Abstract
Factorial treatments of entomopathogenic nematodes (EPN) and composted, manure mulches were evaluated for two years in a central Florida citrus orchard to study the post-application biology of EPN used to manage the root weevil, Diaprepes abbreviatus. Mulch treatments were applied once each year to study the effects of altering the community of EPN competitors (free-living bactivorous nematodes) and antagonists (nematophagous fungi (NF), predaceous nematodes and some microarthro-pods). EPN were augmented once with Steinernema riobrave in 2004 and twice in 2005. Adding EPN to soil affected the prevalence of organisms at several trophic levels, but the effects were often ephemeral and sometimes inconsistent. EPN augmentation always increased the mortality of sentinel weevil larvae, the prevalence of free-living nematodes in sentinel cadavers and the prevalence of trapping NF. Subsequent to the insecticidal effects of EPN augmentation in 2004, but not 2005, EPN became temporarily less prevalent, and fewer sentinel weevil larvae died in EPN-augmented compared to non-augmented plots. Manure mulch had variable effects on endoparasitic NF, but consistently decreased the prevalence of trapping NF and increased the prevalence of EPN and the sentinel mortality. Both temporal and spatial abundance of NF were inversely related to the prevalence of Steinernema diaprepesi, whereas Heterorhabditis zealandica prevalence was positively correlated with NF over time. The number of weevil larvae killed by EPN was likely greatest in 2005, due in part to non-target effects of augmentation on the endemic EPN community in 2004 that occurred during a period of peak weevil recruitment into the soil.
Keywords: Diaprepes abbreviatus, entomopathogenic nematodes, food webs, IPM, nematophagous fungi, post-application biology, survival, trophic cascades
Diaprepes abbreviatus is a serious root weevil pest of numerous food and ornamental crops in the Caribbean Basin. The insect was first detected in Florida citrus groves in 1967 and became a highly destructive citrus pest when the halogenated hydrocarbon soil insecticides were deregistered in the 1970s. Adult weevils feed and oviposit on expanding leaves of citrus and other plants. The neonate larvae fall to the soil, where they complete development during several months, feeding first on fibrous roots and later on the cortex of major roots. Wounding of the major roots by these insects facilitates infection by Phytophthora spp. The resulting pest-disease complex can kill trees, and orchards are sometimes abandoned within several years after the insect is first detected. Managing these weevils with the non-persistent pesticides currently available is difficult because of a continual exchange of adults emerging from soil and neonate larvae entering soil, except during the coldest months. Efforts at classical biological control have, thus far, failed to introduce agents that establish widely in Florida (Pena et al., 2006). Augmentation biological control using entomopathogenic nematodes (EPN) is widely used to kill larvae in soil but, as with pesticides, the residual effectiveness is short (McCoy et al., 2000; Duncan et al., 2003b).
Diaprepes abbreviatus and Phytophthora spp. population densities tend to be highest in coastal and inland ‘flatwoods’ regions that are characterized by shallow, poorly drained soils (Futch et al., 2005). Orchards growing on the deep, well drained, sandy soils of Florida's Central Ridge incur less damage from lower population densities of these pests. Poorly drained soils provide ideal conditions for oomycetes such as Phytophthora spp.; however, the cause(s) of regional variation in D. abbreviatus pressure is unknown. Duncan et al. (2003b) reported that the average mortality during two years of sentinel weevil larvae buried beneath trees in an orchard on the central ridge was 49%/wk compared to just 11%/wk in an orchard in the flat-woods. Most of the mortality in the central ridge orchard was attributed to EPN which were more species-diverse and prevalent than in the flatwoods orchard, suggesting the possible involvement of EPN and other soilborne natural enemies in regional variation of the weevil.
Greater knowledge of EPN population dynamics and food web interactions could provide insights for conservation biological control in orchards where endemic EPN cause significant weevil mortality. For example, the numbers of sentinel weevils killed by EPN are sometimes seasonal, declining throughout the summer and early autumn months in spite of temperature and soil moisture levels conducive for EPN activity (Duncan et al., 2003b). Such a pattern of EPN prevalence could result from changes in the availability of insect hosts and/or density-dependent population growth by competitors and natural enemies of EPN (Jaffee and Strong, 2005). Population growth of EPN antagonists in response to EPN augmentation for weevil control might also temporarily reduce EPN numbers and weevil mortality below natural equilibrium levels (Duncan et al., 2003b; El-Borai et al., 2007). Understanding the relative importance of different habitats to EPN, their natural enemies and the resulting spatial patterns of EPN and their insect hosts in Florida is a further reason to study food web interactions (Strong, 2002).
Here we report the results of an experiment to measure the effects of EPN augmentation and composted manure mulches on the mortality of D. abbreviatus larvae and the prevalence of organisms in the soil food web: nematophagous fungi, Paenibacillus spp., soilborne nematodes (bacterivores, fungivores, omnivores, phyto-parasitic and entomopathogenic), microarthropods (mites and collembola) and enchytraeid worms. Mulches were studied because of their potential to increase the numbers of free-living bactivorous nematodes that sometimes compete with EPN for resources in the insect cadaver (Peters, 1996; Duncan et al., 2003a). We measured temporal responses to our treatments in order to detect patterns consistent with direct or indirect interactions between organisms in the food web. We sought support for the hypotheses that (i) endemic EPN are regulated in part by density-dependent natural enemies and (ii) EPN augmentation can initiate a brief trophic cascade that favors natural enemies to the detriment of EPN and weevil biological control.
Materials and Methods
Forty experimental plots were delineated within six rows of trees in a 0.6 ha area of a commercial citrus orchard near Bartow, on Florida's central ridge. The plots were arranged within an untreated control plot of a previous experiment (Duncan et al., 2003b). The orchard was irrigated by under canopy micro-sprinklers and fertilized four times annually with granular fertilizer; the soil texture was sandy (96% sand, 2% silt, 2% clay). Herbicides were the only pesticides applied to the trees during the experiment, and no other tactics were employed to manage D. abbreviatus that were present in the grove for more than 10 yr. Each experimental plot consisted of three adjacent trees (Valencia orange on Carrizo citrange rootstock, planted in 1990) within a row. A single funnel trap was installed under canopy, midway between the trunk and dripline of each of 2 trees/plot, to monitor the rate at which neonate weevil larvae fell from the tree canopy to develop in soil. Traps consisted of plastic centrifuge tubes containing 10 ml ethylene glycol that were attached to the bottoms of 15-cm-diam. plastic funnels and fitted within PVC tubes that supported them 1 m above the ground. They were monitored approximately biweekly, and weevils and other unidentified insect larvae were counted with the aid of a dissecting microscope.
Ten replications of four treatments were assigned completely randomly to the plots in a 2 x 2 factorial design: untreated control, EPN augmentation, composted manure mulch and EPN + mulch. A 10-cm layer of a composted horse manure and stable straw mixture (Organic Matters, Bartow, FL) was applied to the undercanopy areas of all trees in 20 plots in March 2004. In June 2005, the same trees were treated similarly with chicken manure that had been aged for more than 3 mon in 5-m-high mounds that were turned at monthly intervals (Boyd Brothers, Branford, FL). Steinernema rio-brave Cabanillas, Poinar and Raulston were applied to the irrigated zones beneath all trees of 20 plots on 10 August 2004 and 17 June and 8 September 2005. The nematodes were commercially formulated (BioVector, Becker Underwood, Littlehampton, UK) and applied in 1.0 liter of water/tree with a pressurized hand sprayer at rates of 30 IJ/cm2 soil surface at the initial and final treatment date and 300 IJ/cm2 soil surface on the second treatment date. Plots were irrigated from 30 min prior to the treatment until 1 hr after treatment. During the warmest months between March and October each year, several assays (below) were employed to monitor the population densities of some of the organisms presumed to function in the Diaprepes food web.
EPN and free-living bacterivorous nematode (FLBN) assay: Five caged sentinel weevil larvae were buried 25-cm deep within a 0.5 m2 area midway between the trunk and dripline of 1 tree/plot (Duncan et al., 2003b). Cages were recovered after 7 d, weevil mortality was determined and insect cadavers were incubated on white traps for up to 30 d to identify emerging EPN, FLBN or combinations of the two. When EPN were recovered, a subsample of 100 IJ specimens was examined at x400 to determine the percentage with 0, 1 to 10, 10 to 100, or >100 spores of Paenibacillus spp. visibly attached to the cuticle.
