Abstract
The importance of plant-parasitic nematodes as yield-limiting pathogens of cotton has received increased recognition and attention in the United States in the recent past. This paper summarizes the remarks made during a symposium of the same title that was held in July 2007 at the joint meeting of the Society of Nematologists and the American Phytopathological Society in San Diego, California. Although several cultural practices, including crop rotation, can be effective in suppressing the populations of the important nematode pathogens of cotton, the economic realities of cotton production limit their use. The use of nematicides is also limited by issues of efficacy and economics. There is a need for development of chemistries that will address these limitations. Also needed are systems that would enable precise nematicide application in terms of rate and placement only in areas where nematode population densities warrant application. Substantial progress is being made in the identification, characterization and mapping of loci for resistance to Meloidogyne incognita and Rotylenchulus reniformis. These data will lead to efficient marker-assisted selection systems that will likely result in development and release of nematode-resistant cotton cultivars with superior yield potential and high fiber quality.
Cotton (Gossypium hirsutum) is the most important fiber crop in the world, and current lint production in the US accounts for nearly one quarter of world supply. The land devoted to cotton production in the US peaked in 1926 at approximately 18 million hectares. The advent of mechanized farming and the availability of effective, relatively low-cost fertilizers, pesticides and improved cotton cultivars after World War II allowed the production of significantly greater yields per unit area and total hectares planted declined. United States production of cotton lint in the past 5 years has varied from 3.0 x 109 kg to 4.4 x 109 kg from approximately 5 million hectares. Additionally, cotton seed is a valuable source of vegetable oil, ruminant animal feed and other feed products.
Since World War II, cotton cultivation has been increasingly dependent on inputs of pesticides for weed and insect control. Historically, the cotton boll weevil, Anthonomus grandis, was the most costly pest of cotton in the US. Until recently, the combination of crop loss due to this insect directly and the expense of insecticides for control amounted to several billion dollars annually. The success of the Boll Weevil Eradication Program coordinated by the US Department of Agriculture has resulted in a major reduction in insecticide usage and improved profitability for growers and has led to a resurgence of cotton production in the southeastern US. In addition, the widespread use of transgenic cotton cultivars (currently 92.7% of the crop) with resistance to herbicides and/or lepidopteran insects has further reduced total pesticide usage on the crop (USDA-Agricultural Marketing Service, 2007). Reductions in losses from weeds and insects as a result of the deployment of transgenic traits and the boll weevil eradication program have allowed the cotton industry to focus on other pest problems, especially nematodes.
Modern cotton production in the US is intensive, highly mechanized and dependent on a local infrastructure to support this industry. Equipment for cotton harvesting and lint processing, including cotton pickers, modules for storing seed cotton, and gins, are highly specialized and generally not used for other crops. The necessity of an exclusive infrastructure to support cotton production has two important implications: (i) cotton is frequently grown in monoculture, and (ii) cotton typically has a greater impact on local economies than grain crops because of the jobs created to serve the industry.
The damage potential of plant-parasitic nematodes to cotton has been recognized since the late 19th century. Classic work by Atkinson demonstrated the pathogenicity of Meloidogyne incognita to cotton and the role of this nematode in Fusarium wilt of cotton (Atkinson, 1892, 1899). Plant-parasitic nematodes, however, received only limited study as cotton pathogens until the 1950s. Currently, the four most damaging species of plant-parasitic nematodes affecting cotton in the US are the southern root-knot (Meloidogyne incognita), reniform (Rotylenchulus reniformis), Columbia lance (Hoplolaimus columbus) and sting (Belonolaimus longicaudatus) (Blasingame, 1993; Koenning et al., 1999; Starr et al., 2005; Blasingame, 2006). Estimated losses of cotton lint yield by these pathogens in the US have increased from 1% to 2% in the 1950s to more than 4% in 2000 (Blasingame, 2006). This increase in estimated losses due to plant-parasitic nematodes can be attributed to several factors: (i) the lack of resistant cultivars, (ii) limited use of crop rotation in many areas, (iii) increased awareness of pathogenic nematodes as production constraints, especially the reniform nematode, (iv) the loss of highly effective, low-cost, fumigant nematicides, and (v) a recent increase in cotton production in the southeastern US.
Management by Cultural Practices
In modern cotton production, cultural practices often have limited use in suppressing nematode population densities and minimizing yield losses. Crop rotation, growing non-host, resistant or antagonistic cover crops, incorporation of plant materials or animal manures, and destruction or removal of cotton stalks and roots to minimize nematode survival and reproduction have been investigated (Barker and Koenning, 1998; Davis et al., 2000, 2003; Koenning et al., 2003a, 2003b). Crop rotation, perhaps the most widely used cultural means of limiting nematode populations, requires sufficient suitable land for the production of alternate crops that are non- or poor hosts to the nematode species in question. For rotation crops to be practical, alternative crops also must provide an adequate income to the grower and result in sufficient yield increases in a subsequent cotton crop to justify removing land from cotton production. Selection of a crop for a rotation with cotton must be done on the basis of the nematode species that is present.
Peanut (Arachis hypogaea) is a nonhost for H. columbus, M. incognita and R reniformis and provides an attractive rotational crop for managing these nematodes in parts of the US where peanut is grown (Koenning et al., 2004). Similarly, tobacco (Nicotiana tobacum) can be used effectively in rotation with cotton for suppression of H. columbus and B. longicaudatus population densities where this crop is economically feasible (Robbins and Barker, 1973; Fassuliotus, 1974). Corn (Zea mays) and grain sorghum (Sorghum bicolor) are generally susceptible to M. incognita, whereas soybean (Glycine max) cultivars vary from highly susceptible to immune (Hussey, 1977). Vegetable crops, in general, and tobacco should not be included in rotations with cotton where management of root-knot or reniform nematode is the primary objective. In contrast to M. incognita, R. reniformis has little or no reproduction on grain crops such as corn or grain sorghum (Robinson et al., 1997). Reniform nematode-resistant soybean cultivars and winter grain crops can be included in rotation with cotton to reduce the population density of R. reniformis and improve cotton yield (Fig. 1) (Davis et al., 2003). Rotation for suppression of reniform nematode with nonhost crops, however, is only effective for one year. Rotation is not generally an option in fields infested with H. columbus because many crops typically grown in the same region as cotton are susceptible to this nematode (Fassuliotus, 1974). All corn and soybean cultivars tested were excellent hosts for H. columbus. Peanut and tobacco can be used in rotation with cotton to achieve reductions in the population density of H. columbus. Only tobacco is considered to be resistant to B. longicaudatus, although some vegetables such as watermelon (Citrullus vulgaris) allow only limited reproduction. Populations of B. longicaudatus have been reported to vary in their ability to reproduce on peanut (Robbins and Barker, 1973). Vegetable or other crops may actually be grown in the same year as cotton in the southernmost areas of the US, and cotton is severely affected by B. longicaudatus in Florida following potato (Solanum tuberosum) (Crow et al., 2000).
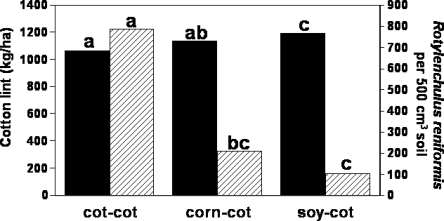
Fig. 1.
Effects of rotation with a soybean cultivar resistant to Rotylenchulus reniformis, (soy-cot), non-host corn (corn-cot), or continuous cotton (cot-cot) on cotton lint yield and initial population densities of R. reniformis (Davis et al., 2003).
