Abstract
Differences in activity between infective juveniles (IJ) of the entomopathogenic nematode Steinernema carpocapsae that emerged directly from cadavers onto either a sand or agar substrate compared with those emerging from a cadaver into water and then being placed on the same substrate are known to occur. Differences between S. carpocapsae IJ that emerged directly from a cadaver vs. those that emerged from a cadaver and held in water were further elucidated. Dispersed and non-dispersed IJ from a cadaver were compared with those held in water between two time periods designated as early- (first two days) or late-emerging IJ (seventh day). A significantly greater proportion of early-emerging IJ from the cadaver treatment dispersed, compared with late-emerging IJ from a cadaver or either group of emerging IJ held in aqueous suspension. Moreover, IJ from cadavers were more infectious than those from the aqueous suspensions, and IJ that dispersed were less infectious than those that did not disperse. IJ that emerged early were mostly males, whereas those that emerged late were mostly females. For the non-dispersed IJ, most that emerged early were males, and those that emerged later were females, but among dispersing IJ, there was no difference in sex ratio between early- and late-emerging nematodes.
Keywords: Ambush forager, foraging behavior, insect-parasitic nematode, Steinernema carpocapsae, Steinernematidae
Entomopathogenic nematodes in the genera Steinernema and Heterorhabditis are lethal pathogens of soil insects and can actively seek and infect their insect host, killing it within 48 hours of infection (Kaya and Gaugler, 1993). The free-living infective juveniles (IJ) enter the host through natural openings (mouth, anus and spiracles) and penetrate into the hemocoel; in the case of heterorhabditids, they can also penetrate directly through soft cuticle into the hemocoel. Once inside the hemocoel, the IJ release a mutualistically associated bacterium (Xenorhabdus spp. for steinernematids and Photorhabdus spp. for heterorhabditids) that kills the host and, along with host sterols, provides nutrition for one to three generations of nematodes. As host resources are depleted, IJ are formed, exit the host cadaver and forage for new hosts.
The foraging behaviors of free-living parasite stages, including the infective stage of entomopathogenic nematode species, greatly influence their probability of finding a host. Thus, these behaviors should be shaped by natural selection to maximize the probability of an individual IJ to successfully infect a host. To find their host, the IJ of steinernematid species adopt foraging behaviors that vary along a continuum between ambush and cruise foraging strategies (Lewis et al., 1992, 1993; Campbell and Gaugler, 1993; Grewal et al., 1994; Campbell and Gaugler, 1997; Lewis et al., 2006), whereas those of heterorhabditid species primarily adopt a cruise foraging strategy (Grewal et al., 1994). Our focus in this paper is on the ambushing IJ of the steinernematid Steinernema carpocapsae (Weiser). It ambushes by standing on its tail and lifting up to 95% of its body to reduce the surface-tension forces holding it to the substrate (i.e., standing behavior), which enables it to attach to a passing insect (Campbell and Kaya, 1999, 2000). This standing behavior for an ambush forager is considered a stationary scanning or ambushing bout separated by movements such as crawling or jumping. Thus, although S. carpocapsae is an ambushing nematode, the IJ do disperse on, in, or above the soil.
In earlier studies, a number of researchers demonstrated that when the IJ of S. carpocapsae were suspended in water and placed on or in soil, a majority of them stayed approximately where they were placed (Moyle and Kaya, 1981; Schroeder and Beavers, 1987). Subsequently, Shapiro and Glazer (1996) reported that Heterorhabditis bacteriophora Poinar or S. carpocapsae IJ that emerged directly from cadavers onto either sand or agar dispersed farther than those that emerged from a cadaver into water and were then placed on the same substrate. Infectivity of H. bacteriophora was 10 times greater for the IJ that emerged from cadavers naturally, compared with IJ that emerged into water and were then artificially applied (Shapiro and Lewis, 1999). There was no significant difference in infectivity of S. carpocapsae between IJ from the cadavers and those held in water suspension and applied artificially, but those that emerged into water developed more slowly once they infected a host (Lewis et al., 2002).