NF assay: A single polyvinyl chloride tube (5-cm-diam. x 30-cm-long) was pounded 20-cm deep into the soil midway between the trunk and dripline of 1 tree/plot. Fifty milliliters of water was poured into the tube, 4 ml of water containing 8,000 IJ of either S. riobrave or Steinernema diaprepesi Nguyen and Duncan was pipetted onto the soil surface and an additional 50 ml of water was added to aid EPN penetration into the soil. After 72 hr, tubes were removed from the soil and emptied into plastic bags (approximately 400 cm3 soil). The sample location was marked with a small flag to avoid resampling the site. The samples were stored at room temperature overnight, and nematodes were recovered the following day by sugar centrifugation (Jenkins, 1964) and further purified by density gradient centrifugation (Kaplan and Davis, 1990). The suspension was reduced to 1 ml, and nematodes were pipetted onto water agar (5-cm-diam. x 1 cm petri dishes) containing 2% streptomycin sulfate. EPN, mites, collembola, enchytraeid worms and the plant-parasitic nematode Tylenchulus semipenetrans were counted, and plates were sealed with parafilm and stored at 28°C. EPN were recounted after 5 d, and cadavers were examined (x50-x1000 magnification) for evidence of NF. Nearly all EPN were killed, and most contained either sporangia typical of Cate-naria spp. or vegetative hyphae without fruiting bodies after 5 d on the agar. Accordingly, the procedure was modified in September 2004 in an effort to increase detection of trapping NF. On d 5, we added 1,000 live S. riobrave or S. diaprepesi to the dishes. Plates were re-sealed, and 48 hr later EPN were counted. Sporulation was abundant and cadavers were again examined for evidence of predation by NF which were identified from spore and trap morphology.
Nematode community samples: Ten soil cores (2.5-cm-diam. x 30-cm-deep) were collected as a single sample midway between the trunk and dripline of 1 tree/plot on 8 June and 27 August 2004 and 15 June and 28 September 2005. Samples were mixed, and 100 cm3 subsamples were refrigerated for up to 2 wk before extracting by sieving twice (43-um-pore) and sucrose cen-trifugation. All nematodes in a sample were counted and identified to the level of genus or family (Rhabditi-dae). Soil moisture content was determined from approximately 10 g soil/sample that was dried at 100°C.
Efficacy estimate: The effectiveness of augmenting with S. riobrave in reducing populations of D. abbreviatus larvae was estimated using the model
Where Sn = number live weevil larvae at end of monthn; Pn = number neonate weevils recruited from the tree canopy in monthn; and Rn = survival rate in monthn = (survival rate of sentinel insects during 7 d in monthn)4, except for EPN-augmented plots during treatment months when Sn = (survival rate of sentinel insects during 7 d following treatment in monthn)2 x (survival rate of sentinel insects in untreated plots during 7 d in monthn)2. We assumed that larvae resided in soil for 3 mon before emerging as teneral adults, that the weekly mortality rates inferred from sentinels in untreated plots each month remained constant for 4 wk, that effects of EPN treatment on weekly survival remained constant for 2 wk and that the rate of larvae survival was independent of the population density.
Long-term treatment effects: Routine sampling of EPN and their natural enemies was discontinued in October 2005. At that time, tw ground traps (Duncan et al., 2001)/plot were installed in-line with the tree row to measure treatment effects on the emergence of adult D. abbreviatus. The cone-shaped traps were made of hardware cloth (0.3-cm mesh) with a base diameter of 90 cm, a 1-cm hole at the apex and a boll weevil trap (Great Lakes IPM, Vestaburgh, MI) attached to the apex. Soil was mounded around the trap bases to prevent the escape of teneral adult weevils emerging from soil circumscribed by the trap. Traps were monitored about every 2 wk.
Soil samples as described previously were taken from each plot on 8 February 2006 to measure treatment effects on citrus fibrous roots and Tylenchulus semipen-etrans. After mixing the soil, two Baermann funnels (60 cm3 soil each) were used to recover and enumerate the nematodes. The remaining soil was rinsed through a 0.25-mm-pore sieve, and citrus fibrous roots were separated by hand from organic debris and weighed.
The number of 0.41-kg boxes of citrus fruit from all trees in each plot was determined on 2 June 2006.
Statistical methods: The effects of mulch and EPN augmentation were tested with two-way ANOVA. Data from each sampling event were analyzed individually (i.e., time was not a variable) because we expected responses to vary at different times after the treatments. Data expressed as population counts and percentages were transformed (log (X + 1) and arcsin-square root, respectively) before analysis, but arithmetic means and standard errors are reported. Population change was calculated as (log time2) − (log time1) prior to analysis of variance. Linear correlation coefficients between the prevalence of NF, mites, collembola and enchytraeid worms recovered each month in 2005 and the prevalence of EPN measured in the subsequent sampling period were determined for EPN that were found to occur in more than two-thirds of the plots. Data from plots in which a particular EPN species was never detected were excluded from the analyses for that species. The relative prevalence of Paenibacillus spp. among either S. diaprepesi or Heterorhabditis zealandica Poinar was measured as the proportion of cadavers killed by S. diaprepesi or H. zealandica that also contained Paenibacillus spp. These values were correlated with numbers of cadavers containing S. diaprepesi or H. zealandica the following month. Area under the curve (AUC) was estimated by summing population counts over time in individual plots before analyzing treatment effects and interactions with two-way ANOVA.
Results
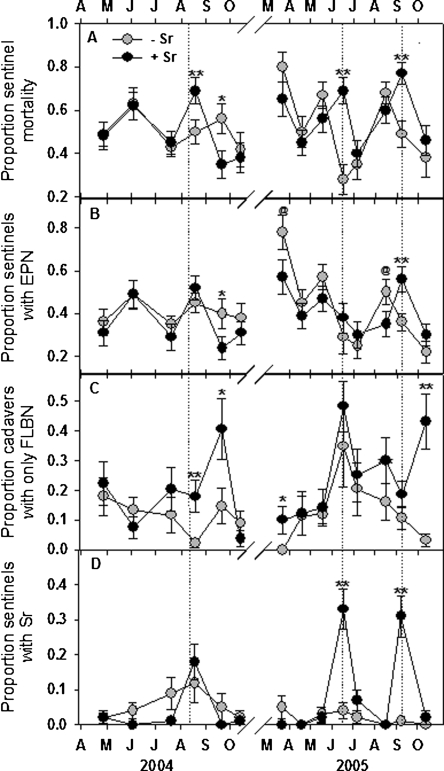
Effects of EPN augmentation: Each of the three applications of S. riobrave increased (P < 0.01) the rate of sentinel weevil mortality (Fig. 1A). The average mortality rate of sentinel weevils that were buried on the day of treatment (2005) or 10 d after treatment (2004) and recovered 7 d later ranged from 38% to 146% higher in EPN-augmented, compared to non-augmented plots. The exotic species S. riobrave killed 44%, 81% and 66% of insects in bare plots and 32%, 42% and 37% in mulched plots that would not otherwise have been killed by endemic predators on the three successive augmentation dates (data not shown; Abbott, 1925). Other than during the treatment months, there were no beneficial residual effects of augmentation on sentinel mortality. Indeed, 5 wk following EPN augmentation in 2004, significantly fewer sentinels died (P < 0.05) in the EPN-treated plots. EPN (endemic species and the exotic S. riobrave) recovered from weevil cadavers were more prevalent (P < 0.01) in augmented plots only in September 2005, whereas they were less prevalent (P < 0.10) on several sampling dates (Fig. 1B). By contrast, EPN augmentation greatly increased the proportion of cadavers containing FLBN (Fig. 1C). Steinernema riobrave was detected in EPN-augmented and non-augmented plots, but S. riobrave prevalence did not differ among the treatments except during the two treatment months in 2005 (Fig. 1D). After EPN augmentation began in 2004, on average 15% fewer sentinels died (NS, P > 0.10) and 20% fewer sentinels supported EPN (P = 0.07) in augmented compared to non-augmented plots during the months between treatment.
Fig. 1.
Effects of augmenting the entomopathogenic nematode community beneath citrus trees with Steinernema riobrave (Sr) on the mortality of Diaprepes abbreviatus sentinel larvae (A) and the prevalence of insect cadavers containing entomopathogenic nematodes (EPN) (B), free-living, bactivorous nematodes (FLBN) (C) and S. riobrave (D). A composted animal manure mulch was applied to half of the plots in each treatment, and the remaining plots remained bare. Treatment differences according to two-way analysis of variance of transformed data (arcsin, square-root) are denoted by @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Error bars are standard errors of means. Dotted lines indicate S. riobrave application dates.
