Abstract
Root knot (Meloidogyne spp.) and cyst (Heterodera and Globodera spp.) nematodes infect all important crop species, and the annual economic loss due to these pathogens exceeds $90 billion. We screened the worldwide accession collection with the root-knot nematodes Meloidogyne incognita, M. arenaria and M. hapla, soybean cyst nematode (SCN-Heterodera glycines), sugar beet cyst nematode (SBCN-Heterodera schachtii) and clover cyst nematode (CLCN-Heterodera trifolii), revealing resistant and susceptible accessions. In the over 100 accessions evaluated, we observed a range of responses to the root-knot nematode species, and a non-host response was observed for SCN and SBCN infection. However, variation was observed with respect to infection by CLCN. While many cultivars including Jemalong A17 were resistant to H. trifolii, cultivar Paraggio was highly susceptible. Identification of M. truncatula as a host for root-knot nematodes and H. trifolii and the differential host response to both RKN and CLCN provide the opportunity to genetically and molecularly characterize genes involved in plant-nematode interaction. Accession DZA045, obtained from an Algerian population, was resistant to all three root-knot nematode species and was used for further studies. The mechanism of resistance in DZA045 appears different from Mi-mediated root-knot nematode resistance in tomato. Temporal analysis of nematode infection showed that there is no difference in nematode penetration between the resistant and susceptible accessions, and no hypersensitive response was observed in the resistant accession even several days after infection. However, less than 5% of the nematode population completed the life cycle as females in the resistant accession. The remainder emigrated from the roots, developed as males, or died inside the roots as undeveloped larvae. Genetic analyses carried out by crossing DZA045 with a susceptible French accession, F83005, suggest that one gene controls resistance in DZA045.
Keywords: root-knot nematode, cyst nematode, resistance, Medicago truncatula, host range, genetics
It has been estimated that the average yield loss due to parasitic nematodes is around 12% annually (Sasser and Freckman, 1987), reaching as high as 20% in certain crops (Koenning et al., 1999). Among the parasitic nematodes, root-knot nematodes (RKN) and cyst nema-todes are the most important and wide spread. Every crop species grown is susceptible to one or more RKN species (Sasser, 1980). RKN (Meloidogyne spp.) are obligate sedentary endo-parasites and are known to occur across a broad range of climatic conditions. While Meloidogyne contains more than 70 described species, four species (M. incognita, M. arenaria, M. javanica and M. hapla) are responsible for 95% of infestations (Sasser et al., 1983). The genus Heterodera includes several economically important nematodes. For instance, the yield loss due to SCN (Heterodera glycines) in soybean is estimated to be $430 million in US alone for the year 1995 (Wrather et al., 1997). Similarly, the annual economic loss due to sugar beet cyst nematode (SBCN-Heterodera schachtii) is estimated to be $95 million in Europe alone (Muller, 1999). While no clear estimates are available for yield losses caused by H. trifolii, it has been reported that it can infect a number of crops, including sugar beet and other vegetable crops (Wang and Riggs, 1999), and also suppresses nitrogen fixation in many leguminous plants (Yeats et al., 1977).
RKN initiates a feeding site after the infective second-stage larva (L2) has penetrated the host root, generally near the root tip, and migrated to the developing vascular cylinder. The nematode induces formation of five to seven giant cells within or near the developing vascular cylinder, which become the permanent feeding site (Hussey, 1989). The nematode is dependent upon the giant cells for its survival and reproduction because it becomes immobile soon after giant cell induction. RKN gets its common name from the classic symptom of heavy root galling in the areas of infection. These external symptoms are pronounced and diagnostic.
Infective second-stage larvae of cyst nematodes penetrate young roots directly and move intra-cellularly to the cortex, where they initiate specialized feeding sites. The cyst nematode feeding site is called a synctium and is formed by the degradation of cell walls and cell hypertrophy (Hussey, 1989). Once the nematode establishes a syncytium, it undergoes three molts and become an adult. During the development of the third-stage larvae, cortical cells surrounding the female larvae are crushed by its expanding body, exposing the nematode to soil. Although H. schachtii and H. glycines reproduce by amphimixis, H. trifolii reproduces by apomixis (Triantaphyllou and Hirschmann, 1978).
Compared to recent advances in other plant-pathogen interactions, less is known about the interaction between the nematode and its host. Although Arabidopsis thaliana (Sijmons et al., 1991; Boiteux et al., 1999; Vercauteren et al., 2001; Gheysen and Fenoll, 2002) and Lotus japonicus (Lohar and Bird, 2003; Lohar et al., 2004) have been used to probe susceptible interactions with RKN, virtually no work has been performed on RKN or cyst resistance in these plants, largely because no Arabidopsis or Lotus ecotypes have been demonstrated to be fully resistant to either nematode (Niebel et al., 1994). Consequently, we explored using Medicago truncatula as a model to understand resistant interactions between legume hosts and root-knot/cyst nematodes. Medicago truncatula is a diploid legume and a close relative of alfalfa. It has a small, completely sequenced genome (450Mb) and has proven to be an effective model to study the interactions between legumes and rhizobial bacteria (Barker et al., 1990; Dhandaydham, 2000; Frugoli and Harris, 2001, www.Medicago.org). Apart from its suitability as a model host, M. truncatula is widely grown as a pasture legume in Australia, although the geographical centre of origin for M. truncatula is the Mediterranean basin and the Near East (Crawford et al., 1989). Numerous accessions have been cataloged from wild isolates, and substantial genetic variability revealed (Auricht et al., 1999). Here we report the establishment of M. truncatula as a model to study the resistant interactions between legumes and nematodes.
