Abstract
Use of resistant cultivars is a desirable approach to manage the peanut root-knot nematode (Meloidogyne arenaria). To incorporate resistance into commercially acceptable cultivars requires reliable, efficient screening methods. To optimize the resistance screening protocol, a series of greenhouse tests were done using seven genotypes with three levels of resistance to M. arenaria. The three resistance levels could be separated based on gall indices as early as two weeks after inoculation (WAI) using 8,000 eggs of M. arenaria per plant, while four or more weeks were needed when 1,000–6,000 eggs/plant were used. High inoculum densities (over 8,000 eggs/plant) were needed to separate the three resistance levels based on eggs per gram of root within eight WAI. A gall index based on percentage of galled roots could separate the three resistance levels at lower inoculum levels and earlier harvest dates than other assessment methods. The use of eggs vs. second-stage juveniles (J2) as inoculum provided similar results; however, it took three to five more days to collect J2 than to collect eggs from roots. Plant age affected gall index and nematode reproduction on peanut, especially on the susceptible genotypes AT201 and D098. The genotypes were separated into their correct resistance classes when inoculated 10 to 30 days after planting, but were not separated correctly when inoculated on day 40.
Keywords: Arachis hypogaea, assessment date, host-plant resistance, inoculation date, inoculum level, inoculum type, Meloidogyne arenaria, method, peanut, resistance evaluation, root-knot nematode
The root-knot nematode Meloidogyne arenaria race 1 causes significant economic losses throughout the peanut (Arachis hypogaea) production regions of the world. In the southern U.S. peanut-producing areas (AL, FL, GA, SC and TX), this nematode decreases peanut yield by 3 to 15% annually (Koenning et al., 1999). Management of root-knot nematode can include crop rotation, application of nematicides and use of resistant cultivars. Few profitable rotation crops are available because of the wide host range of M. arenaria. Use of nematicides is problematic because of the short-term efficacy (Dickson and Hewlett, 1989; Culbreath et al., 1992) and the cost to growers. There is a need for improved root-knot nematode management strategies, and the use of nematode-resistant peanut cultivars would be an effective and inexpensive approach to prevent yield and quality losses to M. arenaria.
Over the past two decades, progress has been made in identification and breeding for nematode resistance in peanut. Several sources of moderate and high resistance have been identified from germplasm of A. hypogaea and wild species of peanut (Holbrook and Stalker, 2003). High levels of resistance in wild species have been introgressed into A. hypogaea, which led to registration of interspecific germplasm TxAG-6 and TxAG-7 (Simpson et al., 1993), GP-NC WS 5 and GP-NC WS 6 (Stalker et al., 2002) and NR 0812 and NR 0817 (Anderson et al., 2006). A backcrossing program was used to introgress the root-knot nematode resistance from TxAG-7 into peanut breeding populations (Starr et al., 1995) and resulted in the release of COAN and NemaTAM, which are highly resistant to M. arenaria and M. javanica (Simpson and Starr, 2001; Simpson et al., 2003). However, neither COAN nor NemaTAM has been widely grown by farmers due to their susceptibility to tomato spotted wilt virus (TSWV) and low oleic content (Simpson and Starr, 2001; Simpson et al., 2003).
The development of new peanut cultivars with resistance to root-knot nematodes will require reliable and efficient resistance screening techniques for identifying resistant progeny within segregating breeding populations. Greenhouse screening techniques to identify peanut germplasm with resistance to M. arenaria are available (Hussey and Boerma, 1981; Holbrook et al., 1983); however, the current evaluation methods can take up to 100 days before results are available (Holbrook et al., 2000a, 2000b) and are subject to high experimental error (Choi et al., 1999; Zhang et al., 2006). Thus, the objectives of this study were to: (i) determine the effects of inoculum type, inoculum level, inoculation date and assessment date on evaluating M. arenaria resistance in peanut, and (ii) optimize the resistance screening protocol used to identify root-knot nematode resistant peanut genotypes in the greenhouse.
Materials and Methods
Peanut genotypes: Seven peanut genotypes with different levels of resistance to M. arenaria were used in all experiments. The genotypes included two highly resistant cultivars, COAN and NemaTAM; three moderately resistant breeding lines, C209–6-37, C209–6-60 and D099; one susceptible cultivar, AT201; and one susceptible breeding line, D098.