Tillage has long been recommended as a means of incorporating crop residue and for destruction of residual roots. This may be especially important for cotton because it is a perennial that can support reproduction of plant-parasitic nematodes when soil temperatures remain above the nematode's activity threshold for extended periods following harvest of the crop. Destruction of cotton root systems or removal with a stalk puller, however, had only a minimal effect on population densities of H. columbus and did not increase the yield of subsequent cotton crops (Davis et al., 2000; Koenning et al., 2003a). In-row subsoiling and/or some other form of deep tillage appear to be necessary to optimize yields in fields infested with lance nematodes if a soil hardpan is present (Fig. 2) (Hussey, 1977). Deep tillage allows the tap roots to reach the clay layer where higher levels of water and nutrients are present than in the sandy top soils of the coastal plain. Many farmers, however, have adopted reduced tillage practices that eliminate or restrict routine subsoiling. The impact of reduced tillage practices on population densities of nematodes parasitic on cotton is not known.
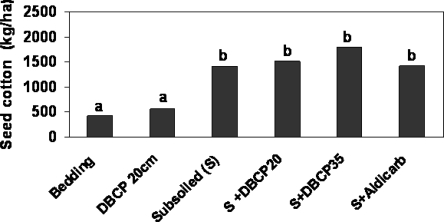
Fig. 2.
Influence of tillage practices on cotton lint yield in the presence of Hoplolaimus columbus in field plots (Hussey, 1977). Bedding (conventional bed), 10 kg/ha 1, 2-dibromo-3chloropropane (DBCP) injected 20 cm deep, subsoiled (S), subsoiled plus DBCP injected 20 or 35 cm deep or aldicarb at 0.8 kg/ha (S + DBCP20, S + DBCP35, S+Aldicarb).
Additional cultural practices that have been suggested for suppressing nematode population densities include planting date, the use of organic amendments, and cover crops. Unfortunately, little information is available on the effects of planting dates on cotton nematode populations. Later spring planting dates for Upland cotton in California were found to lessen the root-knot nematode infection potential and the extent of Fusarium wilt symptoms in infested fields (Jeffers and Roberts, 1993). Cotton planting date had no impact on H. columbus in North Carolina, and planting dates are not flexible enough in many areas because of the relatively long season needed to produce cotton (Koenning et al., 2003b). Application of poultry litter at rates suppressive to M. incognita or H. columbus may be impractical or exceed environmental regulations (Riegel et al., 1996; Koenning et al., 2003b). Winter cover crops, particularly the small grains wheat (Triticum aestivum), rye (Secale cereale) and oats (Avena sativa), are commonly used in the southeast to prevent soil erosion, but their impact on plant-parasitic nematodes in cotton is controversial. Incorporation of cover crop residues improves water retention in sandy soils, and decomposing residues of rye may be toxic to nematodes (Barker and Koenning, 1998). However, many winter cover crops, including small grains, are hosts for M. incognita, H. columbus and B. longicaudatus, but generally not for R. reniformis. Winter wheat or rye cover crops had no impact on population densities of H. columbus in Georgia, and only limited information is available about the influence of small grains on southern root-knot and sting nematodes (Davis et al., 2000). A winter rye cover crop suppressed winter weeds in North Carolina that were hosts for R. reniformis, thus minimizing reproduction of this nematode during winter periods (unpublished, S. R. Koenning). Although small grain winter cover crops are hosts for many nematode species, in most instances nematode reproduction is suppressed by low soil temperatures. In California, wheat planted in soils with temperatures above 18°C supported reproduction of M. incognita, whereas the decline of this nematode did not differ between fallow soil and wheat planted after soil temperatures were below 18°C (Roberts et al., 1981). In the coastal plain soils of the eastern and gulf coast states where H. columbus and B. longicaudatus are most common, the impact of cover crops would be expected to have variable effects on nematode population densities based on local soil temperatures, sowing date and time of destruction of the cover crop.
Management with Nematicides
With only a limited number high yielding nematode-resistant cultivars available and the economic and practical limitations to crop rotation, nematicides continue to be the primary means of managing nematodes in cotton in the US. Practical use of nematicides actually began after WWI, driven by the need to dispose of large quantities of surplus chloropicrin that remained at the close of the war (Johnson and Godfrey, 1932). The concept of applying a volatile material such as chloropicrin to the soil for control of soilborne pests was targeted originally for high value crops, but the success of these treatments was instrumental in focusing attention on the importance of nematodes as plant pests (Johnson and Feldmesser, 1987). By the time the surpluses were exhausted, the concept of soil fumigation as a practical means of nematode control had become established, and a search for more effective and convenient materials was underway, leading to the discovery of the nematicidal properties of the mixture of 1,3-dichloropropene and 1,2-dichloropropane (Carter, 1943) and of ethylene dibromide (Christie, 1945). The discovery of 1,2-dibromo-3-chloropropane in 1954 (McBeth, 1954; Raski, 1954) increased the interest in the application of nematicides to cotton because it was less phytotoxic than other fumigants and was easier to apply.
Although fumigant nematicides were highly effective, the difficulty and expense required for application and safety and environmental concerns associated with their use limited their utility in cotton. During the 1960s, two new classes of chemicals, the organophosphates and the carbamates, were synthesized and screened primarily in a search for more effective insecticides. They were soon recognized as having nematicidal activity (Christie and Perry, 1958; Weiden et al., 1965). Many of the individual chemicals in both classes were much less phytotoxic than most fumigants, and they were active against nematodes in the soil either in their original form or initial degradation products. These insecticide/nematicides rapidly gained in popularity because they could be applied to the soil at planting using relatively simple equipment and they were considerably less expensive than soil fumigants. One of these materials, aldicarb, has been the most widely used nematicide in cotton in the US for more than 20 years (Koenning et al., 2004).
Currently, there are three basic strategies for nematode management using nematicides in the US (Koenning et al., 2004). The most widely used strategy consists of the application of aldicarb at rates of 0.8–1.2 kg/ha in the planting furrow. A second, more expensive strategy is preplant soil fumigation using either 1,3-dichloropropene or metam-sodium. A third strategy is the supplemental use (in addition to an at-planting application of aldicarb) of either aldicarb applied as a side-dress during the first third of the season, or a foliar application of the carbamate oxamyl (Lawrence and McLean, 2000, 2002). The popularity of these strategies varies across the country. At-planting application of aldicarb is perhaps the most universal nematicide strategy in the US and is applied on 20% to 30% of the cotton hectarage each year (Koenning, et al., 2004), whereas soil fumigation is most common in the southeastern states.
Recently, the concept of applying a low concentration of nematicides as a seed dressing has shown promise in protecting emerging roots from nematode infection for a limited period of time (Monfort et al., 2006). Abamectin, one of a number of avermectins produced by Streptomyces avermictilus (Putter et al., 1981), received registration for use on cotton in 2006. A second compound, thiodicarb, was registered for use as a seed-treatment nematicide in 2007. Protection of developing roots during the first few days or weeks after germination may be critical to the establishment of optimum yield potential (Penteado et al., 2005). However, seed treatment alone may not be sufficient to provide protection from nematode damage to cotton plants in fields with high population densities of economic nematode species.
Biorational approaches to nematode control have not been thoroughly explored in cotton production systems. Harpin, a protein from Erwinia amylovora (Wei and Beer, 1996) that may elicit a systemic acquired resistance (SAR) response in certain plants, has been suggested as a way to mitigate nematode infection in cotton. Although laboratory investigations have shown promise, yield in field trials has been disappointing (Bednarz et al., 2002). A second material, acibenzolar-S-methyl, also induces SAR and enhances resistance to certain fungal pathogens (Allen et al., 2004), but its efficacy against nematodes has not been studied.
