Abstract
Somatotopic maps in the cortex and the thalamus of adult monkeys and humans reorganize in response to altered inputs. After loss of the sensory afferents from the forelimb in monkeys because of transection of the dorsal columns of the spinal cord, therapeutic amputation of an arm or transection of the dorsal roots of the peripheral nerves, the deprived portions of the hand and arm representations in primary somatosensory cortex (area 3b), become responsive to inputs from the face and any remaining afferents from the arm. Cortical and subcortical mechanisms that underlie this reorganization are uncertain and appear to be manifold. Here we show that the face afferents from the trigeminal nucleus of the brainstem sprout and grow into the cuneate nucleus in adult monkeys after lesions of the dorsal columns of the spinal cord or therapeutic amputation of an arm. This growth may underlie the large-scale expansion of the face representation into the hand region of somatosensory cortex that follows such deafferentations.
Keywords: primate, somatosensory, sprouting, plasticity, dorsal columns
In adult monkeys and other mammals, a loss of afferents from the skin is followed by reorganization of the somatosensory cortex so that lost inputs are replaced by intact inputs in the representation (1, 2). Massive losses of inputs lead to large-scale reorganizations such that the somatotopic boundaries in the cortex may shift by more than 10 mm (3, 4). Such reorganizations in sensory representations probably depend on multiple mechanisms, including the potentiation of remaining synapses, the unmasking of latent connections by disinhibition, and possibly the growth of axon arbors and dendrites (5–10). However, there is little direct evidence for the mechanisms that mediate large-scale reorganizations. We presumed that neuronal growth may play an important role in reorganizations where response to the face inputs expands into the hand region of area 3b because the expansion of the receptive fields is beyond any known limits of normal spread of thalamocortical or corticocortical arbors (11–15). Moreover, our experiments showed that the emergence of responses to the stimulation of the chin in the deprived hand cortex takes 6–8 mo (3), a time compatible with the growth of new connections. Finally, at the lower brainstem levels, the chin representation in the trigeminal nucleus lies adjacent to the hand representation in the cuneate nucleus. A limited growth of horizontal connections is known to occur within deprived visual cortex of adult cats (10) and deprived somatosensory cortex of monkeys (9). In addition, in monkeys with arm amputations, there is evidence that afferents from the stump of the arm can grow a short distance from their normal terminations in the dorsal part of the cuneate nucleus of the brainstem to the nearby ventral part, where digit inputs normally terminate (16). The question that we address here is whether the growth of new connections in the brain is a critical component of the massive cortical reorganizations that follow a complete or nearly complete loss of afferents from an arm and result in expansion of the face responsive areas into the hand cortex. More specifically, because inputs from the face activate large portions of deprived somatosensory cortex and thalamus after the loss of arm afferents, do face afferents sprout and grow into the deprived forelimb portion of the somatosensory brainstem? This question is important in that it starts to address the role of the growth of new connections in the repair and reorganization of mature primate and human brains and examines the potential for growth across nuclear boundaries in adult brains.
Materials and Methods
Dorsal Column Lesions.
One adult macaque monkey (Macaca nemestrina), female, 4.7 kg, and one adult owl monkey (Aotus trivirgatus), female, 900 g, were used for this study. The monkeys were anesthetized with a mixture of ketamine (15 mg/kg, i.m.) and xylazine (0.4 mg/kg, i.m.). Dorsal column lesions were made at a level between C3 and C5 on one side using a pair of sharp fine forceps (for details see ref. 3). The macaque monkey was studied for brain reorganization and growth of new connections 22 mo after the dorsal column lesion, and the owl monkey after 18 mo. All animal protocols were reviewed and approved by the Vanderbilt Animal Care and Use Committee and followed National Institutes of Health guidelines.
Monkeys with Amputations.
Three macaque monkeys (Macaca mulatta) were obtained from other primate facilities where they had undergone a therapeutic amputation of the arm by veterinary doctors for treatment of injuries. We injected the tracer and analyzed monkey A1 10 yr after the loss of the forearm and hand at the age of 7 yr. Monkey A2 was analyzed 11 yr after the loss of an arm at shoulder level at 5 mo of age, and monkey A3 6 yr after the loss of an arm at the elbow at 14 mo of age.