Nematode inocula: Meloidogyne arenaria race 1, originating from a peanut field in Tifton, GA, was cultured alternately on tomato (Lycopersicon esculentum cv. Rutgers) or eggplant (Solanum melongena cv. Blackbeauty) and peanut (Arachis hypogaea cv. Georgia Green). Eggs for inoculum were extracted from tomato or eggplant roots by agitating in 0.05% NaOCl for 2 to 3 min (Hussey and Barker, 1973). The eggs were then collected and rinsed with tap water on nested 150- and 25-μm-pore sieves. To collect the second-stage juveniles (J2) for use as inoculum, infected tomato or eggplant roots were placed in hatching dishes and incubated in a mistchamber. The J2 were then collected using 150- and 25-μm-pore sieves once a day for 3 to 5 d. During the collection period, J2 were stored in a 1-cm aqueous suspension at 5°C prior to inoculation of peanut plants.
Resistance assessment: For all assessment methods, peanut plants were uprooted and washed clean of soil 2 to 10 wk after inoculation (WAI). Criteria used to evaluate resistance levels in peanut were: gall number, gall index 1, gall index 2, egg mass number, egg mass index and egg number per gram root. Gall index 1 was on a scale of 0 to 5 (Taylor and Sasser, 1978), where 0 = no galls; 1 = 1 to 2; 2 = 3 to 10; 3 = 11 to 30; 4 = 31 to 100; and 5 = more than 100 galls. Gall index 2 was also on a scale of 0 to 5, but it was based on the percentage of the root system with galls (Hussey and Janssen, 2002), where 0 = no galling; 1 = trace infection with a few small galls; 2 = ≤ 25% roots galled; 3 = 26 to 50%; 4 = 51 to 75%; and 5 = >75% roots galled. For the assessments based on root galling, the numbers of galls were counted, and the root systems were rated using the two indices. The roots were then placed in beakers containing approximately 300 ml of 0.05% phloxine B solution for 3 to 5 min to stain egg masses a bright red color so the number of egg masses per root system could be determined visually (Holbrook et al., 1983). Egg mass index was on a scale of 0 to 5 as described for gall index 1. Fresh root systems were weighed and then agitated in 1% NaOCl solution for 5 min to extract eggs. Eggs were collected on nested 150- and 25-μm-pore sieves and counted. Egg number per gram root was then calculated.
Inoculum level and harvest date: The experiment was a 4 × 7 × 4 factorial arrangement of treatments. There were four peanut genotypes in these experiments, including NemaTAM, C209–6-37, C209–6-60 and AT201. The seven inoculum levels were 0, 1,000, 2,000, 4,000, 6,000, 8,000 and 16,000 eggs/pot. The four assessment dates were 2, 4, 6 and 8 WAI. A split-plot treatment design was used with assessment dates as main plots. Subplots of inoculum level × genotype were randomized within six replicate main plots. Two seeds were planted in each 10 × 10-cm2 plastic pot filled with 800 cm3 steam-pasteurized (132°C for 6 hr) loamy sand (texture: 85% sand, 11% silt, 4% clay). After emergence, plants were thinned to 1 seedling/pot. Two holes about 5-cm deep and 1-cm wide were made in the soil around each 2-wk-old peanut seedling. A 2.5 ml aliquot of inoculum suspension was applied to each hole with a pipette. Unless otherwise noted, the plants were maintained in a greenhouse at 20 to 35°C and watered as needed. At harvest dates 1 (2 WAI) and 2 (4 WAI), resistance was assessed by gall number, gall index 1 and gall index 2. At harvest date 3 (6 WAI), gall number, gall index 1, gall index 2 and egg number per gram root were evaluated. At harvest date 4 (8 WAI), two additional variables, egg mass number and egg mass index, were also assessed. The entire experiment was repeated.
Inoculum type: The experiment was a 4 × 2 × 2 factorial arrangement of treatments. Four peanut genotypes, COAN, C209–6-37, C209–6-60 and AT201, were evaluated at two inoculum levels and two harvest dates. The peanut genotypes were grown and inoculated 2 wk after planting with either 2,000 J2 or 8,000 eggs of M. arenaria as previously described. A randomized complete block design with six replications was used. Gall index 2 was used to evaluate the resistance level in the selected peanut genotypes 2 WAI, whereas gall index 2 and egg number per gram root were used to evaluate resistance 10 WAI. The entire experiment was repeated one time under similar conditions.