Materials and Methods
Biological material: Medicago truncatula seeds were scarified in concentrated H2SO4 for 6 min, washed for 3 hr with several changes of sterile tap water and incubated at 4°C for 24 hr to synchronize germination. Seeds were washed again in sterile water for 3 hr and left in a germination chamber for 2 d at 26–28°C. Newly emerged seedlings were planted in 606 Com-Packs (Hummert International, Earth City, MO) that contained a pasteurized 4:1 mixture of sand and soil, and plants were maintained in the greenhouse or growth chamber. Three to four weeks after planting, plants were inoculated with nematodes by making four 1-inch-deep holes around the plant and placing approximately 250 nematode eggs in each hole. Six weeks after inoculation, plants were harvested, washed and evaluated using a galling index (Hussey and Janssen, 2002). For each accession, two to six plants were inoculated.
Nematode culture maintenance: H. glycines OP50 was maintained on soybean cv. Lee 74, H. schachtii was maintained on beet root cv. Monohikari, and H. trifolii 12JI (single J2 descendent generated from pine tree, Arkansas, provided by Dr. R.D. Riggs) was maintained on white clover. Nematode inoculum from the susceptible host plants was isolated as follows: first, the plant roots were soaked in water for few seconds to remove the soil from the root ball. Cleaned roots were later sprayed with high-pressure water over a sieve arrangement of 25/60 mesh. In addition, the cysts in the soil were collected by stirring the soil in water and decanting over the same sieve arrangement. Cysts collected over the 60 mesh sieve were crushed using a rubber stopper over a sieve arrangement of 60/200/500 mesh. The eggs collected over the 500 mesh sieve were counted and adjusted to a concentration of 1,500 eggs/ml for H. trifolii, 4,000 eggs/ml for H. schachtii and 8,000 eggs/ml for H. glycines.
Populations of M. incognita race 1, M. arenaria race 1 and M. hapla race A were maintained in greenhouse on Lycopersicon esculentum cv. Rutgers Large Red. Nematodes were extracted from roots with 0.5% NaOCl (Hussey and Barker, 1973). Eggs collected from a 500 mesh sieve were washed and resuspended at 10,000 eggs/ml.
Evaluation of response to root-knot nematodes: RKN egg masses were stained by placing galled roots in phloxine B solution (0.15 g/liter tap water) for 15–20 min. After staining, excess stain was removed by washing the roots in tap water, and the egg masses were counted using a stereo microscope (Daykin and Hussey, 1985). For rapid RKN screening, each plant was rated for galling and assigned a root galling index value with 0 = no galling, 1 = trace infections with a few small galls, 2 = <25% roots galled, 3 = 25–50% galling, 4 = 50–75% galling, and 5 = > 75% of roots galled. We consider a score of 2 or more to indicate susceptibility and one of less than 1 to indicate resistance. Evaluation of response to cyst nematodes was similar to the evaluation criteria set by Noel et al. (1990) enumerating cysts produced, and the accessions were grouped into resistant (0–15 cysts), mildly susceptible (16–50 cysts) and susceptible (> 50 cysts).
Penetration and emigration experiments for root-knot nematodes on resistant accessions: One-week-old M. truncatula seedlings grown on Pro-Trays containing 2:1 sand and soil mixture were inoculated with 500, 1,000 or 2,000 hatched L2. To determine the number of nematodes that had entered into the plant, five to eight seedlings of DZA045 (resistant) and F83005 (susceptible) were removed at 24, 48 and 96 hr after inoculation and stained with acid fuchsin. Acid fuchsin stains both nematodes and dead plant cells, including those resulting from an HR.
For emigration experiments, plants were harvested 1 wk after inoculation, washed free of soil and debris and maintained in a hydroponic culture in small cups containing 100 ppm of nitrogen:phosphorous:potassium (NPK 20:20:20). Once every 2 d, the hydroponic solution was filtered using a 10-μm-pore sieve, and the nematodes collected were counted using a stereo microscope. Larvae that left the root were collected from the hydroponic solution and counted every 2 d for 6 wk, although L2 were no longer observed outside the roots by 3 wk after inoculation. Nematode development was monitored during staining of root systems and the developmental stage noted.
Genetics: Recombinant inbred lines (RIL) are single-seed descendents of a cross between two homozygous parents that have been selfed for at least five generations (F6). RIL are nearly homozygous and homogenous and provide large numbers of individuals in which the parental alleles are expected to be distributed equally (Burr and Burr, 1991). We initially screened 42 F6 RIL with M. incognita race 1. Two sets of six plants were inoculated with 1,000 and 2,000 eggs, respectively, and analyzed for a visible resistance phenotype 6 wk later (Fig. 1). The reactions of the RIL were scored based on a galling index (0–5 scale). If the average galling score of an inbred line was ≤ 1, then that line was classified as resistant, and if the score was ≥ 3, then the inbred line was classified as susceptible. Lines that had an average score of 1.1 to 2.9 were classified as intermediate.
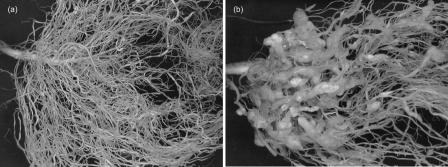
Fig. 1.
Whole roots of Medicago accessions 12 weeks after inoculation with M. incognita. DZA045, the resistant plant (A) shows no symptoms, whereas root knots (galls) are clearly evident on a susceptible plant (F83005, gall score = 5) (B).
