Abstract
Laboratory experiments were conducted to study non-target effects of augmenting entomopathogenic nematode (EPN)communities in soil. When raw soil from a citrus orchard was augmented with either 2,000 Steinernema riobrave or S. diaprepesi, fewer EPN (P ≤ 0.05) survived if the soil had also been treated with 2,000 S. riobrave 7 d earlier (i.e., two augmentation events rather than one). EPN survival was unaffected by treatment (P ≤ 0.05) in soil that was air-dried to disrupt antagonist activity prior to the experiment. When S. diaprepesi, S. riobrave, Heterorhabditis zealandica or no EPN were added to raw soil and S. diaprepesi was added 5 d later, the survival of both S. diaprepesi and of total EPN was greater (P ≤ 0.05) in soil that received no pretreatment than in soilpre treated with S. riobrave. Pretreatment of soil with H. zealandica or S. diaprepesi had less or no affect on survival of S. diaprepesi or total EPN. When nematodes were recovered from soil and placed on water agar, the number of S. diaprepesi that were killed by endoparasitic and trapping nematophagous fungi was greater (P ≤ 0.05) if soil was pretreated with steinernematid species than if the soil was not pretreated or was pretreated with H. zealandica. The adverse effects of pretreating soil on EPN survival were density dependent within a range of pretreatment dosages (20–100 IJ/cm2 soil surface), and the treatment effects required more time to become evident at lower than at higher dosages. These experiments suggest that non-target effects of augmenting the EPN community in soil vary among EPN species and have the potential to temporarily reduce EPN numbers below the natural equilibrium density.
Keywords: Antagonism, nematophagous fungi, numerical response, post-application biology, predation, survival
Entomopathogenic nematodes (EPN) are used in classical and augmentation biological control programs in Florida to manage insect pests of turf, forage crops and citrus. Steinernema scapterisci Nguyen and Smart was discovered in Uruguay, near the presumed center of origin of the mole cricket (Scapteriscus spp.) and introduced in Florida as a potential biological control agent of several invasive mole cricket species (Nguyen and Smart, 1990, 1991). Research is ongoing to determine whether S. scapterisci has established in Florida at levels needed to provide acceptable mole cricket suppression, or whether it is necessary to periodically augment the numbers of the nematode (Adjei et al., 2003). Commercial formulations of four EPN species have also been used at various times since 1991 for augmentation biological control of Diaprepes abbreviatus L., a polyphagous pest in the Caribbean Basin that was introduced into Florida more than 40 years ago (Shapiro-Ilan et al., 2005). The use of EPN to manage the subterranean we evil larvae was adopted because available chemical pesticides are ineffective. An exotic species, Steinernemariobrave Cabanillas, Poinar and Raulston, and an endemic strain of Heterorhabditis indica Poinar, Karunakar and David are currently sold in Florida for control of D.abbreviatus. Estimates of the efficacy and profitability of using EPN for weevil control in citrus vary widely and probably reflect variation in factors such as product quality, application rates, suitability of edaphic conditions for EPN and experimental methods (Adair, 1994;Duncan and McCoy, 1996; Duncan et al., 1996b; Stansly et al., 1997; Bullock et al., 1999; McCoy et al., 2000, 2002; Duncan et al., 2002, 2003b).
Despite their widespread use for pest control in Florida, relatively little is known about the post-application biology of EPN or the effects of augmentation on soil food webs (Duncan et al., 2003a, 2003b;El-Borai et al., 2005). Periodic augmentation of the EPN communities in citrus orchards with S. riobrave effectively killed sentinel weevil larvae; however, following treatment, the prevalence of EPN and the mortality of sentinel larvae sometimes declined temporarily in treated compared to untreated plots (Duncan et al., 2003b, 2007). Duncan et al. (2003b) speculated that competition between long-lived endemic EPN species(i.e., S. diaprepesi Nguyen and Duncan) and the shorter-lived exotic S. riobrave could eventually diminish the number of EPN in soil. Equally plausible is the possibility that density-dependent antagonists of nematodes increased following EPN augmentation, thereby reducing EPN numbers below the previous equilibrium density (Ishibashi and Kondo, 1986; Koppenhofer et al.,1996; Kaya, 2002). Numerically, EPN are a minor part of nematode communities in soils; however, they attain numbers high enough in the vicinity of parasitized insect larvae to stimulate a localized numerical response by some species of nematophagous fungi (NF) (Jaffee and Strong, 2005). Entomopathogenic nematodes are typically augmented at rates equivalent to 500 to 2,000IJ/100 cm3 in the top 5 cm soil, which are in the range of total nematode abundance reported for many soil habitats. Therefore, it seems possible that augmenting EPN could initiate a trophic cascade in which the NF and other natural enemies proliferate and temporarily reduce the numbers of endemic and exotic EPN enough to suppress the natural control of insect larvae.
