Abstract
A lack of diversity and durability of resistant soybean varieties complicates management of the soybean cyst nematode (SCN), Heterodera glycines, exemplified by the current overdependence on the PI 88788 source of resistance. Of interest is the effect of adaptation of a SCN population to a source of resistance on its subsequent ability to develop on others. Female indices (FI) from virulence assays (race, HG Type and SCN Type tests) for SCN field populations and inbred lines were analyzed. Female indices on PI 88788, PI 209332 and PI 548316 were highly correlated, as were those of PI 548402, PI 90763, PI 89772 and PI 438489B. Previous studies on resistant SCN-infected soybean roots indicated that the cellular resistance response was similar within these two groups of soybean genotypes. In field populations, highly significant correlations were also found between FI on PI 88788 and PI 548402 and those on PI 89772 and PI 437654. In inbred lines, FI on PI 437654 were correlated with PI 90763 and PI 438489B. To avoid further adaptation, rotation of cultivars with resistance from these groups should be carefully monitored, including those from the most promising source of resistance, PI 437654, such as CystX. In a separate test, 10 soybean varieties developed from CystX were tested against HG Type 0, HG Type 2.5.7 and HG Type 1–7. Female development occurred in all tests but one. Although identification and deployment of unique resistance is needed, management strategies to prevent and detect adaptation should be emphasized.
Keywords: Adaptation, genetic diversity, Heterodera glycines, management, resistance, soybean cyst nematode
The co-evolution of the soybean cyst nematode (Heterodera glycines Ichinohe) and soybeans (Glycine max [L.] Merr.) has resulted in a complex problem for soybean producers. Once established in a soybean field, eradication of an infestation of H. glycines is unlikely, and careful management is required if soybean cultivation is to remain profitable. Although the use of resistant cultivars is a component of recommended control measures (Riggs and Niblack, 1999), consistent use of soybean cultivars with the same sources of resistance can lead to adaptation of the existing H. glycines population to cultivars with that source of resistance (Triantaphyllou, 1975; Riggs et al., 1977; McCann et al., 1982; Anand and Brar, 1983; Luedders and Dropkin, 1983; Young, 1984; Luedders, 1985; Young et al., 1986; Cloud et al., 1988; Young and Hartwig, 1988; Luedders and Anand, 1989). Use of a single source of resistance is not a good long-term strategy. Diversification of the source of resistance in deployed cultivars is a good management plan. However, the soybean varieties that are available to soybean producers have limited diversity of resistance genes (Gizlice et al., 1994; Rao-Arelli, 1994; Diers et al, 1997; Concibido et al., 2004). Consequently, soybean producers are faced with both a lack of durability and diversity of resistance. Typically, this lack of resistant gene diversity becomes obvious when resistant cultivars are no longer effective in commercial fields where H. glycines populations were inadvertently selected for virulence.
Genetically variable H. glycines populations can present challenges to both researchers and producers. The inherent variability distinctive of H. glycines can be difficult to assess as it must be measured in populations rather than individuals. Race and HG Type tests measure the ability of H. glycines isolates to develop on standard sets of resistant soybean lines relative to a standard susceptible cultivar (Riggs and Schmitt, 1988; Niblack et al., 2002). Results of these assays are useful to characterize greenhouse isolates maintained for variety evaluations, other research, and the identification of field populations, in order to monitor and maximize the effectiveness of deployed resistance. Characterization of populations has been improved through development and use of the HG Type test (Niblack et al., 2002). A truncated version of the HG Type test is the SCN Type test that includes only regionally important indicators (e.g., Plant Introduction [PI] 548402 (‘Peking’), PI 88788 and PI 437654 for Illinois) and is used predominantly for producer samples (Niblack, 2005). The HG and SCN Type numbering system clarifies SCN population virulence descriptions and improves communication of results (Niblack et al., 2002).
