Abstract
Commercial plant essential oils from 26 plant species were tested for their nematicidal activities against the pinewood nematode, Bursaphelenchus xylophilus. Good nematicidal activity against B. xylophilus was achieved with essential oils of ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba). Analysis by gas chromatography-mass spectrometry led to identification of 12, 6 and 16 major compounds from ajowan, allspice and litsea oils, respectively. These compounds from three plant essential oils were tested individually for their nematicidal activities against the pinewood nematode. LC50 values of geranial, isoeugenol, methyl isoeugenol, eugenol, methyl eugenol and neral against pine wood nematodes were 0.120, 0.200, 0.210, 0.480, 0.517 and 0.525 mg/ml, respectively. The essential oils described herein merit further study as potential nematicides against the pinewood nematode.
Keywords: ajowan, allspice, litsea, nematicidal activity, pine wood nematode, plant essential oils
Pine wilt disease, caused by the pinewood nematode (PWN), Bursaphelenchus xylophilus, is the most serious problem in Korean forests (Park et al., 2005). This disease was first reported in Busan, Gyeongsangnam-do province, in 1988 (Yi et al., 1989) and has spread to several areas of the Korean peninsula. As Pinus densiflora and P. thunbergii are predominant tree species in Korean forests and are very susceptible to the pine wood nematode, ecological and economical damage is substantial (Korea Forest Service, 2004). Recently, infected Pinus koreansis has been found for the first time in Korea.
Control of this disease depends primarily on fumigation of disease-infected trees with metham-sodium, aerial application of synthetic pesticides, such as fenitrothion and thiacloprid, against Monochamus alternatus, the insect vector of the pine wood nematode, or injection of nematicides, such as morantel tartrate, emamectin benzoate and levamisole hydrochloride (Kishi, 1995; Korea Forest Service, 2003; Lee et al., 2003). However, there are environmental and human health concerns with synthetic pesticides. To avoid these environmental and health problems, there is a trend to search for naturally occurring toxicants from plants. Plant essential oils may provide potential alternatives to currently used PWN control agents because they constitute a rich source of bioactive chemicals and are commonly used as fragrances and flavoring agents for foods and beverages (Isman, 2006). Furthermore, plant essential oils and their components have been reported to have nematicidal activity against PWN (Park et al., 2005; Kong et al., 2006; Choi et al., 2007a, 2007b, 2007c).
In this study, we investigated the nematicidal activity of commercial plant essential oils and their components against PWN to find potential alternatives to currently used PWN control agents or model compounds for the development of chemically synthesized derivatives with enhanced activity or environmental safety.
Materials and Methods
Collection of PWN: Bursaphelenchus xylophilus was isolated from chips of infected pine wood collected in Haman area (in March 2004), Gyoungsangnam-do province, Korea, and extracted by the Baermann funnel method (Chawla and Prasad, 1975). Details of isolation and culture of PWN are well described by Park et al. (2005).
Chemicals: Plant essential oils were purchased from Oshadhi Ltd. (Cambridge, UK) (Table 1). Isoeugenol (purity 98%), 1,8-cineole (purity 99%), (+)-limonene (purity 97%), verbenol (purity 95%) and myrcene (purity 95%) were purchased from Sigma-Aldrich (Milwaukee, WI). Eugenol (purity 99%), p-cymene (purity 95%), α;-humulene (purity 98%), γ-terpinene (purity 97%), terpinen-4-ol (purity 99%), geraniol (purity 96%), α;-terpinene (purity 85%) and thymol (purity 99%) were purchased from Fluka (Buchs, Switzerland). Methyl isoeugenol (purity 98%), α;-pinene (purity 95%), camphene (purity 80%), α;-terpineol (purity 95%), β;-caryophyllene (purity 90%), carvacrol (purity 95%) and β;-pinene (purity 94%) were purchased from Tokyo Kasei (Tokyo, Japan). Acetyl eugenol (purity 97%), methyl eugenol (purity 98%) and linalool (purity 98%) were purchased from Wako (Osaka, Japan). Triton X-100 was purchased from Sigma (St. Louis, MO). All other chemicals were of reagent grade. Nematicidal activities of isoeugenol, methyl isoeugenol and acetyl eugenol were tested in this study to learn the relationship between nematicidal activity and chemical structure.