In the absence of significant new chemical nematicides, improved precision in utilizing existing materials may enhance crop profitability and environmental stewardship. Of particular interest is the adaptation of precision agriculture technology to more effective placement of nematicide within fields rather than the current practice of applying a single rate of nematicide field-wide (Evans et al., 2002). Accurately determining the spatial variability of most nematode species of concern in cotton has been an impediment to practical adoption of this concept (Wheeler et al., 1999; Wrather et al., 2002; Wyse-Pester et al., 2002). However, ongoing investigations using aerial imagery and/or measurable edaphic factors, such as soil electrical conductivity, may lead to improved strategies both for mapping nematode population distribution within fields and in site-specific delivery of nematicides to specific problem areas (Wolcott et al., 2006; Monfort et al., 2007; Overstreet et al., 2007).
Until effective nematode-resistant cotton cultivars or other tools for mitigating nematode damage in cotton are available, nematicide application is likely to remain a cornerstone of nematode management in cotton in the US. Escalating production costs and heightened environmental and health concerns make it imperative that more sustainable and profitable strategies for nematode control with nematicides are developed.
Management with Host Resistance
Among the nematode management strategies in cotton, host-plant resistance has great potential to be an economic and highly effective approach. The use of resistant cultivars is easy to implement and risk-free to use compared to nematicides and more predictable in effect than cultural tactics such as multi-year rotations. Because resistance in cotton suppresses nematode reproduction resulting in reduced nematode population densities (Fig. 3), resistant cotton cultivars have the added advantage of being able to protect susceptible crops grown in rotation (Ogallo et al., 1999). A primary challenge for advancing nematode resistance implementation in cotton is introducing effective resistance into elite cotton cultivars. Cotton breeding programs have had only moderate success in attaining this goal, in large part because the genetics of the resistance is complicated and the phenotyping protocols are diflficult, time-consuming and expensive. The heavy reliance on nematicides over the last several decades has also influenced priorities for cotton breeders. Consequently, many cotton improvement programs, especially in the private sector, have not placed emphasis on nematode resistance until recently. Further, no resistance to B. longicaudatus or H. columbus has been reported in cotton germplasm collections.
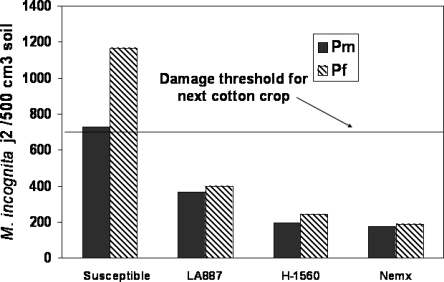
Fig. 3.
Influence of resistant cotton cultivars Acala NemX, LA 887 and H1560 on midseason and final population densities of M. incognita compared to susceptible cotton cultivars Deltapine 90, Deltapine 51 and Suregrow 125 in North Carolina (Koenning et al., 2001).
Resistance to Rotylenchulus reniformis: Rotylenchulus reniformis is an increasing problem in cotton production in the eastern half of the US cotton belt (Gaur and Perry, 1991; Lawrence and McLean, 2001; Starr et al., 2005). It is estimated to cause annual losses of approximately $130M, with major impact in the states of Mississippi, Louisiana and Alabama (Koenning et al., 2004; Blasingame, 2006; Robinson, 2007). Reniform nematode reproduction as a measure of resistance has been evaluated on more than 3,000 accessions of the genus Gossypium to discover sources of resistance (Carter, 1981; Yik and Birchfield, 1984; Beasley and Jones, 1985; Muhammad and Jones, 1990; Stewart and Robbins, 1995, 1996; Robinson and Percival, 1997; Robinson et al., 1999, 2001; Robinson, 2002; Robinson et al., 2004, 2006; Weaver et al., 2007). Only weak to moderate resistance has been reported in G. hirsutum, but high to very high levels of resistance have been found in other Gossypium species, including G. anomalum, G. arboreum, G. barbadense, G. herbaceum, G. longicalyx, G. raimondii, G. somalense, G. stocksii and G. thurberi (Yik and Birchfield, 1984; Stewart and Robbins, 1995; Robinson et al., 2004).
Reniform nematode resistance in accessions of G. barbadense, which hybridizes freely with G. hirsutum, usually suppresses nematode populations by approximately 70% to 90% (Robinson et al., 2004). In contrast, many accessions of G. arboreum, from which genes are introgressed via bridging species, are highly resistant to the reniform nematode (Stewart and Robbins, 1995), and the most resistant G. arboreum accessions suppress nematode reproduction by 95% or more compared to susceptible G. hirsutum. As the extreme case, G. longicalyx, from which genes can be transferred only with great difficulty, is virtually immune to R. reniformis. This apparent inverse relationship between compatibility with G. hirsutum and resistance within Gossypium greatly confounds strategies and funding for developing resistant cultivars.
About 20 G. hirsutum accessions with weak to moderate levels of resistance to the reniform nematode have been reported. Resistance within G. hirsutum appears highly sensitive to environment and/or nematode population. Resistance that was reproducible in one environment has not been observed consistently in a different environment or against a different population of reniform nematode. Seven accessions scored by Yik and Birchfield (1984) in replicated experiments as moderately resistant were later scored by Robinson et al. (1997) as susceptible, because in the latter study they supported 17- to 64-fold increases in nematode populations within a 7-week period. Of six primitive G. hirsutum accessions scored by Robinson et al. (2004) as moderately resistant, only TX1828 and TX 1586 also were classified by Weaver et al. (2007) as resistant. Of six accessions observed by Weaver et al. (2007) to consistently support lower nematode populations than the control, only TX 1565 had been scored as possibly resistant by Robinson et al. (2004). In some cases, moderately to highly resistant primitive accessions of G. hirsutum from the USDA Cotton Collection have been found to have flower and leaf traits similar to those of G. barbadense. Thus, the question remains as to whether some of the resistant accessions in this collection are G. hirsutum or G. barbadense. Nonetheless, several breeding efforts are in progress to develop cotton cultivars with improved levels of resistance to the reniform nematode from these various sources of resistance.
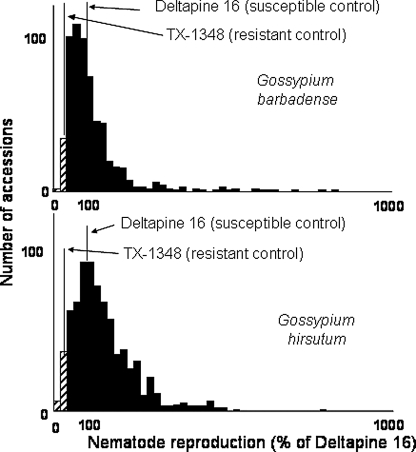
Comparison of reniform nematode reproduction on 850 accessions of G. barbadense and 1,419 of G. hirsutum (Robinson et al., 2004) clearly showed that although there is great variation in the ability of accessions in both species to support reniform nematode reproduction, susceptible G. barbadense accessions on average supported less reproduction than most G. hirsutum accessions and useful levels of resistance were more common in G. barbadense. In G. barbadense, 2.1% of the accessions supported less than one-third the reniform nematode reproduction of the susceptible cultivar Deltapine 16, compared with 0.4% of the G. hirsutum accessions (Fig. 4) (Robinson et al., 2004).
Fig. 4.
Frequency of accessions with resistance to Rotylenchulus reniformis among accessions of Gossypium barbadense and G. hirsutum (Robinson et al., 2004).