Of the more than 100 known sources of resistance (Rao-Arelli et al., 1997), only a few are used for cultivar and germplasm development in the US (Shannon et al., 2004). These include PI 548402 (Peking), 437654, 209332, 90763 and, the most utilized source of resistance in the midwest, PI 88788 (Diers and Arelli, 1999). Inheritance studies indicate that several genes confer resistance to SCN, including rhg1, rhg2 and rhg3 (Caldwell et al., 1960), Rhg4 (Matson and Williams, 1965) and Rhg5 (Rao-Arelli et al., 1992; Rao-Arelli, 1994). Attempts by breeders to exploit novel sources of H. glycines resistance are complicated by several factors, such as unadapted resistance sources, multigenic resistance, inheritance of undesirable agronomic traits (shattering, low yield and susceptibility to other pathogens) and, also, a reduction of the levels of resistance caused by a loss of the full complement of resistance genes during breeding. Subsequent screening of progeny for resistance is time- and space-consuming, expensive and can be complicated by the inherent variability of SCN populations, particularly if test conditions are not adequately controlled (Colgrove et al., 2002). To circumvent reliance on the bioassay of phenotype and increase breeding efficiency, interest in the development of reliable, tightly linked genetic markers for SCN resistance in soybean has led to many reports of mapped quantitative trait loci (QTL) for resistance in soybean (Concibido et al., 1996; Vierling et al., 1996a; Concibido et al., 2004; Guo et al., 2006). Marker assisted selection shows promise as an efficient method to identify genotypes without the constraints of phenotype assessment (Concibido et al., 2004) and is widely used by the soybean breeding community. However, phenotypic assays of selected genotypes are still needed to verify the marker results.
The need for greater resistance diversity in available soybean varieties has increased due to the lack of durability of deployed resistance following overuse of and adaptation to deployed resistance. Soybean cultivars developed from underused resistant sources have potential for effective H. glycines control. These include cultivars with the highly resistant PI 437654 as the original source of resistance. If cultivars developed from this PI prove to be effective, populations virulent on currently used resistance sources could be more easily managed. However, populations able to develop on PI 437654 and varieties developed from it have been found (Young, 1998; Niblack et al., 2003) including varieties with resistance from CystX (Niblack, unpublished). The CystX line was derived from a cross of the resistant soybean Hartwig (PI 437654 source of resistance) and Williams 82 to generate the proprietary soybean germplasm lines that have been used by crop breeders in crosses with elite varieties to develop nematode-resistant soybeans (Faghihi, et al., 1995; Vierling et al., 1996b).
A better understanding of the nature of soybean resistance is needed in order to address the lack of diversity and durability of soybean resistance. The introduction and use of PI in breeding programs has occurred with limited knowledge of their relation to other PI and complement of resistance genes. In addition, reports of the effects of different resistant soybean genotypes on nematode development are limited to just a few studies. Based on similarities of cellular resistance response and reaction in tests, soybean resistance to H. glycines was classified into two main groups: the PI 88788 group, including PI 209332 and 548316; and the PI 548402 (Peking) group, including PI 90763, PI 89772 and, perhaps, partially, PI 437654 (Endo, 1965; Kim et al., 1987; Halbrendt et al., 1992; Kim and Riggs, 1992; Mahalingham and Skorupska, 1996). In a recent evaluation of near isogenic lines (NIL) that segregated for rhg1, different rhg1 alleles were found for PI 88788 and PI 437654 (Breucker et al., 2005). The lineage of the PI, and any ancestral relationship between soybean genotypes, is not known. This is an important issue in the event that adaptation of field populations to deployed sources of resistance reduces the effectiveness of resistant cultivars derived from soybean genotypes within these groups. Observations have been made of FI increases on PI within the above groups following selection of SCN greenhouse isolates on a PI within that group; results of tests on field samples also seemed to follow this trend (Colgrove and Niblack, unpublished). In order to investigate this observation, an analysis of results of virulence assays, including race, HG Type and SCN Type tests, conducted on field samples and on inbred lines maintained in the greenhouse was undertaken to observe relationships in female indices among types of soybean resistance. In addition, a separate test investigated the development of populations on varieties derived from CystX available to Illinois producers.