To explore this possibility, we conducted laboratory experiments to compare the survival of several EPN species and the prevalence of nematophagous fungi in soil with and without prior EPN augmentation. We tested the hypotheses that augmentation of EPN in soil at commercial application rates can (i) increase the prevalence of nematode antagonists, thereby (ii) increasing mortality rates in EPN communities in soil and that (iii) these effects will be less pronounced for heterorhabitid than steinernematid EPN because heterorhabditid IJ more often remain ensheathed in the protective second-stage cuticle (Timper and Kaya, 1989, 1992).
Materials and Methods
Nematode inoculum: Steinernema diaprepesi (Sd), S. rio-brave (Sr) and Heterorhabditis zealandica Poinar (Hz)were isolated from Diaprepes abbreviatus sentinel larvae buried in a commercial citrus orchard near Bartow, FL. Steinernema riobrave were descendents of commercially formulated EPN that were applied periodically for more than 4 yr to manage the insect. The other two species are endemic. Nematodes were maintained in culture by periodically infecting D. abbreviatus larvae in moist (10%) autoclaved sand. Nematodes recovered from insect cadavers in White traps were stored in shallow water in transfer flasks (15°C) for up to 15 d before re-transfer. Nematodes used in these experiments were stored in this manner for between 1 and 5 d before use.
Assay for survival of IJ in soil: A bioassay was developed to determine whether augmenting EPN in soil can result in soil that is temporarily suppressive to EPN. Surface litter was removed, and 500 cm3 of soil from 0 to 30cm depth was obtained from the under canopy of each of eight mature citrus trees in a commercial orchard near Bartow, FL. Soil texture in the orchard is a fine sand (97% sand, 2%, silt, 1% clay; pH 6.7; organic matter <1.0%). To reduce the prevalence of nematode antagonists, the soil was mixed, spread in a thin layer, air-dried in the laboratory for 2 wk and stored in aplastic container. Forty-eight hours prior to initiatingan experiment, additional soil (hereafter referred to asraw soil) was collected from beneath the same trees, mixed and sampled for moisture determination (24 hr at 70°C). In all experiments, the moisture content of the air-dried and raw soil was adjusted to 8% (wt water: wt dry soil) with tap water. Experimental units consisted of petri dishes (53-mm-diam. × 12-mm-deep) filled with either type of soil. Predetermined numbers of EPN IJ in600 μl tap water or tap water without nematodes were pipetted onto the soil surface, and the dishes were sealed with parafilm and stored in the dark at 25°C to allow population growth of natural enemies. After 7 d(unless otherwise indicated), 2,000 EPN IJ of the same or different species were added in the same manner to all of the dishes. The dishes were then resealed and stored for either 3 or 7 additional d to determine the treatment effects on the survival of EPN and the prevalence of natural enemies. The assay was terminated by recovering nematodes from soil with sugar centrifugation (Jenkins, 1964; nematodes recovered on 500 μm sieve) or Baermann funnels (48 hr). IJ on the dish inner surfaces were rinsed either directly into glass tubes (experiment 1) or back into the soil prior to extraction. IJ in aliquots (5 cm3 of 25 cm3) of the recovered nematodes were identified and counted using an inverted compound microscope (×20–×400). In some experiments, nematodes in the remaining 20 cm3water in the tubes were processed to recover nematophagous fungi (NF) as described in the following section.
Assay for prevalence of nematophagous fungi: The IJ and endemic nematodes extracted from soil in the IJ survival assay were further purified by magnesium sulfate centrifugation (Kaplan and Davis, 1990), concentrated in 500 μl tap water and spread across 2% water agar containing 2 ppm streptomycin sulfate in 5-cm-diam.petri dishes. Excess water was allowed to evaporate, and dishes were then sealed with parafilm and incubated for 6 d at 25°C. At this point, sporulation by trapping fungi frequently was not initiated, even though most or all IJ were killed. Therefore, 6 to 8 d after placing then ematodes on agar, 1,000 additional, healthy S. riobrave or S. diaprepesi from the stock cultures in 500 μl tap water were added to the dishes, and excess water allowed to evaporate. After 48 hr, sporulation of some species was prevalent, and dishes were examined to determine the numbers of live and dead IJ and to classify NF associated with nematode cadavers as either trappers or endoparasites, based on the morphology of spores or specialized hyphae. The cause of death for cadavers without evidence of NF was denoted as unknown.
Effect of augmenting with Sr on survival of Sr, Sd and Hz:Fifteen replications of the following treatments were established in both air-dried and raw soil: 0-Sr; 0-Sd;0-Hz; Sr-Sr; Sr-Sd; and Sr-Hz, in which the first termindicates the initial treatment (±2,000 IJ Sr) and the second term indicates the species added to the soil 7 d later. Seven days after the second augmentation, nematodes were extracted from soil using Baermann funnels. Nematodes on the dish surfaces were rinsed into tubes and counted separately. The experimental design was a 2 × 2 × 3 factorial (air-dried vs. raw soil x pretreatment or no pretreatment with Sr x Sr or Sd or Hz, 7 d after adding Sr).