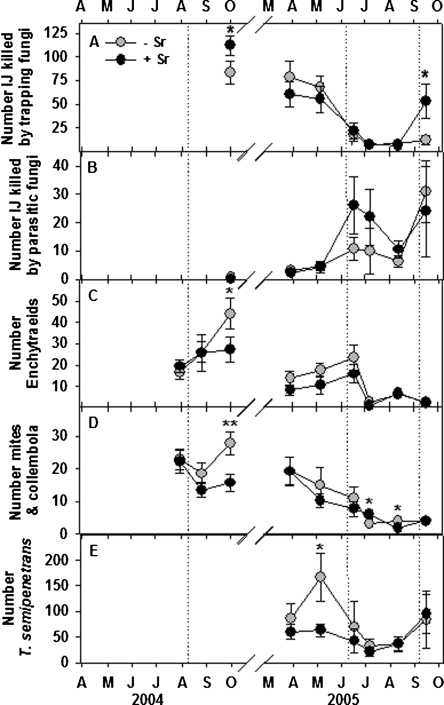
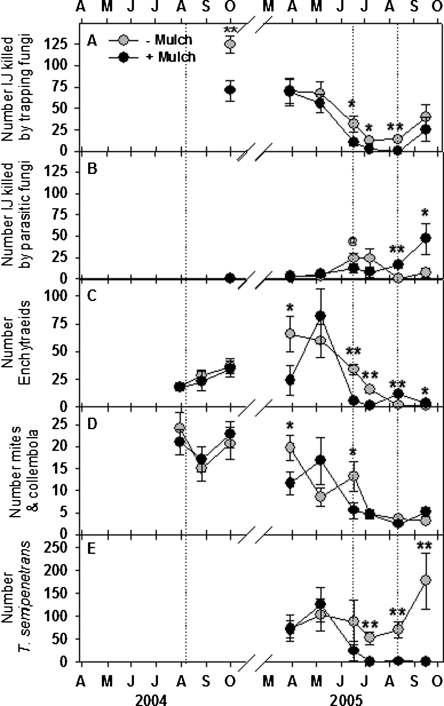
Trapping NF identified as Arthrobotrys oligospora, A. musiformis and Gamsylella gephyropaga (=Monacrosporium cionopagum) killed only 74% as many EPN (P < 0.05) in bioassays of samples from untreated compared to EPN-augmented plots 7 wk after EPN treatment in 2004 (Fig. 2A). EPN augmentation did not again affect the prevalence of NF until the week following the final EPN treatment, when trapping NF killed 4.4 times as many EPN (P = 0.05) in bioassays of treated compared to untreated plots. EPN augmentation did not affect en-doparasitic NF, although more than twice as many EPN were killed by Catenariaspp. (NS; P = 0.11), in bioassays of augmented compared to non-augmented plots during 2 mon following an EPN treatment in June 2005 (Fig. 2B). Fewer enchytraeid worms and microarthro-pods (mites and collembola) were recovered in bioas-says of EPN-augmented plots 7 wk after treatment in 2004 (P = 0.05), but the effect of EPN on microarthro-pods in 2005 varied (Fig. 2C-D). Numbers of T. semi-penetrans recovered in the NF bioassays were approximately 3-fold higher (P < 0.05) in untreated plots than in S. riobrave-treated plots during a peak of population growth in May 2005 (Fig. 2E).
Fig. 2.
The effects of augmenting the entomopathogenic nematode community beneath citrus trees with Steinernema riobrave (Sr) on bioassay measurements of the numbers of entomopathogenic nematodes killed by trapping nematophagous fungi (A) or endoparasitic NF (B), and the numbers of enchytraeid worms (C), mites and col-lembola (D) and Tylenchulus semipenetrans (E) in bioassay dishes. A composted animal manure mulch was applied to half of the plots in each treatment, and the remaining plots remained bare. Treatment differences according to two-way analysis of variance of transformed data (arcsin, square-root) are denoted by @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Error bars are standard errors of means. Dotted lines indicate S. riobrave application dates.
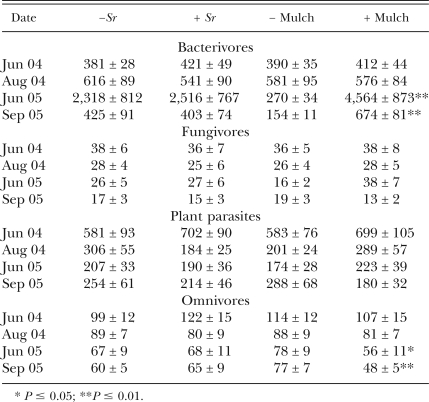
Among 58 nematode genera identified in the study, 22 were bactivorous, 8 mycophagous, 12 phytoparasitic and 12 omnivorous. In addition, several species were identified only to the level of the family Rhabditidae. The application of EPN had few measurable effects on the numbers of nematodes at any trophic level (Table 1). Between June (pre-treatment) and August (post-treatment) 2004, the average number of nematodes in plots treated with S. riobrave declined by 35% (1,281 to 829) compared to just 6% (1,102 to 1,040) in untreated plots (P < 0.05). Plant-parasitic nematodes accounted for much of the difference, declining by 23% vs. 61% (P < 0.06) in untreated and S. riobrave-treated plots, respectively (Table 1). A similar trend was observed for omnivorous nematodes that declined by 10% in control plots compared to 35% in S. riobrave-treated plots (P = 0.10). Bactivorous nematodes increased by 44%, and mycophagous nematodes declined by 29% during this time, but without evidence of effects by S. riobrave augmentation (P > 0.10). The numbers of T. semipenetrans, which represented 93% of the plant parasites in August 2004, were 35% lower in S. riobrave-treated than in untreated plots that month (NS; P > 0.10); by May 2005, following a period of citrus nematode population growth, S. riobrave-treatraent had reduced the numbers of this nematode by 63% (P < 0.05; Fig. 2E).
Table 1.
Effects of augmenting soil with Steinernema riobrave (Sr) and mulching with composted animal manures on the numbers of nematodes per 100 cm3 soil (mean ± standard error) in different trophic groups during 2 yr.
Effects of mulch: The average soil moisture, measured on two dates each year, was 60% (gram water per 100 gram dry soil) higher in the mulched plots (7.7% ± 0.37%; mean and standard error) than that in the bare plots (4.8% ± 0.47%; P < 0.001) in 2005, but moisture was unaffected by mulching in 2004 (mulch = 6.0% vs. bare = 5.7%).
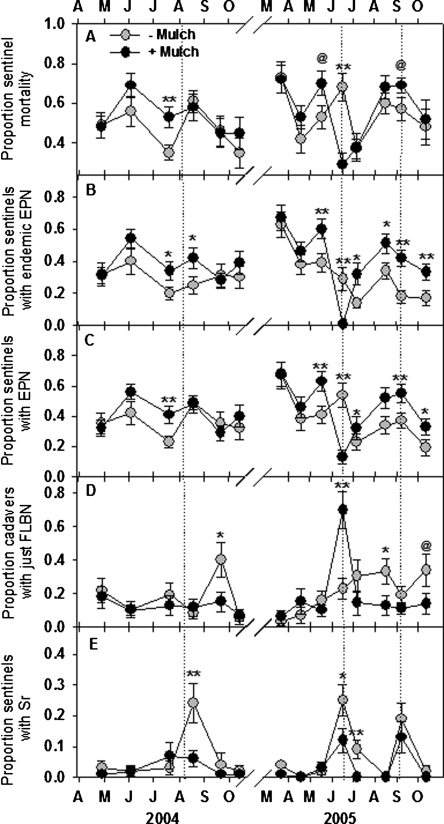
The application of composted chicken manure in June 2005 reduced the prevalence of EPN dramatically and decreased the mortality of sentinel weevils to less than half that in bare plots (P < 0.01; Fig. 3A-C). However, the effect was brief, and sentinel mortality tended to be higher in mulched plots on several other sampling dates. The AUC for percent sentinel mortality, excluding June 2005, was 16% greater (P = 0.05) in mulched than in non-mulched plots. Effects of mulch on EPN may have contributed to increased sentinel mortality in mulched plots. The prevalence of endemic EPN (S. riobrave excluded) was consistently greater in mulched plots (Fig. 3B), as was the prevalence of all EPN (S. riobrave included; Fig. 3C), even though mulch impeded infection of sentinels by exotic S. riobrave (Fig. 3D). Excluding June 2005, the AUC for total EPN and endemic EPN was 28% (P < 0.05) and 43% (P < 0.01) greater, respectively, in mulched than in non-mulched plots. The proportion of cadavers containing FLBN increased immediately following the application of chicken manure (P < 0.01), but otherwise was lower in mulched plots (P < 0.05) on several occasions across both years (Fig. 3E). The mean proportion of cadavers containing FLBN in each plot during yr 2 was higher (P < 0.01) in bare soil treated with S. riobrave (proportion = 0.28) than in either non-treated bare soil (0.12), or mulched soil treated (0.10) or not treated (0.16) with S. riobrave (data not shown).