Because the galling index is to a degree subjective, we compared its reliability with that of egg mass production (which is a quantitative measure of relative nematode fecundity) by counting the number of egg masses present on the roots of the same plants by staining them with phloxine B. For resistant phenotypes, the galling index measure was highly reliable and comparable with that of egg mass numbers, so we used the galling index for subsequent experiments, as it is easier and faster to perform. To ensure the reliability of plant gall index phenotypes, we allowed the nematodes to complete two life cycles in the host. However, only 93.75% of the lines would be expected to have reached homozygosity in the F6 generation. To test the amount of heterozygosity in these RIL, we determined the segregation ratio for the phenotypic character pod coiling in these lines.
Results
Medicago truncatula is non-host for H. glycines and H. schachtii: A need for new and novel sources of resistance to SCN in soybean, the availability of numerous accessions of model legume, M. truncatula and the difficulty in carrying out genetic studies with tetraploid soybean prompted us to test whether M. truncatula can be used as a model to understand SCN-host interactions. In our experiments, we infected the plants with SCN inbred line OP50 at 1,000 eggs/plant. OP50 carries a large suite of necessary parasitism genes for infection of a variety of soybean cultivars (Dong and Opperman, 1997). Even 6 wk post-inoculation, no developing cysts were observed in any of the plants tested. We tested the accessions again with a larger inoculum (8,000 eggs/plant) during summer to verify this result. While developing nematodes were observed in the control soybean plants, no cysts were observed in any of the M. truncatula plants. A subset of these accessions was tested again by Dr. Terry Niblack, University of Illinois, Urbana, with SCN inbred line TN2, which has a host range which includes tomato. While most of the accessions were non-host to this SCN inbred as well, a few nematodes did develop on the accessions E39, E103 and F20025–2. However, the nematodes that grew on these lines could not be increased despite maintaining them for several months. Since M. truncatula was not a host for SCN, we tested the same accessions by infecting with SBCN at an inoculum of 4,000 eggs/plant. Similar to SCN, no SBCN cysts grew in any of the M. truncatula plants. In contrast, nematodes did develop on the susceptible cabbage plants, suggesting that M. truncatula was not a host for SBCN.
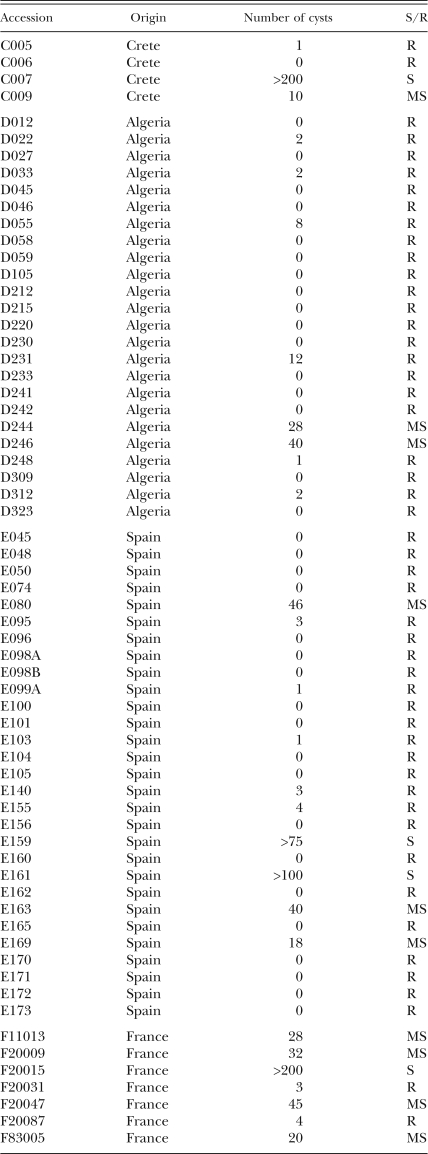
Medicago truncatula is a host for H. trifolii: Six weeks after infecting the plants with 1,500 CLCN eggs, the roots were checked for the presence of developing nematodes. Out of the 74 M. truncatula accessions tested, 16 supported growth of CLCN, the remaining 58 (∼80%) were resistant (Table 1), indicating that M. truncatula is a host for CLCN (Table 1). However, out of the 11 M. truncatula commercial cultivars (distinct from accessions) tested, only four were resistant to the nematode. In fact, the cultivar Paraggio was the most susceptible (> 500 cysts) among all the cultivars and accessions together tested. Accessions C009, D231, E163, F11013 and F20009 showed high variability for CLCN infection, confirming that these accessions were indeed mixtures. Out of the three Medicago littoralis accessions tested, all the accessions were resistant to CLCN (Ta-ble 1).
Table 1.