Because foraging (i.e., dispersal) is an essential function of the transmission stage of parasites, the existence of biological differences between dispersed and non-dispersed IJ of S. carpocapsae was investigated. Accordingly, we report herein the dispersal, infectivity and sex ratio of S. carpocapsae when the IJ emerged from a cadaver and were (i) collected in water, then applied to a sand substrate, allowed to disperse (or not disperse), extracted from the sand and then exposed to an insect host or (ii) allowed to directly disperse (or not disperse) from a cadaver on the sand substrate, extracted from the sand and then exposed to an insect host. Comparisons of dispersed and non-dispersed nematodes were made between two time periods designated as early- or late-emerging IJ.
Materials and Methods
Insect source and nematode maintenance: Galleria mellonella (L.) larvae were obtained from Rainbow Mealworms, Inc. (Compton, CA). Only larvae weighing between 200 and 260 mg were used in the experiments.
Steinernema carpocapsae: All Strain was cultured in G. mellonella larvae (Kaya and Stock, 1997). Briefly, 200 IJ in 1 ml of water were pipetted onto a filter paper in a 100 x 15-mm petri dish containing 10 G. mellonella larvae. Nematode-killed larvae were placed on White traps (White, 1927), and IJ were collected daily and stored in water at 15°C (Kaya and Stock, 1997).
IJ from water suspension: To assess the dispersal, infectivity and sex ratio of S. carpocapsae IJ in a water suspension, a nematode-killed G. mellonella larva was placed on a White trap as described above and maintained at room temperature (23 ± 2°C). When IJ started to emerge, they were collected daily. IJ collected on the first 2 d (designated as early-emerging) and d 7 (designated as late-emerging) after initial emergence from the cadavers were kept for the experiment. The IJ from the first 2 d or d 7 were rinsed three times with deionized water, counted and diluted to 3,000 IJ/ml, placed in 250-ml tissue culture flasks and stored at 15°C. These IJ were used within 2 wk after emergence as a previous study showed that neither S. carpocapsae (All Strain) movement nor infectivity decreased in the first 2 wk of storage in water (Lewis et al., 1995b). Comparisons were made with each group of early- and late-emerging IJ from the same cadavers.
Before the dispersal experiments were initiated, the infectivities and sex ratios of the early- and late-emerging IJ were checked via the one-on-one assay (Kaya and Stock, 1997). This infectivity assay was done by placing 1 IJ suspended in 25 μl water on a filter paper (1.5 cm) in a well of a 24-well plate. One G. mellonella larva was added to the well. The plates were incubated at 25°C for 72 hr. At that time, larval mortality due to nematode infection was assessed. Each cadaver was dissected to confirm nematode infection by the typical signs (i.e., cadaver that was ocher to tan and flaccid with no putrid odor) associated with S. carpocapsae infection, and the nematode, if present, was sexed. To assess the quality of G. mellonella used, 25 μl deionized water alone was applied to the filter paper in the well, and a host larva added. These were incubated as above and mortality in the absence of added IJ measured. Forty-eight nematodes were individually tested for each early- and late-emerging treatment.
To examine the dispersal behavior, a square plastic container (19 × 19 cm2) (Rubbermaid, Wooster, OH) containing 700 g of sand (<2 mm diam.) to a depth of 1.4 cm was used as the experimental arena. The sand was moistened with deionized water to 10% moisture (−2.75 kPa), and 5,000 IJ (early- or late-emerging) were pipetted onto the sand in the center of the container and incubated at 25°C for 72 hr. After this period, the container was removed, and the sand in the container was divided into 25 3.8 cm squares with a spatula. The center square (the point of IJ placement) and the squares on the edge of the arena were placed individually into 250-ml beakers. The sand was carefully washed 3 times with deionized water to extract the IJ. The number of non-dispersed IJ extracted from a center section and the furthest dispersed IJ from the squares at the edge were counted. The IJ from the squares from the edges were combined, and the proportion of dispersed and non-dispersed IJ was calculated.