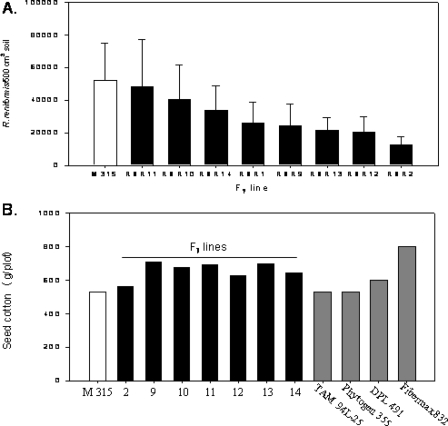
Current efforts (C. W. Smith and J. L. Starr, unpublished data) with progeny from a cross between root-knot nematode-resistant G. hirsutum M-315 RNR and reniform nematode-resistant G. barbadense TX 110 (Yik and Birchfield, 1984) show promise. Numerous F1 plants tested separately against M. incognita and R. reniformis had resistance equivalent to the resistant parent, suggesting dominant inheritance of resistance to each nematode. Based on failure of an F2 population to fit either a one- or a two-gene model, resistance to R. reniformis was assumed to be a polygenic trait. Using a pedigree breeding program, several lines were advanced to the F7 generation with selection for nematode resistance in several generations. Three lines have resistance to both root-knot (data not shown) and reniform nematodes (Fig. 5A). In a single test for seed cotton yield, all of the nematode-resistant selections had yields that were slightly better than M-315 RNR but not equal to those of three high-yielding cultivars (Fig. 5B).
Fig. 5.
Resistance to Rotylenchulus reniformis and yield potential of F7 lines of cotton with resistance introgressed from Gossypium barbadense ‘TX110’. A) Nematode reproduction on eight F7 lines compared to reproduction on the susceptible parent M.315. B) Seed cotton yield in replicated, single-row plots (not infested with R. reniformis) of seven F7 lines compared with M315 and four high-yielding cultivars.
USDA scientists in Texas and Mississippi are working to introgress resistance from G. barbadense GB-713 into several root-knot nematode-resistant breeding lines as well as the once extensively planted susceptible cultivar Deltapine 16. GB-713 was by far the most resistant G. barbadense accession identified in an extensive evaluation of G. barbadense in the USDA Cotton Collection (Robinson et al., 2004), consistently suppressing reniform nematode reproduction by 90% to 98% compared to Deltapine 16. Preliminary analysis of parental, F1, F2 and BC1F1 populations using SSR markers identified three markers linked to the resistance locus. Nematode reproduction on F1 plants was uniform and intermediate between that on the two parents. Generation means analysis of nematode reproduction data from parents, F1, F2 and BC1F1 populations indicated genetic control by a single partially dominant gene with additive effects (Robinson, unpublished data). Thus inheritance indicated the trait was amenable to backcrossing into a root-knot nematode-resistant recurrent parent by selecting for resistance to reniform but not root-knot in progeny from each generation. It may be necessary to self the plants after each backcross and select for reniform nematode resistance in F2 progeny, where highly resistant homozygous plants are expected.
A project under the direction of E. Sacks (pers. commun.) to introgress resistance to the reniform nematode from G. arboreum into cotton was initiated by crossing accession A2 190 (Burma C19) (Stewart and Robbins, 1995) with a 2[(AD1)D4] hexaploid bridging line named G 371. A single hybrid plant was obtained and was subsequently crossed with Deltapine 16 and MD51ne to develop pseudo-backcross populations for nematode screening. Rotylenchulus reniformis populations per gram soil from controlled environment tests confirmed that resistance in A2 190 was similar to that of G. barbadense GB-713. Nematode reproduction in the backcross population, expressed as a percentage of the controls, had a bimodal distribution, suggesting the action of a dominant gene. The peak of the resistant class of the backcross population (heterozygous for resistance) was at about 15% of the cultivar controls.
Virtual immunity to the reniform nematode in G. longicalyx (Yik and Birchfield, 1984) has been confirmed in various laboratories. Two tri-species hybrids of G. hirsutum, G. longicalyx and either G. armourianum or G. herbaceum (Bell and Robinson, 2004; also see Brown and Menzel, 1950 and Konan et al., 2007) were utilized as bridges to introgress this resistance from G. longicalyx into G. hirsutum. Introgression was accomplished by backcrosses to G. hirsutum with cytogenetic analysis of early backcross generations to assess progress toward the euploid state (2n = 52), selection for nematode resistance at each generation and examination of selfed progeny at the first, third, sixth and seventh backcross to identify and eliminate lineages with undesired recessive traits (Robinson et al., 2007). The resistance trait segregated (resistant:susceptible) in a 1:1 ratio in backcross progeny and 3:1 in self progeny from putatively heterozygous resistant plants. There was no obvious diminution of the resistance across backcross generations. Advanced backcross plants were indistinguishable from elite cotton genotypes under greenhouse conditions. Comparisons of 240 homozygous resistant BC6S2 plants with heterozygous, susceptible and recurrent parent plants in field plantings in 2006 showed normal lint quality and quantity. Two reniform nematode-resistant BC7 lines, LONREN-1 and LONREN-2, were released by USDA in April of 2007. In multiple location field tests, these resistant breeding lines suppressed population densities of R. reniformis by 85% to 98% (Robinson, unpublished data).
Other research on the G. longicalyx source of reniform resistance has focused on mapping of the responsible gene(s) and identification of markers linked to resistance genes. Marker discovery initially emphasized representation of all A-subgenome linkage groups, a wide separation of loci and more than 1,000 phenotyped plants spanning seven backcross and three selfed generations (Dighe, 2007). Resistance was found to be linked to the SSR marker BNL1066 and linkage group A03 (chromosome 11), which led to testing of 14 additional markers from public maps of A03 and its homeolog, D02. The results indicated that markers BNL3279_114, BNL1066_156 and BNL836_215 mapped on one side of the resistance locus within 1.4, 2.0 and 4.4 cM, respectively, whereas Fzlon mapped on the opposite side of the resistance locus with a linkage estimate of 4.5 cM (Dighe, 2007). Release of the resistant germplasm and marker information should facilitate incorporation of this trait into new cotton cultivars.
Resistance to M. incognita: Unfortunately, few root-knot resistant cotton cultivars with yield potential and fiber quality comparable to popular susceptible cultivars have been developed. Currently, the only root-knot nematode-resistant cotton cultivars available are Acala NemX, which is adapted to western cotton production areas, and Stoneville 5599BR. The obsolete cultivars LA 887 and H1560, which had levels of resistance comparable to NemX, had gained popularity and were grown in the southeastern US. Final nematode population densities following resistant cultivars in field trials were below the damage threshold for the next cotton crop (Fig. 1). Acala NemX provides increased yields in fields with moderate to severe infestations of M. incognita and suppresses nematode population densities (Ogallo et al., 1997). When Acala NemX was planted in the same infested plots for three consecutive years, the yield was stable, while the yield in plots planted to a root-knot susceptible cotton cultivar declined approximately 30% from the first year to the third year of the test (Ogallo et al., 1999). In addition to protecting the yield potential of the crop in infested fields, resistance to root-knot nematodes also suppressed final nematode population densities. The decline in nematode population densities after production of Acala NemX was beneficial for crops planted in the field after cotton. Yields of lima bean following 2 years of susceptible cotton in a field infested with M. incognita were only 25% of the yields following 2 years of the resistant Acala NemX (Ogallo et al., 1999). Nematode population densities in that study were about 4 times greater following the susceptible cultivar than following Acala NemX.
Despite these successes with cotton resistant to M. incognita, commercial seed producers have been reluctant to pursue the development of improved cultivars resistant to root-knot nematodes. Several recent advances have been made in nematode resistance genetics and gene mapping in cotton that should improve the efficiency and accuracy of incorporating resistance genes into elite cultivars. Further, such markers are highly informative in determining the uniqueness of and relationships between different resistance sources and in optimizing levels of resistance by combining resistance genes in various genetic backgrounds. It is anticipated that with these data, efficient marker-assisted selection systems will be used to develop a larger number of cultivars with high levels of resistance to M. incognita.
