Abstract
The use of entomopathogenic nematodes (EPN) for management of the root weevil, Diaprepes abbreviatus, in Florida citrus groves is considered a biological control success story and typically involves augmentation in which EPN are applied inundatively as biopesticides to quickly kill the pest. However, recent evidence indicates that efficacy of EPN applications in Florida citrus depends on soil type. They are very effective in the well drained coarse sands of the Central Ridge but often less so in poorly drained fine-textured soils of the Flatwoods. Moreover, groves on the Central Ridge can harbor rich communities of endemic EPN that might often suppress weevil populations below economic thresholds, whereas Flatwoods groves tend to have few endemic EPN and frequent weevil problems. Current research is examining the ecological dynamics of EPN in Florida citrus groves, the potential impact of EPN augmentation on soil food webs, especially endemic EPN, and whether habitat manipulation and inoculation strategies might be effective for conserving and enhancing EPN communities to achieve long-term control in problem areas. Conservation biological control could extend the usefulness of EPN in Florida citrus and be especially appropriate for groves with persistent weevil problems.
Keywords: biological control, citrus, conservation, Diaprepes abbreviatus, ecology, entomopathogenic nematode, Florida, food web, Heterorhabditis, management, manure, mulch, root weevil, soil, Steinernema, suppression, trophic cascades
Successful biological control manipulations involve intentional alterations of important variables associated with consumer-resource interactions and induce trophic cascades in which certain natural enemies effectively reduce the abundance of particular pest organisms and thereby provide enhanced protection for crops or other organisms of benefit to man. Sometimes these manipulations involve direct augmentation of populations of parasites, predators, or pathogens (augmentation biological control), but they can also involve manipulations of various biotic or abiotic habitat variables that favor increased populations of natural enemies (conservation biological control), either of which can have the desired effect of suppressing pest populations (Lewis et al., 1998; Stuart et al., 2006).
Entomopathogenic nematodes (EPN) are generally used as biopesticides for short-term inundative or augmentative biological control of insect pests in soil. However, as studies increasingly document the widespread occurrence of EPN in a variety of soil ecosystems and the fundamental importance of EPN as mortality agents of insects in diverse soil food webs, longer-term strategies of conservation biological control are being more widely considered (Lewis et al., 1998; Stuart et al., 2006). Ultimately, when used under appropriate circumstances, conservation biological control strategies focusing on EPN might provide effective long-term control of insect pests and be more practical and cost effective than augmentation. Studies have shown that some EPN are more abundant in undisturbed compared to intensively farmed habitats (Campos-Herrera et al., 2008), and some forms of organic manure mulches have been shown to increase the prevalence of certain EPN (Bednarek and Gaugler, 1997; Duncan et al., 2007). However, to date, methods to enhance the natural control of arthropods using a conservation biological control approach involving EPN remain undeveloped and largely a matter of speculation (Lewis et al, 1998; Stuart et al., 2006)
In Florida citrus groves, augmentation of EPN is considered one of the most effective ways of reducing populations of the Diaprepes root weevil, Diaprepes abbreviatus (L.), and growers have been applying commercially produced EPN in their groves to control root weevils for many years (Shapiro-Ilan et al., 2002, 2005; McCoy et al., 2006, 2007). However, the efficacy of augmentation is apparently reduced in certain soil types, and control failures have been reported (Duncan et al., 2001; McCoy et al., 2002, 2007). Increasing the rate of augmentation might alleviate this problem to some extent, but another strategy, making the soil habitat itself more favorable for the nematodes, could provide a more practical and effective long-term solution for weevil problems in certain areas. In this paper, we discuss the ecological dynamics of root weevil control in Florida citrus groves, examine the influence of EPN augmentation on soil food webs, and provide some initial results of research involving manipulations of soil habitats as a strategy for EPN conservation biological control in this particular agroecosystem.
