Abstract
This study assessed the potential impact of various Fusarium strains on the population development of sugarbeet cyst nematodes. Fungi were isolated from cysts or eggs of Heterodera schachtii Schmidt that were obtained from a field suppressive to that nematode. Twenty-six strains of Fusarium spp. were subjected to a phylogenic analysis of their rRNA-ITS nucleotide sequences. Seven genetically distinct Fusarium strains were evaluated for their ability to influence population development of H. schachtii and crop performance in greenhouse trials. Swiss chard (Beta vulgaris) seedlings were transplanted into fumigated field soil amended with a single fungal strain at 1,000 propagules/g soil. One week later, the soil was infested with 250 H. schachtii J2/100 cm3 soil. Parasitized eggs were present in all seven Fusarium treatments at 1,180 degree-days after fungal infestation. The percentage of parasitism ranged from 17 to 34%. Although the most efficacious F. oxysporum strain 471 produced as many parasitized eggs as occurred in the original suppressive soil, none of the Fusarium strains reduced the population density of H. schachtii compared to the conducive check. This supports prior results that Fusarium spp. were not the primary cause of the population suppression of sugarbeet cyst nematodes at this location.
Keywords: biological control, Beta vulgaris, egg parasitism, Fusarium spp, Heterodera schachtii, nematophagous fungi, population suppression, sugarbeet cyst nematode, Swiss chard
Sugarbeet cyst nematodes have tremendous potential to cause acute and sustained crop damage (Whitehead, 1998). However, there have been various reports that initially large and damaging levels of this nematode can rapidly decline and remain small despite frequent cropping to host plants and otherwise disease-conducive environmental conditions (Thielemann and Steudel, 1973; Müller, 1982; Heijbroek, 1983; Crump and Kerry, 1987). Such inhospitable conditions are generally thought to be related to the presence of pathogen-antagonistic microorganisms and have been termed nematode suppressiveness (Kerry, 1987). Identification of the cause of this decline phenomenon is highly desirable, as it might result in the discovery of novel biocontrol agents. Even more important, in-depth analysis of such sites might advance our understanding of biological control of plant-parasitic nematodes (Borneman and Becker, 2007).
For a number of years, we have focused on the sugarbeet cyst nematode suppressive field 9E at the Agricultural Experiment Station on the UC Riverside campus. The population suppressive to Heterodera schachtii Schmidt was eliminated with broad-spectrum biocides such as soil fumigants and heat (Westphal and Becker, 1999). Adding small amounts of the suppressive soil (Westphal and Becker, 2000), or nematode cysts (Westphal and Becker, 2001) obtained from that soil, to conducive soil transferred and established beet cyst nematode population suppression within one growing season. Identification of the putative causal organisms was aided by exploiting selective sensitivity to chemical, physical and biological exclusion methods combined with isolations from parasitized nematodes on agar (Westphal and Becker, 2001) and media-independent rRNA microbial community analysis (Yin et al., 2003). Fungal parasitism of various life stages of H. schachtii by Dactylella oviparasitica and Fusarium spp. pointed to those organisms as putative major factors in nematode population suppression (Westphal and Becker, 2001). The relative abundance of the fungal rRNA genes derived using various experimental approaches suggested that D. oviparasitica was the key component (Yin et al., 2003). Furthermore, when strains of D. oviparasitica and F. oxysporum each were reintroduced into fumigated, conducive 9E soil, only D. oviparasitica produced the same level of suppressiveness as the original 9E soil (Olatinwo et al., 2006b, 2006c).
However, isolations from fungal-colonized H. schachtii cysts or parasitized eggs obtained from 9E field soil resulted in Fusarium strains that varied widely in colony appearance and growth characteristics on agar media. The genetic diversity among such strains might also influence their saprotrophic competitiveness or efficacy of parasitism. In this study, 26 Fusarium strains were subjected to phylogenic analysis of their rRNA ITS nucleotide sequences. Seven strains, representing the spectrum of Fusarium strains in our collection, were individually evaluated in greenhouse trials for their ability to parasitize beet cyst nematode eggs and to cause H. schachtii population suppression after two nematode generations.
