG, Lee DW, Choo HY, Park CG. Toxic effects of some insecticides on the Japanese pine sawyer, Monochamus altematus . Journal of Korean Forestry Society. 2003a;92:305–312. (in Korean; English summary). [Google Scholar]
- Lee SM, Chung YJ, Moon YS, Lee SG, Lee DW, Choo HY, Lee CK. Insecticidal activity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus altematus) larvae. Journal of Korean Forestry Society. 2003b;92:191–198. (in Korean; English summary). [Google Scholar]
- Linit MJ. Nematode-vector relationships in the pine wilt disease system. Journal of Nematology. 1988;20:227–235. [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Kimura M, Komai K, Hamada M. Nematicidal activities of (−)-N-methylcytisine and (−)-anagyrine from Sophora flavescens against pine wood nematodes. Agricultural and Biological Chemistry. 1989;53:2287–2288. [Google Scholar]
- Matsuda K, Yamada K, Kimura M, Hamada M. Nematicidal activity of matrine and its derivatives against pine wood nematodes. Journal of Agricultural and Food Chemistry. 1991;39:181–191. [Google Scholar]
- Mun, SG, and Woo, Y T, 2001. The ecological study for the rest period of Mt. Gumjeong. Busan, Korea: Busan Metropolitan City (in Korean).
- Suga T, Ohta S, Munesada K, Ide N, Kurokawa M, Shimizu M, Ohta E. Endogenous pine wood nematicidal substance in pines, Pinus massioniana, P. strobes and P. palustris . Phytochemistry. 1993;33:1395–1401. [Google Scholar]
- Takai K, Soejima T, Suzuki T, Kawazu K. Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus . Pest Management Science. 2000;56:937–941. [Google Scholar]
- US EPA. U.S. Environmental Protection Agency; 1996. Exemption of certain pesticide substances from federal insecticide, fungicide, and rodenticide act requirements. Final Rule, 40 CFR 152.25 (g), May 6, 1996. [Google Scholar]
- US EPA. 2004. Biopesticides—25b Minimum risk pesticides. http://www.epa.gov/oppbppdl/biopesticides/regtools/25b_list.htm.
- Viglierchio DR, Schmitt RV. On the methodology of nematode extraction from field samples: Baermann funnel modifications. Journal of Nematology. 1983;15:438–444. [PMC free article] [PubMed] [Google Scholar]
- Zhao BG. Nematicidal activity of quinolizidine alkaloids and the functional group pairs in their molecular structure. Journal of Chemical Ecology. 1999;25:2205–2214. [Google Scholar]