Materials and Methods
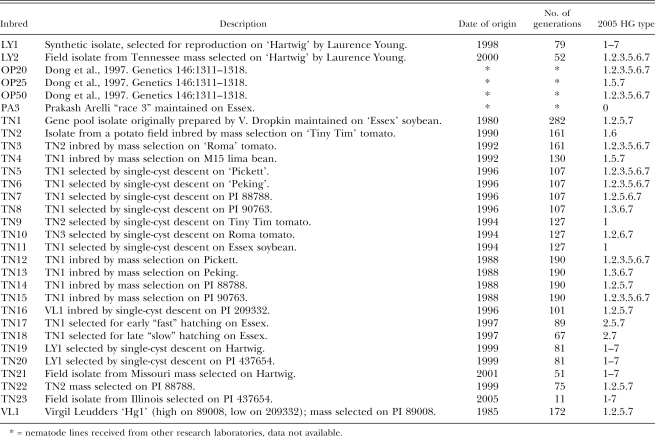
Nematode isolates for virulence assays (race, HG Type and SCN Type tests) were inbred H. glycines lines or soybean field populations. A collection of inbred H. glycines lines initiated at the University of Missouri and continued at the University of Illinois-Urbana for research purposes is tested annually for Race or HG Type (Table 1). These inbred lines are greenhouse cultures maintained under specific selection protocols, including inbreeding by single-cyst descent or by mass selection on specific hosts, at a frequency of approximately 30 d. For single-cyst descent, inoculum for a new culture consists of one cyst transferred from a 30-d-old culture (“mature culture”) to a non-infected host. For mass selection, a subsample of the eggs harvested from a mature culture is used as inoculum. In addition, two lines are selected for early (fast) and late (slow) hatching of nematode eggs. The collection of inbred lines consists of 30 H. glycines lines and the oldest line originated in 1980 (Table 1). Tests were performed from 1993 to 2005 and included 229 tests. Field populations from several states within the US and also several different countries (data not shown) were assayed for virulence from 1989 to 2005 and included 1,053 tests. A high degree of inbreeding distinguishes the inbred lines from the collected field populations.
Table 1.
Inbreeding protocols, dates of origin, numbers of generation and 2005 HG Type of Heterodera glycines inbred lines maintained at the University of Illinois Nematology Laboratory for research purposes.
To produce a sufficient amount of eggs to perform tests of both inbred lines and field populations, eggs extracted from composite samples of cysts were used to infest soil in 400-ml pots planted with H. glycines-susceptible seeds. Initially, cysts from inbred lines were collected from greenhouse cultures, and cysts from field soil were extracted from soil by elutriation or by hand over nested sieves. For female or cyst collection, an 850-μm sieve was nested on top of a 250-μm aperture one, and cysts were collected from the lower sieve. To release eggs for inoculum, cysts were ground with a rubber stopper on a 150-μm aperture sieve over nested 75/25-μm aperture sieves (Faghihi and Ferris, 2000). Cultures were maintained in the greenhouse in 27°C water tables under 16 hr light regimens. After 30 d, contents of the 400-ml pots were released into water-filled containers to gently remove soil from the roots, and cysts and females were washed off of the roots by high-pressure spray of water onto sieves of 850/250-μm aperture. Cysts were then ground to release the egg inoculum for race or HG Type testing.
Race and HG Type tests were performed as previously described (Riggs and Schmitt, 1988; Niblack et al., 1993, 2002). Eggs were collected on a 25-μm-aperature sieve, concentrated by sucrose centrifugation (Niblack et al., 1993) and diluted in suspensions of 1,000–2,000 eggs/ml. The egg suspension (inoculum) was used to infest soil (1,000–2,000 eggs/100 cm3) subsequently planted with uniform seedlings of the appropriate soybean line. For all tests, ‘Lee 74’ was used as the standard susceptible check. Resistant soybean differentials in race tests included ‘Pickett’, ‘Peking’, PI 88788, and PI 90763 and, in later tests, PI 437654. Each test had three replications, and their means were rounded to whole numbers. For HG Type tests, resistant soybean indicators included PI 548402 (‘Peking’, USDA Soybean Germplasm Collection), PI 88788, PI 90763, PI 437654, PI 209332, PI 89772 and PI 548316; PI 438489B was included in many of the tests, and Pickett was often included to allow comparison with race test results. Each test had six replications that were rounded to whole numbers. Results of the truncated Illinois SCN Type tests performed in 2004–05 on producer samples were also included. The IL SCN Type test includes only PI 548402, PI88788 and PI 437654 as the resistant indicators (Niblack, 2005). For all tests, the female indices (FI) used for data analysis were calculated by dividing the mean number of females that developed on a differential or indicator line by the mean number of females on the susceptible check ‘Lee 74’, multiplied by 100.