The Diaprepes root weevil as a major citrus pest
The Diaprepes root weevil, Diaprepes abbreviatus, was first detected in Florida in 1964, but is widely distributed in the Caribbean where it has long been considered a serious agricultural pest (Wolcott, 1936; Woodruff, 1964). In Florida, it has become a major pest of citrus, ornamentals and other crops (Simpson et al., 1996; McCoy, 1999; Peña et al., 2000; McCoy et al., 2006, 2007), and it has recently spread to southern Texas and southern California, where it is considered a major threat to agriculture and is the subject of quarantine and eradication programs (French and Skaria, 2000; Grafton-Cardwell et al., 2004; Stuart and Rogers, 2006). In citrus, larval feeding damages roots, reduces yield and kills trees by girdling or by facilitating infection by plant pathogens such as Phytophthora spp. (Graham et al., 2003; McCoy et al., 2004). Diaprepes abbreviatus can lead to rapid tree decline and devastate entire citrus groves within a few years of infestation. Adults feed on foliage, especially new growth, and can be detected by the characteristic notching of leaf margins. Mating occurs in the canopy, and eggs are laid in masses between leaves that are glued together by the female during oviposition (Adair et al., 1999). The larvae hatch, escape from the sealed leaf envelope, drop to the soil and burrow down to the roots where they begin feeding (Nigg et al., 2003; Stuart et al., 2003). Larvae move to larger roots as they grow and pupate after 9 to 11 instars (Quintela et al., 1998; McCoy, 1999). In Florida, annual losses and cost of control for the Diaprepes root weevil in citrus are estimated at $72 million, whereas losses in ornamentals and vegetables are estimated at $2 million (Peña et al., 2000)
Difficulties in controlling the Diaprepes root weevil arise from various factors. It is highly polyphagous, with 270 known crop, ornamental and weedy plants used for adult feeding, egg laying and/or larval development, and it is easily spread long distances through the transport of ornamentals and nursery stock (Simpson et al., 1996; Stuart and Rogers, 2006). Moreover, it has a markedly asynchronous, complex and variable life history (McCoy et al., 2003). In central Florida, adults emerge from the soil throughout the year, but emergence generally peaks following spring rains (March-June), with an additional peak sometimes occurring in the fall (September-October). Females lay egg masses every one to two days, can live almost a year in laboratory culture, and can produce up to 20,000 eggs (Nigg et al., 2004). In the field, average life span is probably more limited, but individuals might be expected to lay up to 2,000 eggs in the first six weeks of reproduction (Nigg et al., 2004). Since egg masses are laid between sealed leaves, they tend to be protected against various natural enemies, but certain predators and egg para-sitoids are not deterred (Peña et al., 2000). Nonetheless, natural enemies (including EPN) that are effective against various life stages of the weevil tend to have patchy or otherwise limited distributions and can be scarce in certain areas, certain habitats or during certain times of the year (Duncan et al., 2003b; Stuart et al., 2003, 2006; Lapointe et al., 2007). Many foliar pesticides are effective against the adults, but chemical application costs can be prohibitive for the frequent applications required to suppress populations throughout the long period of seasonal activity, and such repeated applications can disrupt natural enemies and lead to additional pest problems (McCoy et al., 2007; but see McCoy et al., 2004; Stuart et al., 2008). The high water table in Florida limits the use of certain soil pesticides, and the only recommended control for larvae that have established themselves in groves is the application of EPN twice per year (Duncan and McCoy, 1996; Duncan et al., 1996; Bullock et al., 1999; McCoy, 1999; McCoy et al., 2000, Duncan et al., 2007; McCoy et al., 2007).
Entomopathogenic nematodes as biological control agents
Entomopathogenic nematodes in the families Steinernematidae and Heterorhabditidae are lethal obligatory parasites of insects and are found in soils throughout the world (Kaya and Gaugler, 1993; Kaya et al., 1993; Stuart et al., 2006). The only life stage typically found outside the host cadaver is the third-stage infective juvenile (IJ), a non-feeding, environmentally resistant “dauer” larva. The IJ exhibits species-specific foraging strategies that range from ambushing to cruising and actively responds to potential insect hosts, which it invades (Lewis et al., 2006). The IJ ultimately enters the host's hemolymph, where it releases symbiotic bacteria (Enterobacteriaceae) carried in its alimentary tract, and the bacteria are largely responsible for overwhelming the insect's immune system and killing the host through septicemia (Boemare, 2002; Forst and Clarke, 2002). The bacteria associated with the Steinernematidae are in the genus Xenorhabdus, whereas those with the Heterorhabditidae are Photorhabdus. The bacteria proliferate rapidly and soon dominate the insect cadaver. The nematodes feed on the bacterial biomass and insect tissue, develop to the adult stage, mate and reproduce, often completing multiple generations, before producing another generation of IJ that leave the cadaver in search of new hosts.
Current interest and advances in our knowledge of the biology of EPN can be attributed to their great potential as effective, practical and relatively inexpensive augmentative biological control agents that can be used in agriculture and other managed environments (Kaya and Gaugler, 1993; Kaya et al., 1993; Shapiro-Ilan et al., 2002). EPN are efficacious against a broad range of insect pests, and mass-produced nematodes have achieved commercial success in various niche markets and high-value crops (Shapiro-Ilan et al., 2002; Georgis et al., 2006). Consequently, a small international industry has developed around the production and merchandising of nematode products for augmentation. However, as research continues on improving the effectiveness of EPN and broadening their usefulness for biological control, numerous questions remain to be answered. Specifically, with respect to the use of EPN for root weevil control in Florida citrus, we are especially concerned with determining the best strategy for augmentation (i.e., the best species, strain, rates, frequency, timing) to maximize control of root weevils across a range of soil habitats, how augmentation of natural populations with commercially produced native or exotic nematodes influences endemic nematode communities and season-long root weevil control, and when, where, and with what strategies the conservation of endemic nematodes might be a viable alternative to augmentation for long-term weevil management.