Table 1.
List of plant essential oils tested.
Synthesis of neral, geranial and 6-Methyl-5-hepten-2-one: 6-Methyl-5-hepten-2-one, geranial and neral were obtained from corresponding alcohols by Pyridinium dichromate (PDC) oxidation (Corey and Schmidt, 1979). The products were confirmed by comparison of the MS spectral data and the retention time with those of authenticated samples. PDC oxidation of 6-methyl-5-hepten-2-ol gave the corresponding ketone in >99.9% purity. However, oxidation of geraniol and nerol gave 85.8% pure geranial (main impurity was neral at 12.1%) and 75.4% pure neral (main impurity was geranial at 21.8%), respectively. Because geraniol and nerol are allyl alcohols, PDC oxidation of those alcohols gave geometric isomers, as reported by Corey and Schmidt (1979). Synthesized geranial and neral were used in bioassay without further purification.
Nematicidal activity: Concentrations of plant essential oils and their components were prepared by serial dilution with distilled water containing Triton X-100 (5,000 ppm). Test solutions were introduced into in wells of 96-well plates. In each well, the concentration of nematodes was between 50 and 150 nematodes (mixtures of juvenile and adult nematodes, male:female: juvenile ≈ 1:1:2) per 100 μl of water. Controls received a distilled water-Triton X-100 solution. Treated and control nematodes were held under the same conditions as used for colony maintenance. Mortality of nematodes was recorded after 24 hr under a microscope. Nematodes were defined as dead if their bodies were straight and they did not move, even after transferral to clean water.
Gas chromatography (GC-FID): Gas chromatography analysis was performed on the Agilent 6890N equipped with flame ionization detector. Retention times for comparison with authentic compounds were measured with a DB-1MS and a DB-FFAP column (30m × 0.25 mm i.d., 0.25 um film thickness, J&W Scientific, Folsom, CA). The oven temperature was programmed as: isothermal at 40°C for 1 min, then raised to 250°C at 6°C/min and held at this temperature for 4 min. Helium was used as the carrier gas at the rate of 1.5 ml/min.
Gas chromatography-mass spectrometry: The essential oils of ajowan, allspice and litsea were analyzed on a gas chromatograph (Agilent 6890N)-mass spectrometer (Agilent 5973N MSD) (GC-MS) equipped with a DB-5MS column (30 m × 0.25 mm i.d., 0.25 um film thickness, J&W Scientific, Folsom, CA). The oven temperature was programmed as for the previous analysis. Helium was used as the carrier gas at the rate of 1.5 ml/min. Effluent of the GC column was introduced directly into the source of the MS via a transfer line (280°C). Ionization was obtained by electron impact (70eV, source temperature 230°C). Scan range was 25–800 amu. Compounds were tentatively identified by comparison of mass spectra of each peak with those of authentic samples in the NIST MS library.
Statistical analysis: Proportional nematode mortality was transformed to arcsine square root values for analysis of variance (ANOVA). Treatment means were compared and separated by Scheffe's test, and LC50 values were calculated by probit analysis (SAS, 1999). Six concentrations for ajowan (1, 0.7, 0.6, 0.5, 0.4 and 0.3 mg/ml) and litsea oil (2, 1, 0.7, 0.5, 0.3 and 0.2 mg/ml) and five concentrations for allspice (0.9, 0.7, 0.6, 0.5 and 0.4 mg/ml) were used to obtain the LC50 values. Four concentration for geranial (0.6, 0.4, 0.2 and 0.1 mg/ml), five concentrations for neral (1, 0.8, 0.6, 0.4 and 0.2 mg/ml), isoeugenol (0.4, 0.3, 0.2, 0.15 and 0.1 mg/ml) and methyl isoeugenol (0.4, 0.3, 0.2, 0.15 and 0.1 mg/ml) and six concentrations for eugenol (0.8, 0.6, 0.5, 0.4, 0.3 and 0.2 mg/ml) were used to obtain the LC50 values. Three replicates were completed for each concentration.