Plant age effect: Six peanut genotypes were evaluated: COAN, C209–6-37, C209–6-60, D099, AT201 and D098. The genotypes were planted in 10 × 10-cm2 plastic pots filled with 800 cm3 loamy sand/pot (texture: 85% sand, 11% silt, 4% clay) on five dates with 10-d intervals between dates. All plants were inoculated at the same date with 8,000 eggs/pot. The ages of the peanut plants at the time of inoculation were 0 to 40 d after planting (DAP). The experimental design was a split plot, with genotypes randomized within six replicate main plots (planting date). Plants were harvested at 8 WAI. Gall index 2 and egg number per gram root were used to assess resistance. The experiment was repeated one time.
Statistical analysis: Data from the two trials of each experiment were combined for analysis. Data were analyzed using Proc MIXED with ddfm = satterth option (a general Satterthwaite approximation for the denominator degrees of freedom) on the model statement (SAS v.9.1) (SAS Institute, Cary, NC), unless otherwise stated. Any interaction effects that were not significant were removed, and the reduced model evaluated again. Main effects were considered significant when P ≤ 0.05 and adjusted with any significant interactions. Fisher's least significant difference (LSD) values at P = 0.05 were computed using standard error and t values of adjusted degrees of freedom from the LSMEAN statement in Proc MIXED.
Results
Inoculum level and harvest date: Galls on peanut roots were observed at 2 WAI at inoculation levels 1,000 to 16,000 eggs/plant. Eggs were extracted from infected roots at 6 WAI, although egg masses were not obvious until 8 WAI.
Initial inoculum level of M. arenaria affected gall index 2 in peanut (Table 1). For all four selected genotypes, gall index 2 increased as the inoculum level increased. However, the magnitude of the increase was not the same for all the genotypes (inoculum × genotype interaction, P < 0.0001). From 1,000 to 16,000 eggs/plant, the rate of increase in gall index 2 was greater for the susceptible genotype AT201 than for the moderately and highly resistant genotypes. On AT201, 4,000 eggs/plant caused greater (P = 0.05) gall index 2 than 2,000 eggs/plant did, while 8,000 eggs/plant were needed to cause gall index 2 to be greater than that for 2,000 eggs/plant on NemaTAM (P = 0.05). Across the harvest dates, the four genotypes could be separated into the appropriate resistance categories using 2,000, 6,000, 8,000 and 16,000 eggs/plant based on gall index 2. Low inoculum level (1,000 eggs/plant) could separate the susceptible genotype AT201 from others, but it could not separate the highly resistant genotype NemaTAM from the moderately resistant genotypes.
Table 1.
Effect of inoculum level of Meloidogyne arenaria from 0 to 16,000 eggs/plant on gall index 2a on four peanut genotypes when tested in two greenhouse trials.
Harvest date affected galling and egg production in peanut roots (P < 0.0001); however, there was a significant interaction of harvest date × genotype (P ≤ 0.01) (Fig. 1A, B). From 2 to 8 WAI, the increase of gall index 2 was greater for AT201 than for the other three genotypes. The gall indices did not differ between 4 and 6 WAI for AT201, C209–6-37 and C209–6-60, whereas they did for the highly resistant genotype NemaTAM. Eggs were obtained from all four genotypes by 6 WAI, but egg numbers increased dramatically by 8 WAI. The increase of egg number for NemaTAM was much lower than for the moderately resistant and susceptible genotypes.
Fig. 1.
Effect of harvest dates on root galling (A) and egg production (B) in different peanut genotypes. Gall index 2: 0 = no galling, 1 = trace infection with a few small galls, 2 = ≤ 25% roots galled, 3 = 25–50%, 4 = 51–75% and 5 = ≥ 75% of root galled. Bars within a genotype with the same letter are not significantly different (P > 0.05).