Mapping.
Standard multiunit procedures using low impedance (1 MΩ at 1 kHz) tungsten microelectrodes were used to map receptive fields under ketamine and xylazine anesthesia. For details of mapping procedures and reconstruction of the somatotopic maps, see Jain et al. (17).
B-HRP Injections.
Three to four days before the terminal mapping experiments, cholera toxin B subunit linked to horseradish peroxidase (B-HRP) (0.1% or 0.5% in sterile water; List Biological Laboratories, Campbell, CA) was injected at multiple sites (up to 30 sites, 4–30 μl per site) on the chin ipsilateral to the deprived side. Larger volumes were injected when the number of injection sites was small. The injections extended beyond the midline. In some animals, the injections were made on both sides of the chin for comparison.
Histology.
The monkeys were perfused transcardially with buffered 2–3% paraformaldehyde (phosphate buffer, pH 7.5), followed by buffered paraformaldehyde with 10% sucrose, and finally with 10% buffered sucrose. The brain was removed, blocked, and cryoprotected in 30% sucrose. The brainstem was cut on a freezing sliding microtome in coronal plane into 40-μm sections. A series of alternate sections was processed for HRP by tetramethylbenzidine reaction (18). The other series of sections was processed for cytochrome oxidase activity by the procedure of Wong–Riley (19) to define architectural boundaries. For monkeys with dorsal column transections, somatosensory cortex was separated from rest of the brain, flattened between glass slides, and cut parallel to the pial surface into 40-μm-thick sections. The sections were stained for myelin to reconstruct electrode penetration sites and to define the anatomical boundaries of the hand and face representations (20). For the monkeys with amputations, the somatosensory cortex was cut in a plane perpendicular to the central sulcus into 50-μm-thick sections. A three-dimensional view of the posterior bank of the central sulcus and the surrounding cortex was reconstructed.
Results
Neuronal Growth Following Spinal and Peripheral Injuries.
In the first set of experiments, we deprived large parts of the somatosensory system of sensory inputs from the arm by transecting the dorsal columns (3) at upper cervical levels (C3–C5). Most of the afferents from the arm enter the spinal cord below this level. Such lesions leave other ascending pathways in the lateral and ventral funiculi intact, including the spinothalamic tract. Dorsal column lesions produce only limited impairments in the sensory capabilities and the sensory control of the motor functions (21, 22). The owl monkey was studied for cortical reorganization and the growth of new connections 18 mo later, and the macaque monkey, 22 mo later. In the second set of experiments, we investigated cortical reorganization and axon growth in three macaque monkeys 6–11 yr after an injured arm had been amputated as part of veterinary treatment.
We investigated the possibility of the growth of the face afferents from their normal targets in the trigeminal nucleus into the deprived cuneate nucleus by injecting a transganglionic tracer B-HRP into the skin of the chin (Fig. 1a). The monkeys were perfused, and the brains were processed for HRP 3–4 days after the injection of the tracer.
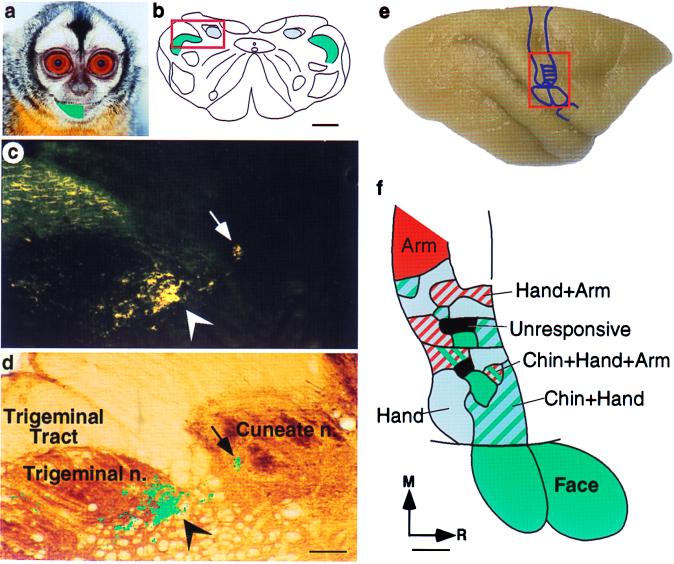
Figure 1.