Results
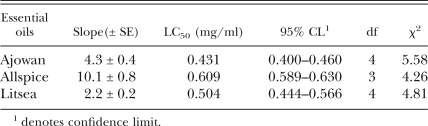
Nematicidal activity of plant essential oils: Nematicidal activity of plant essential oils are shown in Table 2. LC50 values of ajowan, allspice and litsea were 0.431, 0.609 and 0.504 mg/ml, respectively. LC50 values of the other plant essential oils were >2.0 mg/ml.
Table 2.
Nematicidal activity of ajowan, allspice and litsea essential oils.
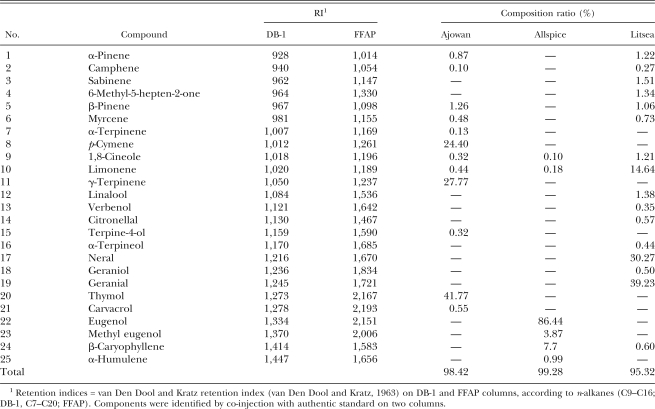
Chemical components of plant essential oils: Total ion chromatograms and chemical compositions of three active essential oils, ajowan, allspice and litsea, are shown in Table 3. Retention indices were obtained with an equation proposed by van Den Dool and Kratz (1963).
Table 3.
Chemical composition of ajowan, allspice and litsea essential oils.
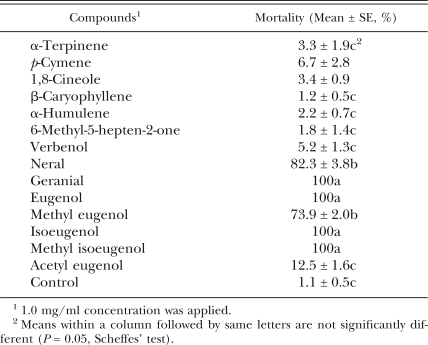
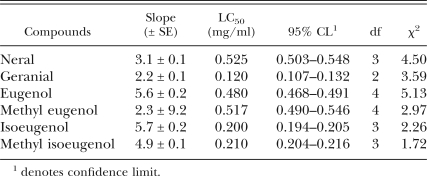
Nematicidal activity of individual compounds: Nematicidal activity of compounds identified in ajowan, allspice and litsea are shown in Table 4. Geranial, eugenol, isoeugenol and methyl isoeugenol showed 100% nematicidal activity against PWN at 1.0 mg/ml concentration. Neral and methyl eugenol produced moderate activity. Nematicidal activity of other compounds was less than 10%. LC50 values of active compounds are shown in Table 5. Geranial was the most active (LC50 = 0.120 mg/ml), followed by isoeugenol (LC50 = 0.200 mg/ml), methyl isoeugenol (LC50 = 0.210 mg/ml), eugenol (LC50 = 0.480 mg/ml), methyl eugenol (LC50 = 0.517 mg/ml) and neral (LC50 = 0.525 mg/ml).
Table 4.
Nematicidal activity of components from ajowan, allspice and litsea esstial oils.
Table 5.
Nematicidal activity of active compounds from ajowan, all spice and litsea essential oils.
Discussion
Many plant essential oils and phytochemicals are known to possess nematicidal activity (Chitwood, 2002). Essential oils of Carum carvi, Foeniculum vulgare, Mentha rotundifolia and Mentha spicata (Oka et al., 2000), three Lamiaceae (Ocimum basilicum, O. sanctum and Mentha piperatum) and two Myrtaceae (Callistemon lanceolatus and Eugenia caryophyllata) (Sangwan et al., 1990) have been reported to show nematicidal activity. Nematicidal activity of plant essential oils and their components against PWN has also been reported (Park et al., 2005; Choi et al., 2007a, 2007b, 2007c). In our study, a total of three plant essential oils, ajowan, allspice and litsea, showed nematicidal activity at 2 mg/ml. Among essential oils tested, ajowan oil was the most toxic, followed by litsea and allspice.