Effect of augmenting with Sr, Sd and Hz on survival of Sd: The following treatments were established in raw soil ina completely randomized design: 0-Sd; Sd-0; Sd-Sd; Sr-Sd; Hz-Sd. Either 600 or no IJ were added initially. Fifteen replicates were prepared for three treatments and 30 replicates for treatments 0-Sd and Sr-Sd. Seven days after the second augmentation, nematodes in soil from 15 replicates of all treatments were extracted for72 hr on Baermann funnels. The IJ in the remaining 15replicate dishes of treatments 0-Sd and Sr-Sd were extracted by sugar centrifugation and assayed for nematophagous fungi. Steinernema diaprepesi were the sentinel IJ added to the fungus bioassays after 8 d.
In a second experiment, 15 replicates of the treatments Sd-Sd, Sr-Sd or Hz-Sd (600 IJ) were established in both air-dried and raw soil. The experimental design was a 2 × 3 factorial (soil type × nematode species).Nematodes were recovered from soil by sugar centrifugation 7 d after the second augmentation. IJ that were not enumerated after extraction from soil were bioassayed for nematophagous fungi. Steinernema riobrave were used as bait in the second part of the NF bioassay.
Temporal responses to EPN augmentation: One hundred-twenty IJ survival assay dishes were prepared with raw soil. Sixty plates were augmented with 600 IJ Sr and the remaining plates received only 600 μl tap water. After 7,14 and 28 d, 2,000 additional Sr were added to 20 dishes each with or without prior augmentation. Baermann funnels were used to recover nematodes 7 d after the second augmentation when numbers of IJ Sr were counted. We also counted endemic, second-stage juvenile (J2) Tylenchulus semipenetrans Cobb, which were especially numerous at this time of year, to determine whether augmenting EPN affected this plant-parasiticnematode. The experiment was repeated, but with an additional augmentation time interval (21 d). Each experiment was arranged in a completely randomized 3 × 2 or 4 × 2 factorial design (augmentation time interval × number of augmentations).
Effects of EPN augmentation density: Eighty IJ survival assay dishes were prepared using raw soil, and 20 replicates of four treatments (0, 500, 1,000 and 2,000 IJ S.riobrave) were arranged completely randomly. Two thousand S. diaprepesi were added to the dishes after 14d. Three days later, nematodes were recovered by sugar centrifugation. An aliquot was counted, and the remaining IJ were used in the NF assay. Steinernema diaprepesi were used in the second part of the NF assay.
Statistical tests: Data from factorial experiments were subjected to ANOVA with the General Linear Model Procedure (Minitab Inc., Release 13). Tukey's Honestly Significant Difference Test and Fisher's LSD test were used to compare treatment means. Pair wise comparisons were by Student's t-test. Proportions and counts were transformed (arcs in-square root and log (X + 1),respectively) prior to analysis, but untransformed means are reported.
Results
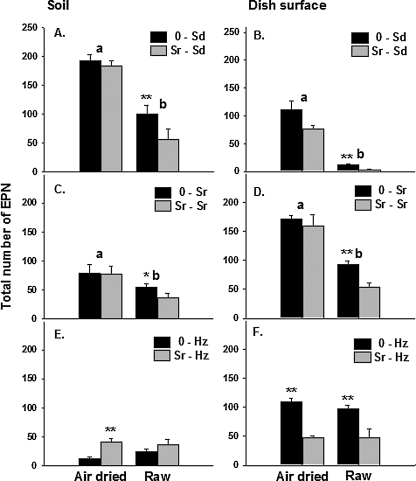
Effect of augmenting with Sr on survival of Sr, Sd and Hz: All two-way interactions between the main factors were at or near a significant level (P ≤ 0.07; Table 1). Accordingly, each factor was analyzed separately. For the steinernematid species, recovery of live IJ from soil was138% higher from air-dried than from raw soil for Sd (F = 43.31; df = 1, 56; P = 0.001) and 73% higher for Sr (F = 10.12; df = 1, 56; P = 0.002) (Fig. 1A,C). The same trends occurred for the steinernematid nematodes recovered from dish surfaces (Fig. 1B,D). However, IJ were recovered in greater numbers from soil than from dish surfaces (P = 0.001) in treatments containing Sd, whereas fewer IJ were recovered from soil than from dish surfaces (P = 0.001) in treatments with only Sr. Pretreatment of raw soil with Sr caused fewer total IJ to be recovered from soil (Sr-Sd, P = 0.01; Sr-Sr, P = 0.03) and dish surfaces (Sr-Sd, P = 0.01; Sr-Sr; P = 0.01), but IJ recovery was unaffected by pretreatment with Sr (P > 0.10) when dishes contained air-dried soil.
Table 1.
Analysis of variance for recovery of three EPN speciesfrom soil that was raw or air-dried and either pretreated or not pre-treated with Steinernema riobrave.
Fig. 1.