Previous and current resistance breeding work in cotton indicates a rich source of M. incognita-resistance genes present among Gossypium germplasm, especially in the allotetraploid species G. hirsutum and G. barbadense and in the A2 genome donor diploid species G. arboreum (Robinson et al, 2001; P. Roberts, M. Ulloa and C. Wang, unpublished). Several highly resistant breeding lines have been made available for cotton breeders, including Auburn 623 RNR (G. hirsutum), a transgressive segregant for resistance from a cross of Clevewilt 6-3-5 and Wild Mexico Jack Jones (Shepherd, 1974), and Auburn 634 RNR, developed from the cross Auburn 623 RNR x Auburn 56, which was used to develop the M-line series (M-120 RNR, M-315 RNR, etc.) of resistant genotypes (Shepherd, 1982; Shepherd et al., 1988; 1996). Resistant breeding lines including LA RN 4-4 and LA RN 1032 and the released cultivar Stoneville LA 887 were developed from crosses emanating from Clevewilt 6 as the likely resistance donor (see Robinson et al., 2001). In California, Acala NemX (Oakley, 1995) and Acala NemX HY (Anonymous, 2005) were released, with resistance derived from line N6072 for which the pedigree source of the resistance is not clear (Hyer and Jorgenson, 1984; Oakley, 1995; Robinson et al., 2001).
Genetic analysis of root-knot nematode resistance in these materials indicated the presence of multiple genes, both dominant and additive, and the occurrence of transgressive segregation for resistance (Shepherd, 1974). McPherson et al. (2004) reported a two-gene model for resistance in M-315 RNR derived from Auburn 623 RNR, and one recessive gene was indicated for moderate resistance in ‘Clevewilt 6-1’ (Bezawada et al., 2003). The first major resistance determinant to be mapped in cotton, rkn1 in Acala NemX, is a single, incompletely recessive gene identified using both SSR (Wang et al., 2006c) and AFLP and CAPS markers (Wang and Roberts, 2006). These markers, tightly linked to rkn1, are informative for comparing resistant genotypes, and the same molecular patterns were amplified with SSR marker CIR316 and CAPS marker GHACCl in resistant Acala NemX, Clevewilt 6, Auburn 623 RNR, Auburn 634 RNR, M-120, M-315, LA RN 4-4 and LA RN 1032 (Wang and Roberts, 2006). These results suggested that Acala NemX may have the same resistance source as Clevewilt 6, and a more detailed account of these germplasm source relationships is provided in Roberts et al. (2007). The SSR marker CIR316 is especially useful because its co-dominance enables the differentiation of heterozygous from homozygous individuals in progeny screening and selection (Wang et al., 2006c).
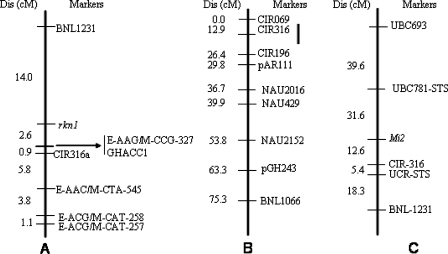
In the gene mapping strategy for rkn1 in Acala NemX using existing SSR markers placed throughout the cotton genome, SSR markers linked to M. incognita resistance were identified using segregating progenies and recombinant inbred lines from intraspecific (G. hirsutum) crosses and an interspecific cross with G. barbadense Pima S-7 (Wang et al., 2006a, 2006c). The rkn1 gene mapped to cotton LG A03 (Wang et al., 2006c), and A03 was subsequently assigned to chromosome 11 (Wang et al., 2006b). Markers CIR316 and BNL1231 in particular were highly informative for mapping, with CIR316 within 2–4 cM of rknl depending on the population used (Wang et al., 2006c). AFLP markers were found linked to rknl, and one was converted to a CAPS marker (GHACCl) and ultimately a SNP marker for high-throughput screening (Wang and Roberts, 2006). These and other markers linked to rknl on chromosome 11 are shown in Figure 6A. Subsequently, Shen et al. (2006) reported that one major dominant resistance gene in M-120 RNR (ex., Auburn 634 RNR) was also linked to SSR marker CIR316 on chromosome 11 (Fig. 6B), and one minor gene influencing resistance mapped to chromosome 7. In one cross within G. hirsutum of resistant (from Auburn 634 RNR) x susceptible near-isolines, Ynturi et al. (2006) used SSR markers to identify one additive and dominant gene on chromosome 14 and an additive gene on chromosome 11 contributing to M. incognita resistance. These studies are consistent with the RAPD and STS marker associations with Auburn 634 RNR-derived resistance on chromosome 11 determined by Nui et al. (2007) (Fig. 6C). In addition, there is evidence that at least one transgressive factor interacting with rknl also maps to this same region (Wang et al., 2007).
Fig. 6.
Location of root-knot nematode resistance genes on cotton chromosome 11. A) the resistance gene rknl in Acala NemX on chromosome 11 relative to four AFLP, one CAPS (GHACCl) and two SSR markers (CIR316a and BNL1231) in an F2:7 (Acala NemX x Acala SJ-2) segregating RIL population (from Wang and Roberts, 2006); B) location of a QTL for resistance (vertical bar) from the Auburn-634 RNR source in the vicinity of marker CIR316 in a combined F2 (M-120 RNR x Pima S-6) (from Shen et al., 2006); C) a resistance gene (putatively Mi2) from Auburn 634 RNR mapped to the CIR316 and UCR-STS (GHACCl in Fig. 1A) marker region in a F2 (ST 474 x Auburn 634 RNR) (from Nui et al., 2007). Distances are reported in Kosambi cM..
The emerging picture is the identification of a suite of genes for root-knot resistance, several of which map to the same region of chromosome 11, although their relationships to one another are unclear. Work is in progress to map BAC-end sequence-derived SSR markers into the chromosome 11 map, providing a good start to saturation mapping and providing a physical map for this region. Chromosome 11 is especially interesting because it also contains other resistance genes. Three large-effect QTL for resistance to Verticillium wilt were mapped to chromosome 11 (Bolek et al., 2005) in an interspecific cross with Acala 44. The reniform nematode (R. reniformis) resistance from G. longicalyx introgressed into upland cotton by Robinson et al. (2007) also is based on a trait that maps to chromosome 11 (Dighe, 2007), and Fusarium wilt resistance also maps to this chromosome (Roberts, unpublished data). Thus chromosome 11 represents a rich resource for resistance gene exploitation.
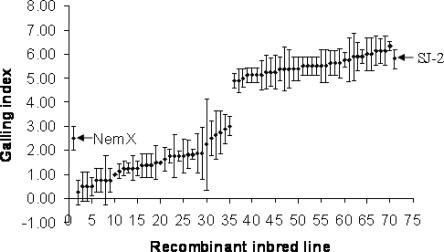
Higher levels of M. incognita resistance in cotton resulting from transgressive segregation were first reported by Shepherd (1974) and can be obtained with factors contributed by susceptible parents in intraspecific and interspecific crosses (Roberts et al., 2007; Wang et al., 2008). Transgressive segregation for resistance was found in a RIL population of the cross Acala NemX x Acala SJ-2, in which susceptible Acala SJ-2 contributed to the level of resistance (Wang et al., 2006a). In this example (Fig. 7), galling reaction phenotypes of the individual RIL form resistant and susceptible classes based on presence and absence of gene rknl, respectively, and also form ‘Acala NemX equivalent’ and ‘higher than Acala NemX’ resistant sub-classes due to absence/presence of the transgressive factor from Acala SJ-2. Analysis of the rknl gene in interspecific crosses between Acala NemX and susceptible G. barbadense Pima S-7 also revealed transgressive segregation (Wang et al., 2007). The F1 plants were much more resistant than the resistant Acala NemX parent, and distinct transgressive segregants with resistance phenotypes beyond the range of the parent were found in test-crosses and advanced segregating progenies (Wang et al., 2007). The transgressive segregation effects on cotton nematode-resistance traits present a valuable resource for cotton improvement because extreme genotypes beyond the parent range are generated, and these can be selected in breeding programs, especially with markers available for the trait determinants.