Various compounds, including alcohols, aldehydes, fatty acid derivatives, terpenoids and phenolics exist in plant essential oils. Jointly or independently, they contribute to insecticidal or nematicidal activity. In this study, the nematicidal constituents of oils were identified by GC-MS analysis. Among identified components of ajowan oil, nematicidal activity of α;-pinene, camphene, β;-pinene, myrcene, limonene, γ-terpinene, terpinen-4-ol, thymol and carvacrol against PWN have been reported in a previous study (Choi et al., 2007a). Thymol and carvacrol were very effective against PWN. The present and previous studies confirm that nematicidal activity of ajowan oil was mainly attributed to the activity of thymol and carvacrol. Eugenol was the most abundant component, followed by β;-caryophyllene, methyl eugenol, α;-humulene, limonene and 1,8-cineole in allspice oil. To investigate the structure-activity relationships, isoeugenol, methyl isoeugenol and acetyl eugenol were also tested in this study. Among the test compounds, acetyl eugenol showed the weakest activity against PWN at 1.0 mg/ml concentration. This result indicates that the functional group at the C1 position of the benzene ring is very important for nematicidal activity. Nematicidal activity of compounds with hydroxyl (-OH) or methoxy (OCH3) groups were stronger than those with an acetyl group. Among active compounds, there were differences in nematicidal activities. Nematicidal activities of isoeugenol and methyl isoeugenol were stronger than eugenol and methyl eugenol. This result indicates that the position of the double bond of the propenyl group is very important for nematicidal activity. Nematicidal activity of citral (a mixture of neral and geranial, 3:7) against PWN has been reported in a previous study (Choi et al., 2007a). In this study, we synthesized two isomers to determine the activity of each compound. Purities of synthesized geranial and neral were 85.8% and 75.4%, respectively. Nematicidal activity of geranial was about 4.3 times greater than neral. Structure-activity relationships of cis- and trans-isomers of various compounds have been well studied. Park (2000) reported that the insecticidal activity of cis-asarone was more pronounced against adults of Sitophilus oryzae (Coleoptera: Rhynchophoridae), Callosobruchus chinensis (Coleoptera: Bruchidae) and Lasioderma serricorne (Coleoptera: Anobiidae) than that of trans-asarone. Lee et al. (2002) also reported that there was a difference in insecticidal activity between cis- and trans-asarone. These and our results indicate that the position of the substituent in geometrical isomer is very important for insecticidal or nematicidal activity.
Elucidation of the mode of action of oils and their constituents is of practical importance for nematode control, because it may give useful information on the most appropriate formulation and delivery means. In the current study with B. xylophilus, the dead nematode body was generally extended and without movement. Kong et al. (2006) reported that PWN bodies treated with the muscle activity blockers levamisole hydrochloride and morantal tatrate usually exhibited semicircular and coiling shapes, respectively. These results suggest that the nematicidal mode of action between the essential oils and commercial nematicides might be different. Amino and hydroxyl groups have been hypothesized as target sites of methyl isothiocyanate in nematodes (Wright, 1981). Some essential oils have been reported to interfere with the neuromodulator octopamine (Kostyukovsky et al., 2002) or GABA-gated chloride channels of insect pests (Priestley et al., 2003). However, the exact mode of action of essential oils and its components against PWN is unclear.
In conclusion, ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils and their components described appear to be useful as natural nematicides for B. xylophilus. For the practical use of the three essential oils and their components as novel nematicides to proceed, further study is necessary on systemic action, phytotoxicity and formulation for improving nematicidal potency and stability and reducing cost.
Footnotes
This paper was edited by Ed Lewis.
