Abstract
Greenhouse experiments with two susceptible hosts of Meloidogyne incognita, a dwarf tomato and wheat, led to the identification of a soil in which the root-knot nematode population was reduced 5- to 16-fold compared to identical but pasteurized soil two months after infestation with 280 M. incognita J2/100 cm3 soil. This suppressive soil was subjected to various temperature, fumigation and dilution treatments, planted with tomato, and infested with 1,000 eggs of M. incognita/100 cm3 soil. Eight weeks after nematode infestation, distinct differences in nematode population densities were observed among the soil treatments, suggesting the suppressiveness had a biological nature. A fungal rRNA gene analysis (OFRG) performed on M. incognita egg masses collected at the end of the greenhouse experiments identified 11 fungal phylotypes, several of which exhibited associations with one or more of the nematode population density measurements (egg masses, eggs or J2). The phylotype containing rRNA genes with high sequence identity to Pochonia chlamydosporia exhibited the strongest negative associations. The negative correlation between the densities of the P. chlamydosporia genes and the nematodes was corroborated by an analysis using a P. chlamydosporia-selective qPCR assay.
Keywords: biological control, dwarf tomato, Meloidogyne incognita, Pochonia chlamydosporia, root-knot nematode, Solanum lycopersicon, suppressive soil, Triticum aestivum, wheat
Root-knot nematodes (Meloidogyne spp.) parasitize a wide range of annual and perennial crops, often impacting both the quantity and quality of marketable yields. These pests are also considered to be the most economically important plant-parasitic nematodes (Whitehead, 1998). Annual crop losses caused by plant-parasitic nematodes have been estimated to exceed $US 100 billion (Bird and Kaloshian, 2003), with more than half caused by Meloidogyne spp. Use of nematicides is one of the most reliable means of managing root-knot nematodes. However, their negative impact on the environment and human health has led to regulatory restrictions in the use of many nematicides. In addition, their use is expensive and typically does not provide long-term nematode suppression. New and more sustainable management strategies for plant-parasitic nematodes are clearly needed.
Biologically suppressive soils hold considerable potential for managing soilborne pests. Such soils have been defined as “soils in which the pathogen does not establish or persist, establishes but causes little or no damage, or establishes and causes disease for a while but thereafter the disease is less important, although the pathogen may persist in the soil” (Cook and Baker, 1983). The initial step in realizing this potential is to discover a location in which a pathogen population has declined or does not increase despite a susceptible host and suitable environmental conditions. This is followed by providing evidence that the suppression is of biological nature and not due to physical or chemical limitations of the soil environment. This is typically accomplished by infesting sterilized, pasteurized or fumigated portions of a soil as well as non-treated samples of the same soil with the nematode pathogen. Treated and non-treated soils are planted with a susceptible host of the nematode and incubated long enough for population differences to occur. In such experiments with nematodes, soils that possess biological suppression exhibit lower nematode population densities in the non-treated portion, due to microbial antagonism or predation. Identification of potential causal organism(s) might result in the discovery of novel biocontrol microorganisms. Moreover, gaining an understanding of the ecological factors enabling these organisms to persist, compete and function will support the practical implementation of biological control in the future. Armed with such knowledge, it may be possible to develop effective and sustainable nematode management strategies through the application of beneficial organisms and/or agronomic practices affecting their populations.
There are fewer descriptions in the literature of soils that biologically suppress root-knot nematodes compared to such occurrences with cyst nematodes. A few examples of root-knot nematode studies include the comparison of vineyard soils from two different locations in South Australia (Bird and Brisbane, 1988) where soils from Cooltong exhibited biological suppressiveness against M. javanica and soils from Loxton did not. In the suppressive soils, the numbers of root-knot nematode egg masses were reduced, and the inhibition was removed by soil autoclaving. In all cases showing egg mass number reductions, the bacterium Pasteuria penetrans was detected in mature females without egg masses. This obligate parasite of root-knot nematodes was also implicated in the M. incognita and M. javanica suppressiveness exhibited by a Florida field soil, which had been cropped to tobacco for 7 years (Weibelzahl-Fulton et al., 1996).
In central California, unexpectedly low population densities of M. incognita were observed in old peach orchards, despite the occurrence of suitable environmental conditions and a susceptible rootstock (Ferris et al., 1976). This observation led to the discovery of the fungus Dactylella oviparasitica as the potential suppressive agent (Stirling and Mankau, 1978; Stirling et al., 1979). In a survey of 20 California tomato field soils, five were discovered that reduced M. incognita J2 densities below the levels observed in their sterilized counterparts (Gaspard et al., 1990a). Finally, a survey of 12 soils from agricultural fields in California led to the identification of three that biologically suppressed M. incognita population development (Pyrowolakis et al., 2002). In the latter two cases, the organisms responsible for the nematode population decline were not identified.
In this report, we describe an approach to detect suppressive activity of soils against M. incognita, provide evidence for the presence of a biological suppressiveness in one of the tested soils and describe fungal rRNA genes associated with this phenomenon.
Materials and Methods
Soil survey: Six California soils were assessed for their abilities to biologically suppress M. incognita population densities. These soils were sandy loams from locations near Reedley, CA, that appeared to be inhospitable to one or more plant-parasitic nematode populations (McKenry, unpublished). From each location, 24 samples of approximately 2,500 cm3 soil were taken from the top 25 cm, pooled and thoroughly mixed in a cement mixer. Greenhouse experiments were performed as follows with non-fumigated and methyl iodide-fumigated portions of each soil (Becker et al., 1998). Three-week-old dwarf tomato seedlings (Solanum lycopersicon cv. Tiny Tim) were planted in 18-cm × 5-cm plastic tubes (Ray Leach Conetainers, Stuewe & Sons Inc., Corvallis, OR) filled with each soil. In a second set of soil-filled tubes, 5 wheat seeds (Triticum aestivum cv. Yecora Rojo) were sown. Each tube was infested with approximately 280 M. incognita J2/100 cm3 soil. The tubes were arranged in the racks in a randomized complete block design with 5 replications/treatment. The racks were incubated in a greenhouse in ambient light at 26 ± 2°C. After emergence, the number of wheat seedlings was thinned to 3 plants/tube. The plants were fertilized with approximately 2 g slow-release fertilizer (Osmocote, 17–6-10; Scotts, Marysville, OH) and watered as needed. After 2 mon, plant tops were cut off at soil level and weighed after oven drying at 80°C for 2 d. Eggs of M. incognita were recovered by agitating each root system in 10% commercial bleach solution (0.5% sodium hypochlorite) for 1 min and rinsing with tap water on a 38-μm aperture sieve. The material retained was washed back into the appropriate soil tube. Each root system was carefully rinsed with water, blotted dry and weighed. The soil in each tube was carefully mixed in a plastic bag. A 50 cm3 subsample from each bag was placed on a Baermann funnel and incubated for 5 d at 26°C. The recovered nematodes were enumerated under low power magnification (x30–40 magnification). Tomato roots were rated for the degree of galling on a scale of 0–10 (0 = no galling) (Zeck, 1971). Each experiment was repeated once.