M. truncatula accessions and their reaction to clover cyst nematode (H. trifolii) infection.
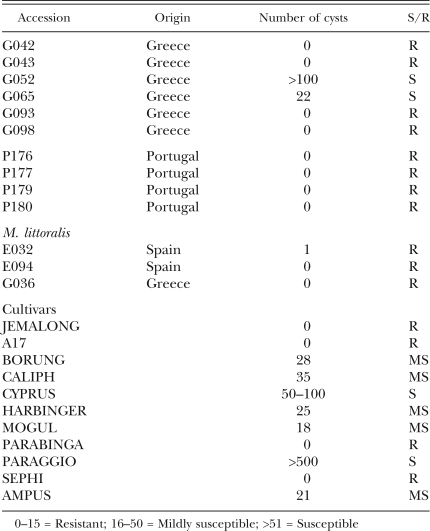
Medicago truncatula accessions are genetically diverse and responded differentially to distinct RKN species: We initially inoculated M. truncatula with M. incognita race 1, revealing 50 of the 80 accessions scored to be resistant, 21 lines to be highly susceptible and the remaining nine accessions to be moderately susceptible to this RKN isolate. As shown in Figure 1, resistant and susceptible accessions are clearly discernable. The spectrum of genetic diversity between accessions we observed is shown in Table 2. To ensure that the differential resistance was not a consequence of growth conditions or RKN inoculum size, subsets of these accessions (40) were independently re-infected with 1,000 or 4,000 M. incognita race 1 eggs in a different greenhouse; the results (data not shown) were consistent with the previous findings. Although individual accessions were collected from single sites, they correspond to a sample of seeds collected from several individuals (Bonnin et al., 1996). Thus, because M. truncatula is an autogamous species, it is expected that each accession is a mixture of several individual inbred lines. Nevertheless, we found that many of the accessions were uniformly resistant to M. incognita race 1, suggesting that these accessions are under constant nematode pressure to retain resistance. This was not unexpected, since a previous survey (Taylor et al., 1982) had demonstrated that RKN is a common root pathogen in the Mediterranean basin. However, we observed substantial variation within each accession to infection and susceptibility to powdery mildew (data not shown), suggesting heterogeneity for traits not under continuous selection.
Table 2.
M. truncatula accessions and their responses to RKN ifection. Lines with prefix DZA are from Algeria, CRE are from Crete, ESP are from Spain, GRC are from Greece, and P are from Portugal. Gall Index Scroes: S = Susceptible (>2.0), MS = Mildly Susceptible (1.1–1.9), R = Resistant (<1), ND = Not Determined.
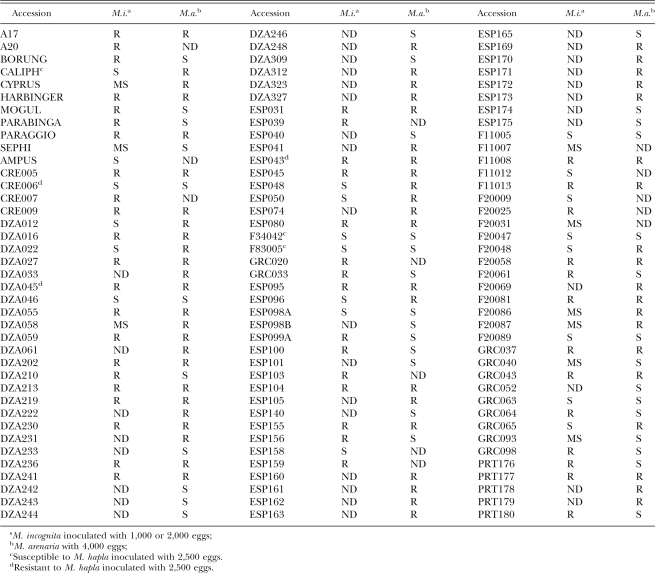
Because the tomato Mi gene confers resistance to three different nematode species (Williamson, 1998), we further tested the M. truncatula accessions with M. arenaria and M. hapla (Table 2). As was the case for M. incognita, resistant and susceptible accessions were found for both species, but, strikingly, resistance to one RKN species was not predictive of resistance to another (Table 2); Medicago accessions show different combinations of resistance and susceptibility to different Meloidogyne species.
One accession (DZA045) collected from Algeria was broadly resistant to all three RKN species tested, and one French accession (F83005) was broadly susceptible. These were chosen for further analysis using M. incognita. Because the accessions retain some heterogeneity, we selected individual DZA045 (DZA045–5) and F83005 (F83005–5) plants and selfed them for two generations. Homozygosity of these progeny was confirmed by simple sequence repeat analysis (data not shown). Individual plants were inoculated with 1,000 M. incognita L2, and gall indices obtained 6 wk later (sufficient for one nematode life cycle). To further quantify the resistance/susceptibility of these accessions, we determined the reproduction factor, which is the ratio between the final nematode counts (Pf) to that of initial inoculum (Pi). For F83005–5, Pf/Pi = 20, confirming the high RKN-susceptibility of this accession indicated by the gall index. In contrast, we obtained Pf/Pi = 0.8 for the resistant accession, DZA045–5. Collectively, these data confirm that the plants that were chosen accurately represented the response of the accession.
Resistant accession DZA045 does not mount a Hypersensitive Response: The hypersensitive response (HR) is a rapid response that resistant plants commonly mount against invading pathogens and usually results in programmed cell death of the plant cell at the initial point of infection. In tomato, the Mi gene conditions resistance to RKN infection and within 24 hr kills the cell where the nematode has initiated its feeding site (Dropkin, 1969). To determine whether the resistance in DZA045 is due to a HR, we inoculated 3-wk-old plants with 1,000 M. incognita L2 and stained the roots with acid fuchsin 24, 48 and 67 hr later; nematodes and dead plant cells acquire color from the stain, but live plant cells do not (Daykin and Hussey, 1985; Isghougi Kaloshian, personal communication). We observed multiple independent plants for evidence of a HR, but none showed any obvious symptoms (Fig. 2). It has been shown in tobacco that a HR can occur even after giant cell formation (Powell, 1962), so we stained the inoculated plants at various time points for up to 3 wk after inoculation, but at no point was a HR observed (not shown).