Experimental units were 3.0-cm-diam. × 15-cm PVC tubes filled with infested soil (100 cm3) planted with one seedling per tube; tubes were contained within plastic crocks suspended in water baths maintained at 27°C, under 16 hr of light. After 30 d, females were washed off soybean roots with a high-pressure spray of water over nested 850/250-μm-aperture sieves and counted.
In a separate test, development on varieties whose source of resistance was CystX was evaluated. The original source of resistance of CystX soybean is ‘Hartwig’, and Hartwig's resistance originated from PI 437654 (Anand, 1992). The CystX germplasm provides genes from the highly resistant PI 437654 for use in crosses with high-yielding soybean lines (Vierling et al., 1996b). Ten soybean varieties developed from CystX germplasm were tested for resistance to three H. glycines greenhouse populations. These included an HG Type 0 (FI < 10 on all HG Type indicator lines), HG Type 2.5.7 (FI ≥ 10 on PI 88788, PI 209332 and PI 548316) and HG Type 1–7 (FI ≥ 10 on all HG Type indicator lines). Also included were Lee 74 and ‘Essex’ as susceptible checks and PI 437654. Means of three replications were rounded to whole numbers and used to calculate female indices.
Data analysis: Tests performed on H. glycines inbred lines and field populations were analyzed separately. Race and HG Type test results used for this study were from tests performed annually on inbred lines from 1993–2005 (data not shown) and from tests on field populations from 1989–2005, and total numbers of test results were 1,053 field tests and 229 inbred line tests. The number of tests performed each year was dependent on the number of inbred lines included at the time in the collection and the number of field samples received for virulence testing. Female indices from race and HG Type tests were analyzed for Pearson's Product-moment correlations (CORR) (SAS Institute, Cary, NC).
Results
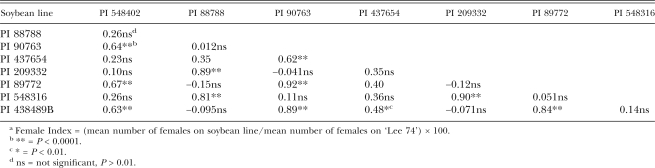
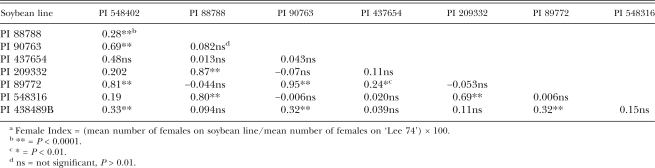
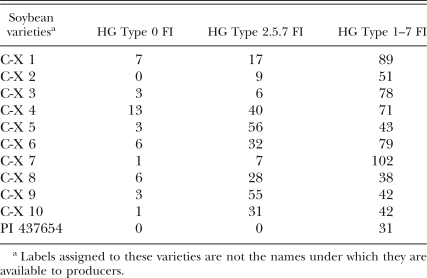
The analyses of both inbred lines and field populations showed significant correlations among female indices (Tables 2,3). For inbred lines, FI on PI 548402, PI 90763, PI 89772 and PI 438489B were highly correlated (R = 0.63–0.67), as were correlations among FI on PI 88788, PI 209332 and PI 548316 (R = 0.81–0.89). Significant correlations with PI 437654 were found with PI 90763 (R = 0.62) and PI 438498B (R = 0.49). Overall, a lack of significant correlation was found between members of the PI 88788 group and the PI 548402 (Peking) group (P > 0.01). For field populations (Table 3), there were again highly significant correlations among FI on PI 548402, PI 90763, PI 89772 and PI 438489B (R = 0.33–0.81) and among PI 88788, PI 209332 and PI 548316 FI (R = 0.80–0.87). Additional correlations were found between FI on PI 548402 and PI 88788 (R = 0.28) and PI 89772 and PI 437654 (R = 0.24). In tests on CystX-derived varieties, cysts from each H. glycines population developed on all soybean varieties tested except for the HG Type 0 population on CX-2 (Table 4).
Table 2.
Correlation coefficients among soybean lines with resistance to H. glycines based on female indices (FI)a from 229 virulence assays on inbred lines of H. glycines performed from 1993 to 2005.
Table 3.