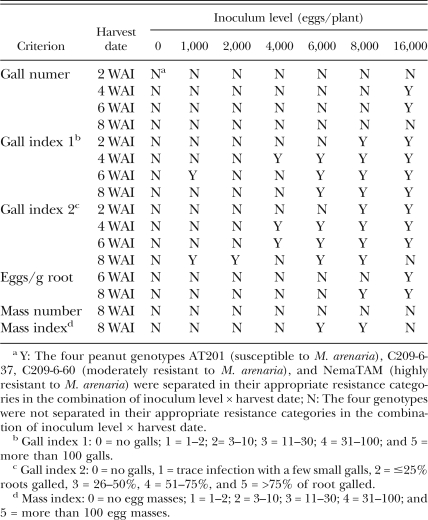
Among all the combinations of seven inoculum levels × four harvest dates, use of gall index 2 could separate the three resistance levels correctly in 14 combinations (Table 2). Based on gall index 2, different resistance levels could be separated successfully as early as 2 WAI at high inoculation levels (8,000 to 16,000 eggs/plant) and could be separated at low inoculation level (1,000 eggs/plant) at the final harvest date (8 WAI). Based on eggs per gram root, the four peanut genotypes with three levels of resistance to M. arenaria were separated at the inoculation rate of 16,000 eggs/plant by 6 WAI and at 8,000 and 16,000 eggs/plant by 8 WAI (Table 2). At low inoculation levels (1,000 to 6,000 eggs/plant) and an early harvest date (6 WAI), the four genotypes were not separated into their appropriate resistance classification due to the high variability of eggs per gram root.
Table 2.
Gall index 2 and numbers of eggs per gram root on four genotypes of peanut using seven inoculum levels of eggs of Meloidogyne arenaria at four harvest dates when tested in two greenhouse trials.
In addition to gall index 2 and eggs per gram root, gall number, gall index 1, egg mass number and egg mass index were also used to assess the resistance levels in the peanut genotypes. The ability of these assessment methods to accurately separate the different levels of resistance is summarized in Table 3. Gall number could only separate the four genotypes correctly by 4 and 6 WAI at the highest inoculum level. Egg mass number was not adequate to separate the four genotypes in this study, whereas egg mass index was a good measure to discriminate between resistance levels with inoculum levels of 6,000 to 8,000 eggs/plant at 8 WAI. Generally, the higher the inoculum level used, the less time was needed to separate the genotypes correctly based on gall index 1 or 2. Both gall index 1 and 2 were positively correlated (P < 0.0001) with eggs per gram root (r = 0.6047 and 0.6773, respectively); however, gall index 2 was the most sensitive method of all measures used for assessing resistance. It provided more choices on combinations of inoculum level × harvest date to separate the four genotypes successfully than gall index 1.
Table 3.
Summary of the evaluation results under seven inoculum levels of Meloidogyne arenaria at four harvest dates by six assessment criteria when tested in two greenhouse trials.
Inoculum type: The hatch rate of the eggs used in this test was 24.7% after 6 d (144 hr, data not shown), thus inoculum levels of 8,000 eggs and 2,000 J2 were approximately equivalent. Eight thousand eggs and 2,000 J2 did not result in significant differences in gall index 2 and egg number at the two harvest dates (Table 4). The resistance classification was also similar between the two inoculum types. The three resistance levels in the four genotypes were distinguished from each other by 2,000 J2 and 8,000 eggs at 2 and 10 WAI, based on gall index 2 or eggs per gram root, except C209–6-37 was not separated from AT201 by 2,000 J2 at 2 WAI.
Table 4.
Gall index 2a and eggs per gram root of four peanut genotypes inoculated either with Meloidogyne arenaria eggs (E) or juveniles (J2).
The coefficients of variation (CV) of gall index 2 for 2,000 J2 and 8,000 eggs were similar at both 2 and 10 WAI. The CV for eggs per gram root for 2,000 J2 (49.2%) was lower than that for 8,000 eggs (76.2%) at 10 WAI, which suggested that the inoculum of 8,000 eggs showed higher variability than 2,000 juveniles.
Plant age at inoculation: Among the six genotypes tested, D098 and AT201 were susceptible, C209–6-37, C209–6-60 and D099 were moderately resistant and COAN was highly resistant. Plant age at time of inoculation affected gall development on the six genotypes (Fig. 2); however, the effects on C209–6-60, D099 and COAN were not as great as on D098 and AT201. Inoculation on d 10 resulted in the highest gall index on all the genotypes except for C209–6-37, which had greatest galling when inoculated on d 20. Inoculation on d 40 could not separate the six genotypes in their correct resistance classifications due to the reduced gall indices of the susceptible genotypes D098 and AT201.