The extracted IJ were held in deionized water for no more than 2 d before the infectivity and sex ratio tests were done. The one-on-one infectivity assay as described above was followed, and 48 nematodes were individually assessed for each early- and late-emerging treatment. This experiment was repeated over time using IJ from eight different cohorts of cadavers.
IJ from cadaver: G. mellonella cadavers with S. carpocapsae were kept at room temperature until IJ began to emerge from them. A cadaver with emerging IJ (early-emerging) was placed on the sand surface at the center of the plastic container arena as described above and incubated at 25°C for 6 hr. After this time period, the cadaver was removed and placed on a White trap. The arena with the early-emerging IJ from the cadavers was placed at 25°C for 72 hr and then removed, and the sand was divided into 25 squares as described above. The sand squares were handled as described for the “IJ from water suspension” experiment. The IJ were then assayed for infectivity and sex ratio as described above. Because the IJ from the cadavers could not be collected, the infectivity and sex ratio of the early- and late-emerging IJ could not be checked.
Six days later, the same cadaver with emerging IJ (late-emerging) was placed on the sand surface in a new arena for 6 or 12 hr. After this time period, the cadaver was removed, the plastic container was incubated at 25°C for 72 hr and the proportion of dispersed IJ, infectivity and sex ratio of early- and late-emerging IJ were examined in the same way as above. This experiment had between 8 and 10 repetitions per treatment.
Statistical analyses: Three separate analyses were performed on the data collected: treatment effects on dispersal, infectivity and sex ratio. The first compared the proportions of nematodes dispersing from the central release point to the edge of the containers with a 2-factor ANOVA (SAS, 2003, v9.1, Cary, NC). The main effects (explained in detail above) were: nematodes released directly from their host cadavers vs. nematodes applied to sand in water, and nematodes that emerged early vs. nematodes that emerged late from their cadaver. Proportions of dispersing nematodes were transformed by arcsine for normalization before analysis. When significant interaction between the main effects occurred, least squared means analysis was performed to separate means.
The second analysis compared the infectivity of IJ in a 3-factor ANOVA. Main effects on infectivity (explained in detail above) were: nematodes released directly from their host cadavers vs. nematodes applied to sand in water, early-emerging vs. late-emerging and dispersed vs. non-dispersed. There was a significant two-way interaction between dispersal and IJ from cadaver vs. IJ stored in an aqueous suspension; thus least squared means analysis was conducted for means separations at this level. No other significant interactions were found.
Finally, the sex ratio of IJ that infected G. mellonella in the one-on-one bioassay was compared among treatments by contingency table analysis. Totals of males and females that were found in the nematode-killed insects were compared. The overall 2 column by 8 row analysis was significant, so pair-wise comparisons were made among treatments.
Results
The quality of the G. mellonella larvae used in the experiments was excellent, with no mortality of the untreated controls during the 2-d infectivity assay period. The sex ratio of the stock nematodes was 45% male. One-on-one infectivity tests showed that 70.1% of the stock nematodes infected their host.
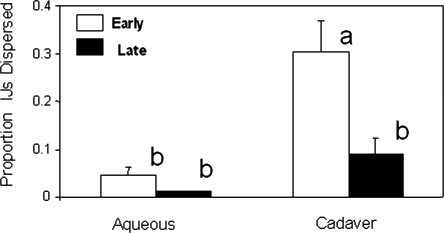
Dispersal: The overall analysis of variance showed significant differences among the various treatment combinations (F = 11.12; df = 3, 32; P < 0.0001) with respect to IJ dispersal. Both main effects, cadaver vs. aqueous (F = 17.03; df = 1, 32; P < 0.0002) and early vs. late (F = 9.45; df = 1, 32; P < 0.0043) demonstrated differences in the proportion of IJ that dispersed. There was also a significant interaction between the main effects (F = 5.13; df = 1, 32; P < 0.0304). Thus, pair-wise comparisons were conducted by least squared means, and this analysis showed that a greater proportion of early-emerging IJ from the cadaver treatment dispersed compared with the rest of the treatments (Fig. 1).
Fig. 1.