Fig. 3.
The effects of composted animal manure applied as a mulch beneath citrus trees on the mortality of Diaprepes abbreviates sentinel larvae (A) and the prevalence of endemic entomopatho-genic nematodes (EPN) (B), endemic plus exotic EPN (C), sentinel cadavers containing only FLBN (D) and prevalence of Steinernema riobrave (Sr) (E). Half of the plots in each treatment were treated with S. riobrave, and the remaining plots remained untreated. Treatment differences according to two-way analysis of variance of transformed data (arcsin, square-root) are denoted by @ (P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Error bars are standard errors of means. Composted horse manure was applied in March 2004, and composted chicken manure was applied in the same plots on 10 June 2005. Dotted lines indicate S. riobrave application dates.
The composted manure mulches consistently suppressed trapping NF (Fig. 4A), but effects on endopara-sitic NF varied over time (Fig. 4B). The prevalence of endoparasitic NF increased from 2004 to 2005 and tended to be lower (P < 0.10) in mulched plots until late in the 2005 summer when endoparasitic NF from mulched plots infected greater numbers of EPN (P < 0.01) in the bioassays. A similar pattern occurred for enchytraeid worms which were consistently suppressed in mulched plots until the trend was reversed after midsummer 2005 (Fig. 4C). Fewer microarthropods (both mites and collembola) were recovered from mulched plots (P = 0.05) in April and June 2005 (Fig. 4D). Between July and September 2005, 99% fewer T. semipenetrans (P < 0.05) were recovered from mulched than from bare plots (Fig. 4E).
Fig. 4.
The effects of composted animal manure applied as a mulch beneath citrus trees on bioassay measurements of the numbers of entomopathogenic nematodes killed by trapping nematophagous fungi (NF) (A) or endoparasitic NF (B), the numbers of enchytraeid worms (C) and mites and collembola (D) and Tylenchulus semipen-etrans (E) in assay plates. Half of the plots in each treatment were treated with S. riobrave, and the remaining plots remained untreated. Treatment differences according to two-way analysis of variance of transformed data (arcsin, square-root) are denoted by @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Error bars are standard errors of means. Composted horse manure was applied in March 2004, and composted chicken manure was applied in the same plots on 10 June 2005. Dotted lines indicate S. riobrave application dates.
Horse manure mulch did not affect total numbers of nematodes in soil samples in 2004, but the application of composted chicken manure in 2005 increased the numbers of bacterivorous nematodes by more than 16-fold and total nematodes by 9-fold in June 2005 (P = 0.01; Table 1). Total nematode numbers and numbers of bacterivorous nematodes remained 70% and 338% higher (P = 0.01), respectively, in mulched than in bare plots in September 2005. Omnivorous nematodes in mulched plots were 72% (P = 0.05) and 63% (P = 0.01) as numerous as in bare plots in June and September 2005, respectively. Despite the effects of the chicken manure mulch on T. semipenetrans, mulches did not affect total numbers of plant-parasitic nematodes or my-cophagous nematodes on any measurement date.
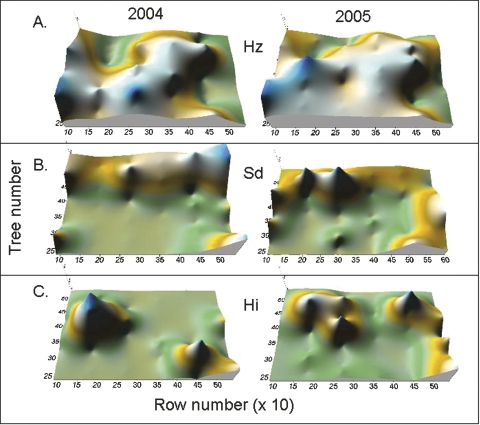
Spatial and temporal patterns of EPN and NF: The endemic EPN community was dominated by Heterorhabdi-tis zealandica which infected 25 ± 3% (95% CI) and 27 ± 3% of sentinels during 2004 and 2005, respectively, compared to 5 ± 1 % and 5 ± 1 % by Steinernema diaprepesi and 4 ± 2% and 4 ± 1% by H. indica Poinar, Karunaka and David. Heterorhabditis zealandica, S. diaprepesi or H. indica were detected in sentinel weevils in 37, 28 and 18 plots, respectively, during the experiment. The endemic EPN species tended to predominate in mutually exclusive areas within the boundaries of the experiment (Fig. 5A-C). The species-specific enclaves were relatively stable over time; correlation coefficients for the average prevalence of a species in a plot in 2004 compared to 2005 were 0.62 (n = 40; P < 0.001), 0.45 (P = 0.004) and 0.19 (NS) for H. zealandica, S. diaprepesi and H. indica, respectively.
Fig. 5.
Contour plots of the average prevalence during 2004 and 2005 of Heterorhabditis zealandica (Hz); Steinernema diaprepesi (Sd) and H. indica (Hi) in six rows of trees contained within a 0.6 ha area of citrus grove near Bartow, FL. Axes are not drawn to scale in order to emphasize spatial patterns.
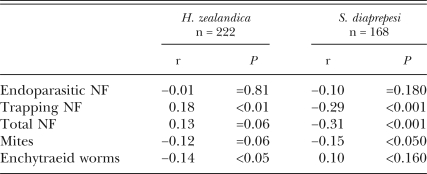
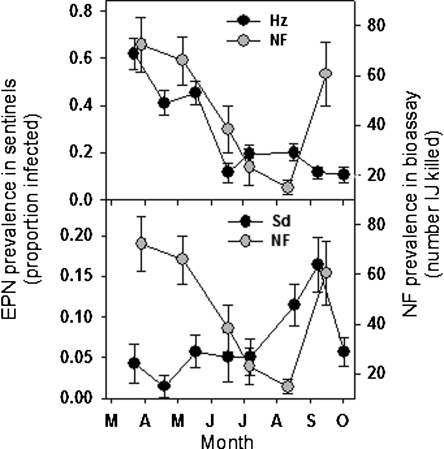
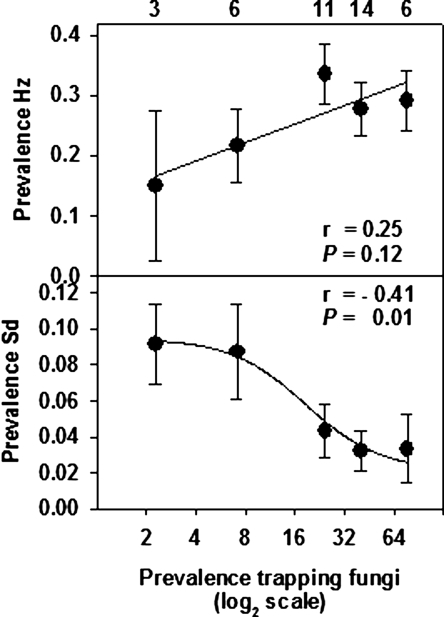
The prevalence per plot of H. zealandica, measured on six sampling dates in 2005, was directly related (P ≤ 0.008) to the prevalence of trapping NF on the preceding sampling date, whereas S. diaprepesi prevalence was inversely related (P ≤ 0.001) to prior NF prevalence (Table 2; Fig. 6A,B). The prevalence of both H. zealandica and S. diaprepesi was inversely related to the numbers of mites in preceding samples, and there was also a negative relationship between enchytraeid worms and the subsequent prevalence of H. zealandica. The average prevalence per plot of NF in 2005 was also spatially related to that of S. diaprepesi (r = −0.41; n = 40; P = 0.01), but not to that of H. zealandica (Fig. 7A,B).
Table 2.
Linear correlation coefficients between the prevalence of species of nematophagous fungi (NF), enchytraeid worms and soilborne mites recovered each month in 2005 and the prevalence of Heterorhabditis zealandica and Steinernema diaprepesi measured in the subsequent sampling period. All numbers were transformed to log (X + 1) and proportions to arcsin, square-root, prior to analysis. Data from plots in which an entomopathogenic nematode species was never detected (three plots for H. zealandica and 12 plots for S. diaprepesi) were excluded from the analyses for that species.