Materials and Methods
Fungal strains: All fungal strains were isolated from cysts or eggs of H. schachtii obtained from field 9E at the Agricultural Experiment Station, University of California, Riverside, CA. Cysts were surface decontaminated in 1.0% sodium hypochlorite for 3 min and triple rinsed in sterile water. The cysts were placed onto water agar (0.75%), and incubated at 25°C for 7 to 10 d. For isolation of fungi from eggs, cysts were carefully opened with forceps and dissecting needles. Recovered eggs were exposed to 1.0% sodium hypochlorite for 3 min, triple rinsed in sterile water and transferred onto water agar. Isolated fungal strains were hyphal-tipped, subcultured and identified. Fusarium spp. strains 440, 446, 447, 448, 459, 463, 464 and 469 were obtained from colonized cysts, while strains 470 to 472 were isolated from parasitized H. schachtii eggs. Fusarium spp. strains 410 to 421, 423, 424 and 427 were isolated from colonized H. schachtii cysts during a previous 9E study (Westphal and Becker, 2001). Arthrobotrys oligospora strain 291, a trapping fungus originally isolated by R. Mankau, was included as a known nematophagous fungus which is a non-egg parasitic antagonist of the sugarbeet cyst nematode.
Phylogenetic analysis of fungal strains: DNA was extracted from each isolate (0.5 g fungal hyphae) by using the BIO 101 FastDNA Kit as described by the manufacturer (Qbiogene, Inc., Carlsbad, CA). PCR was used to amplify a portion of the small-subunit rRNA gene, ITS 1, 5.8S rRNA gene, ITS 2, and a portion of the large-subunit rRNA gene. Ten microliter amplification reactions were performed using a 1002 RapidCycler (Idaho Technologies, Idaho Falls, ID). These reactions contained the following reagents: 50 mM Tris (pH 8.3), 500 μg/ml BSA, 2.5 mM MgCl2, 250 μM of each dNTP, 400 nM of each primer (ITS1-F, CTTGGTCATTTAGAGGAAGTAA and ITS4, TCCTCCGCTTATTGATATGC) (White et al., 1990), 0.1 μl fungal DNA and 0.5 U Taq DNA polymerase. The cycling parameters were: 94°C for 2 min; 35 cycles of 94°C for 10 sec, 52°C for 20 sec, and 72°C for 60 sec; followed by 72°C for 2 min. The PCR products were resolved on a 1% agarose gel, excised from the gel, purified with the QIAquick PCR Purification Kit (Qiagen, Chatsworth, CA), ligated into pGEM-T (Promega, Madison, WI), transformed into competent Escherichia coli JM109 (Promega) and plated on Luria-Bertani agar plates containing 100 μg/ml ampicillin and were surface spread with X-GAL and IPTG. Plasmid purifications were performed on randomly selected white colonies using the QIAprep Spin Miniprep Kit (Qiagen). Nucleotide sequences of the fungal rRNA-ITS clones were obtained by using the ABI PRISM BigDyeTM Terminators v3.0 Cycle Sequencing Kit (Foster City, CA) and a 3100 Genetic Analyzer (ABI). Nucleotide sequences from both strands were obtained for each clone and assembled using ContigExpress (Vector NTI, Informax Inc., Frederick, MD). The rRNA clones ranged from 540 to 564 nucleotides in length. The nucleotide sequences identified in this study and reference sequences from GenBank were aligned using PILEUP (GCG, Madison, WI). Trees and bootstrapping values were obtained using maximum likelihood and maximum parsimony provided by PHYLIP (Felsenstein, 1989). The nucleotide sequences of the following rRNA-ITS clones identified in this work have been deposited in the GenBank database (accession number in captions): Fusarium sp. 410 (AY729051), Fusarium sp. 411 (AY729052), Fusarium sp. 412 (AY729053), Fusarium sp. 413 (AY729054), Fusarium sp. 414 (AY729055), Fusarium sp. 415 (AY729056), Fusarium sp. 416 (AY729057), Fusarium sp. 417 (AY729058), Fusarium sp. 418 (AY729059), Fusarium sp. 419 (AY729060), Fusarium sp. 420 (AY729061), Fusarium sp. 421 (AY729062), Fusarium sp. 423 (AY729063), Fusarium sp. 424 (AY729064), Fusarium sp. 427 (AY729065), Fusarium sp. 440 (AY729066), Fusarium sp. 446 (AY729067), Fusarium sp. 447 (AY729068), Fusarium sp. 448 (AY729069), Fusarium sp. 459 (AY729070), Fusarium sp. 463 (AY729071), Fusarium sp. 464 (AY729072), Fusarium sp. 469 (AY729073), Fusarium sp. 470 (AY729074), Fusarium sp. 471 (AY729075), Fusarium sp. 472 (AY729076).