Correlation coefficients among soybean lines with resistance to H. glycines based on female indices (FI)a from 1,053 virulence assays on field populations of H. glycines conducted from 1989 to 2005.
Table 4.
Female indices of H. glycines populations on ten CystX-derived varieties (CX-1 through CX-10) available to Illinois producers.
Discussion
The correlations found among female indices, in both inbred lines and field populations, revealed a pattern of relationships among the resistant PI tested. In both population types, this grouping according to correlated female indices among PI 548402, 90763, 89772 and 438489B and among PI 88788, 209332 and 548316 was consistent with the PI 548402 (Peking) and PI 88788 groups previously discussed. In inheritance studies, results indicated similar relationships among resistant soybeans. Young (1982) reported that PI 88788 and ‘Bedford’ (derived from PI 88788) showed a resistance reaction that was different than that of PI 89772, PI 90763 and Peking (PI 548402), which were similar to each other. In Luedders and Anand (1989), Peking resistance appeared similar to PI 89772 and PI 90763, but different than PI 88788 and PI 209332. Anand and Brar (1983) found that H. glycines populations responded similarly to PI 88788, PI 209332 and Cloud (PI 548316), but differently to PI 89772 and PI 90763. Additional evidence for similarity in female development between PI 88788 and PI 209332 has been reported (McCann et al., 1982; Luedders, 1983).
Observations of the histological and ultrastructural resistance response similarly groups H. glycines resistance responses. In descriptions by Endo (1965), Kim et al. (1987), Kim and Riggs (1992) and Mahalingham and Skorupska (1996) of the Peking-type, PI 88788-type and PI 437654 resistance responses, the Peking-type was mainly an early reaction of plant tissue where necrosis of cells was evident, PI 88788-type was slower and involved nuclear degeneration and PI 437654 had features of both types. An observation by Halbrendt et al. (1992) of a stage-related effect of resistance on juvenile development supports the observations that the resistance response may be different between the groups. He found that Peking-type resistance affects earlier stages than that of the PI 88788 group, where development to more mature juvenile stages was allowed. In a study on the effect of SCN resistance on male and female development, resistance from the Peking groups prevented maturity of both males and females, while resistance from the PI 88788 group allowed males to develop (Colgrove and Niblack, 2005). In inbred lines maintained in the University of Illinois Nematology lab, selection of H. glycines isolates on specific PI has resulted in the increased ability to develop on PI within the group to which that PI is suspected to belong based on resistance response studies (Niblack, unpublished). Overall, in this study, the strongest correlations were found between members within the two groups. An exception to this was the correlation between FI on PI 548402 and PI 88788 from field populations. This result differed in tests on inbred lines, where the PI were not correlated. The result is probably due to the higher degree of genetic diversity in field populations relative to the inbred lines. Because the correlation coefficient was not high with field populations, this suggests that these two PI are still useful in differentiating H. glycines populations.
As adaptation to PI 88788 increases, so does the importance of the availability of unique sources of resistance. The PI 437654 shows promise as an important source of resistance for use against populations adapted to PI 88788. Use of this PI as a source of SCN resistance in soybean cultivars began relatively recently after the release of Hartwig (Anand, 1992) and with the development of the promising CystX germplasm (Faghihi, et al., 1995; Vierling et al., 1996b), and new varieties with this resistance adapted to northern locations are becoming more common. In tests on both inbred lines and field populations, correlations were found between PI 437654 and other PI (e.g., 89772) in field populations, PI 438489B, PI 89772 and PI 88788, and PI 438489B in inbred lines. These correlations were weaker than those found among members within the two groups, but potential associations should still be considered. The observance of the PI 437654 cellular resistance response suggests a combination of the resistance responses found with Peking and PI 88788 (Mahalingham and Skorupska, 1996). Although PI 437654 is considered highly resistant to most populations of H. glycines (Anand, 1992), cultivars with this PI as the original source of resistance lack this high level of resistance, and adaptation can occur just as it has with PI 88788. For example, the inbred line TN23 originated from cysts collected from PI 437654 from an HG Type test on a soil sample from a field planted to a CystX-derived cultivar. This population was then mass-selected on PI 437654 and quickly adapted to it. After four generations, a large number of cysts was found on PI 437654 roots; when tested for HG Type at eight generations, the FI was at a near-susceptible level of 87 (Colgrove, unpublished). The adaptation of this population was accelerated by greenhouse selection; how quickly adaptation would occur in the field would depend on the SCN population density and the specific planting regimen used. In this study, when the CystX-derived cultivars were screened for female development, all varieties allowed female development in every test except for one variety in one test. These and similar data should serve as a warning to soybean producers that use of PI 437654-type varieties requires careful management in order to restrict population adaptation and eventual loss of effectiveness of this promising source of resistance.