Effects of augmenting the numbers of Steinernema riobrave (Sr) in raw or air-dried soil on the numbers of EPN recovered from Baermann funnels 2 wk later. A second EPN cohort (Sr, S. diaprepesi[Sd] or Heterorhabditis zealandica [Hz]) was added to all experimental units 7 d after Sr were added to half of the units. Error bars are standard errors of means. Significant differences between means within a soil type (air dried or raw) are denoted by *(P ≤ 0.05) and **(P ≤ 0.01) according to Tukey's Honestly Significant Difference Test. Treatment differences between the soil types (P ≤ 0.05) are indicated by different letters.
Unlike the treatments containing steinernematid species, in treatments containing Hz the recovery of IJ from soil or container surfaces was unaffected by the type of soil (raw vs. air-dried; Fig. 1E,F). More IJ were recovered from dish surfaces than from soil (P = 0.001) in treatments containing Hz, and there was an interaction between the location of the nematodes (soil vs. dish surface) and pretreatment with Sr. On dish surfaces, fewer IJ were recovered using both types of soil(air-dried vs. raw) if they were pre-inoculated with Sr(P = 0.01). However, pretreatment of air-dried soil with Sr resulted in higher recovery of Sr+Hz from soil (P = 0.01), and a similar non-significant trend was observed in raw soil.
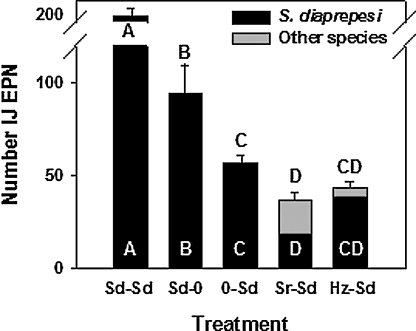
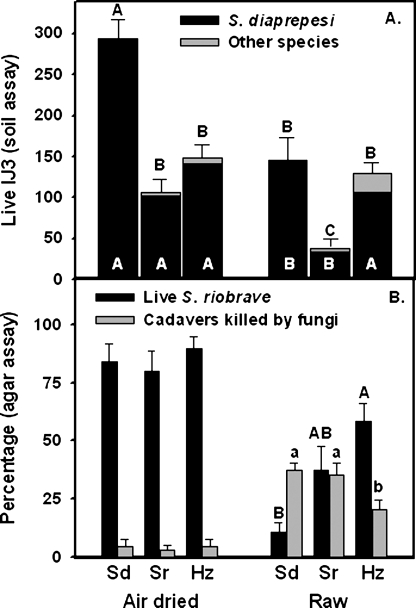
Effect of augmenting with Sr, Sd and Hz on survival of Sd: In the first experiment, significantly fewer Sd were recovered from soil pretreated with Sr compared to nonpretreated soil (Fig. 2). Pretreatment with Hz did not significantly affect recovery of Sd compared to recovery from non-pretreated soil, and pretreatment with Sd increased recovery of Sd to levels numerically higher (ns)than the combined recovery from treatments Sd-0 and0-Sd. The same trend occurred for total EPN recovered from these treatments. Fewer total live EPN were recovered from soil pretreated with Sr than from soil that received no pretreatment with EPN (Fig. 2). The proportion of dead Sd among the Sd recovered from Baer-mann funnels was higher (P < 0.001, Tukey's HSD) in soil pretreated with Sr (39%) than in the other treatments (range 9.8–19.1%) which did not differ from one another (data not shown).
Fig. 2.
Effect of augmenting the numbers of Steinernema diaprepesi(Sd), S. riobrave (Sr) or Heterorhabditis zealandica (Hz) in raw soil, followed by augmentation of S. diaprepesi 7 d later on numbers of entomopathogenic nematodes recovered from Baermann funnels. Error bars are standard errors of means. Bars with the same letter do not differ from each other according to Tukey's Honestly Significant Difference Test (P ≤ 0.05). White letters represent differences between numbers of S. diaprepesi and black letters represent differences between total numbers of EPN.
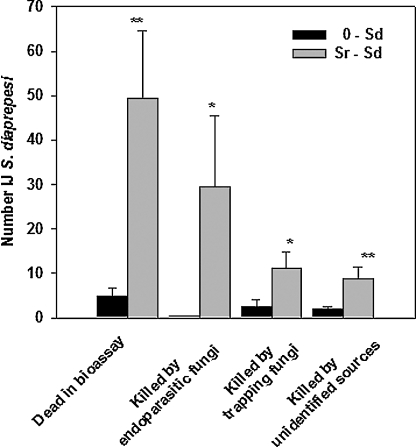
Pretreatment of soil with Sr resulted in a 10-fold increase in Sd cadavers in the NF assay (Fig. 3). More Sd were killed by endoparasitic fungi, trapping fungi and unidentified sources if soil was pretreated with Sr than if no pretreatment occurred.
Fig. 3.
Effect of augmenting the numbers of Steinernema riobrave (Sr) in raw soil on the survival of sentinel S. diaprepesi in the NF assay and on the relative prevalence of nematophagous fungi recovered from soil and transferred to water agar (NF assay). Error bars are standard errors of means. Significant differences between means within a category are denoted by *(P ≤ 0.05) and **(P ≤ 0.01) according to Tukey's Honestly Significant Difference Test.