Greenhouse evaluation of biological control activity of fungal strains: Soil samples from field 9E were randomly collected to a depth of 15 cm, pooled and screened with a 0.32-cm-pore sieve. The soil type was a Hanford fine sandy loam (60.8% sand, 29.7% silt, 9.5% clay; pH 7.9). The soil was thoroughly mixed 1:5:1 (v/v/v) with steam-pasteurized sand and vermiculite in a cement mixer. This improved water drainage and soil aeration without changing its suppressive properties (Westphal and Becker, 2000). One part of the soil mix was fumigated with methyl iodide to eliminate soil suppressiveness (Westphal and Becker, 2000), while the rest remained nontreated. All fungal strains were cultured on 9-cm-diam. potato dextrose agar plates at 25°C and ambient light. Macro- and microconidia from each strain were washed off the surface of 7-d-old PDA cultures with sterile water and filtered through two layers of sterile cheesecloth. The fumigated soil mix of each treatment was amended with one Fusarium strain by thoroughly mixing it with a conidia suspension in a polyethylene bag (50 cm × 45 cm) to a density of 1,000 propagules/g. This resembled the density of Fusarium spp. in the nonfumigated check as detected by soil plating on Komada's semi-selective medium (Komada, 1975). Soil moisture was adjusted to 8% prior to amendments. Four-week-old Swiss chard (Beta vulgaris cv. Large white ribbed, Lockard Seeds, Stockton, CA) seedlings were transplanted into 15-cm-diam. pulp pots (Western Pulp Pots Comp., Corvallis, OR) filled with 2,000 cm3 fungus-amended soil mix. Nonfumigated and fumigated soil mixes without fungal amendments served as suppressive or conducive checks, respectively. The pots were arranged in a randomized complete block design with 10 treatments (Table 1) and five replications in a greenhouse at 23 ± 2°C. The trial was irrigated with tap water as needed. One week after transplanting, each pot was infested with sugarbeet cyst nematodes. Cysts of H. schachtii were obtained from greenhouse cultures using a modified Fenwick extraction method (Caswell et al., 1985) and placed onto Baermann funnels containing 10−4 M zinc chloride at 26°C to stimulate the hatch from eggs (Caswell and Thomason, 1991). Each pot was infested with 5,000 H. schachtii J2. The trial was fertilized with 6 g slow-releasing fertilizer/pot (Sierra N-P-K [17-6-10], Scotts-Sierra Horticultural Products Company, Marysville, OH). The first trial was conducted in spring, during which supplementary light was provided. The second trial was conducted during early summer under ambient light. Soil temperature was monitored by a data logger with a temperature probe (HOBO H8, Onset Computer Corporation, Bourne, MA) to calculate nematode generation time by degree-days (DD, base temperature of 10°C [Thomason and Fife, 1962]). At approximately 1,180 DD after infestation, the plant tops were cut off at soil level, and weights were determined after oven-drying at 65°C for 2 d. Soil and cysts were washed off the roots and back into the soil. The soil of each pot was thoroughly mixed in a polyethylene bag (50 cm × 45 cm), and subsamples were assayed in one of three ways. The first method involved one 350 cm3 subsample, which was used for a modified cyst extraction (Caswell et al., 1985). Eggs were enumerated, and a subsample of 100 eggs was observed using a high-power microscope to determine fungal parasitism. Eggs containing fungal mycelium and/or spores were recorded as parasitized. In the second method, one 50 g subsample was used for moisture content determination. One 10 g subsample was used to enumerate colony forming units (CFU) of Fusarium spp. on a semi-selective medium (Komada, 1975). There were three replicates for each 10-fold dilution of each treatment.
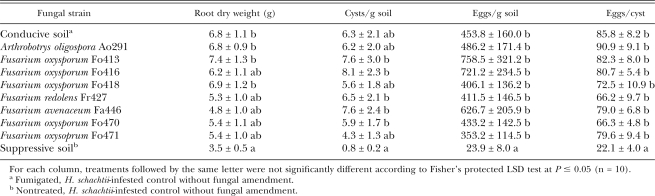
Table 1.