Mean proportion of early- and late-emerging infective juveniles (IJ) of Steinernema carpocapsae held in an aqueous suspension or from a cadaver dispersing on a sand substrate. The same lowercase letters above the bars indicate no significant difference among means at P = 0.05. Bars show standard error of the means.
Infectivity and sex ratio: The overall analysis of variance comparing infectivity measures showed significant differences among the various treatment combinations (F = 6.41; df = 3, 71 = 8; P < 0.0001). The main effects of dispersed vs. non-dispersed IJ (F = 28.81; df = 1, 71; P < 0.0001) and cadaver vs. aqueous application (F = 4.83; df = 1, 71; P < 0.0312) had significant effects on infectivity. The main effect of early- vs. late-emerging IJ was not significant (F = 3.65; df = 1, 71; P < 0.0601). IJ from cadavers were more infectious than those from the aqueous suspensions, and IJ that dispersed were less infectious than those that did not disperse. There was a significant interaction between dispersed vs. non-dispersed IJ and cadaver vs. aqueous application (F = 16.41; df = 1, 71; P < 0.0001). This interaction shows that infection by non-dispersed IJ was greater than infection by dispersed IJ for the aqueous applied IJ (Fig. 2A), but not for the cadaver IJ (Fig. 2B). No other interactions between the main effects were significant.
Fig. 2.
Mean proportion of mortality of Galleria mellonella larvae using an infectivity assay in which one dispersed or non-dispersed infective juvenile (IJ) of Steinernema carpocapsae is exposed to one G. mellonella larva. The source of the dispersed or non-dispersed IJ is from a sand substrate either from an early- or late-emerging nematode held in an aqueous suspension (A) or from a cadaver (B). The same lowercase letters above the bars indicate no significant difference among means at I = 0.05 for (A). Bars show standard error of the means.
The overall contingency table analysis showed that among all treatments, the sex ratio was not what one would predict based upon random distribution (χ2 = 15.87; df = 7; P < 0.05). Therefore, a further breakdown of treatments was conducted. The time of emergence was the only the main effect mentioned above that affected sex ratio (χ2 = 6.53; df = 1; P < 0.025); nematodes that emerged early had a sex ratio of 51.1% males to 49.9% females, whereas those that emerged late were biased towards females (55.1%). For the non-dispersed IJ, most that emerged early were males (52.5%), and those that emerged later were females (56%) (χ2 = 7.18; df = 1; P < 0.01), but among dispersing IJ, there was no difference in sex ratio between early- and late-emerging nematodes.
Discussion
Our goal is to understand the bases of variability in parasite infection behavior, especially among IJ that emerge from the same cadaver. An important aspect of this variability is the differential behavior of dispersed and non-dispersed individuals. The species chosen for this series of experiments, S. carpocapsae, is without a doubt the best known of the entomopathogenic nematodes, especially with reference to its ambushing foraging behavior. This species ambushes its hosts by standing on its tail, sometimes for prolonged periods (Campbell and Kaya, 1999, 2000). It is not attracted to volatile cues from hosts (Lewis et al., 1993) unless first exposed to the host's cuticle (Lewis et al., 1995a). It forages, for the most part, near the soil surface and is usually associated with mobile lepidopteran hosts in nature (Lewis et al., 1996; Campbell et al., 1998).
We are obtaining a deeper understanding of the foraging behavior of S. carpocapsae, and researchers have noted that there are substantial differences among individual IJ, even when they emerge from the same host cadaver. For example, Shapiro and Glazer (1996) showed that a greater proportion of S. carpocapsae IJ emerging early from cadavers that had been placed directly onto a sand substrate dispersed, compared with those IJ that emerged from cadavers early or late into water and were then placed on the sand substrate. Our data confirmed the observation by Shapiro and Glazer (1996) that IJ emerging early from cadavers that had been placed on a sand substrate had a greater propensity to disperse than those that emerged from cadavers, migrated into water and were then allowed to disperse in a sand substrate. However, there was no significant difference in dispersal among S. carpocapsae IJ emerging late from cadavers directly onto a sand substrate, compared to IJ that emerged early or late from cadavers into water and were then placed onto sand. We hypothesize that when a cadaver with nematodes is placed directly on the sand, the first few hundred early-emerging IJ move away quickly from the cadavers, whereas the later emerging IJ are less apt to disperse. When the cadaver with nematodes is placed on a White trap and the IJ emerge into water, the first few hundred early-emerging IJ are mixed with the later emerging IJ. Even though the time lapse is only a few hours, these later emerging IJ are not as dispersive as the initial emerging ones. Thus, between “dispersers” being diluted by capture in the White trap and the negative effect of being captured in water (Shapiro and Lewis, 1999), the resolution to differentiate them is lost.