In the second experiment, pretreatment of raw soil with Sd or Hz resulted in recovery of more than three times as many EPN than did pretreatment with Sr (P = 0.01) (Fig. 4A). In air-dried soil, pretreatment with Sr and Hz had similar effects on recovery of EPN which were approximately half as numerous as in soil pre-treated with Sd (P = 0.01). Heterorhabditis zealandica appeared to be less affected by antagonists than was eithersteinernematid species because fewer EPN (P = 0.01)were recovered from raw soil than from air-dried soil pretreated with steinernematids, whereas pretreatment with Hz had the same effect in both soils.
Fig. 4.
Effects of sequentially augmenting air-dried or raw soil, first with Steinernema diaprepesi, S. riobrave or Heterorhabditis zealandica, followed 7 d later by S. diaprepesi on A) the survival of entomopathogenic nematodes (EPN) and B) the prevalence of nematophagousfungi (NF). Error bars are the standard error of the mean. Different letters above bars denote significant treatment differences (P ≤ 0.05;Tukey's Honestly Significant Difference) for both S. diaprepesi and total EPN within a soil type. Different letters at the base of bars denote significant differences in recovery of both S. diaprepesi and total EPN for a specific pretreatment across soil types.
The survival of Sr in the nematophagous fungi assay was unaffected by pretreatment of air-dried soil with any species, but pretreatment of raw soil with Sd resulted in fewer surviving Sr than did pretreatment with Hz (Fig. 4B). The prevalence of trapping or endoparasitic fungi was not significantly different among treatments, but when considered together these fungi were less prevalent (P = 0.02) when raw soil was pretreated with Hz than with Sr or Sd.
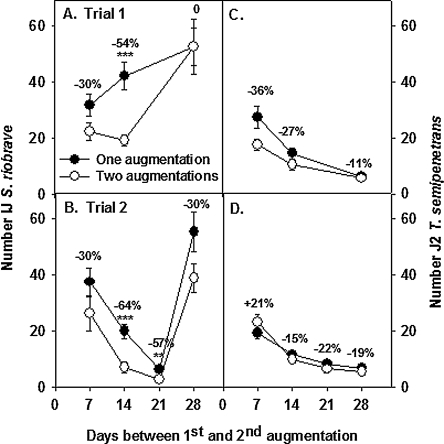
Temporal response to EPN augmentation: The numbers of EPN recovered from soil treated once or twice with600 Sr differed (P < 0.01) when the interval between treatments was 2 to 3 wk (Fig. 5A,B). The effect of augmenting EPN in soil on EPN population density was ephemeral; no differences were detected when 28 d separated the first and second application of Sr.
Fig. 5.
Effects of interval between the first and second augmentation of soil with Steinernema riobrave on numbers of S. riobrave (A–B) or Tylenchulus semipenetrans (C–D) recovered from soil that was augmented once or twice with the nematode. Numbers above symbols represent the percentage reduction in numbers of nematodes from soil augmented twice compared to once. Significant treatment differences according to Student's t-test are denoted by **(P ≤ 0.01) and ***(P ≤ 0.001).
Although there was a tendency to recover fewer T.semipenetrans from soil that was augmented twice compared to once, the trend was not significant in either experiment (Fig. 5C,D).
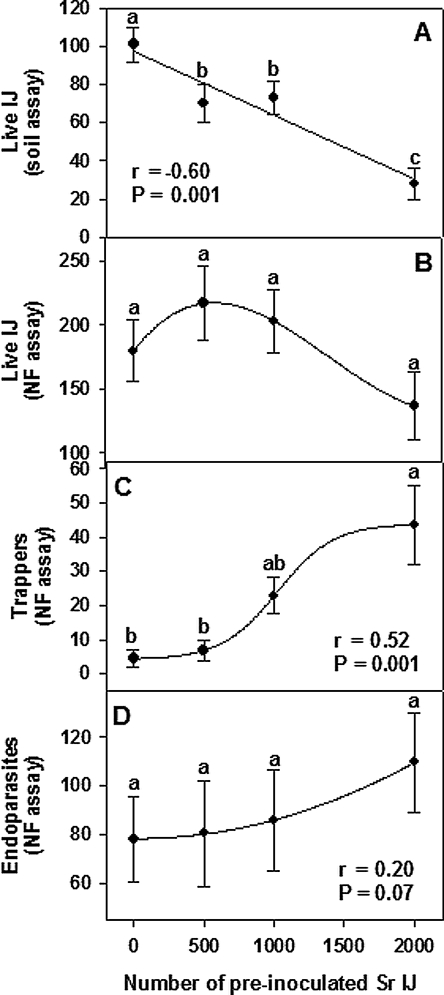
Effects of EPN augmentation density: Fewer EPN (P = 0.05) were recovered from soil in all treatments that received two EPN augmentations compared to that receiving a single augmentation (Fig. 6A). There was no significant treatment effect on numbers of live Sd at the end of the NF assay (Fig. 6B). The numbers of Sd killed by trapping fungi increased with increasing dosage of IJ Sr used in the initial augmentation, and a similar non-significant trend occurred for IJ killed by endoparasitic fungi (Fig. 6C,D).