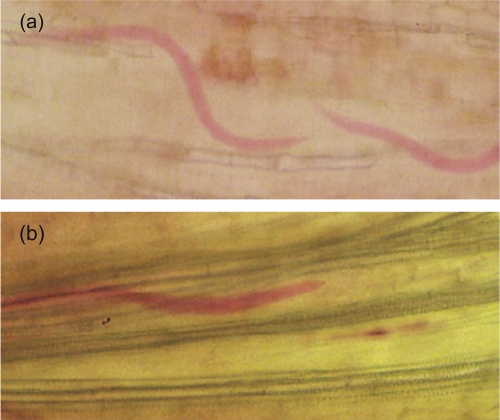
Fig. 2.
Neither RKN-resistant DZA045 (A) nor RKN-susceptible F83005 (B) accessions of Medicago appear to mount a hypersensitive response to invading M. incognita L2. Roots were stained with acid Fuchsin 67 hr after inoculation. Nematodes (stained red) are migrating between cells. Because it is in the vasculature system and slightly swollen, it is likely that the L2 in the susceptible host is beginning to establish a feeding site. .
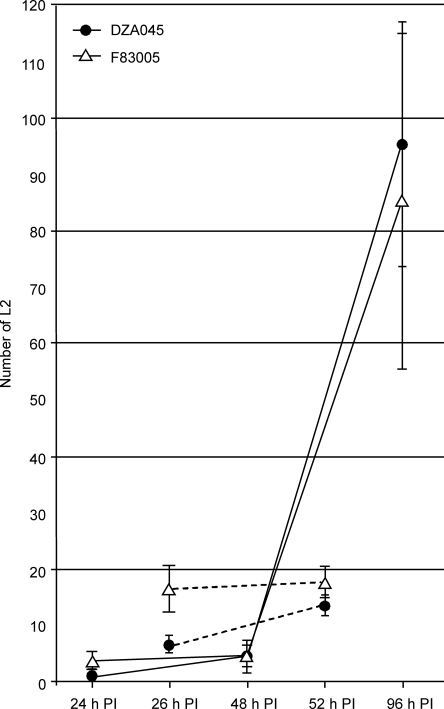
RKN penetrate susceptible and resistant accessions equally: Because resistance in DZA045 did not appear to correspond to a HR-based resistance mechanism, we tested whether it was due to lack of nematode penetration into the roots, which has been found to be the basis for RKN-resistance in cucumber (Haynes and Jones, 1976). As is the case on other hosts, RKN penetrate Medicago just behind the root tip. No meaningful difference in nematode penetration between resistant and susceptible accessions was observed (Fig. 3).
Fig. 3.
No difference in penetration by RKN was observed between resistant and susceptible accessions. Number of nematodes within the root-tips of 3-week-old plants inoculated with 1,000 L2 and scored at various times post infection (PI). Error bars represent standard deviation for five replications per time point.
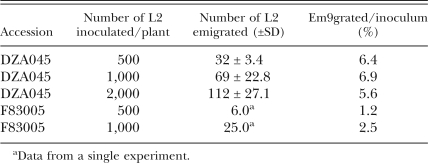
More nematodes emigrate from the resistant accession than from the susceptible accession: In grape root-stocks, resistance to RKN is positively correlated with the exit of nematodes from the root (Anwar and McKenry, 2002). Although approximately twice as many L2 emigrated from the resistant accession compared to the susceptible accession (Table 3), most nematodes remained in the roots (data not shown). Consequently, larval emigration does not explain the resistance in DZA045.
Table 3.
Twice the numbers of M. incognita L2 emigrate from the resistant accession (DZA045) than from the susceptible accession (F83005).
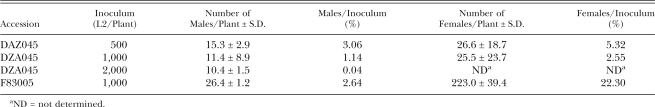
RKN develop poorly on the resistant accession: RKN are sexually undifferentiated when they initially infect a host. Although the larvae generally develop as females, stress conditions such crowding or lack of sufficient nutrients may redirect sexual development into males (Triantaphyllou, 1973). Because males are non-feeding and leave the roots at approximately 4 wk post-infection, they are considered to be non-pathogenic. As shown in Table 3, only a few percent of the inoculum developed into males on both resistant and susceptible cultivars, suggesting that DZA045 resistance is not manifested through the redirecting of development towards males. To examine female development, we stained the entire root mass and counted the nematodes in the root (Table 3). Whereas 22% of the initial inoculum developed as females in the susceptible accession, only 3 to 5% developed as females in the resistant accession. The remainder died as L2 inside the root. Collapsed or abnormally developed giant cells were not observed near these dead nematodes, suggesting that they had not established feeding sites before their death (data not shown). Collectively, these results point to a failure of the invading L2 to establish functional feeding sites in the resistant accession DZA045.
Genetic analysis of nematode resistance in DZA045: Of the 177 F6 lines tested, 120 lines showed clockwise coiling (dominant), and 57 lines showed anti-clockwise coiling (recessive). Although this trait is known to be controlled by a single gene, SPC, the segregation ratio of F6 did not fit a single gene hypothesis with χ-square analysis. This data suggest that either the F6 lines retain significant heterozygosity or that a bottleneck had occurred during the single-seed descent. Furthermore, in many inbred lines, plants were segregating for resistance. To reduce the effect of heterozygosity in determining the number of genes controlling resistance, we generated F7 lines by selfing the F6 plants. Out of the 143 F7 lines tested, only 36 lines showed the intermediate phenotype (instead of the expected 71 if two genes were involved), suggesting that a single gene controls resistance in DZA045. Although the intermediate phenotypes strongly indicate that the resistance gene is semi-dominant, we did not pursue this area further.