We have also confirmed the observation by Shapiro and Lewis (1999) that there is no effect of emergence time on the infectivity of non-dispersing S. carpocapsae IJ from cadavers and those held in water. On the other hand, when we assayed the infectivity of IJ from cadavers vs. IJ held in water that did disperse, the IJ that emerged from cadavers onto the sand substrate had significantly greater infectivity. Our data support those of Campbell and Kaya (2000), who showed that S. carpocapsae IJ that had jumped were more infectious than those that had not jumped. We hypothesize that jumping (a form of dispersal) and dispersing IJ in or on sand are from the same sub-population and possess similar infectivity.
Our data showed that for the non-dispersed IJ, the early-emerged IJ were biased towards males and those that emerged later were mostly females. However, for dispersing IJ, there was no difference in sex ratio between early- and late-emerging IJ. Lewis and Gaugler (1994) examined the sex ratios of S. carpocapsae (and S. feltiae (Filipejev) and S. glaseri (Steiner)) and showed that S. carpocapsae IJ emerging on the first day of emergence were more female-biased (85% female) than were those that emerged on the fourth day (67% female). Our results differ for two potential reasons: first, Lewis and Gaugler (1994) did not consider dispersal behavior in their investigation and second, while we exposed hosts to single IJ, Lewis and Gaugler (1994) exposed hosts to batches of 15 IJ.
Working with two isolates of another entomopathogenic nematode species, S. feltiae, Rolston et al. (2006) demonstrated that for both isolates dispersing IJ were more infectious than non-dispersing IJ. These authors observed that 11 to 22% of non-dispersing IJ were mature (i.e., fully formed IJ), in comparison to 78 and 82% of dispersing IJ. In addition, one of the two isolates had a sex bias. For this one isolate, the IJ that emerged from an insect cadaver that were allowed to disperse had a female bias, whereas those that did not disperse had a male bias. Based on our data, we speculate that the proportion of S. carpocapsae that disperses is so small that we are more likely to see differences in sex ratio, etc., in the dispersed and non-dispersed group, just because the “non-dispersers” comprise almost the whole population. However, for S. feltiae, this is not the case, as a greater proportion of the IJ population dispersed.
Shapiro and Glazer (1996) showed that the presence of the host cadaver had a significant effect on S. carpocapsae IJ behavior; more IJ migrated away from their point of application when they were allowed to emerge directly from their cadaver into the arena, compared with those captured in a White trap. Infectivity was not influenced by the cadaver (Shapiro and Lewis, 1999), but development rate of the IJ after infection was faster when they emerged directly from host cadavers rather than being White trapped and held in water before infection (Lewis et al., 2002). These aspects of S. carpocapsae foraging ecology are well-documented in the literature, but it is important to realize that not all IJ in a population, or even all IJ from the same infection, behave in the same way. The bases of these differences among IJ are currently the subject of further research.
Footnotes
We thank the Genetic Resources Conservation Program, UC Davis, for financial support in maintaining the nematode cultures.
This paper was edited by Brian Kerry.