Fig. 7.
Distribution of different classes of RKN resistance reaction of F2:7 RIL (Acala NemX x Acala SJ-2) based on galling index. Mean values of four plants per line plus SD bar. Mean scores of the resistant (Acala NemX) and susceptible (Acala SJ-2) parents are indicated (Wang et al., 2006a). Galling index: 0 to 10 scale; 0 = no galling, and 10 = severe galling.
Conclusions
Plant-parasitic nematodes are increasingly recognized as economically important pests of cotton. Research and development efforts, in both the public and private sectors, to improve management of these pathogens have increased substantially since 1990. Because the economics of cotton production rather than ability to suppress nematode population densities largely govern the strategies used by most growers, many effective approaches to nematode management are under-utilized. As evidenced by the emphasis in this review, there is much hope that the development and deployment of high-quality cotton cultivars with effective levels of genetic resistance will find widespread acceptance among growers and reduce the impact of M. incognita and R. reniformis on cotton yields. Further, it is generally acknowledged that greater effort on the part of private sector breeders for nematode resistance will be dependent on development of efficient, high-throughput marker-assisted selection protocols. However, in the final analysis it will likely be the yield potential and fiber quality of these resistant cultivars, rather than the level of resistance itself, that will determine grower acceptance and whether host resistance plays a more important role in future nematode management systems in cotton than it does today.
Recent advances in engineered resistance to M. incognita in Arabidopsis based on RNA interference (Huang et al., 2006) and the recent announcement by a company that it was working to develop RNA interference technology to engineer nematode resistance in corn (http://www.divergence.com/press/20070917.html) bring the hope of additional sources of resistance to several nematodes.
New, more efficacious and environmentally safe nematicides are needed along with technologies for more efficient application. The clustered distribution of most nematodes within a field poses a serious challenge to efficient placement of nematicides. Cotton production in the US is an intensive production system with narrow profit margins. Corn and soybean have traditionally had even lower profit margins, thus nematode management using rotation with either of these crops is likely to be limited. An increase in the economic value of these or other potential rotation crops would affect the use of rotation as a management tactic. Regardless, such practices as crop rotations or organic soil amendments will be used profitably by some producers.
Footnotes
This manuscript is a summary of information presented in a symposium at the joint APS/SON meetings in San Diego, CA 31 July 2007. The authors are grateful to all persons who have shared their data with us for presentation in that symposium and in this manuscript. This work was supported in part by cooperative research agreements from Cotton Incorporated (to AFR, PAR, JLS) and a University of California Discovery Grant (to PAR).
Mention of a trademark, warranty, proprietary product or vendor does not constitute a guarantee by the U. S. Department of Agriculture and does not imply approval or recommendation of the product to the exclusion of others that may be suitable.
This paper was edited by David Bird.
Literature Cited
- Allen SJ, Nehl DB, Mondal AH, Jhoran O. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 2004. Seed treatments to induce resistance to Fusarium wilt and black root rot in cotton; pp. 365–367. [Google Scholar]
- Anonymous. Washington, DC: Plant Variety Protection Office, Agricultural Marketing Service, US Department of Agriculture; 2005. Plant variety protection certification no. 200500113 for cotton Acala NemX HY. [Google Scholar]
- Atkinson GF. Some diseases of cotton. III. Frenching. Bulletin Alabama Agricultural Experiment Station. 1892;41:19–29. [Google Scholar]
- Atkinson GF. Nematode root-galls. Alabama Polytech. Institute and Agricultural Experiment Station. Bulletin. 1899;9:177–226. [Google Scholar]
- Barker KR, Koenning SR. Developing sustainable systems for nematode management. Annual Review of Phytopathology. 1998;36:165–205. doi: 10.1146/annurev.phyto.36.1.165. [DOI] [PubMed] [Google Scholar]
- Beasley JP, Jones JE. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 1985. The current status in the development of resistance to the reniform nematode in cotton in Louisiana; pp. 23–25. [Google Scholar]
- Bednarz CW, Brown SN, Fanders JT, Tankersley TB, Brown SM. Effects of foliar applied harpin protein on cotton lint yield, fiber quality, and crop maturity. Communications in Soil Science and Plant Analysis. 2002;33:933–945. [Google Scholar]
- Bell AA, Robinson AF. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 2004. Development and characteristics of triple species hybrids used to transfer reniform nematode resistance from Gossypium longicalyx to Gossypium hirsutum ; pp. 422–426. [Google Scholar]
- Bezawada C, Saha S, Jenkins JN, Creech RG, McCarty JC. SSR marker(s) associated with root-knot nematode resistance gene(s) in cotton. Journal of Cotton Science. 2003;7:179–184. [Google Scholar]
- Blasingame DC. Memphis, TN: The National Cotton Foundation; 1993. Cotton Nematodes: Your Hidden Enemies. [Google Scholar]
- Blasingame D. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 2006. 2005 Cotton Disease Loss Estimate; pp. 155–157. [Google Scholar]
- Bolek Y, El-Zik KM, Pepper AE, Bell AA, Magill CM, Thaxton PM, Reddy OUK. Mapping of verticillium wilt resistance genes in cotton. Plant Science. 2005;168:1581–1590. [Google Scholar]
- Brown MS, Menzel MY. New trispecies hybrids in cotton. Journal of Heredity. 1950;41:291–292. [Google Scholar]
- Carter W. A promising new soil amendment and disinfectant. Science. 1943;97:383–384. doi: 10.1126/science.97.2521.383. [DOI] [PubMed] [Google Scholar]
- Carter WW. Resistance and resistant reaction of Gossypium arboreum to the reniform nematode, Rotylenchulus reniformis . Journal of Nematology. 1981;13:368–374. [PMC free article] [PubMed] [Google Scholar]
- Christie JR. Some preliminary tests to determine the efficacy of certain substances when used as soil fumigants to control the root-knot nematode, Heterodera marioni (Cornu) Goodey. Proceedings of the Helminthological Society of Washington. 1945;12:14–19. [Google Scholar]
- Christie JR, Perry VG. A low phytotoxic nematicide of the organic phosphate group. Plant Disease Reporter. 1958;42:74–75. [Google Scholar]
- Crow WT, Weingartner DP, Dickson DW. Effects of potato-cotton cropping systems and nematicides on plant-parasitic nematodes and crop yields. Journal of Nematology. 2000;32:297–302. [PMC free article] [PubMed] [Google Scholar]
- Davis RF, Baird RE, McNeill RD. Efficacy of cotton root destruction and winter crops for suppression of Hoplolaimus columbus . Supplement to the Journal of Nematology. 2000;32:550–555. [PMC free article] [PubMed] [Google Scholar]