Characterization of test soil: Based on the outcome of the aforementioned survey, one soil, obtained from a field site at the Kearney Research and Extension Center in Parlier, CA, was used in this test. The soil, was a sandy loam (65% sand, 23% silt, 11% clay; 0.5% OM; pH 7.3). Over the years, the site had been planted with various perennial crops such as grape, peach, walnut and others. Soils from other field sites of this station have been previously shown to harbor microorganisms deleterious to nematodes (Mankau and McKenry, 1976; Stirling et al., 1979).
Greenhouse trials: The moist soil, approximately 50% field capacity, was treated in three different ways to confirm the biological nature of the suppressiveness and to establish gradients of suppressiveness for microbial community analysis. In a first soil transfer trial (A), ratios of non-treated suppressive soil to fumigated non-suppressive soil were established: 100:0, 50:50, 10:90, 1:99 and 0:100. Soil fumigation with methyl iodide was performed as described (Becker et al., 1998). Non-treated and fumigated portions were thoroughly mixed in large plastic bags.
In a second temperature trial (B), the soil was exposed to one of the following treatments: room temperature, 30°C, 40°C, 50°C, and 60°C. Soil samples (approximately 1 kg) were placed in double plastic bags and submerged in a water bath. After the center of the sample reached the target temperature, it was incubated for 30 min at that temperature. The bags were then cooled to room temperature under running tap water. All samples of the same treatment were pooled and thoroughly mixed.
In a third soil fumigation trial (C), soil samples were exposed to various amounts of methyl iodide in 1.9 dm3 fumigation jars. After being chilled on dry ice, methyl iodide (D.S.M. Fine Chemicals, Saddle Brook, NJ) was pipetted into a glass vial inserted into the soil contained within the fumigation jar. The jars were immediately sealed. The treatments were 0, 12, 24, 48 and 96 μl per jar; three jars were used for each treatment. After 48 hr, the jars were vented in a fume hood for 5 d. Soils from the same treatment were pooled and thoroughly mixed.
Pasteurized silica sand was mixed into all soils (4:1 soil to sand) to facilitate water drainage and aeration during the greenhouse trials. Soils were infested with root-knot nematodes (1,000 M. incognita eggs/100-cm3 soil) and placed in 1,500-cm3 fiber pots. Each pot was planted with 1 3-wk-old dwarf tomato seedling (cv. Tiny Tim). Each trial (A, B and C) was arranged in a greenhouse under ambient light at 26 ± 2°C in a randomized complete block design and included five treatments, with six replications of each. Plants were watered daily as needed, and each pot was fertilized with 6 g slow-release fertilizer (Sierra 17–6-10 plus Minors, Scotts-Sierra Horticultural Products Company, Marysville, OH). The experiments were terminated 8 wk after infestation. Plant tops were cut off at soil level, and roots were carefully removed from soil. Fresh and dry weights of shoots and roots were measured. Root galling was rated on a scale of 0–10 (0 = no galling). The roots were placed in erioglaucine solution overnight, and the stained egg masses of root-knot nematodes were counted (Omwega et al., 1988). The roots were also processed for egg extraction and enumeration (Hussey and Barker, 1973). A soil subsample (50 cm3) from each pot was incubated on a Baermann funnel for 5 d at 26°C. The collected J2 were counted under low power magnification (x30–40 magnification). The egg mass, egg and J2 numbers for each greenhouse experiment were transformed by x = log10 (x + 1) before being subjected to ANOVA and Fisher's least significant difference (LSD) tests (Minitab 15, State College, PA). Each trial was repeated once. Nematode population density data are presented separately due to variation between the replicate trials.
DNA extraction from M. incognita egg masses: DNA was extracted from egg masses collected at the end of the greenhouse experiments. For each replicated pot in the greenhouse experiments, DNA was extracted from 10 randomly collected M. incognita egg masses. Extractions were performed using the FastDNA Spin Kit for Soil as described by the manufacturer, with a 30 sec bead-beating step at a FastPrep Instrument setting of 5.5 (Qbiogene, Carlsbad, CA). DNA was further purified and size-fractionated by electrophoresis in 1% agarose gels. DNA larger than 3 kb was excised without exposure to UV or ethidium bromide and recovered using the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) following the manufacturer's instructions, except that the gel pieces were not exposed to heat and the DNA was eluted in 1 mM Tris-Cl (pH 8.5).
Oligonucleotide fingerprinting of rRNA genes (OFRG): OFRG, which is a method that sorts rRNA gene clones into operational taxonomic units (OTUs) by a series of hybridization experiments that leads to each clone being designated by a fingerprint, was performed as previously described (Bent et al., 2006) with the following exceptions. One fungal rRNA gene clone library was produced from egg mass DNA for each of the 30 soil treatments. The template DNA for each soil treatment type contained an equal volume of DNA from all of the replicate samples. PCR amplifications were performed using the HPLC-purified fungal rRNA gene primers FunfOFRGpUSER (GGAGACAUTTAGCATGGAATA-ATRRAATAGGA) and FunrOFRGpUSER (GGGAAA-GUATTGCAATGCYCTATCCCCA). These primers are modified versions of nu-SSU-0817–5’ and nu-SSU-1536–3’ (Borneman and Hartin, 2000), which contain eight nucleotide sequences (underlined) allowing directional cloning with the USER Friendly Cloning Kit (New England Biolabs, Beverly, MA). Thermal cycling parameters were 94°C for 2 min; 36 cycles of 94°C for 20 sec, 55°C for 30 sec, and 72°C for 2 min; followed by 72°C for 2 min.
Sixty 11 × 7 cm macroarrays on nylon membranes were produced, each containing 230 or 231 clones from each of the soil treatment libraries and 384 control clones.