Literature Cited
- Chawla ML, Prasad SK. Techniques in nematology. II.Comparative efficiency of sampling tools and nematode extraction methods. Indian Journal of Nematology. 1975;4:115–123. [Google Scholar]
- Chitwood DJ. Phytochemical based strategies for nematode control. Annual Review of Phytopathology. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- Choi IH, Kim JH, Shin SC, Park IK. Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchus xylophilus) Russian Journal of Nematology. 2007a;15:35–40. [Google Scholar]
- Choi IH, Park JY, Shin SC, Kim JH, Park IK. Nematicidal activity of medicinal plant essential oils against the pine wood nematode (Bursaphelenchus xylophilus) Applied Entomology and Zoology. 2007b;42:397–401. [Google Scholar]
- Choi IH, Shin SC, Park IK. Nematicidal activity of onion (Allium cepa) oil and its components against the pine wood nematode (Bursaphelenchus xylophilus) Nematology. 2007c;9:231–235. [Google Scholar]
- Corey EJ, Schmidt G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in approtic media. Tetrahedron Letters. 1979;20:399–402. [Google Scholar]
- Isman MB. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Kishi Y. The pine wood nematode and the Japanese pine sawyer. Tokyo: Thomas Company Limited; 1995. [Google Scholar]
- Kong JO, Lee SM, Moon YS, Lee SG, Ahn YJ. Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) Journal of Asia-Pacific Entomology. 2006;9:173–178. [Google Scholar]
- Korea Forest Service. Guideline for the control of forest diseases and insect pests. Daejeon: Korea Forest Service; 2003. [Google Scholar]
- Korea Forest Service. Statistical yearbook of forestry. Daejeon: Korea Forest Service; 2004. [Google Scholar]
- Kostyukovsky M, Rafaeli A, Gileadi C, Demchenko N, Shaaya E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Management Science. 2002;58:1101–1106. doi: 10.1002/ps.548. [DOI] [PubMed] [Google Scholar]
- Lee SM, Chung YJ, Moon YS, Lee SG, Lee DW, Choo HY, Lee CK. Insecticidal activity and fumigation conditions of several insecticides against Japanese pine sawyer (Monochamus alternatus) larvae. Journal of Korean Forestry Society. 2003;92:191–198. [Google Scholar]
- Lee HK, Park C, Ahn YJ. Insecticidal activities of asarones identified in Acorus gramineus rhizome against Nilaparvata lugens (Homoptera: Delphacidae) and Plutella xylostella (Lepidoptera: Yponomeutoidae) Applied Entomology and Zoology. 2002;37:459–464. [Google Scholar]
- Oka Y, Nacar S, Putievsky E, Ravid U, Yaniv Z, Spiegel Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology. 2000;90:710–715. doi: 10.1094/PHYTO.2000.90.7.710. [DOI] [PubMed] [Google Scholar]
- Park C. Suwon: Seoul National University; 2000. Insecticidal activity of β;-asarone derived from Acorus gramineus rhizome against insect pests. MS thesis. [Google Scholar]
- Park IK, Park JY, Kim KH, Choi KS, Choi IH, Kim CS, Shin SC. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus) Nematology. 2005;7:767–774. [Google Scholar]
- Priestley CM, Williamson EM, Wafford KA, Sattelle DB. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homooligomeric GABA receptor from Drosophila melanogaster . British Journal of Pharmacology. 2003;140:1363–1372. doi: 10.1038/sj.bjp.0705542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan NK, Verma BS, Verma KK, Dhindsa KS. Nematicidal activity of some essential plant oils. Pesticide Science. 1990;28:331–335. [Google Scholar]
- SAS INSTITUTE. SAS/STAT User's Guide, release 8.0. Cary, North Carolina: SAS Institute; 1999. [Google Scholar]
- van Den Dool H, Kratz PD. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. Journal of Chromatography A. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- Wright DJ. Nematicides: Mode of action and new approaches to chemical control. In: Zukerman BM, Rhode RA, editors. Plant Parasitic Nematodes. Vol. III. New York: Academic Press; 1981. pp. 421–449. [Google Scholar]
- Yi CK, Byun BH, Park JD, Yang SI, Chang KH. First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle, and its insect vector in Korea. Research Reports of Forestry Research Institute. 1989;38:141–149. [Google Scholar]