Discussion
Colbran (1958) first reported annual medic as a host for RKN. Emergence of M. truncatula as a model to study plant-symbiont interactions, availability of worldwide collections of M. truncatula accessions for scientific studies and lack of an incompatible interaction between Arabidopsis and RKN or cyst nematodes prompted us to test whether M. truncatula could be used as a model to understand plant-nematode interactions. Our results confirm not only that M. truncatula is an excellent and typical host for RKN and CLCN, but that substantial natural variability among the accessions in their reaction to infection exists. At the extremes, some accessions were resistant to all species tested (M. incognita, M. arenaria, M. hapla and CLCN), whereas some were susceptible to all. Further, accessions with varying combinations of RKN resistance were also present in the collection.
The mechanism of resistance to RKN in crop plants seems to vary between crop plants and may manifest either pre- or post-infection. Pre-infection resistance, such as is observed in cucumber (Haynes and Jones, 1976) and peanut (Bendezu and Starr, 2003), is due to lack of nematode entry into the plant and is possibly due to the presence of pre-formed chemicals in the plant that are toxic or antagonistic to the nematodes (Huang, 1985). However, in the RKN-resistant Medicago accession DZA045, nematode resistance is not due to lack of nematode penetration and is thus more generally similar to resistance in soybean (Herman et al., 1991), alfalfa (Griffin and Elguin, 1977) and tomato (Hadisoeganda and Sasser, 1982), where there is no difference in nematode penetration between the susceptible and resistant lines.
Post-infection resistance mechanisms are manifested after the penetration of the nematode in the host and in some cases are associated with a classical hypersensitive response (HR). The HR is typically explained by the gene-for-gene-model in which an avirulence gene product from the pathogen is specifically recognized by the resistance gene product of the host (Bent, 1996). In contrast, no obvious HR was observed in DZA045, even days after inoculation. The fact that nematodes were still migrating inside the root for several days after penetration suggests that they were unable to establish a feeding site inside the root. Consistent with this observation, we detected significant emigration from the root by larvae, with only 3 to 5% developing as females, leading to net low fecundity.
The number of genes controlling resistance to RKN seems to differ among hosts and even among varieties. For example, a single gene controls resistance in soybean cultivar ‘Forrest’ (Luzzi et al., 1994a), whereas multiple genes control resistance in soybean lines PI96354 and PI417444 (Luzzi et al., 1994b). Based on results from screening F7 RIL, it is apparent that a single gene controls resistance in DZA045. Furthermore, mapping suggests that a single gene may control resistance in DZA045 (data not shown).
We screened Medicago accessions with SCN, which is considered to have a broad host range (Riggs, 1992). We chose SCN for the study because it is the most economically damaging nematode in the US, and no single soybean cultivar is resistant to all known SCN races (Sipes, 1992). However, our screening results suggest that M. truncatula is not a host for SCN. Despite development of a few nematodes from the SCN inbred line, TN2, it is likely that M. truncatula is a poor or non-host for SCN. Other annual medics, including M. hispida, M. arabica and M. minima, are also non-hosts for SCN (Riggs, 1992). SBCN, like SCN, has a broad host range and infects a number of economically important crop species (Steele, 1965). However, M. truncatula was not a host for this nematode either.
Because M. truncatula is not a host for either SCN or SBCN, we tested with CLCN, which is the primary cyst nematode affecting forage legume production in the world (Pederson and Quesenberry, 1998). In fact, H. trifolii is the most common cyst nematode in North America, and the economic threshold for this nematode is < 1 egg/ml (Cook and Yeates, 1993). Infection by H. trifolii on white clover can reduce the forage production by 14 to 37% (Stelter and Meinl, 1972) and suppresses the amount of nitrogen fixed in the plant (Yeates et al., 1977). Screening Medicago accessions with H. trifolii identified several accessions that are susceptible and resistant to this nematode. While some accessions were highly susceptible to CLCN, some were mildly susceptible. Accessions that show mild susceptibility to CLCN are still useful, and they can be used to grow in soils that show low initial amounts of inoculum. However, these accessions cannot be used in soils with high nematode inoculum. Accessions that are resistant but poor in their forage yield can be used in breeding programs. Genes conferring resistance to CLCN may be more stable than other nematode resistance genes because CLCN reproduces by obligate mitotic parthenogenesis and therefore it will exhibit lowered genetic variation (Mulvey, 1958; Triantaphyllou and Hirschmann, 1978).
In conclusion, we have established Medicago truncatula as a model to understand plant-nematode interactions. Eventual cloning of the nematode resistance gene(s) from the resistant accession should not only help us in understanding the interaction between the host and the nematode, but would help breeders to introgress quickly these gene(s) into superior yet susceptible Medicago cultivars. Establishing this model system should provide us with opportunities to identify the mechanism underpinning this unique nematode resistance pathway.
The authors wish to thank Dr. J-M. Prosperi for providing Medicago truncatula seeds and the information about the origin of the accessions, Dr. Robert D. Riggs for supplying H. trifolii and Dr. Terry Niblack for testing M. truncatula accessions with SCN inbreds.
Table 4.
Adult M. incognita development on M. truncatula accession.
Footnotes
The authors thank Dr. Fred Gould for assistance with AFLP experiments. We also thank the NCSU Phytotron facility for their help and support during this study. This research was supported by NSF Plant Genome award DBI0077503 and by NC ARS to CHO.
This paper was edited by David Bird.