The fungal probe set used was: F1, AGTTTTTGGG; F2, CAAGCCGATG; F6, CGCTGGCTTC; F8, TGGCCGGAAG, F9, GAAACTCACC; F10, CGTGCGGTTC; F11, GTGGAGCCTG; F12, GGGACTATCG; F13, GGGATCGGGC; F21, TCACCTTGGC; F23, CAGGTCTGTG; F29, CACCACCAGG; F30, CTGGTCGCCG; F31, TTTGCGGGCC; F32, ACCTGCTAAA; F33, GCACCTTAC; F35, AGGGACAGTC; F36, CGGTCCGCAT; F37, CTTTGGCTGG; F40, ACTGCGAAAG; F41, TCTAGGACCG; F42, ATAGCCCGGC; F43, AGTTTTTGG; F44, GGTCCGGGTA; F45, CTGACAGAGC; F46, GTCTGGGTAA; F49, CCAGCGAGTT; S5, GCTTCTTAGA; S7, GGTCTGGGTA; S9, TCCAGACACA; S11, TTATTGAAGA; S13, AGGTCTGGGT; L2, GGGCATTAGT; L3, AACCTTGGC; L4, GTCGGGGGCA; L5, TTTGGGT-TCT; L7, GAGTGGAGCC; L8, AGCGAGTTTA; L12, CAATTGTCAG; L13, GACTATCGGC; L14, GAGAGGTCTG; L25, AGTATGGTCG; L26, GCCGGCTTCT; L28, GTGCGGTTCT; L30, GGTTAATTCC. The reference probe was GGTGAGTTTCCC (Valinsky et al., 2002.). Arrays were washed twice in 1X SSC for 30 min at 11°C.
Background-subtracted intensity values for each spot were divided by the intensities from the reference probe, as usual, and these values were transformed by adding an identical amount to each value so that the smallest one would be 0.0001. This transformation does not alter the variances in the values (Gotelli and Ellison, 2004) and enabled all of them to be used in subsequent fingerprint classifications (Jeske et al., 2007).
A fingerprint UPGMA dendogram was constructed using the program GCPAT (Figueroa et al., 2004), and groups of clones with fingerprints that appeared to be highly similar based on the dendogram distances were identified. Nucleotide sequences of representative clones from each group were obtained, and groups containing clones with 98.5% or greater sequence identity were combined. At this point, each group (combined or not) was called an OTU or phylotype. To focus the subsequent analyses on the most abundant fungal phylotypes and to minimize the possible inclusion of chimeras and heteroduplex molecules, only phylotypes containing 15 or more clones (termed major phylotypes) were analyzed further.
Phylogenetic tree: Several randomly selected fungal small-subunit rRNA gene sequences from each of the major OTU (those containing 15 clones or more) and their closest relatives, determined by an analysis using BLAST (Altschul et al., 1997), were aligned using the ClustalW algorithm (Thompson et al., 1994) in Vector NTI v10 (Invitrogen, Carlsbad, CA); the sequences contained the 729 nucleotide region delineated by the PCR primer sequences TTAGCATGGAATAATRRAATAGGA and ATTGCAATGCYCTATCCCCA (Borneman and Hartin, 2000). A phylogenetic tree was constructed from these aligned sequences using the Phylip v3.66 program Dnapars (Felsenstein, 2005), which produces unrooted parsimony trees (Eck and Dayhoff, 1966; Kluge and Farris, 1969).
Quantitative PCR (qPCR): Genes of P. chlamydosporia were quantified using real-time PCR assays performed in a Bio-Rad iCycler MyiQ Real-Time Detection System (Bio-Rad Laboratories, Inc., Hercules, CA). The amplification reactions were performed in iCycler iQ PCR Plates with Optical Flat 8-Cap Strips (Bio-Rad Laboratories, Inc). Twenty-five microliter reaction mixtures contained the following reagents: 50 mM Tris (pH 8.3), 500 μg/ml BSA, 2.5 mM MgCl2, 250 μM of each dNTP, 400 nM of each primer, 1 μl of egg mass DNA from individual replicate pots, 2 μl of 10X SYBR Green I (Invitrogen) and 1.25 U Taq DNA polymerase. The P. chlamydosporia-selective primers were PochSSUF5 (TGCTTTGGCAGTACGCC) and PochSSUR4 (CTTCCGGCCAAGGG), which target a 149-bp fragment of the small-subunit rRNA gene from P. chlamydosporia. Sequence-selective primers were designed using PRISE software (Fu et al., 2008). The thermal cycling conditions were 94°C for 5 min; 42 cycles of 94°C for 20 sec, 64°C for 30 sec, and 72°C for 30 sec; followed by 72°C for 2 min. At each cycle, accumulation of PCR product was measured by monitoring the increase in fluorescence of the double-stranded DNA-binding SYBR Green dye. rRNA gene levels in the egg mass DNA were quantified by interpolation from a standard curve comprised of a dilution series of cloned P. chlamydosporia rRNA genes. To increase the likelihood that the real-time signals were produced by amplification of the target sequences, PCR fragments from egg mass DNA were cloned into pGEM-T (Promega, Madison, WI), and the nucleotide sequences of two clones were determined; these experiments confirmed that the target sequences were being amplified (data not shown). For the P. chlamydosporia reactions, the average R2 and amplification efficiencies were 0.995 and 85.4%, respectively. The qPCR analysis was performed on individual egg mass DNA from individual replicate pots. The number of replicates from each of the greenhouse trials used in the correlation analyses were: trial one mixture: 18; trial one temperature: 20; trial one methyl iodide: 20; trial two mixture: 28; trial two temperature: 22; trial two methyl iodide: 23; and pooled: 131. Values outside of those from the standard curve were not used in the correlation and regression analyses. The scatter plots of the qPCR values were created using SigmaPlot (San Jose, CA).
Associations between rRNA genes and M. incognita population densities: Correlation and linear regression analyses between the number of fungal rRNA genes and M. incognita population densities (egg masses, eggs or J2) were performed using Minitab 15.
Nucleotide sequence analysis of rRNA gene clones: Nucleotide sequences of fungal rRNA gene fragments were determined using the ABI BigDye Terminator v3.1 Cycle Sequencing Kit and an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA). Sequence identities were determined by an analysis using BLAST (NCBI) (Altschul et al., 1997).
Nucleotide sequence data: The nucleotide sequences of clones from each of the major fungal phylotypes identified by the OFRG analysis were deposited in GenBank (NCBI) under accession numbers EU215391 to EU215415.
Results
Soil survey: Only one out of the six soils tested consistently showed a significant difference in M. incognita population densities between the non-treated and fumigated soils, with both host plants (Table 1). The numbers of J2 per gram root in the non-treated soils were 5 to 16 times lower than in the treated soils. In the other soils, root-knot nematode population differences were highly variable (data not shown). The treatments did not differ in terms of fresh root or dry top weight with either crop, and no difference in the tomato root galling index between treatments was detected (data not shown).