The growth of terminals from trigeminal nucleus into the cuneate nucleus in an adult owl monkey 18 mo after partial transection of the dorsal columns. (a) The green overlay on the face of an owl monkey shows the region of the skin that received 29 5-μl injections of the transganglionic tracer B-HRP. (b) An outline of a coronal section of the lower brainstem showing the locations of cuneate nucleus (blue) and trigeminal nucleus (green). In normal monkeys, all of the tracer from skin injections such as those shown in a is confined to the expected locations in the trigeminal nucleus. The part of the brainstem that is shown at a higher magnification in c and d is boxed. (c) A dark-field photomicrograph of a brainstem section showing presence of the B-HRP label in the cuneate nucleus (arrow) after injections of the tracer in the skin of the chin. This label indicates the growth of trigeminal nucleus terminations into the cuneate nucleus. Normal terminations of the inputs from the face in the trigeminal nucleus are more numerous, and they are more strongly labeled (arrowhead). The labeled fibers in the upper left corner are in the trigeminal tract. (d) A section of the lower brainstem adjacent to that shown in c and stained for cytochrome oxidase activity. The cuneate and trigeminal nuclei and the trigeminal tract are marked, and the locations of the labeled terminals are marked in green. Images of the sections in c and d were digitized and overlaid in photoshop (Adobe Systems, Mountain View, CA) based on surface features and shared blood vessels. The label in c was converted to a channel mask on a separate layer and filled with green color, and the layer containing the section shown in c was deleted. (e) A dorsolateral view of an owl monkey brain showing location of the somatosensory area 3b. The hand and the face regions shown in f are boxed. (f) Reorganized somatotopy in area 3b as a result of the incomplete lesion of the dorsal columns of the spinal cord at C4/C5 level on the right side. In the deafferented hand region, there were responses to the stimulation of the chin in addition to the stimulation of the remaining inputs from the hand and arm. There were regions that responded to the stimulation of only chin (green), chin and hand (green and blue hatch), and the stimulation of the chin, hand, and arm (green, blue and red hatch). Other parts of the hand cortex responded to the stimulation of the hand (blue) or both hand and arm (blue and red hatch). There were small regions that did not respond to the stimulation of any part of the body (gray). The responses in the lateral part of area 3b that normally represents the face remained unaltered. R, rostral; M, medial; n., nucleus. (Scale bar, 1 mm in b and f, 200 μm in d.)
As expected, most of the transported label from the injections in the chin of each experimental monkey was in the portion of the trigeminal nuclear complex where the face is normally represented. This label was similar to that seen after control injections, either in the chin of the normal monkeys or in the normal side of the deafferented monkeys (see Fig. 3a, Right). In addition, there was normal label in the lateral most parts of the pars triangularis of the cuneate nucleus adjacent to the trigeminal nucleus. Similar label has been reported from the injections of WGA-HRP in the trigeminal ganglion (23). This label corresponds to the region where responses to the stimulation of face are observed in electrophysiological mapping experiments (24). However, on the deprived side of all of the monkeys with deafferentations, a much larger extent of label was clearly apparent in the cuneate nucleus ipsilateral to the forelimb that was deafferented (Figs. 1 b–d, 2, and 3). The rostrocaudal extent of the label varied between the animals. The largest extent of the label was in a macaque monkey with limb amputation (Fig. 3 a, Left and b), where the label was found through nearly all of the rostrocaudal extent of the cuneate nucleus, and in the owl monkey with dorsal column lesion (Fig. 1), where the label spanned more than one-third of the cuneate nucleus. In other monkeys with deprivations, the extent of the label was more limited. The presence of the tracer in the cuneate nucleus, which normally gets inputs from the hand and the arm, shows that the axons terminating in the spinal trigeminal nucleus had undergone collateral sprouting and grown into the cuneate nucleus, which was deprived of its normal inputs as a result of the deafferentations.