Literature Cited
- Anwar SA, McKenry MV. Developmental response of a resistance-breaking population of Meloidogyne arenaria on Vitis spp. Journal of Nematology. 2002;34:143–145. [PMC free article] [PubMed] [Google Scholar]
- Auricht GC, Prosperi JM, Snowball R, Hughes J. The characterization and preliminary evaluation of Medicago and Trifolium germplasm. Current Plant Science and Biotechnology in Agriculture. 1999;33:141–149. [Google Scholar]
- Barker DG, Bianchi S, Blondon F, Dattee Y, Duc G, Flament P, Gallusci P, Genier G, Guy P, Muel X, Tourneur J, Denarie J, Huguet T. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Molecular Biology Reporter. 1990;8:40–49. [Google Scholar]
- Bendezu IF, Starr J. Mechanism of resistance to Meloidogyne arenaria in the peanut cultivar COAN. Journal of Nematology. 2003;35:115–118. [PMC free article] [PubMed] [Google Scholar]
- Bent A. Plant disease resistance genes: Function meets structure. Plant Cell. 1996;8:1757–1771. doi: 10.1105/tpc.8.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux LS, Fonseca MEN, Simon PW. Host status and reaction of Arabidopsis thaliana ecotypes to infection by the northern root-knot nematode (Meloidogyne hapla) Plant Breeding. 1999;118:355–358. [Google Scholar]
- Bonnin I, Prosperi JM, Olivieri T. Genetic markers and quantitative genetic variation in Medicago truncatula (Leguminosae): A comparative analysis of population structure. Genetics. 1996;143:1795–1805. doi: 10.1093/genetics/143.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B, Burr FA. Recombinant inbreds for molecular mapping in maize: Theoretical and practical considerations. Trends in Genetics. 1991;7:55–60. doi: 10.1016/0168-9525(91)90232-F. [DOI] [PubMed] [Google Scholar]
- Colbran G. Studies of plant and soil nematodes: Queensland host records of root knot nematode (Meloidogyne spp) Queensland Journal of Agricultural Science. 1958;15:101–136. [Google Scholar]
- Cook R, Yeates GW. Nematode pests of grassland and forage crops. In: Evans K, Trudgill DL, Webster JM, editors. Plant parasitic nematodes in temperate agriculture. Wallingford, UK: CAB International; 1993. pp. 305–350. [Google Scholar]
- Crawford EJ, Lake AWH, Boyce KG. Breeding annual Medicago species for semiarid conditions in southern Australia. Advances in Agronomy. 1989;42:399–437. [Google Scholar]
- Daykin ME, Hussey RS. Staining and histo-pathological techniques in nematology. In: Barker KR, Carter CC, Sasser JN, editors. An Advanced Treatise on Meloidogyne: Volume II. Raleigh, NC: North Carolina State University Graphics; 1985. pp. 39–48. [Google Scholar]
- Dhandaydham M. Model legumes in the limelight. Genome Biology. 2000;1:4016.1–4016.3. [Google Scholar]
- Dong K, Opperman CH. Genetic analysis of parasitism in the soybean cyst nematode Heterodera glycines . Genetics. 1997;146:1311–1318. doi: 10.1093/genetics/146.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropkin VH. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: Reversal by temperature. Phytopathology. 1969;59:1632–1637. [Google Scholar]
- Frugoli J, Harris J. Medicago truncatula on the move. Plant Cell. 2001;13:458–463. doi: 10.1105/tpc.13.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen G, Fenoll C. Gene expression in nematode feeding sites. Annual Review of Phytopathology. 2002;40:191–219. doi: 10.1146/annurev.phyto.40.121201.093719. [DOI] [PubMed] [Google Scholar]
- Griffin GD, Elguin JH. Penetration and development of Meloidogyne hapla in resistant and susceptible alfalfa under differing temperatures. Journal of Nematology. 1977;9:51–56. [PMC free article] [PubMed] [Google Scholar]
- Hadisoeganda WW, Sasser JN. Resistance of tomato, bean, southern pea, and garden pea cultivars to root-knot nematodes based on host suitability. Plant Disease. 1982;66:145–150. [Google Scholar]
- Haynes RL, Jones CM. Effects of the Bi locus in cucumber on reproduction, attraction, and response of the plant to infection by the southern root-knot nematode. Journal of the American Society of Horticultural Science. 1976;101:422–424. [Google Scholar]
- Herman M, Hussey RS, Boerma HR. Penetration and development of Meloidogyne incognita on roots of resistant soybean genotypes. Journal of Nematology. 1991;23:155–161. [PMC free article] [PubMed] [Google Scholar]
- Huang JS. Mechanisms of resistance to root-knot nematodes. An Advanced Treatise on Meloidogyne: Volume II. In: Barker KR, Carter CC, Sasser JN, editors. Raleigh, NC: North Carolina State University Graphics; 1985. pp. 165–174. [Google Scholar]
- Hussey RS. Disease inducing secretions of plant parasitic nematodes. Annual Review of Phytopathology. 1989;27:123–141. [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Hussey RS, Janssen GJW. Root-knot nematodes: Meloidogyne species. In: Starr JL, Cook R, Bridge J, editors. Plant Resistance to Parasitic Nematodes. Wallingford, UK: CABI Publishing; 2002. pp. 43–70. [Google Scholar]
- Koenning SR, Overstreet C, Noling JW, Donald PA, Becker JO, Fortnum BA. Survey of crop losses in response to phytoparasitic nematodes in the United States for 1994. Journal of Nematology. 1999;31:587–618. [PMC free article] [PubMed] [Google Scholar]