Table 1.
Meloidogyne incognita population densities in fumigated and non-treated soil 8 weeks after they were infested with 1,000 M. incognita eggs/100-cm3 soil and planted with tomato.
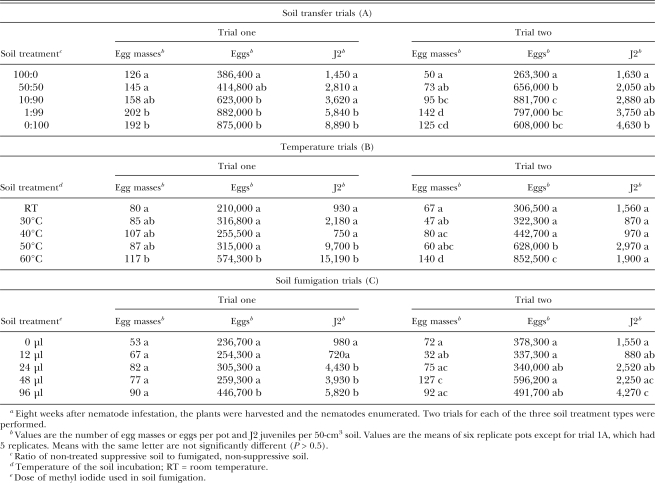
Greenhouse trials: Each of the three soil treatment methods led to significant differences in M. incognita population densities, with the greatest impact on the J2 populations (Table 2). Approximately 10% of the original soil was sufficient to cause a reduction in the J2 population when transferred to fumigated, nematode-infested soil. The population reduction was less pronounced for egg masses and eggs and required a 50:50 mixture ratio between treated and non-treated soil. Eight weeks after nematode infestation, distinct increases in nematode densities were observed in soils incubated at 50°C to 60°C for 30 min before infestation with root-knot nematodes. In the soil fumigation trials, 24 μl methyl iodide resulted in a 3- to 4-fold increase in the J2 population density at the end of the trial one, but there was little proliferation of egg masses or eggs. For each of the six individual experiments, there was no difference in fresh root or shoot weights among the soil treatments (data not shown).
Table 2.
Meloidogyne incognita population densities from portions of a suppressive soil subjected to three soil treatment series, planted with tomato, infested with J2 of M. incognita, and examined 8 weeks after infestationa.
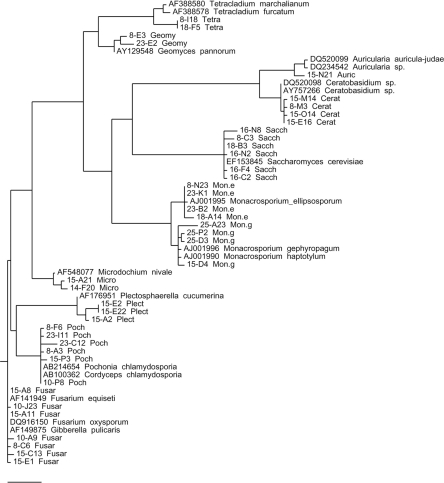
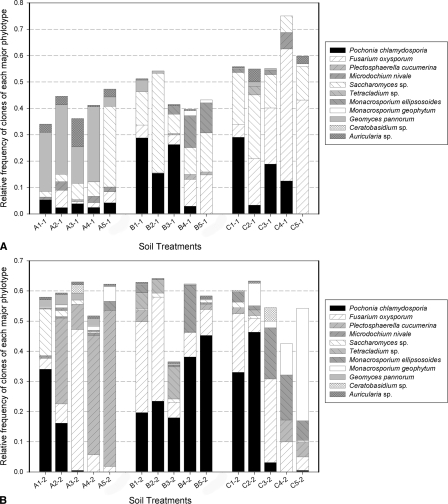
rRNA gene analyses: A fungal OFRG analysis was performed on M. incognita egg masses collected from plant roots at the end of the greenhouse experiments. From the 6,912 rRNA genes analyzed, 11 major phylotypes (OTU containing at least 15 clones) were identified. Nucleotide sequence analyses of representative rRNA genes from each phylotype showed that they had high sequence identity to rRNA genes from Pochonia chlamydosporia (99–100%, 883 clones), Fusarium oxysporum (99–100%, 675 clones), Plectosphaerella cucumerina (99%, 336 clones), Microdochium nivale (99%, 21 clones), Saccharomyces sp. (99–100%, 413 clones), Tetracladium sp. (98%, 53 clones), Monacrosporium ellipso-sporum (99%, 193 clones), Monacrosporium geophytum(99%, 142 clones), Geomyces pannorum (99%, 192 clones),Ceratobasidium sp. (99%, 18 clones), and Auricularia sp. (99%, 59 clones); the numbers in parentheses indicate the % pairwise identities of the sequences from this study and their nearest relatives (determined by an analysis using BLAST) in GenBank, followed by the number of rRNA gene clones in each phylotype. A phylogenetic depiction of the major phylotypes is shown in Figure 1. The relative frequencies of rRNA genes in each phylotype from each of the experiments are also depicted (Fig. 2a,b).
Fig. 1.
Phylogenetic tree of the major fungal phylotypes in the M. incognita egg masses collected at the end of the greenhouse experiments. The unrooted parsimony tree was made using at least one representative small-subunit rRNA gene sequence from each of the major phylotypes (identified by the OFRG analysis) and their closest relatives determined by an analysis using BLAST (1). Sequences from this study are named by their clone number followed by their phylotype designation (Poch = Pochonia chlamydosporia, Fus = Fusarium oxysporum, Plect = Plectosphaerella cucumerina, Micro = Microdochium nivale, Sacch = Saccharomyces sp., Tetra = Tetracladium sp., Geomy = Geomyces pannorum, Mon. e = Monacrosporium ellipsosporum, Mon. g = Monacrosporium geophytum, Cerat = Ceratobasidium sp., Auric = Auricularia sp.). Reference sequences are designated by their accession number and taxon. The scale bar length is 0.1, and it represents the number of nucleotide changes per position.
Fig. 2.