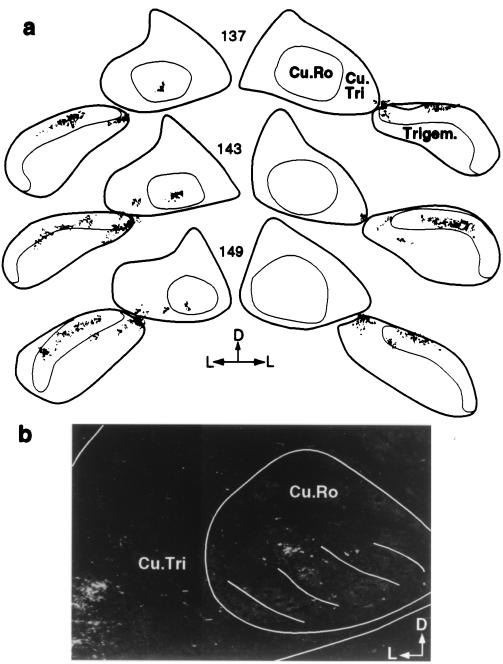
Figure 3.
The growth of arbors into the cuneate nucleus of a macaque monkey 11 yr after the amputation of a forelimb at shoulder level. (a) A series of coronal sections through the lower brainstem showing the label on the deafferented side (Left) and the normal side (Right) from bilaterally matched injections in the chin. Note that there is no label in the pars rotunda of the cuneate nucleus on the normal right side, but a small region of the pars triangularis of the cuneate nucleus adjacent to the trigeminal nucleus is labeled. The fine circular line in the cuneate nucleus defines the pars rotunda, and the fine line in the trigeminal nucleus defines the substantia gelatinosa. (b) A dark-field photomicrograph of a section intermediate to sections number 143 and 149 shown in a. The lines in the ventral part of the pars rotunda mark the territories of the digit representations seen in cytochrome oxidase preparations (43). Cu.Ro., pars rotunda of the cuneate nucleus; Cu.Tri., pars triangularis of the cuneate nucleus; Trigem, trigeminal nucleus; D, dorsal; L, lateral.
Figure 2.
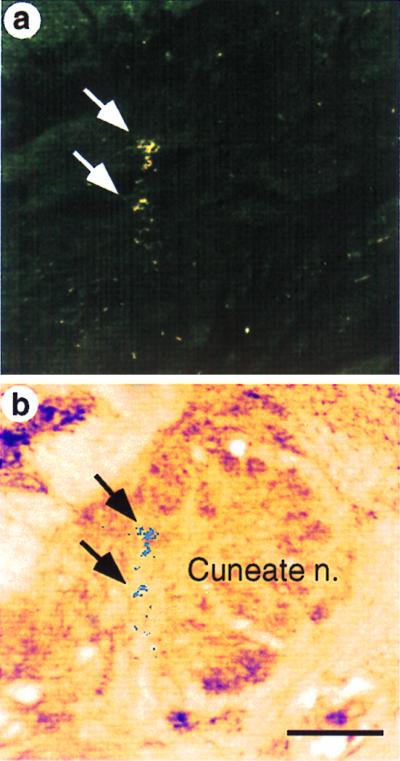
The growth of arbors into the cuneate nucleus (arrows) in a macaque monkey 22 mo after transection of the dorsal columns at C3 level. (a) A dark-field photomicrograph of the section through the lower medulla showing B-HRP tracer in the cuneate nucleus from injections of the tracer in the skin of the chin. (b) A bright-field photomicrograph of the adjacent section stained for cytochrome oxidase activity showing location of the label. For details, see Fig. 1. (Scale bar, 500 μm.)
Cortical Reorganization Accompanies Neuronal Growth.