Literature Cited
- Campbell JF, Gaugler R. Nictation behaviour and its ecological implications in the host search strategies of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) Behaviour. 1993;126:155–169. [Google Scholar]
- Campbell JF, Gaugler R. Inter-specific variation in entomopathogenic nematode foraging strategy: Dichotomy or variation along a continuum? Fundamental and Applied Nematology. 1997;20:393–398. [Google Scholar]
- Campbell JF, Kaya HK. How and why a parasitic nematode jumps. Nature. 1999;397:485–486. [Google Scholar]
- Campbell JF, Kaya HK. Mechanism, kinematic performance, and fitness consequences of jumping behavior in entomopathogenic nematodes (Steinernema spp.) Canadian Journal of Zoology. 2000;77:1947–1955. [Google Scholar]
- Campbell JF, Orza G, Yoder F, Lewis E, Gaugler R. Spatial and temporal distribution of endemic and released entomopathogenic nematode populations in turfgrass. Entomologia Experimentalis et Applicata. 1998;86:1–11. [Google Scholar]
- Grewal PS, Lewis EE, Campbell JF, Gaugler R. Searching behavior as a predictor of foraging strategy for entomopathogenic nematodes. Parasitology. 1994;108:207–215. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Stock SP. Techniques in insect nematology. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. London: Academic Press; 1997. pp. 281–324. [Google Scholar]
- Lewis EE, Campbell JF, Griffin C, Kaya H, Peters A. Behavioral ecology of entomopathogenic nematodes. Biological Control. 2006;38:66–79. [Google Scholar]
- Lewis EE, Gaugler R. Entomopathogenic nematode sex ratio relates to foraging strategy. Journal of Invertebrate Pathology. 1994;64:238–242. [Google Scholar]
- Lewis EE, Gaugler R, Harrison R. Entomopathogenic nematode host finding: Response to host contact cues by cruise and ambush foragers. Parasitology. 1992;105:309–319. [Google Scholar]
- Lewis EE, Gaugler R, Harrison R. Response of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues. Canadian Journal of Zoology. 1993;71:765–769. [Google Scholar]
- Lewis EE, Grewal PS, Gaugler R. Hierarchical order of host cues in parasite foraging strategies. Parasitology. 1995a;110:207–213. [Google Scholar]
- Lewis EE, Ricci M, Gaugler R. Host recognition behavior reflects host suitability for the entomopathogenic nematode, Steinernema carpocapsae . Parasitology. 1996;113:573–579. doi: 10.1017/s0031182000067627. [DOI] [PubMed] [Google Scholar]
- Lewis EE, Selvan S, Campbell JF, Gaugler R. Changes in foraging behaviour during the infective stage of entomopathogenic nematodes. Parasitology. 1995b;110:583–590. [Google Scholar]
- Lewis EE, Shapiro-Ilan DI, McCoy C. Comparison of development rates in entomopathogenic nematodes applied in infected hosts versus aqueous suspension. Journal of Nematology. 2002;34:340–342. [PMC free article] [PubMed] [Google Scholar]
- Moyle PL, Kaya HK. Dispersal and infectivity of the entomogenous nematode, Neoaplectana carpocapsae Weiser (Rhabditida: Steinernematidae), in sand. Journal of Nematology. 1981;13:295–300. [PMC free article] [PubMed] [Google Scholar]
- Rolston AN, Griffin CT, Downes MJ. Emergence and dispersal patterns of two isolates of the entomopathogenic nematode Steinernema feltiae . Journal of Nematology. 2006;38:221–228. [PMC free article] [PubMed] [Google Scholar]
- Schroeder WJ, Beavers JB. Movement of the entomogeneous nematodes of the families Heterorhabditidae and Steinernematidae in soil. Journal of Nematology. 1987;19:257–259. [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Glazer I. Comparison of entomopathogenic nematode dispersal from infected hosts versus aqueous suspension. Environmental Entomology. 1996;25:1455–1461. [Google Scholar]
- Shapiro DI, Lewis EE. Infectivity of entomopathogenic nematodes from cadavers vs. aqueous applications. Environmental Entomology. 1999;28:907–911. [Google Scholar]
- White GF. A method for obtaining infective nematodes larvae from cultures. Science. 1927;66:302–303. doi: 10.1126/science.66.1709.302-a. [DOI] [PubMed] [Google Scholar]