Fig. 6.
Effect of augmenting raw soil with various numbers ofSteinernema riobrave and then adding 2,000 S. diaprepesi 2 wk later on A) numbers of live entomopathogenic nematodes extracted from the soil 1 wk after the second augmentation, B) numbers of S. diaprepesi that survived the NF bioassay, C) numbers of S. diaprepesi killed by trapping fungi in the NF bioassay and D) numbers of S. diaprepesi killed by endoparasitic fungi in the NF bioassay. Error bars represent the standard errors of means (n = 20). Linear correlation coefficients(r) were determined for data in A, C and D. Data in A were fitted by linear regression, whereas arbitrary curves were fitted to data in the other panels. Data points with the same letters within a panel do not differ from each other according to Tukey's Honestly Significant Difference Test (P ≤ 0.05).
Discussion
Augmenting the EPN in these soil microcosms caused species-specific, non-target effects. Within two weeks following the first of two applications of steinernematid nematodes to raw but not air-dried soil, the prevalence of some nematophagous fungi increased, where as numbers of EPN declined compared to treatments that only received a single augmentation treatment. Jaffee and Strong (2005) found that populationdensities of some species of trapping fungi increased as much as 100-fold in localized responses to the large numbers of IJ steinernematids that egress from insect cadavers. The substantial NF numerical responses reported by Jaffee and Strong (2005) resulted in approximately one-third higher EPN mortality, which is of the same order of magnitude that we observed. These results are consistent with the hypothesis that EPN antagonists can respond to EPN augmentation in the field with population growth high enough to temporarily reduce numbers of EPN and rates of sentinel insectmortality compared to those in non-augmented soil (Duncan et al., 2003b, 2007).
As predicted, the survival of Hz was less affected than that of either steinernematid when soil was pretreated with EPN. Moreover, pretreatment of soil with Hz or Sd had less effect on the survival of Sd than if soil was pretreated with Sr, and the prevalence of NF was significantly lower in soil pretreated with Hz than with either steinernematid. These results and a similar pattern of responses by NF to H. marelatus and S. glaseri (Jaffee and Strong, 2005) support the observation of Timper and Kaya (1989, 1992) that, in contrast to steinernematids, the tendency of IJ heterorhabditids to retain the second-stage cuticle as a sheath affords them increased protection against some natural enemies. The reasons for greater survival of EPN in soil pre-treated with Sd compared to Sr are unknown. The innate longevity of Sd in autoclaved soil (>2 yr) is much greater than that of Sr (<3 mon) (Duncan, unpublished); however, the relatively short duration of these experiments would seem to preclude longevity as a reason that augmentation using Sr reduced EPN survival more than did use of Sd. Other possible causes such as differences in nematode cuticular chemistry and adhesion of NF infection structures (Jansson, 1993; DenBelder et al., 1996; Yang et al., 2005) and differences in nematode behavior (Jaffee and Strong, 2005) remain to be investigated. The fact that Sd was less likely than the other species to move out of soil and onto the container surfaces may have contributed to relative differences between species in predation by NF. Nevertheless, the strongest effect of pretreatment on NF and EPN survival occurred with Sr, which had a high propensity in this and other studies to aggregate on the dish surfaces(Duncan et al., 1996a).
Tylenchulus semipenetrans juveniles were occasionally plentiful in the raw soil, but EPN augmentation did not measurably affect their prevalence. Duncan et al.(2007) reported that augmentation of Sr in field plots did not affect numbers of T. semipenetrans measurably for several months until a period of major population growth occurred in untreated, but not Sr-amended plots.
Additional research is needed to evaluate the spatial patterns of antagonist responses to EPN augmentation. The microcosms were amended with EPN within a range of rates recommended for pest control on a surface area basis. Because the EPN were restricted to just one centimeter of downward migration in the assay dishes, the EPN concentration per volume of soil was constrained at a higher level than occurs over time in the field. Therefore, it is likely that our assays amplified the numerical responses of NF and their effects on EPN mortality. For example, an interval of two weeks between EPN augmentations was required to measure significant differences in IJ survival in soil when dishes were treated with 20 IJ/cm2, compared to just one week when treated with 100 IJ/cm2. Differences in numerical responses by NF to one vs. two EPN augmentations were always detected at higher EPN treatment rates, but not at lower rates. These trends suggest that detecting food web cascades at the lower EPN densities typically found at greater depths in the field will require substantially greater replication or more sensitive detection methods. Use of real-time PCR to better quantify low population densities of NF offers promise in this regard (Arora et al., 1996; Atkins et al., 2003).
