Abstract
Pseudomonas fluorescens GcM5-1A, isolated from the pine wood nematode (PWN), Bursaphelenchus xylophilus, was cultured in Luria Broth medium (LB). The clarified culture was extracted with ethyl acetate, and two dipeptides were purified from the extract. The chemical structures of 1 and 2 were identified as cyclo(-Pro-Val-)and cyclo(-Pro-Tyr-), respectively, by MS, 1H NMR, 13C NMR,1H-1H COSY, 1H -13C COSY spectra. Bioassay results showed that the two compounds were toxic to both suspension cells and seedlings of Pinus thunbergii, which may offer some clues to research the mechanism of pine wilt disease caused by PWN.
Keywords: Bursaphelenchus xylophilus, cyclo(-Pro-Tyr-), cyclo(-Pro-Val-), interaction, Pinus thunbergii, Pseudomonas fluorescens GcM5-1A, toxicity
The pine wood nematode (PWN), Bursaphelenchus xylophilus, is the causal agent of pine wilt disease (PWD). It is native to North America and has been introduced to Japan by way of timber trade in the early 20th century, from where it has spread into China and Korea. In 1999, it was reported for the first time in Portugal and in Europe (Mota et al., 1999) and has become a worldwide threat to forest resources. It is essential to know the mechanism of pine wilt caused by PWN, in order to better control the nematode.
Bursaphelenchus xylophilus has been thought to be the only pathogen of PWD (Mamiya, 1983; Myers, 1988; Yang, 2002), though the mechanism of the disease has not been well elucidated. However, there has been an increase in claims that bacteria are associated with PWN, and thus surface-sterilized PWN would lose its pathogenicity (Kawazu and Kaneko, 1997; Oku et al., 1980; Zhao et al., 2000a). Each nematode from naturally infected Japanese black pine trees, P. thunbergii, has been reported to carry an average of 290 bacterial cells (Guo et al., 2002) on its body surface, which can be observed directly by scanning electron microscopy (Zhao et al., 2000b). A survey of species of bacteria carried by PWN isolated from diseased P. thunbergii and P. massoniana in China indicated that 24 bacterial strains were isolated and identified. Bioassays showed that 17 of the 24 strains could produce toxins, and most of the strains belonged to genus Pseudomonas (Zhao et al., 2003). Since it is a common phenomenon for nematodes to carry bacteria in nature, it is possible that these bacteria may interact with nematodes and contribute to pine wilt to some extent. Therefore, we hypothesize that PWD is a complex, induced by both PWN and the bacteria it carries. To further elucidate the role of bacteria carried by PWN in PWD, we report two phytotoxins, cyclo(-Pro-Val-) and cyclo(-Pro-Tyr-), referred to as compounds 1 and 2, respectively, secreted by P. fluorescens GcM5-1A carried by PWN in vitro, which were toxic to both suspension cells and shoot seedlings of P. thunbergii.
Materials and Methods
Bacterium strain: Pseudomonas fluorescens GcM5-1A was isolated and primarily identified from PWN in diseased Japanese black pine, P. thunbergii, in China as described by Zhao et al. (2003).
Culturing of aseptic black pine seedlings and callus: Following the procedure of Zhao et al. (2003), seeds of black pine were washed in running water for 2 hr, dipped in 0.1% potassium permanganate for 3 hr and washed three times with sterile water. The seeds were then treated with 0.1% mercuric chloride for 1 min and washed with sterile water five times. They were then placed on Murashige and Skoog (MS) solid medium for germination at 25°C.When new seedlings sprouted, they were transplanted into large bottles containing autoclaved perlite supplemented with MS liquid medium and cultured under a regime of 14 hr light:10 hr dark at 25°C until approximately 8 cm high.
Calli were induced on the tissue culture medium (½ MS, 10.0 mg/liter 2,4-D [2,4-dichlorophenoxyacetic acid], 4.0 mg/liter KT [kinetin], 4.0 mg/liter 6-BA [6-benzylaminopurine]) from mature embryos of P. thunbergii at 27°C in the dark. The callus was subcultured on the calli-maintaining medium (1/2 MS, 0.5 mg/liter NAA [1-naphthaleneacetic acid], 0.1 mg/liter IBA [indolebutyric acid], 1.0 mg/liter GA3 [gibberellin], 100 mg/liter LH [lactolbumin hydrolysate], 1.0 mg/liter 2,4-D) in the same conditions as described above.