In each of the experimental monkeys, we also demonstrated that somatosensory cortex had undergone reorganization so that much of the deprived hand cortex had become responsive to inputs from the chin. The monkeys were deeply anesthetized, and microelectrodes were used to record from a large number of sites in the somatosensory cortex. An example of such reorganization is shown in Fig. 1f for the owl monkey with dorsal column transection. Because the section was incomplete, some inputs from the hand and upper arm remained, which activated not only their normal zones of cortex in area 3b, but an expanded zone, as has been reported elsewhere (3). In addition, much of the deprived cortex was also responsive to touch on the chin. An expansion of the chin responsive region into the hand cortex was also seen in the macaque monkey with dorsal column lesion (not shown). Similarly, in the monkeys with arm amputations, there were responses to the chin stimulation in the hand region of area 3b adjacent to the normal face representation (9, 16).
Discussion
Large-scale loss of inputs from the arm and hand because of transection of the dorsal columns of the spinal cord (3) or therapeutic amputation of an arm (16, 25–28) leads to cortical reorganization. The deprived hand cortex comes to respond to the stimulation of the face. This study demonstrates that in adult monkeys with such injuries and cortical reorganizations the neurons in the lower brainstem grow significantly.
Neuronal Growth in Adult Primates.
The environment of the adult central nervous system (CNS) has been considered to be unfavorable for the regenerative growth of neuronal processes because of the presence of growth inhibitory molecules (29, 30). However, it is becoming increasingly clear that the adult CNS may have more regenerative capacity than previously believed. Recently, ongoing neurogenesis and neuronal migration to the associative regions of adult monkey cortex has been shown (31). There are only a few reports of the neuronal growth in the CNS of adult primates after injury. Growth cone-like structures were observed in the dorsal horn of the spinal cord of macaque monkeys after sciatic nerve crush (32), although it was not established whether the growth was regenerative or collateral sprouting. Similarly after amputation of the forelimb, sprouting was seen in the somatosensory cortex (9), brainstem, and spinal cord (16) of adult macaque monkeys. However, the extent of growth that we report in the present study extends the known limits of such sprouting in the adult primate CNS. We show that the new growth can cross nuclear boundaries, and it accompanies the physiologically observed reorganization of somatotopy (see below).
Neuronal Growth in Other Adult Mammalian Species.
Neosynaptogenesis has been frequently observed in the CNS of adult animals after injury, for example, in the motor cortex of cats after lesions of deep cerebellar nuclei (33), and in the ventroposterior nucleus of rats after lesion of the dorsal column nuclei (34). There are also a few reports of neuronal growth after injury in nonprimate animals. Darian–Smith and Gilbert (10) reported collateral sprouting in the cat area 17 after binocularly matched retinal lesions. The growth, which was most noticeable in the superficial layers, however, did not extend the normal limits of the horizontal spread of the fibers. In rats, myelinated fibers of sciatic nerve have been shown to undergo collateral sprouting and extend into lamina 2 of the dorsal horn of the spinal cord after chronic constriction injury (35) or after capsaicin treatment (ref. 36, see however ref. 37). A more extensive sprouting, but which had little physiological expression (see below), was seen in rats after transection of the dorsal roots. In these rats, axons from the gracile nucleus grew into the denervated cuneate nucleus (38).
Mechanisms of Cortical Reorganization.
In monkeys with amputation of a limb, limited neuronal growth in the cortex, brainstem, and spinal cord is accompanied by reorganization of the somatotopic representations in cortex (9, 16). Similarly, in the present studies, the axonal growth from the trigeminal nucleus into the cuneate nucleus is seen in monkeys with large-scale cortical reorganization that leads to the emergence of responses to face stimulation in the region of area 3b normally responsive to hand stimulation. However, in rodents, the subcortical neuronal growth that is seen after injuries in adult animals (38) or even early in development (39) is not physiologically expressed in the cortex. This indicates the possibility that rodents and primates differ in the potential for cortical plasticity, mechanisms of brain reorganizations after injury, and physiological expression of neuronal growth.