Fig. 6.
Prevalence in bioassays of nematophagous fungi (NF) and of sentinel weevils infected by Heterarhabditis zealandica (Hz) (A) or Steinernema diaprepesi (Sd) (B) during March through October 2005 in a Florida citrus orchard.
Fig. 7.
Spatial relationships between the average prevalence during 2005 of unidentified nematophagous fungi and heterorhabditid nematodes (A) or Steinernema diaprepesi (B) beneath 40 citrus trees in a 0.6 ha area of a Florida citrus orchard. Error bars are standard errors of means. Data were pooled for illustration (n shown at top of figure) according to unit values of the log-transformed fungal prevalence per plot.
The average proportions of cadavers each month that contained Paenibacillus spp. and either S. diaprepesi or H. zealandica were 0.25 (range 0.0–0.50) and 0.34 (0.13–0.55), respectively. The proportion of cadavers that contained both S. diaprepesi and Paenibacillus sp. was unrelated to the prevalence of S. diaprepesi in the succeeding month (r = 0.03, n = 58, P = 0.80; data not shown). However, prevalence of H. zealandica was positively related (r = 0.16, n = 168, P ≤ 0.05) to the proportion of cadavers the preceding month that contained both H. zealandica and Paenibacillus sp.
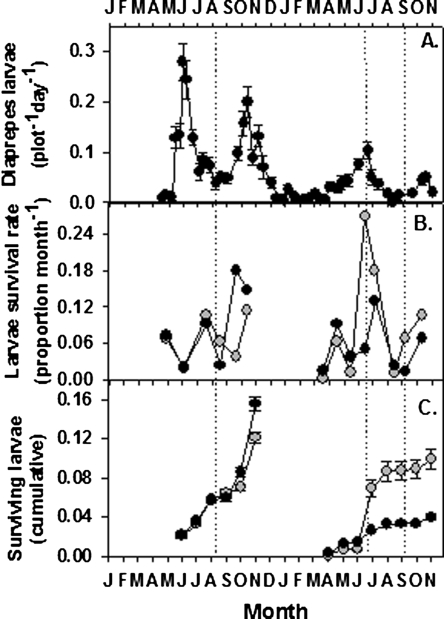
Weevil recruitment and estimated survival: The average number of neonate weevils caught in funnel traps in 2004 (0.091/plot/d) was more than 3-fold the number caught in 2005 (0.028/plot/d) (P ≤ 0.001; Fig. 8A). The rate of weevil recruitment into soil each year was distributed bimodally; numbers began to increase in May and September both years, and peaks were centered on June and October. The numbers of captured weevil larvae were unaffected by the experimental treatments.
Fig. 8.
The average numbers of Diaprepes abbreviatus neonate larvae per plot per day caught in funnel traps during 2004 to 2005 (A) and the estimated survival rates per month of larvae in soil (B) that was used to estimate (from eq. 1) the cumulative numbers of weevils that survive predation by entomopathogenic nematodes in plots augmented or non-augmented with Steinernema riobrave once in 2004 and twice in 2005 (C). Nematode treatment dates are indicated by dotted lines.
The estimated monthly survival rate of weevils following EPN augmentation in 2004 did not differ greatly among EPN-treated and untreated plots except in September (Fig. 8B), when 18% of larvae were estimated to survive in EPN-treated plots compared to only 4% in untreated control plots (Fig. 1A). Despite lower survival of sentinels due to EPN treatment in August 2004 (0.31/wk), survival was also relatively low (0.50/wk) in untreated plots that month (Fig. 1A). The effect of compounding these low weekly survival rates resulted in similar monthly survival rates in both EPN-treated (0.02/mon) compared to untreated (0.06/mon) plots in August (Fig. 8B). The cumulative numbers of weevils estimated to have survived in 2004 was slightly higher in EPN-augmented compared to non-augmented plots (Fig. 8C). By contrast, larvae survival rates in 2005 differed between treatments only during the months when EPN were augmented (Fig. 8B). The two EPN treatments in 2005 were estimated to reduce cumulative larvae survival to less than 40% of that in untreated plots (Fig. 8C).
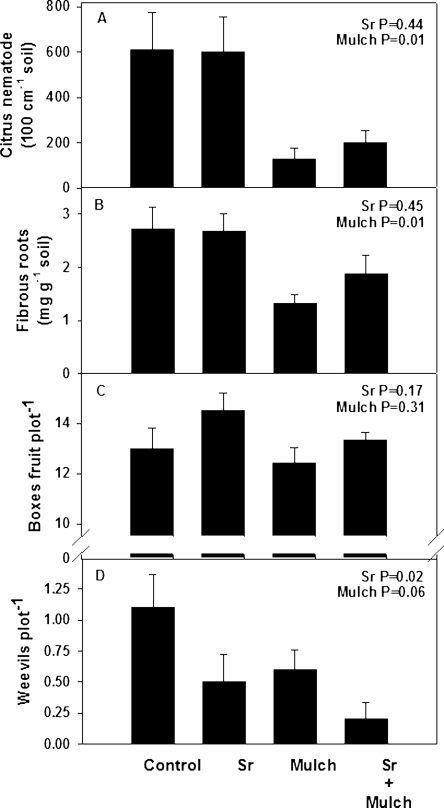
Long-term treatment effects: In February 2006, 8 mon after the application of chicken manure, numbers of juvenile T. semipenetrans remained 3.6-fold higher (P = 0.001) in bare plots (606 ± 110/100 cm3 soil; mean ± s.e.) than in mulched ones (165 ± 25) (Fig. 9A). Much of the mulch effect on the nematode can be attributed to fibrous root mass density, which was 45% less (P ≤ 0.01) in the mulched (1.48 ± 0.20 mg/g soil) than in the bare plots (2.70 ± 0.25 mg/g soil) (Fig. 9B). Neither mulch nor augmentation of EPN affected fruit yield harvested in June 2006 (Fig. 9C). During 12 mon between October 2005 and 2006, only 23 adult weevils emerged from soil to be caught in the ground traps. Half as many adult weevils were trapped in mulched as in bare soil (P = 0.10), and EPN augmentation reduced the recovery of adults by 56% (P = 0.02) (Fig. 9D).
Fig. 9.
The effects of Steinernema riobrave and composted animal manure mulches on the numbers of Tylenchulus semipenetrans in soil (A) and the mass density of citrus fibrous roots (B) on 2 February 2006, the numbers of 41-kg boxes of citrus fruit per plot on 2 June 2006 (C) and the numbers of adult citrus root weevils emerging from soil during one year beginning in October 2005 (D).
Discussion
Adding EPN to soil at rates recommended for control of D. abbreviatus directly or indirectly affected the prevalence of organisms at several trophic levels. Some of the temporal and spatial relationships in the data were consistent with our hypothesis that EPN augmentation can initiate cascades with successive bottom-up (population growth by natural enemies of nematodes) and top-down (greater predation of EPN) non-target effects that result in fewer EPN and reduced biological control. The mortality of sentinel weevils did not differ between the designated EPN treatments until the first application of S. riobrave in August 2004. Despite higher sentinel mortality in EPN-amended plots in August, EPN prevalence did not increase concomitantly. Rather, a disproportionately large number of cadavers in S. riobrave-amended plots produced FLBN, suggesting that S. riobrave was less competitive with FLBN than were some endemic EPN (Duncan et al., 2003a). In addition to interference by nematode competitors, results of the NF bioassay suggest that EPN in the augmented plots suffered higher predation by trapping fungi soon after the application of S. riobrave. One month following S. riobrave application (September 2004), EPN prevalence and sentinel weevil mortality were both reduced by approximately 40% in S. riobrave-amended compared to unamended plots. Non-target effects were also evident among omnivorous and plant-parasitic nematodes, microarthropods and enchytraeid worms, all of which were significantly reduced by the single application of S. riobrave in 2004.