From a practical standpoint, the frequency with which NF respond strongly enough to EPN augmentation to interfere with biological control of insects maybe as important as determining threshold EPN densities needed to elicit a measurable increase in NF. Duncan et al. (2007) reported increased NF prevalence following two of three EPN augmentation events in the field; however, only once did EPN prevalence decline enough to reduce the mortality of buried sentinel insects below that in untreated plots. Perhaps NF responses to EPN augmentation have the greatest potential to affect EPN prevalence during times of high insect recruitment in soil, because the response to increased numbers of exotic EPN could intensify the localized responses to EPN emerging from insect cadavers (Jaffee and Strong, 2005). An additional reason to consider temporal patterns of insect recruitment is that high recruitment soon after EPN augmentation may permit large numbers of insects to escape EPN predation. Ideally, EPN augmentation would be timed to occur immediately following peak insect recruitment and prior to a period of low recruitment to avoid the possibility that non-target effects may reduce the normal mortality rate of young weevils entering the soil (Duncan et al.,2007).
There is some evidence that biological control of D.abbreviatus larvae by endemic EPN is greater on the deep sandy soils of Florida's Central Ridge than in other parts of the state with soils of finer texture and shallower water tables (Duncan et al., 2003b). During a two-year survey in an orchard on the Central Ridge, the mortality of buried sentinel weevil larvae averaged 52%per week, with 81% of cadavers containing endemic EPN (Duncan et al., 2007). Non-target effects caused by EPN augmentation could be important in orchards with vigorous EPN communities, whereas they should be of little concern in habitats less suitable to endemic EPN. Nevertheless, it has been speculated that the Central Ridge may be uniquely suited for use of EPN augmentation because reduced prevalence of D. abbreviatus there, compared to other regions, makes it more feasible to suppress insect numbers below a damaging level (Futch et al., 2005; Shapiro-Ilan et al., 2005).
Our results are consistent with the basic paradigm that population size is maintained in equilibrium with density dependent forces such as food, competitors and antagonists (Jaffee et al., 1993). Augmentation biological control is a tactic employed to temporarily increase the numbers of a biocontrol agent beyond its equilibrium density in order to increase the mortality of a pest. Implicit in this tactic is the expectation that numbers of the biocontrol agent will eventually decline to the equilibrium state, either gradually or in a series of dampening oscillations above and below the equilibrium. The significant bottom-up and top-down cascades that developed from successive EPN augmentations of our microcosms suggest that augmenting EPN in the field may sometimes result in the latter condition and thereby influence biological control.
Footnotes
This research was supported by the Florida Agricultural Experiment Station and awards from Florida Citrus Production Research Advisory Council and the T-STAR Special Grants Program.
This manuscript was edited by Ed Lewis.
Literature Cited
- Adair RC. A four-year field trial of entomopathogenic nematodes for control of Diaprepes abbreviatus in a flatwoods citrus grove. Proceedings of the Florida State Horticultural Society. 1994;107:63–68. [Google Scholar]
- Adjei MB, Frank JH, Gardner CS. Survey of Pestmole crickets (Orthoptera: Gryllotalpidae) activity on pasture in south-central Florida. Florida Entomologist. 2003;86:199–205. [Google Scholar]
- Arora DK, Hirsch PR, Kerry BR. PCR-based molecular discrimination of Verticillium chlamydosporium isolates. Mycolological Research. 1996;7:801–809. [Google Scholar]
- Atkins SD, Clark IM, Sosnowska D, Hirsch PR, Kerry BR. Detection and quantification of Plectosphaerella cucumerina, a potential biological control agent of Potato Cyst Nematodes, by using conventional PCR, real-time PCR, selective media and baiting. Applied and Environmental Microbiology. 2003;69:4788–4793. doi: 10.1128/AEM.69.8.4788-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock RC, Pelosi RR, Killer EE. Management of citrus root weevils (Coleoptera: Curculionidae) on Florida citrus with soil-applied entomopathogenic nematodes (Nematoda: Rhabditida) Florida Entomologist. 1999;82:1–7. [Google Scholar]
- Den Belder E, Jansen E, Donkers J. Adhesive hyphae of Arthrobotrys oligospora: An ultrastructural study. European Journal of Plant Pathology. 1996;102:471–478. [Google Scholar]