Culturing of bacteria: A single colony of P. fluorescens GcM5-1A was inoculated into 30 ml Luria Broth (LB) medium and shake-cultured at 30°C for 24 hr. Then the culture was inoculated into 2 liters LB medium in an 8-liter flask and cultured in the same way as above. The final culture was clarified by centrifugation at 15,000g for 20 min at 4°C, and the supernatant was collected for isolation of toxins.
Extraction and purification of toxins: Ammonium sulfate was added to 2 liters of clarified culture to a saturation of 60%, the precipitated protein was collected by centrifugation at 4°C, 15,000g for 30 min, and the resulting supernatant was used to isolate low molecular weight toxins. The supernatant was extracted three times with 1 volume of ethyl acetate. The combined organic phase was concentrated in vacuum to give a brown oil (0.73 g). The brown oil was applied on a silica gel (200–400 mesh) column (2.0 × 30 cm) and eluted with a solvent gradient of ethyl acetate-methanol (from 100 to 1:1). Twelve fractions were collected guided by TLC (GF254). At the same time, LB medium (2 liters) was extracted with ethyl acetate (3 × 2 liters), and ethyl acetate extract of LB medium was obtained. To establish that the main component of each fraction was in fact from P. fluorescens GcM5-1A, they were analyzed by TLC (GF254) in contrast with ethyl acetate extract of LB medium. According to their TLC profiles, the main components of fractions III, IV, VI, VIII, IX, X and XII were found to be produced by P. fluorescens GcM5-1A instead of from LB medium. Bioassays in toxicities of fractions to both suspension cells and seedlings showed fractions IV and VI had strong toxicities. Active fraction IV (0.03 g) was rechromatographied on a Sephadex LH-20 column (1.6 × 30cm) using ethyl acetate-methanol (10:1) as elution solvent, followed by preparative TLC (GF254) with acetate-methanol (8:1) as developing solvent, to yield compound 1 (1.2 mg), with strong toxicity. Another active fraction, VI (0.05 g), was again subjected to silica gel (200 - 400 mesh) column (1.5 × 20cm) chromatography using an eluent of acetate-methanol (6:1) and then to TLC (GF254) with acetate-methanol (5:1)as developing solvent; finally, compound 2 (2.1 mg), also with strong toxicity, was obtained.
Structure elucidation of toxins: NMR spectra were obtained on a Bruker AV-400 with tetramethylsilane (TMS) as internal standard in CDCl3. EI mass spectra were measured at 70 ev using an Agilent 5973 mass spectrometer. ESI mass spectra were obtained on Waters ZQ-4000 LC-ESIMS.
Assays of toxicity to Japanese black pine suspension cells: Callus (about 0.1 g) was suspended in 0.5 ml 1/2 MS medium; aliquots of 0.19 ml suspension cells were put into sterilized Eppendorf tubes, and 10 μl of different samples were added to the suspension cell solution and mixed gently. For control, 10 μl distilled water was added instead of tested sample. The suspension cells were incubated at 28°C, and lethal rate of the suspension cells was calculated every day under a fluorescence microscope following the procedure of Zhao et al. (2005).
Assays of toxicity to Japanese black pine seedlings: Japanese black pine seedlings approximately 8-cm high were root-cut and put into Eppendorf tubes filled with 0.5 ml of distilled water or sample through the small holes on the lids. The seedlings were then cultured in an incubator at 28°C with light intensity of 1,000 lux; symptoms on the seedlings were observed and recorded every day after treatment. For each test, 100 healthy seedlings were used for treatment, and another 100 seedlings were used for control. Each experiment was repeated three times.
Results
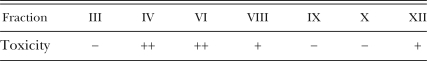
Isolation of components from the culture of P. fluorescens GcM5-1A: The culture of P. fluorescens GcM5-1A was extracted with ethyl acetate. The extract was applied to a silica gel column and eluted with a solvent gradient of ethyl acetate-methanol (from 100 to 1:1), 12 fractions were eluted by TLC (GF254), and only the main components of fractions III, IV, VI, VIII, IX, X and XII were tested to confirm that they had been produced by P. fluorescens GcM5-1A. After the solvents of these seven fractions were evaporated, the fractions were dissolved in distilled water and bio-assayed. The toxicities of the seven components to suspension cells of P. thunbergii 36 hr after treatment were classified at three levels: −, lethal rate less than 10%; +, lethal rate between 10% and 40%; and ++, lethal rate greater than 40%. The bioassay result showed that fractions IV and VI were most toxic to suspension cells (Table 1).
Table 1.