Numerous mapping papers and reports of marker associations for SCN resistance in soybean have been published. Conflicting results and other inconsistencies have been found between studies (Concibido et al., 2004; Guo et al., 2006) that make it difficult to draw conclusions regarding relationships of sources of resistance and the genes that they share. However, areas of consistency among studies, such as the location of Rhg4 and rhg1 on linkage groups A2 and G, respectively, suggest the possibility that most known sources of resistance have resistance genes in common, and this underscores the problem that most cultivars carry similar genes (Gizlice et al., 1994; Rao-Arelli, 1994; Diers et al., 1997; Concibido et al., 2004). The use of marker assisted selection to characterize potentially resistant soybean progeny lines can reduce the number of lines requiring greenhouse screening. In addition, the potential for future identification of resistance genes seems promising. To reconcile marker data with cyst development, the importance of well characterized SCN lines used under controlled environmental conditions cannot be overemphasized.
The reconciliation of QTL data to resistance response is much needed. The reduction of the effectiveness of deployed resistance in soybean is further complicated by a narrow genetic base for SCN resistance in soybean. The highly correlated FI in virulence assays supports speculation that the known sources of resistance have genes in common. These results correspond to observations of similar resistance responses within two groups of SCN resistance (Endo, 1965; Kim et al., 1987; Kim and Riggs, 1992; Mahalingham and Skorupska, 1996). Further, greenhouse selection of SCN isolates on specific PI has resulted in the increased ability to develop on PI within the group to which that PI belongs (Niblack, unpublished). The consequence of overuse of soybean cultivars with similar resistance is adaptation of field populations. Before management techniques such as rotation with different resistant cultivars can be successfully employed, the genetics of soybean resistance, the relationship of resistant genes in different sources of resistance and the complement of resistance genes in cultivars must be better understood.
Footnotes
The authors wish to gratefully acknowledge Robert Heinz and Kamron Colgrove for invaluable contributions to this research.
This paper was edited by Isgouhi Kaloshian.
Literature Cited
- Anand SC. Registration of ‘Hartwig’ soybean. Crop Science. 1992;32:1294. [Google Scholar]
- Anand SC, Brar GS. Response of soybean lines to differentially selected cultures of soybean cyst nematode Heterodera glycines Ichinohe. Journal of Nematology. 1983;15:120–123. [PMC free article] [PubMed] [Google Scholar]
- Bruecker E, Niblack T, Kopisch-Obuch F, Diers B. The effect of rhg1 on reproduction of Heterodera glycines in the field and greenhouse and associated effects on agronomic traits. Crop Science. 2005;45:1721–1727. [Google Scholar]
- Caldwell BE, Brim CA, Ross JP. Inheritance of resistance to soybean cyst nematode, Heterodera glycines . Agronomy Journal. 1960;52:635–636. [Google Scholar]
- Cloud GL, Riggs RD, Caviness CE. Variability in host preference among field populations of Heterodera glycines . Journal of Nematology. 1988;20:417–420. [PMC free article] [PubMed] [Google Scholar]
- Colgrove AL, Niblack TL. The effect of resistant soybean on male and female development and adult sex ratios of Heterodera glycines . Journal of Nematology. 2005;37:161–167. [PMC free article] [PubMed] [Google Scholar]
- Colgrove AL, Smith GS, Wrather JA, Heinz RD, Niblack TL. Lack of predictable race shift in Heterodera glycines-infested field plots. Plant Disease. 2002;86:1101–1108. doi: 10.1094/PDIS.2002.86.10.1101. [DOI] [PubMed] [Google Scholar]