- Duncan LW, Dunn DC, Bague G, Nguyen K. Competition between entomopathogenic and free-living bactivorous nematodes in larvae of the weevil Diaprepes abbreviatus . Journal of Nematology. 2003a;35:187–193. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Dunn DC, McCoy CW. Spatial patterns of entomopathogenic nematodes in microcosms: Implications for laboratory experiments. Journal of Nematology. 1996a;28:252–258. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Dunn DC, Zellers J, McCoy CW, Nguyen K. Incidence of endemic entomopathogenic nematodes following application of Steinernema riobrave for control of Diaprepes abbreviatus . Journal of Nematology. 2003b;35:178–186. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Zellers J. Profitability of applications of Steinernema riobrave, metalaxyl and supplemental fertilisation for management of Diaprepes abbreviatus and Phytophthoranicotianae in a Florida citrus orchard. Fourth International Congress of Nematology. 2002;4:192. (Abstr.). [Google Scholar]
- Duncan LW, Graham JH, Zellers J, Bright D, Dunn DC, El-Borai FE, Porazinska DL. Food web responses to augmenting the entomopathogenic nematodes in bare and animal manure-mulched soil. Journal of Nematology. 2007;39 this issue. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, McCoy CW. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Curculionidae) of two species of entomogenous nematodes (Rhabditida:Steinernematidae; Heterorhabditidae) Environmental Entomology. 1996;25:174–178. [Google Scholar]
- Duncan LW, McCoy CW, Terranova AC. Estimating sample size and persistence of entomogenous nematodes in sandy soils and their efficacy against the larvae of Diaprepes abbreviatusin Florida. Journal of Nematology. 1996b;28:56–67. [PMC free article] [PubMed] [Google Scholar]
- El-Borai FE, Duncan LW, Preston JF. Bionomics ofa phoretic association between Paenibacillus sp. and the entomopathogenic nematode Steinernema diaprepesi . Journal of Nematology. 2005;37:18–25. [PMC free article] [PubMed] [Google Scholar]
- Futch SH, Duncan LW, Zekri M. Validation of an area-wide extension program to estimate the seasonal abundance of adult citrus root weevils with un-baited pyramidal traps. Proceedings of the Florida State Horticultural Society. 2005;117:143–147. [Google Scholar]
- Ishibashi N, Kondo E. Steinernema feltiae (DD-136) and S. glaseri: Persistence in soil and bark compost and their influence on native nematodes. Journal of Nematology. 1986;18:310–316. [PMC free article] [PubMed] [Google Scholar]
- Jaffee BA, Tedeford EC, Muldoon AE. Tests for density-dependent parasitism of nematodes by nematode-trapping and endoparasitic fungi. Biological Control. 1993;3:329–336. [Google Scholar]
- Jaffee BA, Strong DR. Strong bottom-up and weak top-down effects in soil: Nematode-parasitized insects and nematode-trapping fungi. Soil Biology and Biochemistry. 2005;37:1011–1021. [Google Scholar]
- Jansson HB. Adhesion to nematodes of conidia from the nematophagous fungus Drechmeria coniospora . Journal of General Microbiology. 1993;139:1899–1906. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:492. [Google Scholar]
- Kaplan DT, Davis EL. Improved nematode extraction from carrot disk culture. Journal of Nematology. 1990;22:399–406. [PMC free article] [PubMed] [Google Scholar]
- Kaya HK. Natural enemies and other antagonists. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford,UK: CABI Publishing; 2002. pp. 189–204. [Google Scholar]
- Koppenhofer AM, Jaffee BA, Muldoon AE, Strong DR, Kaya HK. Effect of nematode-trapping fungi on an entomopathogenic nematode originating from the same field site inCalifornia. Journal of Invertebrate Pathology. 1996;68:246–252. doi: 10.1006/jipa.1996.0092. [DOI] [PubMed] [Google Scholar]
- McCoy CW, Shapiro DI, Duncan LW, Nguyen K. Entomopathogenic nematodes and other natural enemies as mortality factors for larvae of Diaprepes abbreviatus . Biological Control. 2000;19:182–190. [Google Scholar]
- McCoy CW, Stuart RJ, Duncan LW, Nguyen K. Field efficacy of two commercial preparations of entomopathogenic nematodes against larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in Spodosol type soil. Florida Entomologist. 2002;85:537–544. [Google Scholar]
- Nguyen KB, Smart GC. Steinernema scapterisci n. sp. (Rhabditida: Steinernematidae) Journal of Nematology. 1990;22:187–199. [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Smart GC. Pathogenicity of Steinernemascapterisci to selected invertebrates. Journal of Nematology. 1991;23:7–11. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Duncan LW, Lacey LA, Han R. Orchard crops. In: Grewal P, Ehlers R-U, Shapiro-Ilan D, editors. Nematodes as biological control agents. St. Albans, U.K: CABI Publishing; 2005. pp. 215–230. [Google Scholar]
- Stansly PA, Mizell RF, McCoy CW. Monitoring Diaprepes abbreviatus with Tedder's traps in Southwest Florida citrus. Proceedings of the Florida State Horticultural Society. 1997;110:22–26. [Google Scholar]
- Timper P, Kaya HK. Role of the 2nd-stage cuticle of entomogenous nematodes in preventing infection by nematophagous fungi. Journal of Invertebrate Pathology. 1989;54:314–321. [Google Scholar]
- Timper P, Kaya HK. Impact of a nematode-parasiticfungus on the effectiveness of entomopathogenic nematodes. Journal of Nematology. 1992;24:1–8. [PMC free article] [PubMed] [Google Scholar]
- Yang J, Huang X, Tian B, Wang M, Niu Q, Zhang K. Isolation and characterization of a serine protease from the nematophagous fungus, Lecanicillium psalliotae, displaying nematicidalactivity. Biotechnology Letters. 2005;15:1123–1128. doi: 10.1007/s10529-005-8461-0. [DOI] [PubMed] [Google Scholar]