Toxicities of seven fractions isolated from clarified culture of P. fluorescens GcM5-1A to suspension cells of P. thunbergii 36 hr after treatment. a 10 μg aliquot of concentrated fractions III, IV, VI, VIII, IX, X and XII was added to 0.5 ml suspension cells, respectively. The cells were incubated at 28°C for 36 hr, and lethal rates were calculated under a fluorescence microscope after cells were stained with FDA (fluorescein diacetate) following the procedure of Zhao et al. (2005).
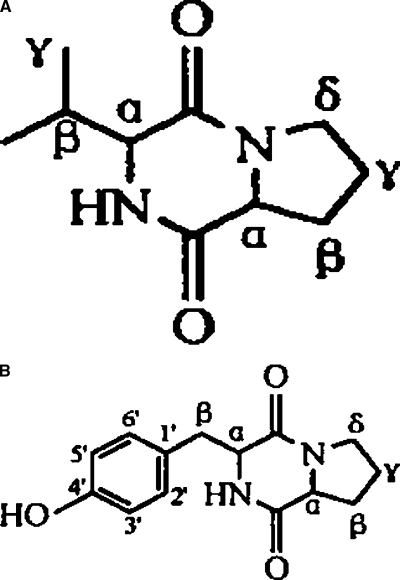
Elucidation of structure of compounds 1 and 2: Compound 1 was isolated from fraction IV through repeated chromatographies on silica gel and Sephadex LH-20 columns and preparative thin-layer GF254 plates. m/z197 of [M+H+] from ESIMS indicated the molecular weight of compound 1 was 196. The chemical structure was identified as a known cyclic dipeptide, cyclo(-Pro-Val-) (Fig. 1), by 1H NMR, 13C NMR, 1H-1H COSY, 1H-13C COSY spectra and comparison with published spectral values (Schmitz et al., 1983; Kang and Chen, 2003; Li et al., 2005).
Fig. 1.
Structure of cyclo(-Pro-Val-) (A) and cyclo(-Pro-Tyr-) (B) isolated from clarified culture of Pseudomonas fluorescens GcM5-1A.
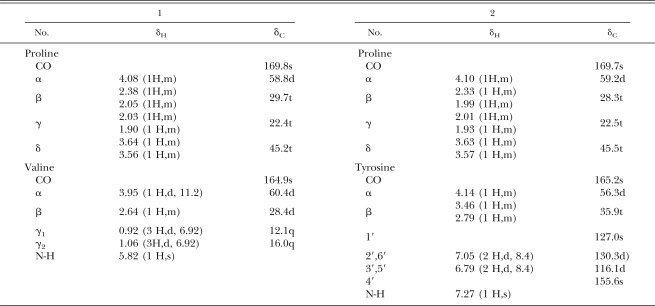
Compound 2 was purified from fraction VI through repeated silica gel column chromatographies followed by preparative TLC (GF254). m/z260 of [M+H+] from EIMS and m/z261 of [M+H+] from ESIMS indicated the molecular weight of compound 2 was 260. Its chemical structure also was identified as a known cyclic dipeptide, cyclo(-Pro-Tyr-) (Fig. 1), by 1H NMR, 13C 1H-1H COSY, 1H-13C COSY spectra and published evidence (Xiong et al., 2002). The data for compounds 1 and 2 are summarized as follows: compound 1, cyclo(-Pro-Val-), white crystals from acetate-methanol (8:1), C10H16N2O2, ESIMS, m/z: 197([M+H]+), 1H NMR and 13C NMR(CDCl3), see Table 2. Compound 2, cyclo(-Pro-Tyr-), white powder from acetate-methanol(5:1), C14H16N2O3, EIMS,m/z:260([M]+,5), 154(100), 107(56), 77(10), 70(28), 41(10); ESIMS, m/z:261([M+H]+), 1H NMR and 13C NMR(CDCl3), see Table 1.
Table 2.
1H NMR and 13C NMR spectra of cyclo(-Pro-Val-) (1) and cyclo(-Pro-Tyr-) (2).