Relative frequencies of the fungal phylotypes from the M. incognita egg masses collected at the end of the greenhouse experiments. Relative frequencies are the number of clones per phylotype divided by the number of total clones per soil treatment. (2a) Trial one; (2b) Trial two. Soil treatments: the first character designates the experiment type [A = Soil transfer trials (ratios of non-treated suppressive soil to fumigated non-suppressive soil), B = Temperature trials (soils were exposed to different temperature treatments), C = Soil fumigation trials (soils were exposed to various dosages of methyl iodide)], the second character designates the treatment type within each experiment (Experiment A: 1 = 100, 2 = 50, 3 = 10, 4 = 1, 5 = 0% suppressive soil; Experiment B: 1 = room temperature, 2 = 30°C, 3 = 40°C, 4 = 50°C, 5 = 60°C; Experiment C: 1 = 0 μl, 2 = 12 μl, 3 = 24 μl, 4 = 48 μl, 5 = 96 μl), and the third character designates the trial number.
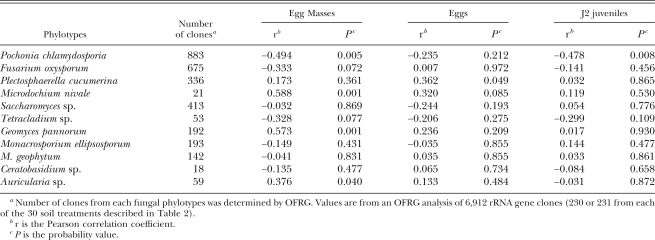
Associations between rRNA genes and M. incognita population densities: Correlation analyses were performed between the number of rRNA gene clones in each phylotype (from the OFRG analysis) and each of three M. incognita population density measurements (egg masses, eggs or J2). When these analyses were performed on data from all six of the greenhouse experiments, several of the phylotypes exhibited associations with one or more of the nematode population densities (Table 3). The phylotype containing rRNA genes with high sequence identity to P. chlamydosporia exhibited the strongest negative associations: egg masses (r = −0.494, P = 0.005), eggs (r = −0.235, P = 0.212) and J2 (r = −0.478, P = 0.008).
Table 3.
Correlations between the number of clones from each fungal phylotypea and M. incognita population densities.
To verify these negative associations, a sequence-selective qPCR assay targeting the P. chlamydosporia phylotype was employed. When correlation analyses were performed on pooled data from all six of the greenhouse experiments, the association values were: egg masses (r = −0.165, P = 0.058), eggs (r = −0.177, P = 0.043) and J2 (r = −0.154, P = 0.078). Plots of these data showed that they exhibited characteristics of an exponential decay curve (Fig. 3), which may suggest a density-dependent association. When the nematode values were log transformed and the correlation analyses repeated, the association values were: egg masses (r = −0.226, P = 0.009), eggs (r = −0.200, P = 0.022) and J2 (r = −0.194, P = 0.027). Linear regression analyses of these log-transformed data corroborated these negative associations (egg masses, P = 0.009; eggs, P = 0.022; J2, P = 0.027).
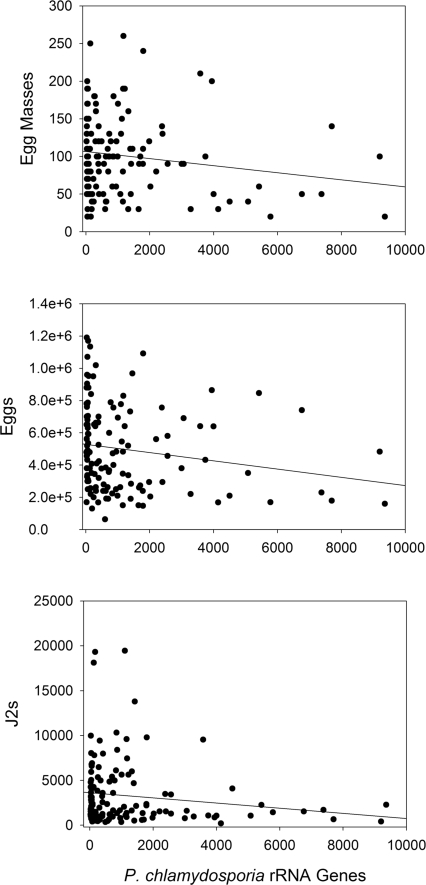
Fig. 3.
Scatter plots of the population densities of three nematode measurements vs. P. chlamydosporia rRNA genes. Nematode values are the number of egg masses or eggs per plant and the number of J2 per 50-cm3 soil. Pochonia chlamydosporia rRNA gene values are copy numbers per egg mass. The trend lines in the plots are from linear regression analyses.
Discussion
This study employed a systematic screening procedure to identify soils that can suppress population densities of M. incognita in the presence of susceptible hosts and disease-conducive physical conditions. Both the dwarf tomato and the wheat grew uniformly well in the small soil containers with minimum interference of neighboring plants. Such a space-saving set-up is suitable for large-scale surveys endeavoring to identify soils suppressive to root-knot or other plant-parasitic nematodes. Based on the number of J2, one test soil proved to be suppressive to the target nematode. Its final nematode population density of more than 100 J2/50 cm3 was still quite high for agronomical considerations. Under suitable environmental conditions, it would likely result in considerable crop damage with many susceptible crops. However, if we consider the high initial M. incognita infestation and the relatively short length of the experiments, a 5- to 16-fold reduction in J2 of M. incognita within 8 weeks is remarkable. This screening approach was intentionally designed to select for biological suppression under high disease pressure. A less suitable host of the pathogen and consequently lower reproduction rate of M. incognita may have improved the biocontrol efficacy (Stirling et al., 1979; Kerry and Bourne, 1996), but it is unknown if such an approach would select for the most efficient biocontrol strains. Stirling (1991) hypothesized that antagonists require time to establish an equilibrium with their nematode hosts and, consequently, naturally occurring suppressiveness usually takes years to develop. More recent trial results indicate that the introduction of suppressive biocontrol agents at sufficient infestation levels can result in substantial nematode population reductions within one cropping season, equal to the population level in the original suppressive soils (Chen and Dickson, 2004; Olatinwo et al., 2006).
In the following three tomato trials, which examined the effects of various soil treatments on M. incognita suppression, the initial observation of J2 suppression was not only confirmed, but these trials also provided evidence that the suppressive nature of this soil was biological. This property was diminished by exposure to heat and soil fumigation. It was also transferred to non-suppressive soil with as little as 10% of the non-treated suppressive soil, which is notable given the short duration (8 weeks) of the greenhouse experiments. Furthermore, these experiments suggested that specific microorganism(s) were causing the suppressiveness, as distinct differences in nematode densities were observed in soil exposed to 50 to 60°C.