We propose that the growth of chin afferents into the deafferented hand nucleus of the brainstem contributes significantly to the activation of hand cortex by chin afferents. The chin afferents in the trigeminal nucleus lie directly adjacent to the cuneate nucleus, whereas afferents from other parts of the face are more lateral and ventral (S.L.F. and J.H.K., unpublished results). Although the spread of axon terminals in the cuneate nucleus due to the new growth is limited, it undoubtedly activates a number of neurons in the cuneate nucleus, which relay to the contralateral ventroposterior nucleus, where an even larger area of the deprived nucleus may be activated (40–42). A relay from ventroposterior nucleus to area 3b probably further amplifies the process, so that an even more extensive reactivation occurs in cortex, undoubtedly with the help of other mechanisms, including unmasking of previously existing connections at different processing levels, and perhaps local growth of new connections in cortex. It is presently not known whether the growth of connections from the face region of area 3b to the hand region contributes to this process.
We conclude that the adult primate CNS is capable of extensive new growth, and that the growth of even a few new connections can have a major impact on the functional organization of the brain. In the case of the growth of face afferents into parts of the brainstem normally devoted to the hand and arm, the new growth may be maladaptive. But new growth in other situations, if it occurs, could mediate significant recoveries.
Acknowledgments
We thank Dr. Christine Collins for her comments on the manuscript. This work is supported by National Institutes of Health Grants NS 16446 (J.H.K.) and NS 36469 (S.L.F.) and Christopher Reeve Paralysis Foundation Grant JB1-9803 (N.J.).
Abbreviations
- HRP
horseradish peroxidase
- CNS
central nervous system
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090572597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090572597
References
- 1.Jain N, Florence S L, Kaas J H. News Physiol Sci. 1998;13:143–149. doi: 10.1152/physiologyonline.1998.13.3.143. [DOI] [PubMed] [Google Scholar]
- 2.Merzenich M M, Kaas J H, Wall J, Nelson R J, Sur M, Felleman D. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 3.Jain N, Catania K C, Kaas J H. Nature (London) 1997;386:495–498. doi: 10.1038/386495a0. [DOI] [PubMed] [Google Scholar]
- 4.Pons T P, Garraghty P E, Ommaya A K, Kaas J H, Taub E, Mishkin M. Science. 1991;252:1857–1860. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 5.Akhtar N D, Land P W. J Comp Neurol. 1991;307:200–213. doi: 10.1002/cne.903070204. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs J L, Salazar E. J Comp Neurol. 1998;395:209–216. [PubMed] [Google Scholar]
- 7.Rema V, Armstrong-James M, Ebner F F. J Neurosci. 1998;18:10196–10206. doi: 10.1523/JNEUROSCI.18-23-10196.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garraghty P E, LaChica E A, Kaas J H. Somatosens Mot Res. 1991;8:347–354. doi: 10.3109/08990229109144757. [DOI] [PubMed] [Google Scholar]
- 9.Florence S L, Taub H B, Kaas J H. Science. 1998;282:1117–1121. doi: 10.1126/science.282.5391.1117. [DOI] [PubMed] [Google Scholar]
- 10.Darian-Smith C, Gilbert C D. Nature (London) 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- 11.Garraghty P E, Pons T P, Sur M, Kaas J H. Somatosens Mot Res. 1989;6:401–411. doi: 10.3109/08990228909144683. [DOI] [PubMed] [Google Scholar]
- 12.Garraghty P E, Sur M. J Comp Neurol. 1990;294:583–593. doi: 10.1002/cne.902940406. [DOI] [PubMed] [Google Scholar]
- 13.Manger P R, Woods T M, Munoz A, Jones E G. J Neurosci. 1997;17:6338–6351. doi: 10.1523/JNEUROSCI.17-16-06338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rausell E, Bickford L, Manger P, Woods T M, Jones E G. J Neurosci. 1998;18:4216–4232. doi: 10.1523/JNEUROSCI.18-11-04216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson R J, Kaas J H. J Comp Neurol. 1981;199:29–64. doi: 10.1002/cne.901990104. [DOI] [PubMed] [Google Scholar]