The responses of the soil food web to the initial application of EPN were dramatic, but also ephemeral and inconsistent. Although the numbers of EPN and T. semipenetrans in S. riobrave-treated plots remained somewhat lower than normal through May 2005, population levels of most organisms affected by treatment with S. riobrave in 2004 returned to background (untreated) levels more quickly. Moreover, significant non-target effects on EPN did not occur following either of two S. riobrave applications in 2005, and the subsequent emergence of adult weevils from soil in S. riobrave-treated plots (October 2005–September 2006) was reduced by more than half, a level similar to previous reports of the effectiveness of S. riobrave against D. abbreviatus (Bullock et al., 1999; Duncan et al., 1999, 2002, 2003b). Although weevil emergence was not monitored in the first year of the study, the between-year variation in non-target effects suggests that EPN antagonists likely modulated treatment efficacy more in some years than others. Thus, despite the short-term effectiveness of S. riobrave against sentinels in both years of the study, net efficacy may have been lower in 2004 because (i) S. riobrave was applied at a time when endemic EPN were highly active, killing 50% of sentinels per week and (ii) a subsequent period of lower than normal EPN prevalence in S. riobrave-treated plots coincided with a peak of neonate weevil recruitment into soil. Biocontrol by endemic EPN appears to be especially important in the deep sandy soils of Florida's central ridge (Duncan et al., 2003b); however, the non-target effect of augmentation noted in this orchard should be of no importance in many agricultural systems that support fewer endemic EPN. Moreover, scheduling EPN augmentation to follow, rather than closely precede, anticipated peaks of larval recruitment would minimize the potential for augmentation to temporarily interfere with biological control.
The post-application biology of EPN merits greater attention given the evident potential of these nema-todes as biocontrol agents and the complexity of soil food webs (Curran, 1993; Kaya and Koppenhofer, 1996). Few studies have documented the relative contributions of competitors and antagonists to the survival and efficacy of EPN. Although augmenting EPN with steinernematids and heterorhabditids in pasture had no effect on NF prevalence (Forschler and Gardner, 1991), Jaffee and Strong (2005) found that some NF species increased by more than 100-fold in the vicinity of insect cadavers following EPN emergence. Similarly, pretreating soil with EPN in laboratory experiments increased NF and reduced the survival of a second cohort of EPN added to the soil seven days later compared to EPN added to non-pretreated soil (El-Borai et al., 2007). The inverse spatial and temporal relationships between the prevalence of NF and S. diaprepesi are additional evidence that NF may have regulated some EPN during the present study. In contrast to S. diaprepesi, H. zealandica was spatially independent of NF and positively correlated with NF in time. These relationships could have resulted if H. zealandica benefited from greater predation by NF on steinernematid competitors of H. zealandica than on H. zealandica itself. The tendency of heterorhabditid IJ to retain the second-stage cuticle as a sheath affords them protection from some NF, whereas steinernematid IJ are more susceptible prey because they readily lose the sheath while migrating in soil (Timper and Kaya, 1989, 1992). The population density of Arthrobotrys oligospora increased 10-fold more in the vicinity of insect cadavers infected by S. glaseri compared to cadavers infected by H. marela-tus (Jaffee and Strong, 2005). As with heterorhabditids in the present study, no spatial relationships were evident between H. marelatus and 12 species of nema-tophagous fungi isolated from sites at Bodega Bay in California (Jaffee et al., 1996b). Nevertheless, the likelihood that NF detection efficiencies are method- and species-dependent and the possibility that growth of fungi in the assays does not reflect predatory activity in the soil are well noted caveats against viewing these correlations as compelling evidence that NF affect heterorhabditid prevalence less than that of steinernematids (Jaffee et al., 1996b; Jaffee and Strong, 2005).
Unlike the numerical responses by some NF to EPN augmentation, it is less evident why the numbers of enchytraeid worms and microarthropods were sometimes temporarily suppressed and occasionally increased by EPN augmentation. Rahayu (1983; as given by Poinar, 1989) found that augmentation of S. carpo-capsae in sugar beet fields reduced the numbers of col-lembola and that the nematode was able to infect the collembolan Onychiurus armatus in the laboratory. Edwards and Oswald (1981) also reported suppression of collembola and mites by S. carpocapsae in sugar beet. They noted that populations returned to normal within one month, which may be why neither S. carpocapsae nor H. bacteriophora affected mite or collembolan numbers measured one month after adding the EPN to turf-grass (Klein and Georgis, 1992). The feeding habits of microarthropods are diverse, and individual species can likely respond to any part of the food web affected by EPN treatments. Inadequate taxonomic resolution of the microarthropod community in this and other studies undoubtedly obscured important interactions. For example, nematophagous mites and collembolans can respond directly to EPN augmentation with population growth (Ishibashi et al., 1987; Epsky et al., 1988). Forschler and Gardner (1991) reported that numbers of nematophagous mites (mainly Rodacaridae) more than doubled during four weeks in response to EPN augmentation in pasture, whereas total numbers of microarthropods were unaffected. Although little is known about the relative contribution of microarthropods as predators of EPN, both mites and collembola can reduce EPN efficacy in microcosms (Epsky et al., 1988; Gilmore and Potter, 1993), and the abundance of both mites and collembola near the soil surface was inversely related to survival of H. bacteriophora applied to turf (Wilson and Gaugler, 2004).
Enchytraeid worms feed on microbe-colonized organic matter, performing a role in decomposition and mineralization similar to that of microbivorous nema-todes (Didden, 1993). EPN augmentation may have affected enchytraeids indirectly through changes in the microbial or microarthropod community. Both enchytraeids and microarthropods were suppressed by EPN augmentation in 2004. Those results suggest that the two communities may have been affected independently because mixed communities of microarthropods reduced enchytraeids and nitrogen mineralization in microcosms at low and moderate but not high water potential (Sulkava et al., 1996; Huhta et al., 1998). Because treatments with just predatory mites did not affect enchytraeids, Huhta et al. (1998) speculated that mite communities competitively displaced enchytraeids in drier soils. Numbers of enchytraeids increased in response to soil amendments of the NF Hirsutella rossilin-iensis, and the fungus grew better in soil from which enchytraeids and other mesofauna were mechanically excluded (Jaffee et al., 1996a, 1997); however, the possibility that enchytraeids suppress NF was not supported by subsequent research (Jaffee, 1999).
El-Borai et al. (2005) and Enright and Griffin (2005) demonstrated that Paenibacillus spp. spore attachment to the EPN cuticle can reduce the motility of steinerne-matids and heterorhabditids and the rate at which hosts are infected. Although cadavers with Paenibacillus adhering to EPN were encountered frequently in this study, we found no evidence that this bacteria-nematode symbiosis impairs the population growth of EPN in the field. Indeed, the positive association between prevalence of cadavers with Paenibacillus and prevalence of H. zealandica one month later is at variance with the hypothesis that the bacterium may adversely affect EPN population growth. These results should be interpreted cautiously, however, because detection of the bacteria depended on detection of the EPN in sentinel larvae. High numbers of Paenibacillus spores in soil with few EPN would not necessarily be detected by our method. Molecular probes or bioassays designed more specifically to detect Paenibacillus independently of resident EPN are required to fully evaluate the potential for these bacteria to regulate EPN populations.
The intended role of the mulch treatment in this study was to produce a habitat with different soil community structure than that of bare soil. The objective was to compare the effects of EPN augmentation under conditions that we predicted would increase the prevalence of EPN competitors and antagonists. Specifically, we predicted that non-target effects of augmenting EPN would increase in mulched plots if mulches increased FLBN and, consequently, NF (Linford et al., 1938; Wang et al., 2002). To insure a sustained effect on the organisms in a soil comprised of >96% sand, we applied unrealistically high rates of mulch. Our soil samples revealed no effect of horse manure mulch on FLBN in 2004, whereas chicken manure had the intended effect of increasing FLBN and, to a lesser extent, total nematodes from June to September 2005. However, despite the long period of elevated nematode numbers in 2005, the bioassay results suggested that trapping NF were consistently less prevalent in mulched plots in both years, in contrast to our expectation. Neither did endoparasitic NF increase in response to elevated numbers of nematodes during the first two months after composted chicken manure was applied. The substantial increase of endoparasitic NF in August and September 2005 in composted but not bare plots may have been a delayed response by the motile zoospore stage of these fungi to elevated soil moisture and/or nematode numbers. Given the dramatic suppression of EPN, T. semipenetrans, enchytraeids and microarthropods immediately following application of chicken manure, it is likely that degradation and leaching of harmful compounds in the composted manure was necessary before endoparasitic NF could respond to favorable aspects of the mulch treatment. Also, in contrast to our expectation, higher numbers of FLBN in mulched plots in 2005 did not result in a greater percentage of cadavers containing only FLBN except in June, immediately after the chicken manure was applied. Indeed, mulch suppressed the two-year average proportion of cadavers containing FLBN. However, the mulch treatment's main effect resulted from a significant partial effect of S. riobrave treatment that increased the incidence of cadavers with FLBN in bare soil, but not mulched soil. This result suggests that the mulch effect was due to the reduced presence of S. riobrave in mulched plots and further supports the possibility that some FLBN compete more effectively with S. riobrave than with some endemic EPN in cadavers of D. abbre-viatus (Duncan et al., 2003a). Deleterious effects of compounds in the chicken manure may have temporarily reduced the competitive abilities of endemic EPN in June 2005.