Fig. 2.
Root galling on six peanut genotypes inoculated with Meloidogyne arenaria infection at different days after planting (DAP). Gall index 2: 0 = no galling, 1 = trace infection with a few small galls, 2 = ≤25% roots galled, 3 = 25–50%, 4 = 51–75% and 5 = ≥75% of root galled. For each genotype, gall index 2 at each date interval that differ (P ≤ 0.05) according to Fisher's least significant difference (LSD) test are indicated by different letters around the symbols.
In this experiment, the genotype, DAP and genotype × DAP effects on nematode reproduction (Table 5) were significant (P < 0.05). The eggs per gram root for all the genotypes decreased on inoculation d 40. In contrast, inoculation at 10, 20 and 30 DAP resulted in lower (P ≤ 0.05) reproduction on the highly resistant genotype COAN than on the susceptible genotypes AT201 and D098. However, such differences between moderately resistant and highly resistant or between moderately resistant and susceptible genotypes were not always apparent (Table 5). The six peanut genotypes could not be separated into their appropriate resistance categories with inoculation at 0 and 40 DAP. Nematode reproduction was not different on the susceptible genotype D098 and the highly resistant genotype COAN at these two inoculation dates.
Table 5.
Effects of plant age at inoculation on reproduction (eggs/g root) of Meloidogyne arenaria a in six peanut genotypes with different levels of resistance to M. arenaria when tested in two green house trials.
Discussion
In plant nematology, resistance is used to describe the ability of a plant to suppress development or reproduction of the nematode (Roberts, 2002). For root-knot nematodes, the symptoms can be evaluated with as sufficient ease, accuracy and precision as for some fungal diseases, such as leaf spot and rust. Therefore, the term resistance is also used to describe the capacity of a host to suppress the disease (Sasser et al., 1984; Roberts, 2002) as in general plant pathology. Peanut breeders, geneticists and nematologists have evaluated peanut genotypes for root-knot nematode resistance based on indices of root galling and/or egg mass production (Holbrook et al., 1983, 2000a, 2000b; Timper et al., 2000) or egg counts (Abdel-Momen et al., 1998; Choi et al., 1999). Others also have used gall counts to evaluate resistance to root-knot nematodes in plants (Harris et al., 2003). Gall number and the degree of galling may be used to reflect the ability of a plant to lessen or overcome the attack by the root-knot nematode. However, they do not indicate nematode reproduction directly, while egg mass number, egg mass index and egg number per gram root do.
In our experiments, numerous eggs were collected from the root before egg masses became readily visible to the naked eye. Use of eggs per gram root also separated the three resistance levels correctly in more combinations of inoculum level × harvest date than the use of egg masses. Therefore, we agree with Luzzi et al. (1987) that, for advanced breeding lines, the quantitative data on egg numbers will give a better indication of root-knot nematode resistance than egg mass numbers. In comparison with gall number or gall index 1 (based on gall number), gall index 2 (based on percentage of infested root) was more robust, in that it separated the genotypes into their appropriate resistance categories. Additionally, it was time-consuming and difficult to count the galls at later harvest dates, since galls are usually conjunct. Therefore, we consider gall index 2 to be a better indicator of the resistance level than gall index 1 and gall number.
Harvest date had significant effects on galling and egg production in peanut roots. Galls on roots were visible two weeks after inoculation, and gall index 2 could be successfully used to separate the different resistance levels in the selected peanut genotypes at that time. The different levels of resistance in this study were not correctly separated by eggs per gram root until eight weeks after inoculation with 8,000 eggs/plant, although nematode eggs in peanut roots have been observed as early as 22 days after inoculation (Timper et al., 2000). This is likely due to the high variability in egg numbers at the earlier harvest dates. Based on these observations, we concluded that 8 WAI is necessary to detect differences in the ability of peanut genotypes to restrict nematode reproduction. Temperature has significant influences on penetration, development and reproduction of nematodes (Noe, 1991). Degree-days would have been more accurate than days after inoculation for determining resistance in plants, especially for early assessment dates. During these experiments, the temperature was at 20 to 35°C in the greenhouse, which is the optimum temperature for nematode infection and development. The soil temperature was recorded by a temperature recorder. The degree-days, which used 12.2°C as the threshold temperature (Trudgill and Perry, 1994), were 150 and 695 at 2 and 8 WAI, respectively (data not shown).