Effect of soil amendment with individual fungal strains to fumigated, Heterodera schachtii-infected soil on plant root weights and nematode populations in greenhouse trials.
Statistical analysis: The greenhouse trial was repeated once. There was no difference between the variances of the two data sets, and therefore the data were pooled. Numbers of cysts and eggs were transformed by log (x + 1) to normalize the data before analysis. The transformed cyst and egg data and the plant weights were subjected to ANOVA and Fisher's protected LSD tests (SuperANOVA, Abacus, Berkeley, CA).
Results
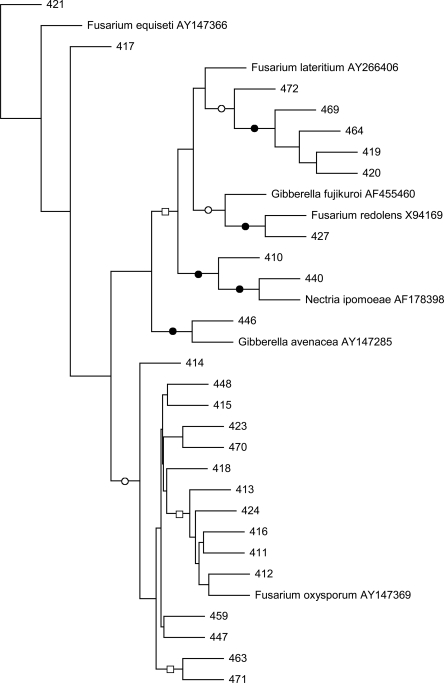
Phylogenetic analysis of fungal strains: All fungal strains, identified by phylogenic analysis of their rRNA-ITS nucleotide sequences, belonged to the genus Fusarium (Fig. 1). Strains 413, 416, 418, 470 and 471 were most closely related to F. oxysporum, while 427 was related to F. redolens and 446 to F. avenaceum.
Fig. 1.
Maximum likelihood phylogram of fungal rRNA-ITS nucleotide sequences. Branch points supported by maximum likelihood and maximum parsimony analyses are indicated: bootstrap values ≥89% (filled circles), bootstrap values between 55 and 83% (open circles). All other branch points had less than 50% bootstrap support. This is an unrooted tree. The scale bar indicates the number of nucleotide substitutions per position.
Greenhouse evaluation of biological control activity of fungal strains: Amendment of the Fusarium strains to conducive 9E soil mix had no effect on the H. schachtii population densities after two nematode generations (Table 1). Neither the number of cysts or eggs per gram soil changed, nor did the fungi influence the number of eggs per cyst in comparison to the non-amended conducive soil. In contrast, the nematode populations in the suppressive control were significantly reduced. While top growth of Swiss Chard did not differ in weight among the treatments (data not shown), root weight was reduced in the suppressive control.
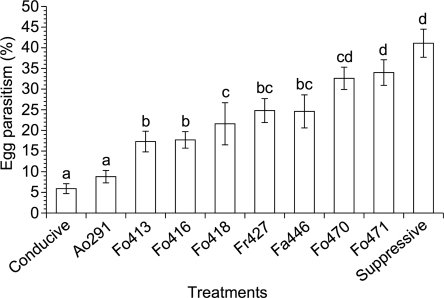
Parasitism of H. schachtii eggs: Parasitized eggs were identified in all treatments. Parasitism was slightly more than 40% in the suppressive check and approximately 6% in the noninfested conducive control (Fig. 2). Hyphae, chlamydospores and microconidia were often visible within the eggs. All tested Fusarium spp. appeared to be egg parasites. The efficacy of the Fusarium strains differed considerably from a low of 17.3% with strain 413 to a high of 34% with strain 471. The latter efficacy of egg parasitism was not different from the one found in the suppressive check.
Fig. 2.
Percent egg parasitism caused by strains of Fusarium spp. after two Heterodera schachtii generations in a replicated greenhouse trial (pooled data). Arthrobotrys oligospora strain 291, conducive and suppressive 9E soil served as controls. Means with the same letters were not significantly different (P ≤ 0.05). Bars represent standard error (n=10).