Whereas fresh manure from cattle and particularly chickens had deleterious effects on the virulence of S. carpocapsae to black cutworm and greater wax moth in the field, composting those materials rendered them non-detrimental (Shapiro et al., 1999). Our results were more similar to those of Bednarek and Gaugler (1997), who reported 3-fold higher numbers of endemic S. feltiae in field plots amended in alternate years for 20 years with 20 to 30 tons of animal manure/ha compared to unamended plots. Bednarek and Gaugler (1997) attributed the positive effect of manure on EPN to a presumed increase in numbers of soil-inhabiting insects in amended plots. Their hypothesis is an equally plausible explanation for the behavior of EPN in the present study. Additionally, the suppression of trapping NF in the mulched plots may have reduced predation on EPN sufficiently to result in higher EPN prevalence. The NF Hirsutella rossiliensis parasitized fewer plant-parasitic nematodes if soil was amended with composted chicken manure in field plots and laboratory assays (Jaffee et al., 1994). However, endoparasitic and trapping NF responded to chicken manure mulch differently from one another in the present study, and our methods could not reveal the relative predation rates by various NF. Some NF respond to environmental factors with strong saprophytic growth, but exhibit little trapping activity (Jaffee, 2004). Our assay was designed to favor the detection of predation rates rather than prop-agule density by transferring nematodes rather than soil or soil suspensions from the field plots onto the agar plates and by quantifying EPN cadavers with identifiable fungi rather than NF colonies. Nevertheless, it is an indirect, qualitative assay of predation that occurs in artificial conditions. If EPN in the present study were partly regulated by NF, the fact that EPN were favored by mulch suggests that an apparent inhibitory effect of mulch on trapping NF may have been more important than an apparent stimulatory effect of mulch on endo-parasitic NF. Whatever the mechanism, EPN in mulched plots appear to have increased sufficiently to provide greater biological control of D. abbreviatus than occurred in plots with bare soil. Our results support the suggestion by Bednarek and Gaugler (1997) that use of animal manures may represent a useful tactic for conservation biological control.
Data from this site revealed the presence of stable, mutually exclusive EPN species enclaves. The 40 plots in this experiment were arranged within a 0.6 ha untreated control plot of a previous experiment (Duncan et al., 2003b). During the two years that endemic EPN were monitored in the prior experiment, S. diaprepesi was the dominant species comprising 53% and 66% of the endemic EPN recovered from all plots each year compared to 30% and 23% that were H. zealandica. However, within the control plot that encompassed the current experiment, 74% and 75% of the recovered EPN were H. zealandica during 2000 and 2001, respectively. During 2004 and 2005, H. zealandica still accounted for 64% and 69%, respectively, of the EPN recovered from the 20 plots that were not treated with S. riobrave. Moreover, the spatial patterns of individual EPN species within the current site were significantly similar from one year to the next. The dominance of H. zealandica in this specific area of the orchard was not due to the absence of other EPN species, because endemic species other than H. zealandica were recovered from all of the plots. Neither are there obvious edaphic factors that might favor H. zealandica because the deep, sandy (>97%) soil is uniform throughout the orchard. The apparent stability of species enclaves at this site should reinforce the point that the treatment effects pertain primarily to a single heterorhabditid species. Had the experiment been comprised of a predominantly S. diaprepesi enclave, trophic cascades in response to our treatments may have affected the EPN community differently.
Inconsistency is often cited as a main impediment to wider adoption of EPN for biological control (Lewis, 2002). The use of Steinernema riobrave to augment EPN communities is promising in this regard. In this and other experiments on Florida's Central Ridge, S. riobrave consistently and effectively killed D. abbreviatus sentinel larvae for a brief time following its application (Duncan and McCoy, 1996; Duncan et al., 1996a, 1996b, 2002, 2003b). Steinernema riobrave also reliably suppressed pink bollworm (Pectinophora gossypiella) in cotton and would likely be utilized for pink bollworm control were it not for the introduction of highly effective, inexpensive cotton cultivars transformed to express toxin genes from Bacillus thuringiensis (Gouge et al., 1997; Shapiro-Ilan et al., 2002). Although EPN augmentation has provided consistent results in these two pest systems, each system responds to augmentation in fundamentally different ways. Augmentation with S. riobrave performed well as a stand-alone tactic against pink bollworm, because a properly timed application can affect the entire pink bollworm population that overwinters in soil. In contrast, use of EPN to manage D. abbreviatus is generally supplemented by other IPM tactics because the root weevil population resides above-and below-ground throughout the year (McCoy et al., 2003). Non-target effects of EPN augmentation would have little impact on pink bollworm control because endemic EPN are not involved in biocontrol after the insects emerge from soil to reside in the foliage and bolls. However, non-target effects that cause a reduction in biological control of Diaprepes weevils entering soil subsequent to EPN augmentation could be a matter for concern in those regions where biological control of weevils by endemic EPN is ordinarily high.
Footnotes
This research was supported by the Florida Agricultural Experiment Station, including CRIS project LAL-04324 and grants from USDA-CSREES T-STAR 2003-34135-14078 and the Florida Citrus Production Research Advisory Coun-cil_042-10E.
This paper was edited by Gregor Yeates.
Literature Cited
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Bednarek A, Gaugler R. Compatibility of soil amendments with entomopathogenic nematodes. Journal of Nematology. 1997;29:220–227. [PMC free article] [PubMed] [Google Scholar]
- Bullock RC, Pelosi RR, Killer EE. Management of citrus root weevils (Coleoptera: Curculionidae) on Florida citrus with soil-applied entomopathogenic nematodes (Nematoda: Rhabditida) Florida Entomologist. 1999;82:1–7. [Google Scholar]
- Curran J. Post-application biology of entomopathogenic nematodes in soil. In: Bedding R, Kaya H, editors. Nematodes and the biological control of insect pests. East Melbourne, Australia: CSIRO Publications; 1993. pp. 225–240. [Google Scholar]
- Didden WAM. Ecology of terrestrial Enchytraeidae. Pedobiologia. 1993;37:2–29. [Google Scholar]
- Duncan LW, Dunn DC, Bague G, Nguyen K. Competition between entomopathogenic and free-living bactivorous nematodes in larvae of the weevil Diaprepes abbreviatus . Journal of Nematology. 2003a;35:187–193. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Dunn DC, McCoy CW. Spatial patterns of entomopathogenic nematodes in microcosms: Implications for laboratory experiments. Journal of Nematology. 1996a;28:252–258. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Dunn DC, Zellers J, McCoy CW, Nguyen K. Incidence of endemic entomopathogenic nematodes following application of Steinernema riobrave for control of Diaprepes abbreviatus . Journal of Nematology. 2003b;35:178–186. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Zellers J. Profitability of applications of Steinernema riobrave, metalaxyl and supplemental fertilisation for management of Diaprepes abbreviatus and Phytophthora nicotianae in a Florida citrus orchard. Fourth International Congress of Nematology. 2002;4:192. (Abstr.). [Google Scholar]
- Duncan LW, McCoy CW. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Cur-culionidae) of two species of entomogenous nematodes (Rhabditida: Steinernematidae; Heterorhabditidae) Environmental Entomology. 1996;25:174–178. [Google Scholar]
- Duncan LW, McCoy CW, Stansly PA, Graham JH, Mizell RF. Estimating the relative abundance of citrus root weevils with modified Tedders traps. Environmental Entomology. 2001;30:939–946. [Google Scholar]