- Davis RF, Koenning SR, Kemerait RC, Cummings TD, Shurley TD. Rotylenchulus reniformis management in cotton with crop rotation. Journal of. Nematology. 2003;35:58–64. [PMC free article] [PubMed] [Google Scholar]
- Dighe ND. Texas A&M University; 2007. Introgression of reniform nematode resistance and other germplasm from Gossypium longicalyx and G. armourianum into G. hirsutum ; p. 149. Ph. D. Dissertation, [Google Scholar]
- Evans K, Webster RM, Halford PD, Baker AD, Russell MD. Site-specific management of nematodes—pitfalls and practicalities. Journal of Nematology. 2002;34:194–199. [PMC free article] [PubMed] [Google Scholar]
- Fassuliotis G. Host range of the Columbia lance nematode Hoplolaimus columbus . Plant Disease Reporter. 1974;58:1000–1002. [Google Scholar]
- Gaur HS, Perry RN. The biology and control of the plant-parasitic nematode Rotylenchulus reniformis . Agricultural Zoology Reviews. 1991;4:177–212. [Google Scholar]
- Huang G, Allen R, Davis EL, Baum TJ, Hussey RS. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proceedings of the National Academy of Sciences. 2006;103:14302–14306. doi: 10.1073/pnas.0604698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey RS. Effect of subsoiling and nematicides on Hoplolaimus columbus populations and cotton yield. Journal of Nematology. 1977;9:83–86. [PMC free article] [PubMed] [Google Scholar]
- Hyer AH, Jorgenson EC. Proceedings Beltwide Cotton Conferences. Memphis, TN: National, Cotton Council of America; 1984. Root-knot nematode resistance in cotton breeding: Techniques and results; pp. 377–379. [Google Scholar]
- Jeffers DP, Roberts PA. Effect of planting date and host genotype on the root-knot nematode-Fusarium wilt disease complex of cotton. Phytopathology. 1993;83:645–654. [Google Scholar]
- Johnson AW, Feldmesser J. Nematicides—a historical review. In: Veech JA, Dickson DW, editors. Vistas on Nematology. Hyattsville, MD: Society of Nematologists; 1987. pp. 448–454. [Google Scholar]
- Johnson OM, Godfrey GH. Chloropicrin for nematode control. Industrial Engineering Chemistry. 1932;24:311–313. [Google Scholar]
- Koenning SR, Barker KR, Edmisten KL, Bowman DT, Morrison DE. Effects of rate and time of application of poultry litter on Hoplolaimus columbus on cotton. Plant Disease. 2003a;87:1244–1249. doi: 10.1094/PDIS.2003.87.10.1244. [DOI] [PubMed] [Google Scholar]
- Koenning SR, Edmisten KL, Barker KR, Morrison DE. Impact of cotton production system on Hoplolaimus columbus . Journal of Nematology. 2003b;35:73–77. [PMC free article] [PubMed] [Google Scholar]
- Koenning SR, Kirkpatrick TL, Starr JL, Walker NA, Wrather JA, Mueller JD. Plant-parasitic nematodes attacking cotton in the U.S.: Old and emerging problems. Plant Disease. 2004;88:100–113. doi: 10.1094/PDIS.2004.88.2.100. [DOI] [PubMed] [Google Scholar]
- Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. Supplement to the Journal of Nematology. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Konan ON, D'Hont A, Baudoin J-P, Mergeai G. Cytogenetics of a new trispecies hybrid in cotton: [(Gossypium hirsutum L. x G. longicalyx Hutch. & Lee] Plant Breeding. 2007;126:176–181. [Google Scholar]
- Lawrence GW, McLean KS. Effect of foliar applications of oxamyl with aldicarb for the management of Rotylelnchulus reniformis on cotton. Supplement to the Journal of Nematology. 2000;32:542–549. [PMC free article] [PubMed] [Google Scholar]
- Lawrence GW, McLean KS. Reniform nematodes. In: Kirkpatrick TL, Rothrock CS, editors. Compendium of Cotton Diseases. 2nd edition. St. Paul, MN: APS Press; 2001. pp. 42–44. [Google Scholar]
- Lawrence GW, McLean KS. Foliar applications of oxamyl with aldicarb for the management of Meloidogyne incognita on cotton. Nematropica. 2002;32:103–112. [PMC free article] [PubMed] [Google Scholar]
- McBeth CW. Some practical aspects of soil fumigation. Plant Disease Reporter Supplement. 1954;227:95–97. [Google Scholar]
- McPherson MG, Jenkins JN, Watson CE, McCarty JC. Inheritance of root-knot nematode resistance in M-315 RNR and M78-RNR cotton. Journal of Cotton Science. 2004;8:154–161. [Google Scholar]
- Monfort WS, Kirkpatrick TL, Long DL, Rideout S. Efficacy of a novel nematicidal seed treatment against Meloidogyne incognita on cotton. Journal of Nematology. 2006;38:245–249. [PMC free article] [PubMed] [Google Scholar]
- Monfort WS, Kirkpatrick TL, Rothrock CS, Mauromoustakos A. Potential for site-specific management of Meloidogyne incognita in cotton using soil textural zones. Journal of Nematology. 2007;39:1–8. [PMC free article] [PubMed] [Google Scholar]
- Muhammad N, Jones JE. Genetics of resistance to reniform nematode in Upland cotton. Crop Science. 1990;30:13–16. [Google Scholar]
- Niu C, Hinchliffe DJ, Cantrell RG, Wang C, Roberts PA, Zhang J. Identification of molecular markers associated with root-knot nematode resistance in upland cotton. Crop Science. 2007;47 In press. [Google Scholar]
- Oakley SR. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 1995. CPCSD Acala G-225: A new nematode-resistant Acala variety for California's San Joaquin Valley; p. 39. [Google Scholar]
- Ogallo JL, Goodell PB, Eckert J, Roberts PA. Evaluation of NemX, a new cultivar of cotton with high resistance to Meloidogyne incognita . Journal of Nematology. 1997;29:531–537. [PMC free article] [PubMed] [Google Scholar]
- Ogallo JL, Goodell PB, Eckert J, Roberts PA. Management of root-know nematodes with resistant cotton cv. NemX. Crop Science. 1999;39:418–421. [Google Scholar]
- Overstreet C, Burris E, Padgett B, Wolcott M. Defining nematode management zones in cotton. Journal of Nematology. 2007;39:84–85. (abstr.). [Google Scholar]
- Penteado M, Kirkpatrick TL, Still JA. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 2005. Effect of delayed infection by the root-knot nematode on damage to cotton; p. 147. [Google Scholar]
- Putter I, MacConnell JG, Preiser FA, Haidri AA, Ristich SS, Dybas RA. Avermectins: Novel insecticides, acaricides and nematicides from a soil microorganism. Experientia. 1981;37:963–964. [Google Scholar]
- Raski DJ. Soil fumigation for the control of nematodes on grape replants. Plant Disease Reporter. 1954;38:811–817. [Google Scholar]
- Riegel C, Fernandez FA, Noe JP. Chicken litter soil amendment effects on soilborne microbes and Meloidogyne incognita on cotton. Plant Disease. 1996;84:1275–1281. doi: 10.1094/PDIS.2000.84.12.1275. [DOI] [PubMed] [Google Scholar]
- Robbins RT, Barker KR. Comparison of host range and reproduction among populations of Belonolaimus longicaudatus from North Carolina and Georgia. Plant Disease Reporter. 1973;57:750–754. [Google Scholar]
- Roberts PA, Ulloa M, Wang C. Proceedings Fourth World Cotton Research Conference. Lubbock, TX: 2007. Host plant resistance to root-knot nematode in cotton. [Google Scholar]
- Roberts PA, VanGundy SD, McKinney HE. Effects of soil temperature and planting date of wheat on Meloidogyne incognita reproduction. Journal of Nematology. 1981;13:338–344. [PMC free article] [PubMed] [Google Scholar]
- Robinson AF. Reniform nematodes: Rotylenchulus species. In: Starr JL, Cook R, Bridge J, editors. Plant Resistance to Parasitic Nematodes. Wallingford, UK: CABI Publishing; 2002. pp. 153–174. [Google Scholar]