The differences in the egg mass counts, which are indicative of the number of fecund females, were relatively minor (less than 2-fold) between treated and non-treated soils. We hypothesize that the small increase in first generation egg masses in the treated soils is mainly due to the frequently observed improvement in overall root health that occurs in pasteurized or fumigated field soil (Cook and Baker, 1983) and not to any significant antagonism in the non-treated soil prior to reproduction. The lack of treatment differences in root galling suggests the mode of action in this suppression is likely occurring at a life cycle stage after the juvenile stages. The relatively large differences in the egg numbers between non-treated and treated soils pointed toward eggs being a potential target in this suppression.
Lower M. incognita population densities in our trials did not coincide with an increase in plant root or shoot weights. This is not unusual in well watered and fertilized experiments under greenhouse conditions. We also suggest that this is in part due to the large initial root-knot nematode infestation. High levels of root-knot nematode pressure at such an early stage in the development of the plant are likely to lead to relatively uniform plant damage.
To identify the microorganism(s) that may contribute to the M. incognita suppressiveness, we employed a population-based approach (Borneman and Becker, 2007). The goal of the initial phase was to identify fungal rRNA genes whose abundances were negatively associated with M. incognita population densities. In phase two, sequence-selective qPCR analyses were used to evaluate the associations identified in phase one.
Several fungal phylotypes detected in the OFRG analysis exhibited an association with one or more of the nematode population density measurements (egg masses, eggs or J2). The strongest negative associations were from the P. chlamydosporia phylotype, suggesting that it was a promising candidate for further study. However, since this negative association was not observed in all of the six individual greenhouse experiments (Fig. 2), these results also suggest that other organisms, including fungi such as the Tetracladium phylotype, or other types of organisms such as bacteria, may be involved in the nematode suppression.
In phase two, a sequence-selective qPCR assay targeting the P. chlamydosporia phylotype was used to evaluate the negative associations identified by the OFRG analysis. The qPCR results corroborated the OFRG data, as negative associations were observed for all three nematode population density measurements. There was however considerable variation in these data, and the relationships between the population densities of the P. chlamydosporia rRNA genes and the nematodes appeared to be nonlinear (Fig. 3).
The possibility that P. chlamydosporia is one of the major factors influencing the M. incognita population in this soil is consistent with data from several other investigations. This fungus is a ubiquitous soil saprotroph (Domsch et al., 1980) with the ability to parasitize various nematode species, in particular sedentary nematodes of the genera Heterodera and Meloidogyne (Kerry, 2001). It is a rhizosphere-competent species, able to grow into egg masses, and it has been shown to parasitize both eggs and females (Kerry and Jaffee, 1997; Kerry, 2001). The fungus has been extensively evaluated as a biological control agent for Meloidogyne spp., applied either on its own (Gaspard et al., 1990b; De Leij and Kerry, 1991; Mertens and Stirling, 1993; Kerry and Bourne, 1996; Atkins et al., 2003b; Sorribas et al., 2003; Van Damme et al., 2005) or in combination with other agents (De Leij et al., 1992; Siddiqui and Shaukat, 2003). Biological control products based on P. chlamydosporia are available or being developed in various countries including the EU, India, China and Cuba (Mo et al., 2005; Brian Kerry, personal communication). However, the diversity in the genus Pochonia and among strains of P. chlamydosporia may account for differences in their ecological behavior, host specificity and efficacy of parasitism (Mauchline et al., 2002; Morton et al., 2003). These factors may be responsible, at least in part, for reports showing that root-knot nematode populations were not reduced in the presence of these egg-parasitizing fungi (Gaspard et al., 1990b; Mertens and Stirling, 1993).
Ten other major fungal phylotypes were identified by the OFRG analysis of the M. incognita egg masses, several of which have been previously reported to be associated with root-knot nematodes. The results support earlier investigations that showed Meloidogyne egg masses are a densely populated microbial niche (Kok et al., 2001). It is notable that such a wide range of microorganisms inhabits egg masses, which have been previously reported to possess antimicrobial properties (Orion and Kritzman, 1991). Prominent among the detected phylotypes, F. oxysporum has been frequently isolated from root-knot nematodes, and some strains have been shown to reduce their populations (Nigh et al., 1980; Hallmann and Sikora, 1994; Sikora et al., 2007). Plectosphaerella cucumerina has been isolated from M. hapla egg masses (Yu and Coosemans, 1998) and investigated for its ability to control potato cyst nematode (Atkins et al., 2003a; Jacobs et al., 2003). Monacrosporium spp. are nematode-trapping fungi, and several species have been shown to parasitize root-knot nematode populations in greenhouse assays (Mankau and Wu, 1985; Gaspard and Mankau, 1987; Stirling, 1991; Jaffee and Muldoon, 1995). Saccharomyces are common saprotropic yeasts. Microdochium nivale is a seed- and soil-borne pathogen that is known to cause diseases in cereals and turf grasses (Wiese, 1987). Tetracladium spp. are often described in literature as aquatic hyphomycetes but were also isolated from various agricultural soils (Domsch et al., 1980). Geotrichum spp. are common fungi with world-wide distribution (Domsch et al., 1980) that have previously been isolated from Meloidogyne eggs (Sun et al., 2006). Some representatives of these fungi are able to produce keratinolytic enzymes (Friedrich et al., 1999), which may be of interest in their apparent association with nematodes. Auricularia spp. and Ceratobasidium spp. are common lower basidiomycetes also known as “jelly fungi” (Wells, 1994). The occurrence of most of these organisms was not correlated to a reduction in nematode population densities. More conclusive information about the causal agent(s) will require pathogenicity tests against M. incognita with individual fungal strains and combinations of fungi. Also, the potential influence of other types of organisms such as bacteria deserves further investigations.
Footnotes
This project was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (CSREES), grant 2005–35302–16150.
We thank J. Darsow for his technical assistance and J. Smith Becker for a critical manuscript review.
This paper was edited by Brian Kerry.