- 16.Florence S L, Kaas J H. J Neurosci. 1995;15:8083–8095. doi: 10.1523/JNEUROSCI.15-12-08083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain N, Florence S L, Kaas J H. J Neurophysiol. 1995;73:1537–1546. doi: 10.1152/jn.1995.73.4.1537. [DOI] [PubMed] [Google Scholar]
- 18.Gibson A R, Hansama D I, Robinson F R. Brain Res. 1984;298:235–241. doi: 10.1016/0006-8993(84)91423-9. [DOI] [PubMed] [Google Scholar]
- 19.Wong-Riley M T T. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 20.Jain N, Catania K C, Kaas J H. Cereb Cortex. 1998;8:227–236. doi: 10.1093/cercor/8.3.227. [DOI] [PubMed] [Google Scholar]
- 21.Leonard C M, Glendinning D S, Wilfong T, Cooper B Y, Vierck Jr C J. Somatosens Mot Res. 1992;9:75–89. doi: 10.3109/08990229209144764. [DOI] [PubMed] [Google Scholar]
- 22.Makous J C, Friedman R M, Vierck C J., Jr Exp Brain Res. 1996;112:253–267. doi: 10.1007/BF00227644. [DOI] [PubMed] [Google Scholar]
- 23.Marfurt C F, Rajchert D M. J Comp Neurol. 1991;303:489–511. doi: 10.1002/cne.903030313. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Wall J T. J Comp Neurol. 1999;411:369–389. [PubMed] [Google Scholar]
- 25.Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W, Taub E. NeuroReport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- 26.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Birbaumer N, Larbig W, Taub E. Nature (London) 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 27.Knecht S, Henningsen H, Elbert T, Flor H, Höling C, Pantev C, Taub E. Brain. 1996;119:1213–1219. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- 28.Yang T T, Gallen C, Schwartz B, Bloom F E, Ramachandran V S, Cobb S. Nature (London) 1994;368:592–593. doi: 10.1038/368592b0. [DOI] [PubMed] [Google Scholar]
- 29.Bregman B S, Kunkel-Bagden E, Schnell L, Dai H N, Gao D, Schwab M E. Nature (London) 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 30.Kapfhammer J P. Anat Embryol. 1997;196:417–426. doi: 10.1007/s004290050109. [DOI] [PubMed] [Google Scholar]
- 31.Gould E, Reeves A J, Graziano M S, Gross C G. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 32.Knyihar-Csillik E, Rakic P, Csillik B. Cell Tissue Res. 1985;239:633–641. doi: 10.1007/BF00219242. [DOI] [PubMed] [Google Scholar]
- 33.Keller A, Arissian K, Asanuma H. Exp Brain Res. 1990;80:23–33. doi: 10.1007/BF00228843. [DOI] [PubMed] [Google Scholar]
- 34.Wells J, Tripp L N. J Comp Neurol. 1987;255:466–475. doi: 10.1002/cne.902550312. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura S, Myers R R. Brain Res. 1999;818:285–290. doi: 10.1016/s0006-8993(98)01291-8. [DOI] [PubMed] [Google Scholar]
- 36.Mannion R J, Doubell T P, Coggeshall R E, Woolf C J. J Neurosci. 1996;16:5189–5195. doi: 10.1523/JNEUROSCI.16-16-05189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodin B E, Sampogna S L, Kruger L. J Comp Neurol. 1983;215:187–198. doi: 10.1002/cne.902150206. [DOI] [PubMed] [Google Scholar]
- 38.Sengelaub D R, Muja N, Mills A C, Myers W A, Churchill J D, Garraghty P E. Brain Res. 1997;769:256–262. doi: 10.1016/s0006-8993(97)00708-7. [DOI] [PubMed] [Google Scholar]
- 39.Rhoades R W, Wall J T, Chiaia N L, Bennett-Clarke C A, Killackey H P. J Neurosci. 1993;13:1106–1119. doi: 10.1523/JNEUROSCI.13-03-01106.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis K D, Kiss Z H, Luo L, Tasker R R, Lozano A M, Dostrovsky J O. Nature (London) 1998;391:385–387. doi: 10.1038/34905. [DOI] [PubMed] [Google Scholar]
- 41.Jones E G, Pons T P. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 42.Pollin B, Albe-Fessard D. Brain Res. 1979;173:431–449. doi: 10.1016/0006-8993(79)90240-3. [DOI] [PubMed] [Google Scholar]
- 43.Florence S L, Wall J T, Kaas J H. J Comp Neurol. 1989;286:48–70. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]