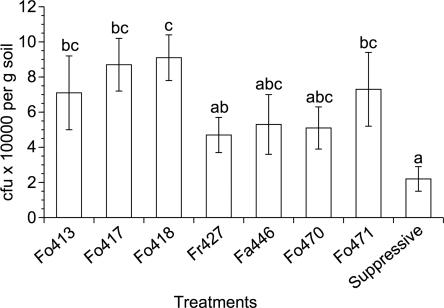
Population density of Fusarium spp. in soil at end of trial: All Fusarium spp.-amended treatments had higher CFU counts than the number of conidia added at the beginning of the trial (Fig. 3). While F. oxysporum strain 427 was detected at approximately 5 × 103 CFU/g soil, nearly twice as many CFU were found for strain 418. During the length of the trial, Fusarium CFU per gram soil doubled in the suppressive control soil.
Fig. 3.
Fusarium spp. population densities in soil after two Heterodera schachtii generations in a replicated greenhouse trial (pooled data). Enumeration of Fusarium spp. in suppressive 9E soil served as a control. Means with the same letters were not significantly different (P ≤ 0.05). Bars represent standard error (n=10).
Discussion
These results support earlier reports that certain Fusarium strains are egg parasites of sugarbeet cyst nematodes (Jorgenson, 1970; Nigh et al., 1980a, 1980b; Crump, 1987; Qadri and Saleh, 1990). After two nematode generations, more than one third of the eggs were parasitized by F. oxysporum strain 471. It is likely that the percentage of parasitism was actually greater because initial stages of the infection are easily overlooked, and eggs weakened by fungal infection tend to disintegrate in the extraction process. The strains in our study were comprised of a wide genetic diversity within the genus Fusarium, with most of them closely related to F. oxysporum. All strains of this species can exist saprotrophically, but some are able to cause root rots and wilt diseases while others remain nonpathogenic to plants (Fravel et al., 2003). Each of the seven Fusarium strains evaluated in our greenhouse trials appeared to be nonpathogenic to Swiss chard but able to parasitize eggs of H. schachtii. We had hypothesized that, as egg parasites, these Fusarium spp. might play a potentially important role in the regulation of the nematode population density. In a survey of 32 sugarbeet fields in California, F. oxysporum was apparently the primary parasite on H. schachtii eggs (Nigh et al., 1980a). While typically 10–20% of the eggs appeared to be parasitized, in five fields, levels of parasitism were greater than 40%. In our trials, parasitism of eggs by strains Fo471 and Fo470 was at similar levels to that observed in the 9E suppressive soil. Nevertheless, this was not correlated with population suppression of H. schachtii. While the field observations were related to sugarbeet crops, in the pot tests we used Swiss chard, which grows better than sugarbeets under our greenhouse conditions. It is conceivable that the crop influenced fungal performance, as plants can have a profound effect on root colonization and efficiency of facultative parasites as biological control agents (De Leij and Kerry, 1991, Bourne et al., 1996, Persmark, and Jansson, 1997).
In prior research, we had reported that F. oxysporum and other Fusarium spp. were frequently isolated from parasitized H. schachtii eggs obtained from 9E soil (Westphal and Becker, 2001). Microscopic examination of H. schachtii eggs from 9E soil often found them filled with hyphae and chlamydospores. More specifically, we observed eggs that exhibited a faint orange-reddish color, presumably due to fungal metabolites, as well as F. oxysporum macroconidia (Gao and Becker, 2000). The loss of suppressiveness after exposure of 9E soil to 55°C coincided with a reduction of Fusarium propagules on Komada's medium (Westphal and Becker, 2001). Also, rRNA gene analyses and consequent sequence-selective PCR assays corroborated that F. oxysporum was a common colonizer of H. schachtii cysts that were obtained from 9E soil (Yin et al., 2003). However, the relative abundance of the fungal rRNA genes derived from various different soil treatments and PCR assays pointed to another fungus, Dactylella oviparasitica.