Literature Cited
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Millier W, Lipman DJ. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins SD, Clark IM, Sosnowska D, Hirsch PR, Kerry BR. Detection and quantification of Plectosphaerella cucumerina, a potential biological control agent of potato cyst nematodes, by using conventional PCR, real-time PCR, selective media, and baiting. Applied and Environmental Microbiology. 2003a;69:4788–4793. doi: 10.1128/AEM.69.8.4788-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins SD, Hidalgo-Diaz L, Kalisz H, Mauchline TH, Hirsch PR, Kerry BR. Development of a new management strategy for the control of root-knot nematodes (Meloidogyne spp.) in organic vegetable production. Pest Management Science. 2003b;59:183–189. doi: 10.1002/ps.603. [DOI] [PubMed] [Google Scholar]
- Becker JO, Ohr HD, Grech NM, Sims ME. Evaluation of methyl iodide as a soil fumigant in container and small field plot studies. Pesticide Science. 1998;52:58–62. [Google Scholar]
- Bent E, Yin B, Figueroa A, Ye J, Fu Q, Liu Z, McDonald V, Jeske D, Jiang T, Borneman J. Development of a 9,600-clone procedure for oligonucleotide fingerprinting of rRNA genes: Utilization to identify soil bacterial rRNA genes that correlate in abundance with the development of avocado root rot. Journal of Microbiological Methods. 2006;67:171–180. doi: 10.1016/j.mimet.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Bird AF, Brisbane PG. The influence of Pasteuria penetrans in field soils on the reproduction of root-knot nematodes. Revue de Nématologie. 1988;11:75–81. [Google Scholar]
- Bird DM, Kaloshian I. Are roots special? Nematodes have their say. Physiological and Molecular Plant Pathology. 2003;62:115–123. [Google Scholar]
- Borneman J, Becker JO. Identifying microorganisms involved in specific pathogen suppression in soil. Annual Review of Phytopathology. 2007;45:153–172. doi: 10.1146/annurev.phyto.45.062806.094354. [DOI] [PubMed] [Google Scholar]
- Borneman J, Hartin RJ. PCR primers that amplify fungal rRNA genes from environmental samples. Applied and Environmental Microbiology. 2000;66:4356–60. doi: 10.1128/aem.66.10.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZX, Dickson DW. Biological control of nematodes with bacterial antagonists. In: Chen ZX, Chen SY, Dickson DW, editors. Nematology: Advances and perspectives. Vol. 2. Nematode management and utilization. Beijing, China/Oxfordshire, UK: Tsinghua University Press/CABI Publishing; 2004. pp. 1041–1082. [Google Scholar]
- Cook RJ, Baker KF. St. Paul, MN: APS Press; 1983. The nature and practice of biological control of plant pathogens. [Google Scholar]
- De Leij FAAM, Davies KG, Kerry BR. The use of Verticillium chlamydosporium Goddard and Pasteuria penetrans (Thorne) Sayre & Starr alone and in combination to control Meloidogyne incognita on tomato plants. Fundamental and Applied Nematology. 1992;15:235–242. [Google Scholar]
- De Leij FAAM, Kerry BR. The nematophagous fungus Verticillium chlamydosporium as a potential biological control agent for Meloidogyne arenaria . Revue de Nématologie. 1991;14:157–164. [Google Scholar]
- Domsch KH, Gams W, Anderson T. Vol. 1. London, UK: Academic Press; 1980. Compendium of soil fungi. [Google Scholar]
- Eck RV, Dayhoff MO. Silver Spring, MD: National Biomedical Research Foundation; 1966. Atlas of protein sequence and structure. [Google Scholar]
- Felsenstein J. Seattle, WA: University of Washington; 2005. PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author. Department of Genome Sciences. [Google Scholar]
- Ferris H, McKenry MV, McKinney HE. Spatial distribution of nematodes in peach orchards. Plant Disease Reporter. 1976;60:18–22. [Google Scholar]
- Figueroa A, Borneman J, Jiang T. Clustering binary fingerprint vectors with missing values for DNA array data analysis. Journal of Computational Biology. 2004;11:887–901. doi: 10.1089/cmb.2004.11.887. [DOI] [PubMed] [Google Scholar]
- Friedrich J, Gradisar H, Mandin D, Chaumont JP. Screening fungi for synthesis of keratinolytic enzymes. Letters in Applied Microbiology. 1999;28:127–130. [Google Scholar]
- Fu Q, Ruegger P, Bent E, Chrobak M, Borneman J. PRISE (PRImer SElector): software for designing sequence-selective PCR primers. Journal of Microbiological Methods. 2008;72:263–267. doi: 10.1016/j.mimet.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Gaspard JT, Jaffee BA, Ferris H. Meloidogyne incognita survival in soil infested with Paecilomyces lilacinus and Verticillium chlamydosporium . Journal of Nematology. 1990a;22:176–181. [PMC free article] [PubMed] [Google Scholar]
- Gaspard JT, Jaffee BA, Ferris H. Association of Verticillium chlamydosporium and Paecilomyces lilacinus with root-knot nematode infested soil. Journal of Nematology. 1990b;22:207–213. [PMC free article] [PubMed] [Google Scholar]
- Gaspard JT, Mankau R. Density-dependence and host-specificity of the nematode-trapping fungus Monacrosporium ellipso-sporum . Revue de Nématologie. 1987;10:241–246. [Google Scholar]
- Gotelli NJ, Ellison AM. Sunderland, MA, USA: Sinauer Associates, Inc; 2004. A Primer of Ecological Statistics. [Google Scholar]
- Hallmann J, Sikora RA. Occurrence of plant-parasitic nematodes and non-pathogenic species of Fusarium in tomato plants in Kenya and their role as mutualistic synergists for biological control of root-knot nematodes. International Journal of Pest Management. 1994;40:321–325. [Google Scholar]
- Hussey RS, Barker K. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Jacobs H, Gray SN, Crump DH. Interactions between nematophagous fungi and consequences for their potential as biological agents for the control of potato cyst nematodes. Mycological Research. 2003;107:47–56. doi: 10.1017/s0953756202007098. [DOI] [PubMed] [Google Scholar]
- Jaffee BA, Muldoon AE. Susceptibility of root-knot and cyst nematodes to the nematode-trapping fungi Monacrosporium ellipsosporum and M. cionopagum . Soil Biology and Biochemistry. 1995;27:1083–1090. [Google Scholar]
- Jeske DR, Liu Z, Bent E, Borneman J. Classification rules that include neutral zones and their application to microbial community profiling. Communications in Statistics—Theory and Methods. 2007;36:1965–1980. [Google Scholar]