- Concibido VC, Diers BW, Rao-Arelli P. A decade of mapping for cyst nematode resistance in soybean. Crop Science. 2004;44:1121–1131. [Google Scholar]
- Concibido VC, Young ND, Lange DA, Denny RL, Danesh D, Orf JH. Targeted comparative genome analysis and qualitative mapping of a major partial-resistance gene to the soybean cyst nematode. Theoretical and Applied Genetics. 1996;93:234–241. doi: 10.1007/BF00225751. [DOI] [PubMed] [Google Scholar]
- Diers BW, Rao-Arelli P. Management of parasitic nematodes of soybean through genetic resistance. In: Kauffman HE, editor. Proceedings of the World Soybean Research Conference, 6th; Aug. 4–7 1999; Chicago, IL. Champaign, IL: Superior Printing; 1999. pp. 300–306. [Google Scholar]
- Diers BW, Skorupska T, Rao-Arelli P, Cianzio SR. Genetic relatedness among soybean plant introductions with resistance to soybean cyst nematode. Crop Science. 1997;37:1966–1972. [Google Scholar]
- Endo BY. Histological responses of resistant and susceptible soybean varieties, and backcross progeny to entry and development of Heterodera glycines . Phytopathology. 1965;55:375–381. [Google Scholar]
- Faghihi J, Ferris JM. An efficient new device to release eggs from Heterodera glycines. Journal of Nematology. 2000;32:411–413. [PMC free article] [PubMed] [Google Scholar]
- Faghihi J, Vierling RA, Halbrendt JM, Ferris VR, Ferris JM. Resistance genes in a ‘Williams 82’ X ‘Hartwig’ soybean cross to an inbred line of Heterodera glycines . Journal of Nematology. 1995;27:418–421. [PMC free article] [PubMed] [Google Scholar]
- Gizlice Z, Carter TE, Jr., Burton JW. Genetic Base for North American Public Soybean Cultivars Released between 1947 and 1988. Crop Science. 1994;34:1143–1151. [Google Scholar]
- Guo B, Sleper A, Lu P, Shannon G, Nguyen T, Rao-Arelli P. QTLs Associated with resistance to soybean cyst nema-tode in soybean: Meta-Analysis of QTL locations. Crop Science. 2006;46:595–602. [Google Scholar]
- Halbrendt J, Lewis S, Shipe E. A technique for evaluating Heterodera glycines development in susceptible and resistant soybean. Journal of Nematology. 1992;24:84–91. [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Riggs RD. Cytopathological reactions of resistant soybean plants to nematode invasion. In: Wrather JA, Riggs RD, editors. Biology and Management of the Soybean Cyst Nematode. St. Paul: APS Press; 1992. pp. 157–168. [Google Scholar]
- Kim YH, Riggs RD, Kim KS. Structural changes associated with resistance of soybean to Heterodera glycines . Journal of Nematology. 1987;19:177–187. [PMC free article] [PubMed] [Google Scholar]
- Luedders VD. Genetics of the cyst nematode-soybean symbiosis. Phytopathology. 1983;73:944–948. [Google Scholar]
- Luedders VD. Selection and inbreeding of Heterodera glycines on Glycine max . Journal of Nematology. 1985;17:400–404. [PMC free article] [PubMed] [Google Scholar]
- Luedders VD, Anand SC. Attempt to select a cyst nematode population on soybean plant introduction 437654. Journal of Nematology. 1989;21:264–267. [PMC free article] [PubMed] [Google Scholar]
- Luedders VD, Dropkin VH. Effect of secondary selection on cyst nematode reproduction on soybeans. Crop Science. 1983;23:263–264. [Google Scholar]
- Mahalingham R, Skorupska HT. Cytological expression of early response to infection by Heterodera glycines Ichinohe in resistant PI 437654 soybean. Genome. 1996;39:986–998. doi: 10.1139/g96-123. [DOI] [PubMed] [Google Scholar]
- Matson AL, Williams LF. Evidence for a fourth gene for resistance to soybean cyst nematode. Crop Science. 1965;5:477. [Google Scholar]
- McCann J, Luedders VD, Dropkin VH. Selection and reproduction of soybean cyst nematodes on resistant soybeans. Crop Science. 1982;22:78–80. [Google Scholar]