The size of galls as well as the number of galls is related to the number of nematodes infecting roots, although the inoculum concentration may have less effect at later evaluation stages (Abdel-Momen et al., 1998; Vovlas et al., 2005). Our results demonstrated that the later the harvest date, the lower was the inoculum level needed to separate the different levels of resistance. Based on gall index 2, the initial inoculum level could be as low as 1,000 eggs/plant to separate the three resistance levels at 8 WAI, or 8,000 to 16,000 eggs/plant could be used to separate the genotypes as early as 2 WAI. Therefore, if a rapid evaluation is required, higher inoculum levels can be used to achieve reliable results, and, if inoculum is a limiting factor, the screening period can be extended. However, to confirm the resistance by egg production level, over 8,000 eggs/plant as initial inoculum and eight weeks from inoculation to harvest are still needed.
J2, intact egg masses or egg suspensions can be used as inoculum for resistance screening tests (Hussey and Janssen, 2002). Intact egg masses are typically not used for inoculum because they are difficult to collect, quantify and disperse in the soil. Only J2 and egg suspensions were compared in our experiments, and both produced similar results. No significant differences in gall index and nematode reproduction were found at 2 and 10 WAI assessments using either type of inoculum. Compared with egg inoculum, no advantages were seen with J2 inoculum. However, three to five additional days were needed to collect the juveniles in the mist chamber.
Infection by root-knot nematodes begins with penetration of the roots by the J2 at the zone of elongation. In small pot tests, root growth is limited at later plant growth stages, which may reduce availability of suitable penetration sites. Our results showed that later inoculation (40 days after planting) resulted in fewer galls and less egg production on peanut, especially on the susceptible genotypes. This reduced the ability to separate susceptible and moderately resistant genotypes.
In summary, we showed that a gall index based on percentage of the root system with galls was a reliable indicator of the level of resistance on early harvest dates (as early as two weeks) after inoculation with 8,000 or more eggs/plant during 10 to 30 days after planting. If the nematode population is the limiting factor, as few as 1,000 eggs/plant could be used to separate the different levels of resistance on late harvest dates (8 WAI) either based on the gall index or eggs per gram root. This is important because we have identified a rapid method for assessing resistance in peanut genotypes. The selected genotypes could then be assessed for eggs per gram root at eight weeks after inoculation with 8,000 eggs/plant to verify the resistance level based on egg production.
Footnotes
The authors wish to express their appreciation to Dannie Mauldin, Jason Golden, Betty Tyler, Vickie Hogan, Brad Buchanan and William H Wilson for assistance in various aspects of this study. The research reported here was funded in part by the National Peanut Board
This paper was edited by Steve Koenning.
Literature Cited
- Abdel-Momen SM, Simpson CE, Starr JL. Resistance of interspecific Arachis breeding lines to Meloidogyne javanica and an undescribed Meloidogyne species. Journal of Nematology. 1998;30:341–346. [PMC free article] [PubMed] [Google Scholar]
- Anderson WF, Holbrook CC, Timper P. Registration of root-knot nematode resistant peanut germplasm lines NR 0812 and NR 0817. Crop Science. 2006;46:481–482. [Google Scholar]
- Choi K, Burow MD, Church G, Burow G, Paterson AH, Simpson CE, Starr JL. Genetics and mechanism of resistance to Meloidogyne arenaria in peanut germplasm. Journal of Nematology. 1999;31:283–290. [PMC free article] [PubMed] [Google Scholar]
- Culbreath AK, Minton NA, Brenneman TB. Response of Florunner and Southern Runner peanut cultivars of chemical management of late leaf spot, southern stem rot, and nematodes. Plant Disease. 1992;76:1199–1203. [Google Scholar]