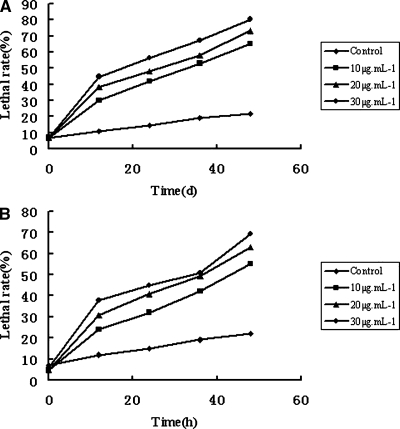
Toxicities of compounds 1 and 2 to suspension cells of Japanese black pine: From the bioassay results it is clear that compounds 1 and 2 were toxic to black pine suspension cells, and the effect was quite related to treatment time and concentration of the compounds. The lethal rates of suspension cells treated with cyclo(-Pro-Val-) at 10, 20, and 30 μg/ml for 48 hr were 65%, 73% and 80%, respectively. Lethal rates of suspension cells treated with cyclo(-Pro-Tyr-) were 55%, 63% and 69%, respectively, while for control, the lethal rate was only 22%. Also, the lethal rates increased with the prolongation of treatment time. The lethal rates of suspension cells treated with cyclo(-Pro-Tyr-) at 10, 20, and 30 μg/ml increased 1.17, 0.92 and 0.78 times from 12 to 48 hr after treatment, respectively. Comparatively, lethal rates for cyclo(-Pro-Val-) increased 1.29, 1.03 and 0.82 times, respectively (Fig. 2). These data indicated that compounds 1 and 2 were both toxic to suspension cells of Japanese black pine and showed a similar toxicity.
Fig. 2.
Lethal rates of suspension cells treated with cyclo(-Pro-Val-) (A) and cyclo(-Pro-Tyr-) (B) of different concentrations (μg/ml) for different times.
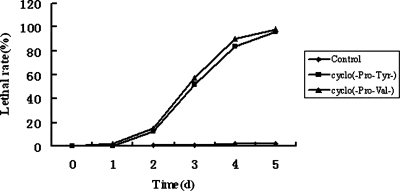
Toxicities of compounds 1 and 2 to Japanese black pine seedlings: Since these two compounds could cause damage to suspension cells, we suggest that they may also affect the physiology of Japanese black pine seedlings. Seedlings were treated with 20 μg/ml of compound 1 or compound 2 for 5 d. At the same time, seedlings were inserted in distilled water for control, and the number of wilted seedlings was recorded every day. As shown in Figure 3, the seedlings treated with the two compounds began to wilt from d 2. On d 3, more than half the seedlings treated with compound 1 and about half the seedlings treated with compound 2 were wilted, and 98% of seedlings treated with compound 1 and 96% of those treated with compound 2 were wilted by d 5. In the control, only 2% of seedlings were wilted at the end of test. Thus, both compounds 1 and 2 were toxic to black pine seedlings.
Fig. 3.
Number of wilted seedlings treated with cyclo(-Pro-Val-) and cyclo(-Pro-Tyr-) (20 μg/ml) for different times.
Discussion
It is a general and natural phenomenon for PWN to carry bacteria. Previous studies showed that the bacteria alone could not invade seedlings and cause PWD, and axenic nematodes that remained free of bacteria were also less pathogenic (Zhao et al., 2003). Bacteria isolated from nematodes in diseased pine could produce toxin in vitro, which led to a conclusion that pine wood nematodes carrying toxin-producing bacteria were necessary to induce to PWD in Japanese black pine (Zhao et al., 2003, 2005). But until now, very little was known about the toxins produced by bacteria or the effect of nematodes on the production of toxins. Kawazu (1998) reported that Bacillus spp. isolated from nematodes could produce phenylacetic acid, which was toxic to callus, suspension cells and black pine seedlings. Here, we report for the first time two toxins synthesized by P. fluorescens GcM5-1A carried by nematodes, cyclo(-Pro-Val-) (1) and cyclo(-Pro-Tyr-) (2). Cyclo(-Pro-Tyr-) has been detected in the plant Phytolacca polyandra (Xiong et al., 2002), bacterium Aristabacter necator (Cain et al., 2003) and fungus Alternaria alternata (Stierle et al., 1988). This compound was phytotoxic to spotted knap-weed and showed synergistic effect of antimicrobial activity against Aspergillus niger with pyrrolnitrin. In this study, this compound as well as cyclo(-Pro-Val-) was found to be toxic to both Japanese black pine suspension cells and seedlings.
We propose that nematode-carrying bacteria might produce various toxins including cyclo(-Pro-Val-) and cyclo(-Pro-Tyr-) which may cause damage to pine trees. More in vivo experiments are needed to ascertain this. An interesting phenomenon was observed during experimental process: water in the Eppendorf tubes of the treated group evaporated faster than that of control (data not shown). To know exactly the role of compounds 1 and 2 in the mechanism of PWD caused by nematodes, first of all, these two compounds must be detected in diseased trees. Zhao's (2005) recent research indicated that PWN promoted the production of toxins by bacterium in host tissue. Therefore, we inoculated 3-year-old Japanese black pine seedlings with nematodes carrying P. fluorescens GcM5-1A and tried to determine whether there was compounds 1 or 2 in vivo; this work is still undergoing. From the above result, we find that Japanese black pine seedlings were very sensitive to the two toxins. Oku et al. (1980) reported that bacteria were associated with PWN and a pine wilt toxin was the metabolite of a bacterium associated with a nematode. If bacteria associated with PWN could indeed produce toxins in seedlings, these toxins could be transported from sites where nematodes intruded to other parts of infected trees and cause cell necrosis of the tissues. Therefore, this study offers a basis for further investigation of the mechanism of PWD caused by PWN.