- Niblack TL. Soybean cyst nematode management reconsidered. Plant Disease. 2005;89:1020–1026. doi: 10.1094/PD-89-1020. [DOI] [PubMed] [Google Scholar]
- Niblack TL, Arelli PR, Noel GR, Opperman CH, Orf JH, Schmitt DP, Shannon JG, Tylka GL. A revised classification scheme for genetically diverse populations of Heterodera glycines . Journal of Nematology. 2002;34:279–288. [PMC free article] [PubMed] [Google Scholar]
- Niblack TL, Heinz RD, Smith GS, Donald PA. Distribution, density, and diversity of Heterodera glycines in Missouri. Journal of Nematology. 1993;25:880–886. [PMC free article] [PubMed] [Google Scholar]
- Niblack TL, Wrather JA, Heinz RD, Donald PA. Distribution and virulence phenotypes of Heterodera glycines in Missouri. Plant Disease. 2003;87:929–932. doi: 10.1094/PDIS.2003.87.8.929. [DOI] [PubMed] [Google Scholar]
- Rao-Arelli AP. Inheritance of resistance to Heterodera glycines Race 3 in soybean accessions. Plant Disease. 1994;78:898–900. [Google Scholar]
- Rao-Arelli AP, Anand SC, Wrather JA. Soybean resistance to soybean cyst nematode race 3 is conditioned by an additional dominant gene. Crop Science. 1992;32:862–864. [Google Scholar]
- Rao-Arelli AP, Wilcox JA, Myers O, Gibson PT. Soybean germplasm resistant to Races 1 and 2 of Heterodera glycines . Crop Science. 1997;37:1367–1369. [Google Scholar]
- Riggs RD, Hamblen ML, Rakes L. Development of Heterodera glycines pathotypes as affected by soybean cultivars. Journal of Nematology. 1977;9:312–318. [PMC free article] [PubMed] [Google Scholar]
- Riggs RD, Niblack TL. Soybean cyst nematode. In: Hartman GL, Sinclair JB, Rupe JC, editors. Compendium of Soybean Diseases. Fourth Edition. St. Paul: APS Press; 1999. pp. 52–53. [Google Scholar]
- Riggs RD, Schmitt DP. Complete characterization of the race scheme for Heterodera glycines . Journal of Nematology. 1988;20:392–395. [PMC free article] [PubMed] [Google Scholar]
- Shannon JG, Arelli PR, Young LD. Breeding for resistance and tolerance. In: Schmitt DP, Wrather JA, editors. Biology and Management of Soybean Cyst Nematode. Second Edition. Marcelline: University of Missouri-Columbia; 2004. pp. 155–180. [Google Scholar]
- Triantaphyllou AC. Genetic structure of races of Heterodera glycines and inheritance of ability to reproduce on resistant soybeans. Journal of Nematology. 1975;7:356–364. [PMC free article] [PubMed] [Google Scholar]
- Vierling RA, Faghihi J, Ferris VR, Ferris JM. Association of RFLP markers conferring broad-based resistance to the soybean cyst nematode (Heterodera glycines) Theoretical and Applied Genetics. 1996a;92:83–86. doi: 10.1007/BF00222955. [DOI] [PubMed] [Google Scholar]
- Vierling, R.A., Faghihi, J., Ferris, V.R., and Ferris, J.M., inventors; Purdue Research Foundation, assignee. 1996b. Methods for conferring broad-based soybean cyst nematode resistance to a soybean line. U.S. Pat. No. 6,096,944. Date issued: 1 Aug. 2000.
- Young LD. Reproduction of Tennessee soybean cyst nematode population on cultivars resistant to race 4. Plant Disease. 1982;66:251–252. [Google Scholar]
- Young LD. Changes in the reproduction of Heterodera glycines on different lines of Glycine max . Journal of Nematology. 1984;16:304–309. [PMC free article] [PubMed] [Google Scholar]
- Young LD. Managing soybean resistance to Heterodera glycines . Journal of Nematology. 1998;30:525–529. [PMC free article] [PubMed] [Google Scholar]
- Young LD, Hartwig EE. Selection pressure on soybean cyst nematode from soybean cropping sequences. Crop Science. 1988;28:845–847. [Google Scholar]
- Young LD, Hartwig E, Aand SC, Widick D. Responses of soybeans and soybean cyst nematodes to cropping sequences. Plant Disease. 1986;70:787–791. [Google Scholar]