- Kerry BR. Exploitation of the nematophagous fungal Verticillium chlamydosporium Goddard for the biological control of root-knot nematodes (Meloidogyne spp.) In: Butt TM, Jackson C, Magan N, editors. Fungi as biocontrol agents: Progress, problems and potential. New York: CAB International; 2001. pp. 155–168. [Google Scholar]
- Kerry BR, Bourne JM. The importance of rhizosphere interactions in the biological control of plant parasitic nematodes—A case study using Verticillium chlamydosporium . Pesticide Science. 1996;47:69–75. [Google Scholar]
- Kerry BR, Jaffee BA. Fungi as biological control agents for plant-parasitic nematodes. In: Wicklow DT, Soderstrom BE, editors. The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic Applied Research Environmental and Microbial Relationships. Vol. 4. Berlin, Germany: Springer; 1997. pp. 203–218. [Google Scholar]
- Kluge AG, Farris JS. Quantitative phyletics and the evolution of anurans. Systematic Zoology. 1969;18:1–32. [Google Scholar]
- Kok CJ, Papert A, Hok-A-Hin CH. Microflora of Meloidogyne egg masses: Species composition, population density and effect on the biocontrol agent Verticillium chlamydosporium (Goddard) Nematology. 2001;3:729–734. [Google Scholar]
- Mankau R, Mc Kenry MV. Spatial distribution of nematophagous fungi associated with Meloidogyne incognita on peach. Journal of Nematology. 1976;8:294–295. Abstr. [Google Scholar]
- Mankau R, Wu X. Effects of the nematode-trapping fungus, Monacrosporium ellipsosporium on Meloidogyne incognita populations in field soil. Revue de Nématologie. 1985;8:147–153. [Google Scholar]
- Mauchline TH, Kerry BR, Hirsch PR. Quantification in soil and the rhizosphere of the nematophagous fungus Verticillium chlamydosporium by competitive PCR and comparison with selective plating. Applied and Environmental Microbiology. 2002;68:1846–1853. doi: 10.1128/AEM.68.4.1846-1853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens MCA, Stirling GR. Parasitism of Meloidogyne spp. on grape and kiwifruit by the fungal egg parasites Paecilomyces lilacinus and Verticillium chlamydosporium . Nematologica. 1993;39:400–410. [Google Scholar]
- Mo MH, Xu CK, Zhang KQ. Effects of carbon and nitrogen sources, carbon-to-nitrogen ratio, and initial pH on the growth of nematophagous fungus Pochonia chlamydosporia in liquid culture. Mycopathologia. 2005;159:381–387. doi: 10.1007/s11046-004-5816-3. [DOI] [PubMed] [Google Scholar]
- Morton CO, Mauchline TH, Kerry BR, Hirsch PR. PCR-based DNA fingerprinting indicates host-related genetic variation in the nematophagous fungus Pochonia chlamydosporia . Mycological Research. 2003;107:198–205. doi: 10.1017/s0953756203007251. [DOI] [PubMed] [Google Scholar]
- Nigh EA, Thomason IJ, Van Gundy SD. Effect of temperature and moisture on parasitization of Heterodera schachtii eggs by Acremonium strictum and Fusarium oxysporum . Phytopathology. 1980;70:889–891. [Google Scholar]
- Olatinwo R, Borneman J, Becker JO. Induction of beet-cyst nematode suppressiveness by the fungi Dactylella oviparasitica and Fusarium oxysporum in field microplots. Phytopathology. 2006;96:855–859. doi: 10.1094/PHYTO-96-0855. [DOI] [PubMed] [Google Scholar]
- Omwega CO, Thomason IJ, Roberts PA. A non-destructive technique for screening bean germplasm for resistance to Meloidogyne incognita . Plant Disease. 1988;72:970–972. [Google Scholar]
- Orion D, Kritzman G. Antimicrobial activity of Meloidogyne javanica gelatinous matrix. Revue de Nématologie. 1991;14:481–483. [Google Scholar]
- Pyrowolakis A, Westphal A, Sikora RA, Becker JO. Identification of root-knot nematode suppressive soils. Applied Soil Ecology. 2002;19:51–56. [Google Scholar]
- Siddiqui IA, Shaukat SS. Combination of Pseudomonas aeruginosa and Pochonia chlamydosporia for control of root-infecting fungi in tomato. Journal of Phytopathology. 2003;151:215–222. [Google Scholar]
- Sikora RA, Schäfer K, Dababat AA. Modes of action associated with microbially induced in planta suppression of plant-parasitic nematodes. Australasian Plant Pathology. 2007;36:124–134. [Google Scholar]
- Sorribas FJ, Ornat C, Galeano M, Verdejo-Lucas S. Evaluation of a native and introduced isolate of Pochonia chlamydosporia against Meloidogyne javanica . Biocontrol Science and Technology. 2003;13:707–714. [Google Scholar]
- Stirling GR. Wallingford, UK: CAB International; 1991. Biological control of plant parasitic nematodes: Progress, problems and prospects. [Google Scholar]
- Stirling GR, Mankau R. Parasitism of Meloidogyne eggs by a new fungal parasite. Journal of Nematology. 1978;10:236–240. [PMC free article] [PubMed] [Google Scholar]
- Stirling GR, McKenry MV, Mankau R. Biological control of root-knot nematodes (Meloidogyne spp.) on peach. Phytopathology. 1979;69:806–809. [Google Scholar]
- Sun M-H, Gao L, Shi Y-X, Li B-J, Liu X-Z. Fungi and actinomycetes associated with Meloidogyne spp. eggs and females in China and their biocontrol potential. Journal of Invertebrate Pathology. 2006;93:22–28. doi: 10.1016/j.jip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valinsky L, Della Vedova G, Jiang T, Borneman J. Oligonucleotide fingerprinting of rRNA genes for analysis of fungal community composition. Applied and Environmental Microbiology. 2002;68:5999–6004. doi: 10.1128/AEM.68.12.5999-6004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme V, Hoedekie A, Viaene N. Long-term efficacy of Pochonia chlamydosporia for management of Meloidogyne javanica in glasshouse crops. Nematology. 2005;7:727–736. [Google Scholar]
- Weibelzahl-Fulton E, Dickson DW, Whitty EB. Suppression of Meloidogyne incognita and M. javanica by Pasteuria penetrans in field soil. Journal of Nematology. 1996;28:43–49. [PMC free article] [PubMed] [Google Scholar]
- Wells K. Jelly fungi, then and now! Mycologia. 1994;86:18–48. [Google Scholar]
- Whitehead AG. Wallingford, UK: CAB International; 1998. Plant nematode control. [Google Scholar]
- Wiese MV. 2nd ed. St. Paul, MN: APS Press; 1987. Compendium of wheat diseases. [Google Scholar]
- Yu O, Coosemans J. Fungi associated with cysts of Globodera rostochiensis, G. pallida, and Heterodera schachtii; and egg masses and females of Meloidogyne hapla in Belgium. Phytoprotection. 1998;79:63–69. [Google Scholar]
- Zeck WM. A rating scheme for field evaluation of root-knot nematode infestations. Pflanzenschutz-Nachrichten. Bayer AG. 1971;24:141–144. [Google Scholar]