- Duncan LW, McCoy CW, Terranova AC. Estimating sample size and persistence of entomogenous nematodes in sandy soils and their efficacy against the larvae of Diaprepes abbreviatus in Florida. Journal of Nematology. 1996b;28:56–67. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Shapiro DI, McCoy CW, Graham JH. Entomopathogenic nematodes as a component of citrus root weevil IPM. In: Polavarapu S, editor. Proceedings of workshop on optimal use of insecticidal nematodes in pest management; Rutgers University; August 28–30; New Brunswick, NJ. 1999. pp. 69–78. [Google Scholar]
- Edwards CA, Oswald J. Proceedings, 1981 British crop protection conference on pests and diseases. vol. 2. Croydon, England: BCPC Publications; 1981. Control of soil-inhabiting arthropods with Neoaplectana carpocapsae ; pp. 467–473. [Google Scholar]
- El-Borai FE, Brentu CF, Duncan LW. Augmenting entomopathogenic nematodes in soil from a Florida citrus orchard: Non-target effects of a trophic cascade. Journal of Nematology. 2007 in press. [PMC free article] [PubMed] [Google Scholar]
- El-Borai FE, Duncan LW, Preston JF. Bionomics of a phoretic association between Paenibacillussp. and the entomopathogenic nematode Steinernema diaprepesi . Journal of Nematology. 2005;37:18–25. [PMC free article] [PubMed] [Google Scholar]
- Enright MR, Griffin CT. Effects of Paenibacillus nema-tophilus on the entomopathogenic nematode Heterorhabditis megidis . Journal of Invertebrate Pathology. 2005;88:40–48. doi: 10.1016/j.jip.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Epsky ND, Walter DE, Capinera JL. Potential role of nematophagous microarthropods as biotic mortality factors of entomogenous nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) Journal of Economic Entomology. 1988;81:821–825. [Google Scholar]
- Forschler BT, Gardner WA. Field efficacy and persistence of entomogenous nematodes in the management of white grubs (Coleoptera: Scarabaeidae) in turf and pasture. Journal of Economic Entomology. 1991;84:1454–1459. [Google Scholar]
- Futch SH, Duncan LW, Zekri M. Validation of an area-wide extension program to estimate the seasonal abundance of adult citrus root weevils with un-baited pyramidal traps. Proceedings of the Florida State Horticultural Society. 2005;117:143–147. [Google Scholar]
- Gilmore SK, Potter DA. Potential role of collembo-lan as biotic mortality agents for entomopathogenic nematodes. Pedobiologia. 1993;37:30–38. [Google Scholar]
- Gouge DH, Smith KA, Payne C, Lee LL, Van Berkum JR, Ortega D, Henneberry TJ. Control of pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) with biocontrol and biorational agents. In: Dugger P, Richter DA, editors. Proceedings of the Beltwide cotton production research conference. Memphis, TN: National Cotton Council of America; 1997. pp. 1066–1072. [Google Scholar]
- Huhta V, Sulkava P, Viberg K. Interactions between enchytraeid (Cognettia sphagnetorum), microarthropod and nematode populations in forest soil at different moistures. Applied Soil Ecology. 1998;9:53–58. [Google Scholar]
- Ishibashi N, Young F, Nakashima M, Abiru C, Haraguchi N. Effects of application of DD-136 on silkworm, Bombyx mori, predatory insect, Agriosphodorus dohrni, parasitoid, Trichomalus apanteloctenus, soil mites, and other nontarget soil arthropods, with brief notes on feeding behavior and predatory pressure of soil mites, tardigrades, and predatory nematodes on DD-136 nematodes. In: Ishibashi N, editor. Recent advances in biological control of insect pests by entomogenous nematodes in Japan. Sago, Japan: Ministry of Education, Culture, and Science, in Japanese, with English abstract and tables; 1987. pp. 158–164. [Google Scholar]
- Jaffee BA. Enchytraeids and nematophagous fungi in tomato fields and vineyards. Phytopathology. 1999;89:398–406. doi: 10.1094/PHYTO.1999.89.5.398. [DOI] [PubMed] [Google Scholar]
- Jaffee BA. Do organic amendments enhance the nematode-trapping fungi Dactylellina haptotyla and Arthrobotrys oligospora? . Journal of Nematology. 2004;36:267–275. [PMC free article] [PubMed] [Google Scholar]
- Jaffee BA, Ferris H, Stapleton JJ, Norton MVK, Muldoon AE. Parasitism of nematodes by the fungus Hirsutella rhossiliensis as affected by certain organic amendments. Journal of Nematology. 1994;26:152–161. [PMC free article] [PubMed] [Google Scholar]
- Jaffee BA, Muldoon AE, Westerdahl BB. Failure of a mycelial formulation of the nematophagous fungus Hirsutella rhossiliensis to suppress the nematode Heterodera schachtii . Biological Control. 1996a;6:340–346. [Google Scholar]
- Jaffee BA, Santos PF, Muldoon AE. Suppression of nematophagous fungi by enchytraeid worms: A field exclosure experiment. Oecologia. 1997;112:412–423. doi: 10.1007/s004420050327. [DOI] [PubMed] [Google Scholar]
- Jaffee BA, Strong DR. Strong bottom-up and weak top-down effects in soil: Nematode-parasitized insects and nematode-trapping fungi. Soil Biology and Biochemistry. 2005;37:1011–1021. [Google Scholar]
- Jaffee BA, Strong DR, Muldoon AE. Nematode-trapping fungi of a natural shrubland: Tests for food chain involvement. Mycologia. 1996b;88:554–564. [Google Scholar]
- Jenkins WR. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Disease Reporter. 1964;48:692. [Google Scholar]
- Kaplan DT, Davis EL. Improved nematode extraction from carrot disk culture. Journal of Nematology. 1990;22:399–406. [PMC free article] [PubMed] [Google Scholar]
- Kaya HK, Koppenhofer AM. Effects of microbial and other antagonistic organism and competition on entomopathogenic nematodes. Biocontrol Science and Technology. 1996;6:357–371. [Google Scholar]
- Klein MG, Georgis R. Persistence of control of Japanese Beetle (Coleoptera: Scarabaeidae) larvae with steinernematid and heterorhabditid nematodes. Journal of Economic Entomology. 1992;85:727–730. [Google Scholar]
- Lewis EE. Behavioral ecology. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI Publishing; 2002. pp. 205–223. [Google Scholar]
- Linford MB, Yap F, Oliveira JM. Reduction of soil populations of the root-knot nematode during decomposition of organic matter. Soil Science. 1938;45:127–141. [Google Scholar]
- McCoy CW, Shapiro DI, Duncan LW, Nguyen K. Entomopathogenic nematodes and other natural enemies as mortality factors for larvae of Diaprepes abbreviatus . Biological Control. 2000;19:182–190. [Google Scholar]
- McCoy CW, Stuart RJ, Nigg HN. Seasonal life stage abundance of Diaprepes abbreviatus in irrigated and non-irrigated citrus plantings in central Florida. Florida Entomologist. 2003;86:34–42. [Google Scholar]
- Peña JE, Ulmer B, Duncan R, McCoy C. Biological control of neotropical citrus root weevils. IOBC, Memorias IV congreso internacional de control biologico; CIAT; May 31-June 2, 2006; Palmira, Colombia. 2006. pp. 98–99. [Google Scholar]
- Peters A. The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Science and Technology. 1996;6:389–402. [Google Scholar]
- Poinar GO., Jr Non-insect hosts for the entomogenous rhabditoid nematodes Neoaplectana (Steinernematidae) and Heterorhabditis (Heterorhabditidae) Revue de Nematologie. 1989;12:423–428. [Google Scholar]
- Rahayu A. Zurich: Swiss Federal Institute of Technology; 1983. Neoaplectana carpocapsae Weiser (Nem. Steinernematidae); behavioral studies and field application. Ph.D. dissertation, [Google Scholar]
- Shapiro DI, Lewis LC, Obrycki JJ, Abbas M. Effects of fertilizers on suppression of black cutworm (Agrostis ipsilon) damage with Steinernema carpocapsae . Supplement to the Journal of Nematology. 1999;31:690–693. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhofer AM. Factors affecting commercial success: Case studies in cotton, turf and citrus. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI Publishing; 2002. pp. 333–355. [Google Scholar]
- Strong DR. Populations of entomopathogenic nematodes in foodwebs. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI Publishing; 2002. pp. 225–240. [Google Scholar]
- Sulkava P, Huhta V, Laakso J. Impact of soil faunal structure on decomposition and N mineralization in relation to temperature and moisture in forest soil. Pedobiologia. 1996;40:505–513. [Google Scholar]
- Timper P, Kaya HK. Role of the 2nd-stage cuticle of entomogenous nematodes in preventing infection by nematopha-gous fungi. Journal of Invertebrate Pathology. 1989;54:314–321. [Google Scholar]
- Timper P, Kaya HK. Impact of a nematode-parasitic fungus on the effectiveness of entomopathogenic nematodes. Journal of Nematology. 1992;24:1–8. [PMC free article] [PubMed] [Google Scholar]
- Wang K-H, Sipes BS, Schmitt DP. Crotalaria as a cover crop for nematode management: A review. Nematropica. 2002;32:35–57. [Google Scholar]
- Wilson M, Gaugler R. Factors limiting short-term persistence of entomopathogenic nematodes. Journal of Applied Entomology. 2004;128:250–253. [Google Scholar]