- Dickson DW, Hewlett TE. Effect of bahiagrass and nematicides on Meloidogyne arenaria on peanut. Journal of Nematology. 1989;21:671–676. [PMC free article] [PubMed] [Google Scholar]
- Harris DK, Boerma HR, Hussey RS, Finnerty SL. Additional sources of soybean germplasm resistant to two species of root-knot nematode. Crop Science. 2003;43:1848–1851. [Google Scholar]
- Holbrook CC, Knauft DA, Dickson DW. A technique for screening peanut for resistance to Meloidogyne arenaria . Plant Disease. 1983;67:957–958. [Google Scholar]
- Holbrook CC, Stalker T. Peanut breeding and genetic resources. In: Janick J, editor. Plant Breeding Review. vol. 22. Westport, CT: AVI Publishing; 2003. pp. 297–356. [Google Scholar]
- Holbrook CC, Stephenson MG, Johnson AW. Level and geographical distribution of resistance to Meloidogyne arenaria in the U.S. peanut germplasm collection. Crop Science. 2000a;40:1168–1171. [Google Scholar]
- Holbrook CC, Timper P, Xue HQ. Evaluation of the core collection approach for identifying resistance to Meloidogyne arenaria in peanut. Crop Science. 2000b;40:1172–1175. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Hussey RS, Boerma HR. A greenhouse screening procedure for root-knot nematode resistance in soybean. Crop Science. 1981;21:794–796. [Google Scholar]
- Hussey RS, Janssen GJW. Root-knot nematode: Meloidogyne species. In: Starr JL, Cook R, Bridge J, editors. Plant Resistance to Parasitic Nematodes. Wallingford, UK: CAB International; 2002. pp. 43–70. [Google Scholar]
- Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA. Survey of crop losses in response to phytoparasitic nematodes in the United States. Supplement to Journal of Nematology. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Luzzi BM, Boerma HR, Hussey RS. Resistance to three species of root-knot nematode in soybean. Crop Science. 1987;27:258–262. [Google Scholar]
- Noe JP. Development of Meloidogyne arenaria on peanut and soybean under two temperature cycles. Journal of Nematology. 1991;23:468–476. [PMC free article] [PubMed] [Google Scholar]
- Roberts PA. Concepts and consequences of resistance. In: Starr JL, Cook R, Bridge J, editors. Plant Resistance to Parasitic Nematodes. Wallingford, UK: CAB International; 2002. pp. 23–41. [Google Scholar]
- Sasser JN, Carter CC, Hartman KM. North Carolina State University, Raleigh and United States Agency for International Development; 1984. Standardization of Host Suitability Studies and Reporting of Resistance to Root-knot Nematodes. [Google Scholar]
- Simpson CE, Nelson SC, Starr JL, Woodard KE, Smith OD. Registration of TxAG-6 and TxAG-7 peanut germ-plasm lines. Crop Science. 1993;33:1418. [Google Scholar]
- Simpson CE, Starr JL. Registration of ‘COAN’ peanut. Crop Science. 2001;41:918. [Google Scholar]
- Simpson CE, Starr JL, Church GT, Burow MD, Paterson AH. Registration of ‘NemaTAM’ peanut. Crop Science. 2003;43:1561. [Google Scholar]
- Stalker HT, Beute MK, Shew BB, Barker KR. Registration of two root-knot nematode-resistant peanut germplasmlines. Crop Science. 2002;42:312–313. doi: 10.2135/cropsci2002.312a. [DOI] [PubMed] [Google Scholar]
- Starr JL, Simpson CE, Lee TA., Jr Resistance to Meloidogyne arenaria in advanced generation breeding lines of peanut. Peanut Science. 1995;22:59–61. [Google Scholar]
- Taylor AL, Sasser JN. Raleigh, N.C: N.C. State University Graphics; 1978. Biology, identification and control of root-knot nematodes (Meloidogyne species) [Google Scholar]
- Timper P, Holbrook CC, Xue HQ. Expression of nematode resistance in plant introductions of Arachis hypogaea . Peanut Science. 2000;27:78–82. [Google Scholar]
- Trudgill DL, Perry JN. Thermal time and ecological strategies - a unifying hypothesis. Annals of Applied Biology. 1994;125:521–532. [Google Scholar]
- Vovlas N, Rapoport HF, Jimenez-Diaz RM, Castillo P. Differences in feeding sites induced by root-knot nematodes, Meloidogyne spp., in chickpea. Phytopathology. 2005;95:368–375. doi: 10.1094/PHYTO-95-0368. [DOI] [PubMed] [Google Scholar]
- Zhang J, Waddell C, Sengupta-Gopalan C, Potenza C, Cantrell RG. Relationships between root-knot nematode resistance and plant growth in upland cotton: Galling index as a criterion. Crop Science. 2006;46:1581–1586. [Google Scholar]