Dactylella oviparasitica was originally described as the primary antagonist of Meloidogyne incognita in a peach orchard soil that had developed suppressiveness against root-knot nematodes (Stirling and Mankau, 1978). The strain isolated from 9E was different but closely related to a peach orchard isolate. In contrast to Fusarium species, D. oviparsitica grows slowly and inconspicuously on common isolation media. When added to fumigated, H. schachtii-reinfested soil, it produced the same high levels of suppressiveness as the naturally suppressive 9E soil (Olatinwo et al., 2006b, 2006c). When D. oviparasitica was added to nonfumigated portions of four soils that possessed different physicochemical characteristics, it reduced population densities of H. schachtii in those soils that did not exhibit preexisting levels of suppressiveness (Olatinwo et al., 2006a). This is in contrast to a similar examination of an egg-parasitic F. oxysporum strain from 9E, which did not reduce H. schachtii population densities when amended to fumigated, nematode-reinfested soil (Olatinwo et al., 2006b). Given this result, and those described in this study, it does not appear that egg parasitism by Fusarium spp. is a major factor in the nematode population suppression exhibited by the 9E soil. The large population density in Fusarium CFU at the end of the trials indicated that all strains became sufficiently established. In fact, the comparison to suppressive 9E soil with its rather moderate increase in Fusarium CFU suggests that establishment of the strains in the fumigated soil was favored by reduced microbial competition. In another scenario, one might speculate that single strains do not realistically reflect strain composition in 9E soil. Perhaps a consortium of strains with various genotypes and ecological abilities could more effectively control nematode populations, as it would fill a wider array of niches in the rhizosphere. Along the same line of reasoning, nematode control may be enhanced by microbial communities that employ a broader spectrum of metabolites deleterious to nematodes such as egg-degrading enzymes or toxins.
Footnotes
This project was financially supported in part by the University of California Statewide IPM Project, the UC Center for Pest Management Research and Extension and the USDA NRI program.
We thank J. Darsow for his technical assistance and J. Smith Becker for her critical review of the manuscript.
This paper was edited by Brian Kerry.
Literature Cited
- Borneman J, Becker JO. Identifying microorganisms involved in specific pathogen suppression in soil. Annual Review of Phytopathology. 2007;45:153–72. doi: 10.1146/annurev.phyto.45.062806.094354. [DOI] [PubMed] [Google Scholar]
- Bourne JM, Kerry BR, de Leij FAAM. The importance of the host plant in the interaction between root-knot nematodes (Meloidogyne spp.) and the nematophagous fungus Verticillium chlamydosporium Goddard. Biocontrol Science and Technology. 1996;6:539–48. [Google Scholar]
- Caswell EP, Thomason IJ. A model of egg production by Heterodera schachtii (Nematoda: Heteroderidae) Canadian Journal of Zoology. 1991;69:2085–2088. [Google Scholar]
- Caswell EP, Thomason IJ, McKinney HE. Extraction of cysts and eggs of Heterodera schachtii from soil with an assessment of extraction efficiency. Journal of Nematology. 1985;17:337–340. [PMC free article] [PubMed] [Google Scholar]
- Crump DH. Effect of time sampling, method of isolation and age of nematode on the species of fungi isolated from females of Heterodera schachtii and H. avenae . Revue de Nématologie. 1987;10:369–373. [Google Scholar]
- Crump DH, Kerry BR. Studies on the population dynamics and fungal parasitism of Heterodera schachtii in soil from sugar-beet monoculture. Crop Protection. 1987;6:49–55. [Google Scholar]
- De Leij FAAM, Kerry BR. The nematophagous fungus Verticillium chlamydosporium Goddard, as a potential biological control agent for Meloidogyne arenaria (Neal) Chitwood. Revue de Nématologie. 1991;14:157–64. [Google Scholar]
- Felsenstein J. PHYLIP - Phylogeny Inference Package (Version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- Fravel D, Olivain C, Alabouvette C. Fusarium oxysporum and its biocontrol. New Phytologist. 2003;157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. [DOI] [PubMed] [Google Scholar]