Footnotes
This work was supported by a key project of Natural Science Foundation of China (No:30430580).
This paper was edited by Andrea Skantar.
Literature Cited
- Cain CC, Lee D, Waldo RH, III, Henry AT, Casida EJ, Jr, Wani MC, Wall ME, Oberlies NH, Falkinham JO., III Synergistic antimicrobial activity of metabolites produced by a nonobligate bacterial predator. Antimicrobial Agents and Chemotherapy. 2003;47:2113–2117. doi: 10.1128/AAC.47.7.2113-2117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DS, Cong PJ, Li L, Zhao BG. Determination of bacterial number carried by a pine wood nematodes and culture of sterilized nematodes on calli of Pinus Thunbergii . Journal of Qingdao University (in Chinese) 2002;15:29–31. [Google Scholar]
- Kang J, Chen RY. Studies on chemical constituents of the mycelia from fermented culture of Flammulina velutipes . China Journal of Chinese Materia Medica (in Chinese) 2003;28:1038. [PubMed] [Google Scholar]
- Kawazu K. Pathogenic toxins of pine wilt disease. Kagaku to Seibutsu. 1998;36:120–124. [Google Scholar]
- Kawazu K, Kaneko N. Asepsis of the pine wood nematode isolate OKD-3 causes it to lose its pathogenicity. Japanese Journal of Nematology. 1997;27:76–80. [Google Scholar]
- Li DH, Gu QQ, Zhu WM. Antitumor components from marine actinomycete 11014 I. Cyclic dipeptides. Chinese Journal of Antibiotics(in Chinese) 2005;30:449–452. [Google Scholar]
- Mamiya Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annual Review of Phytopathology. 1983;21:201–220. doi: 10.1146/annurev.py.21.090183.001221. [DOI] [PubMed] [Google Scholar]
- Mota MM, Braasch HB, Penas AC, Bravo MA, Sousa E, Braasch H. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology. 1999;1:727–734. [Google Scholar]
- Myers RF. Pathogenesis in pine wilt caused by pine wood nematode, Bursaphelenchus xylophilus . Journal of Nematology. 1988;20:236–244. [PMC free article] [PubMed] [Google Scholar]
- Oku H, Shiraishi T, Kurozumi S. Pine wilt toxin, the metabolite of a bacterium associated with a nematode. Naturwissenschaften. 1980;67:198–199. [Google Scholar]
- Schmitz FJ, Vanderah DJ, Hollenbeak KH. Metabolites from the marine sponge Ted aniaignis . Journal of Organic Chemistry. 1983;8:39–41. [Google Scholar]
- Stierle AC, Cardellina JH, II, Strobel GA. Maculosin, a host-specific phytotoxin for spotted knapweed from Alternaria alternate . Proceedings of the National Academy of Science of the United States of America. 1988;85:8008–8011. doi: 10.1073/pnas.85.21.8008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Zhou J, Dai HF, Tan NH, Ding ZT. Chemical constituents from Phytolacca polyandra . Acta Botanica Yunnanica (in Chinese) 2002;24:401–405. [Google Scholar]
- Yang BJ. Advance in research of pathogenetic mechanism of pine wood nematode. Forest Pest and Disease. 2002;1:27–31. [Google Scholar]
- Zhao BG, Guo DS, Gao R, Guo J. A preliminary study on the relationship between the bacterium isolate B19 and pine wilt disease. Journal of Nanjing Forest University (in Chinese) 2000a;24:72–74. [Google Scholar]
- Zhao BG, Guo DS, Gao R. Observation of the site of pine wood nematodes where bacteria are carried with SEM and TEM. Journal of Nanjing Forest University (in Chinese) 2000b;24:69–71. [Google Scholar]
- Zhao BG, Liang B, Zhao LG, Xu M. Influence of pine wood nematode on production of phytotoxins of an accompanying pathogenic bacterial strain. Journal of Beijing Forestry University (in Chinese) 2005;27:71–75. [Google Scholar]
- Zhao BG, Wang HL, Han SF, Han ZM. Distribution and pathogenicity of bacteria species carried by Bursaphelenchus xylophilus in China. Nematology. 2003;5:899–906. [Google Scholar]