- Lohar DP, Bird DMcK. Lotus japonicus: A new model to study root-parasitic nematodes Plant Cellular Physiology. 2003;44:1176–1184. doi: 10.1093/pcp/pcg146. [DOI] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DMcK. Cytokinins play opposite roles in lateral root formation, and nematode and rhizobial symbioses. Plant Journal. 2004;38:203–214. doi: 10.1111/j.1365-313X.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- Luzzi BM, Boerma HR, Hussey RS. A gene for resistance to the southern root-knot nematode in soybean. Journal of Heredity. 1994a;85:484–486. [Google Scholar]
- Luzzi BM, Boerma HR, Hussey RS. Inheritance of resistance to the peanut root-knot nematode in soybean. Crop Science. 1994b;34:1240–1243. [Google Scholar]
- Muller J. The economic importance of Heterodera schachtii in Europe. Helminthologia. 1999;36:205–213. [Google Scholar]
- Mulvey RH. Parthenogenesis in a cyst-forming nematode, Heterodera trifolii . Canadian Journal of Zoology. 1958;36:91–98. [Google Scholar]
- Niebel A, Barthels N, Almeida-Engler J, Karimi M, Vercauteren V, Van Montagu V, Gheysen G. Arabidopsis thaliana as a model host plant to study molecular interactions with root-knot and cyst nematodes. In: Lamberti F, De Giorgi C, Bird D, editors. Advances in Molecular Plant Nematology. New York: Plenum Press; 1994. pp. 161–170. [Google Scholar]
- Noel GR, Franco J, Jatala P. Screening for resistance to cyst nematodes, Globodera and Heterodera species. In: Starr JL, editor. Methods for evaluating plant species for resistance to plant-parasitic nematodes. Hyattsville, MD: The Society of Nematologists; 1990. pp. 24–32. [Google Scholar]
- Pederson GA, Quesenberry KH. Clovers and other forage legumes. In: Barker KR, Pederson GA, Windham GL, editors. Plant and Nematode Interactions. Madison, WI: American Society of Agronomy; 1998. pp. 399–425. [Google Scholar]
- Powell NT. Histological basis of resistance to root-knot nematodes in flue-cured tobacco. Phytopathology. 1962;52:25. [Google Scholar]
- Riggs RD. Host range. In: Riggs RD, Wrather JA, editors. Biology and the management of the soybean cyst nematode. St. Paul: APS Press; 1992. pp. 107–114. [Google Scholar]
- Sasser JN. Root knot nematodes: A global menace to crop production. Plant Disease. 1980;64:36–41. [Google Scholar]
- Sasser JN, Eisenback JD, Carter CC, Triantaphyllou AC. The International Meloidogyne project - its goals and accomplishments. Annual Review of Phytopathology. 1983;21:271–288. [Google Scholar]
- Sasser JN, Freckman DW. A world perspective on nematology: The role of the society. In: Veech JA, Dickson DW, editors. Vistas on Nematology. Hyattsville, MD: U.S.A Society of Nematology, Inc; 1987. pp. 7–14. [Google Scholar]
- Sijmons PC, Grundler FMW, Von Mende N, Burrows PR, Wyss U. Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant Journal. 1991;1:245–254. [Google Scholar]
- Sipes BS. Genetics. In: Riggs RD, Wrather JA, editors. Biology and the management of the soybean cyst nematode. St. Paul: APS Press; 1992. pp. 61–71. [Google Scholar]
- Steele AE. The host range of the sugar beet nematode, Heterodera schachtii Schmidt. Journal of the American Society of Sugar beet Technologists. 1965;13:573–603. [Google Scholar]
- Stelter H, Meinl G. The effects of the infestation of red and white clover by Heterodera trifolii and Heterodera galeopsidis . Archiv für Pflanzenschutz. 1972;8:463–470. [Google Scholar]
- Taylor AL, Sasser JN, Nelson LA. Raleigh, NC: North Carolina State University Graphics; 1982. Relationship of climate and soil characteristics to geographical distribution of Meloidogyne species in agricultural soils. [Google Scholar]
- Triantaphyllou AC. Environmental sex differentiation of nematodes in relation to pest management. Annual Review of Phytopathology. 1973;11:441–462. [Google Scholar]
- Triantaphyllou AC, Hirschmann H. Cytology of the Heterodera trifolii parthenogenetic species complex. Nematologica. 1978;24:418–424. [Google Scholar]
- Vercauteren I, Van Der Schueren E, Van Montagu M, Gheysen G. Arabidopsis thaliana genes expressed in the early compatible interaction with root knot nematodes. Molecular Plant-Microbe Interactions. 2001;14:288–299. doi: 10.1094/MPMI.2001.14.3.288. [DOI] [PubMed] [Google Scholar]
- Wang S, Riggs RD. Variations in host preference among and within populations of Heterodera trifolii and related species. Journal of Nematology. 1999;31:407–417. [PMC free article] [PubMed] [Google Scholar]
- Williamson VM. Root-knot nematode resistance genes in tomato and their potential for future use. Annual Review of Phytopathology. 1998;36:277–293. doi: 10.1146/annurev.phyto.36.1.277. [DOI] [PubMed] [Google Scholar]
- Wrather JA, Anderson TR, Arzsad DM, Gai J, Ploper LD, Porta-Puglia A, Ram HH, Yorinori YT. Soybean disease loss estimates for the top 10 soybean-producing countries in 1995. Plant Disease. 1997;81:107–110. doi: 10.1094/PDIS.1997.81.1.107. [DOI] [PubMed] [Google Scholar]
- Yeats GW, Ross DJ, Bridger BA, Visser TA. Influence of the nematodes Heterodera trifolii and Meloidogyne hapla on nitrogen fixation by white clover under glasshouse conditions. New Zealand Journal of Agricultural Research. 1977;20:401–413. [Google Scholar]