- Gao X, Becker JO. Observations on parasitized eggs from a beet cyst nematode-suppressive field. Journal of Nematology. 2000;32:430. [Google Scholar]
- Heijbroek W. Some effects of fungal parasites on the population development of the beet cyst nematode (Heterodera schachtii Schm.) Mededelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 1983;48:433–439. [Google Scholar]
- Jorgenson EC. Antagonistic interaction of Heterodera schachtii Schmidt and Fusarium oxysporum (Woll.) on sugarbeets. Journal of Nematology. 1970;2:393–398. [PMC free article] [PubMed] [Google Scholar]
- Kerry BR. Biological control. In: Brown RH, Kerry BR, editors. Principles and Practice of Nematode Control in Crops. New York: Academic Press; 1987. pp. 233–263. [Google Scholar]
- Komada H. Development of a selective medium for quantitative isolation of Fusarium solani from natural soil. Review Plant Protection Research. 1975;8:114–125. [Google Scholar]
- Müller J. The influence of fungal parasites on the population dynamics of Heterodera schachtii on soil radish. Nematologica. 1982;28:161. [Google Scholar]
- Nigh EA, Thomason IJ, Van Gundy SD. Identification and distribution of fungal parasites of Heterodera schachtii eggs in California. Phytopathology. 1980a;70:884–889. [Google Scholar]
- Nigh EA, Thomason IJ, Van Gundy SD. Effect of temperature and moisture on parasitization of Heterodera schachtii eggs by Acremonium strictum and F. oxysporum . Phytopathology. 1980b;70:889–891. [Google Scholar]
- Olatinwo R, Becker JO, Borneman J. Suppression of Heterodera schachtii populations by Dactylella oviparasitica in four soils. Journal of Nematology. 2006a;38:345–348. [PMC free article] [PubMed] [Google Scholar]
- Olatinwo R, Borneman J, Becker JO. Induction of beet-cyst nematode suppressiveness by Dactylella oviparasitica and Fusarium oxysporum in field microplots. Phytopathology. 2006b;96:855–859. doi: 10.1094/PHYTO-96-0855. [DOI] [PubMed] [Google Scholar]
- Olatinwo R, Yin B, Becker JO, Borneman J. Suppression of the plant-parasitic nematode Heterodera schachtii by the fungus Dactylella oviparasitica . Phytopathology. 2006c;96:111–114. doi: 10.1094/PHYTO-96-0111. [DOI] [PubMed] [Google Scholar]
- Persmark L, Jansson H-B. Nematophagous fungi in the rhizosphere of agricultural crops. FEMS Microbiology Ecology. 1997;22:303–312. [Google Scholar]
- Qadri AN, Saleh HM. Fungi associated with Heterodera schachtii (Nematoda) in Jordan II. Effect on H. schachtii and Meloidogyne javanica . Nematologica. 1990;36:104–113. [Google Scholar]
- Stirling GR, Mankau R. Dactylella oviparasitica, a new fungal parasite of Meloidogyne eggs. Mycologia. 1978;70:774–783. [Google Scholar]
- Thielemann R, Steudel W. Neunjährige Erfahrungen mit Monokultur von Zuckerrüben auf mit Heterodera schachtii (Schmidt) verseuchtem Boden. Nachrichtenblatt des Deutschen Pflanzenschutzdienstes. 1973;25:145–149. [Google Scholar]
- Thomason IJ, Fife D. The effect of temperature on development and survival of Heterodera schachtii Schm. Nematologica. 1962;7:139–145. [Google Scholar]
- Westphal A, Becker JO. Biological suppression and natural population decline of Heterodera schachtii in a California field. Phytopathology. 1999;89:434–440. doi: 10.1094/PHYTO.1999.89.5.434. [DOI] [PubMed] [Google Scholar]
- Westphal A, Becker JO. Transfer of biological soil suppressiveness against Heterodera schachtii . Phytopathology. 2000;90:401–406. doi: 10.1094/PHYTO.2000.90.4.401. [DOI] [PubMed] [Google Scholar]
- Westphal A, Becker JO. Components of soil suppressiveness against Heterodera schachtii . Soil Biology and Biochemistry. 2001;33:9–16. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. New York: Academic Press; 1990. pp. 315–322. [Google Scholar]
- Whitehead AG. Wallingford, UK: CAB International; 1998. Plant nematode control. [Google Scholar]
- Yin B, Valinsky L, Gao X, Becker JO, Borneman J. Identification of fungal rDNA associated with soil suppressiveness against Heterodera schachtii using oligonucleotide fingerprinting. Phytopathology. 2003;93:1006–1013. doi: 10.1094/PHYTO.2003.93.8.1006. [DOI] [PubMed] [Google Scholar]