- Robinson AF. Reniform in U.S. cotton: When, where, why, and some remedies. Annual Review of Phytopathology. 2007;45:263–288. doi: 10.1146/annurev.phyto.45.011107.143949. [DOI] [PubMed] [Google Scholar]
- Robinson AF, Akridge JR, Bradford JB, Cook CC, Gazaway WS, McGawley EC, Starr JL, Young LD. Suppression of Rotylenchulus reniformis 122 cm deep endorses resistance introgression in Gossypium . Journal of Nematology. 2006;38:195–209. [PMC free article] [PubMed] [Google Scholar]
- Robinson AF, Bell AA, Dighe ND, Menz MA, Nichols RL, Stelly DM. Introgression of resistance to nematode Rotylenchulus reniformis into upland cotton (Gossypium hirsutum) from G. longicalyx . Crop Science. 2007;47:1865–1877. [Google Scholar]
- Robinson AF, Bowman DT, Cook CG, Jenkins JN, Jones JE, May LO, Oakley SR, Oliver MJ, Roberts PA, Robinson M, Smith CW, Starr JL, Stewart JMcD. Nematode resistance. In: Kirkpatrick TL, Rothrock CS, editors. Compendium of Cotton Diseases. St. Paul, MN: APS Press; 2001. pp. 68–79. [Google Scholar]
- Robinson AF, Bridges AC, Percival AE. New sources of resistance to the reniform (Rotylenchulus reniformis Linford and Oliveira) and root-knot (Meloidogyne incognita (Kofoid & White) Chitwood) nematode in upland (Gossypium hirsutum L.) and sea island (G. barbadense L.) cotton. Journal of Cotton Science. 2004;8:191–197. [Google Scholar]
- Robinson AF, Cook CG, Percival AE. Resistance to Rotylenchulus reniformis and Meloidogyne incognita race 3 in the major cotton cultivars planted since 1950. Crop Science. 1999;39:850–858. [Google Scholar]
- Robinson AF, Inserra RN, Caswell-Chen EP, Vovlas N, Troccoli A. Rotylenchulus species: Identification, distribution, host ranges, and crop plant resistance. Nematropica. 1997;27:127–180. [Google Scholar]
- Robinson AF, Percival AE. Resistance to Meloidogyne incognita race 3 and Rotylenchulus reniformis in wild accessions of Gossypium hirsutum and G. barbadense from Mexico. Journal of Nematology. 1997;29:746–755. [PMC free article] [PubMed] [Google Scholar]
- Shen X, Van Becelaere G, Kumar P, Davis RF, May MO, Chee P. QTL mapping for resistance to root-knot nematodes in the M-120 RNR Upland cotton line (Gossypium hirsutum L.) of the Auburn 623 RNR source. Theoretical and Applied Genetics. 2006;113:1539–1549. doi: 10.1007/s00122-006-0401-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RL. Transgressive segregation for root-knot nematode resistance in cotton. Crop Science. 1974;14:872–875. [Google Scholar]
- Shepherd RL. Registration of three germplasm lines of cotton. Crop Science. 1982;22:692. [Google Scholar]
- Shepherd RL, McCarty JC, Jenkins JN, Parrott WL. Registration of twelve nonphotoperiodic lines with root-knot nematode resistant primitive cotton germplasm. Crop Science. 1988;28:868–869. [Google Scholar]
- Shepherd RL, McCarty JC, Jenkins JN, Parrott WL. Registration of nine cotton germplasm lines resistant to root-knot nematode. Crop Science. 1996;36:820. [Google Scholar]
- Starr JL, Carneiro RG, Ruano O. Nematode parasites of cotton and other tropical fiber crops. In: Luc M, Sikora RA, Bridge J, editors. Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. 2nd Ed. Wallingford, UK: CABI Publishing; 2005. pp. 733–750. [Google Scholar]
- Stewart JM, Robbins RT. Evaluation of Asiatic cottons for resistance to reniform nematode. In: Oosterhius DM, editor. Proc. 1994 Cotton Research Meeting, Special Report 166. Fayetteville, AR: Arkansas Agricultural Experiment Station; 1995. pp. 165–168. [Google Scholar]
- Stewart JM, Robbins RT. Proceedings Beltwide Cotton Conferences. Memphis, TN: National Cotton Council of America; 1996. Identification and enhancement of resistance to reniform nematode in cotton germplasm; p. 255. [Google Scholar]
- USDA-Agricultural Marketing Service. Cotton varieties planted, United States 2007 crop. 2007 http://www.ams.usda.gov/cottonrpts/MNPDF/mp_cn833.PDF.
- Wang C, Matthews WC, Roberts PA. Phenotypic expression of rkn1-mediated Meloidogyne incognita resistance in Gossypium hirsutum populations. Journal of Nematology. 2006a;38:250–257. [PMC free article] [PubMed] [Google Scholar]
- Wang C, Roberts PA. Development of AFLP and derived CAPS markers for root-knot nematode resistance in cotton. Euphytica. 2006;152:185–196. [Google Scholar]
- Wang K, Song X, Han Z, Guo W, Yu JZ, Sun J, Pan J, Kohel RJ, Zhang T. Complete assignment of the chromosomes of Gossypium hirsutum L. by translocation and fluorescence in situ hybridization mapping. Theoretical and Applied Genetics. 2006b;113:73–80. doi: 10.1007/s00122-006-0273-7. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulloa M, Roberts PA. Identification and mapping of microsatellite markers linked to a root-knot nematode resistance gene (rkn1) in Acala NemX cotton (Gossypium hirsutum L.) Theoretical and Applied Genetics. 2006c;112:770–777. doi: 10.1007/s00122-005-0183-0. [DOI] [PubMed] [Google Scholar]
- Wang G, Ulloa M, Roberts PA. A transgressive segregation factor (RKN2) in Gossypium barbadense for nematode resistance clusters with gene rkn1 in G. hirsutum . Molecular Genetics and Genomics. 2008;279:41–52. doi: 10.1007/s00438-007-0292-3. [DOI] [PubMed] [Google Scholar]
- Weaver DB, Lawrence KS, Van Santen E. Reniform nematode resistance in upland cotton germplasm. Crop Science. 2007;47:19–24. [Google Scholar]
- Wei ZM, Beer SV. Harpin from Erwinia amylovora induces plant resistance. Acta Horticulturae. 1996;411:223–225. [Google Scholar]
- Weiden MHJ, Moorfield HH, Payne LK. O-(methyl-carbamoyl) oximes: A new class of carbamate insecticide-acaricides. Journal of Economic Entomology. 1965;58:154–155. [Google Scholar]
- Wheeler TA, Kaufman HW, Baugh B, Kidd P, Schuster G, Siders K. Comparison of variable and single-rate applications of aldicarb on cotton yields in fields infested with Meloidogyne incognita . Supplement to the Journal of Nematology. 1999;31:700–708. [PMC free article] [PubMed] [Google Scholar]
- Wolcott M, Overstreet C, Burris E, Burns D, Cook D, Padgett B. Proceedings of the 8th International Conference on Precision Agriculture and Other Precision Resources Management. Madison, WI: ASA/CSSA/SSA; 2006. Site-specific nematode management for cotton in the Mississippi River Alluvial soils of Louisiana. [Google Scholar]
- Wrather JA, Stevens WE, Kirkpatrick TL, Kitchen RN. Effects of site-specific application of aldicarb on cotton in a Meloidogyne incognita-infested field. Journal of Nematology. 2002;34:115–119. [PMC free article] [PubMed] [Google Scholar]
- Wyse-Pester D, Wiles LJ, Westra P. The potential for mapping nematode distributions for site-specific management. Journal of Nematology. 2002;34:80–87. [PMC free article] [PubMed] [Google Scholar]
- Yik C-P, Birchfield W. Resistant germplasm in Gossypium species and related plants to Rotylenchulus reniformis . Journal of Nematology. 1984;16:146–153. [PMC free article] [PubMed] [Google Scholar]
- Ynturi P, Jenkins JN, McCarty JC, Jr, Gutierrez OA, Saha S. Association of root-knot nematode resistance genes with simple sequence repeat markers on two chromosomes in cotton. Crop Science. 2006;46:2670–2674. [Google Scholar]