Entomopathogenic nematodes in Florida citrus
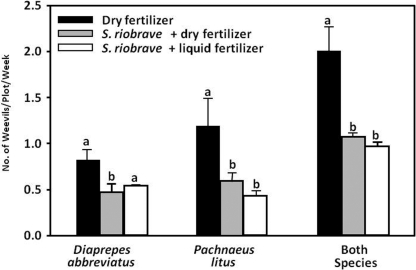
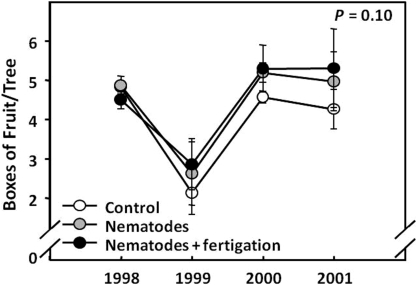
Citrus growers in Florida have been applying commercially produced EPN to control root weevils for over 15 years in what can be considered a biological control success story (Shapiro-Ilan et al., 2002; Georgis et al., 2006). Steinernema carpocapsae (Weiser) was the first nematode to be developed for this purpose but was supplanted by the more recently discovered S. riobrave Cabanillas, Poinar and Raulston, which was found to cause greater D. abbreviatus mortality, with some field studies reporting ≥90% suppression (Schroeder, 1994; Duncan and McCoy, 1996; Duncan et al., 1996; Bullock et al., 1999). In most field studies, application rates have been relatively high compared to the label rate (Shapiro-Ilan et al., 2002), but evidence indicates that the use of S. riobrave twice per year at the label rate (i.e., 20,000 IJ/ft2 = 22 IJ/cm2) can significantly reduce adult root weevil emergence (Fig. 1) and enhance yield (Fig. 2). However, there is growing evidence that EPN field efficacy can be quite variable and that applications sometimes fail to provide weevil control (McCoy et al., 2002; Shapiro-Ilan et al., 2002). Numerous factors could be responsible for these problems, but regional differences in soil habitat associated with different soil types are the most likely causes (Duncan et al., 2001; McCoy et al., 2002). Overall, estimates of the efficacy and profitability of using EPN for weevil control in Florida citrus vary widely and likely reflect variation in factors such as product quality, application rates, suitability of edaphic conditions for EPN, and experimental methods (Adair, 1994; Duncan et al., 1996; Stansly et al., 1997; Bullock et al., 1999; McCoy et al., 2000; Duncan et al., 2002; McCoy et al., 2002; Duncan et al., 2003a, 2007). In 1999, S. riobrave was applied to approximately 19,000 ha of Florida citrus to control root weevils (Shapiro-Ilan et al., 2002) and is currently marketed under the brand name BioVector (Becker Underwood Inc., Ames, IA).
Fig. 1.
Cumulative results of a four-year study in which S. riobrave (BioVector) was applied in a Florida citrus orchard at the label rate twice per year and the emergence of adult citrus root weevils Diaprepes abbreviatus and Pachnaeus litus was monitored weekly during the summer months. Bars with common letters within species groups are not significantly different at the P = 0.05 level based on ANOVA and Tukey's tests (Duncan, personal communication).
Fig. 2.
Fruit yield obtained during a four-year study in which S. riobrave (BioVector) was applied in a Florida citrus orchard at the label rate twice per year to suppress the citrus root weevils Diaprepes abbreviatus and Pachnaeus litus (Fig. 1). The P-value refers to the results of two-way ANOVA (Duncan, personal communication).
Soil variability as a limiting factor for weevils and entomopathogenic nematodes
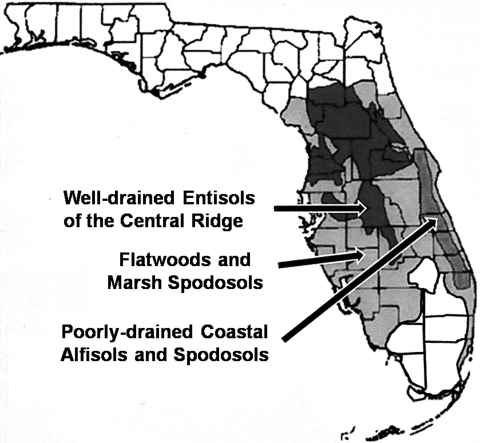
Florida citrus groves occur on soils ranging from coarse-textured well drained Entisols on the relatively high hilly landscapes of the Central Ridge to finer-textured poorly drained Alfisols and Spodosols on low-lying coastal and inland Flatwoods (Obreza and Collins, 2002; Fig. 3). In most cases, the root zones of these soils are dominated by sand (94% and higher) and contain only minor quantities of silt, clay and organic matter. However, these soils can vary widely in pH and soil texture and can be especially challenging for the proper water and nutrient management required for citriculture. Moreover, differences in these soils could contribute to root weevil problems and be responsible for limiting both endemic EPN communities and the effectiveness of EPN augmentations.
Fig. 3.
Map showing the locations of some of the major soil differences in the citrus-growing regions of Florida (from Obreza and Collins, 2002).
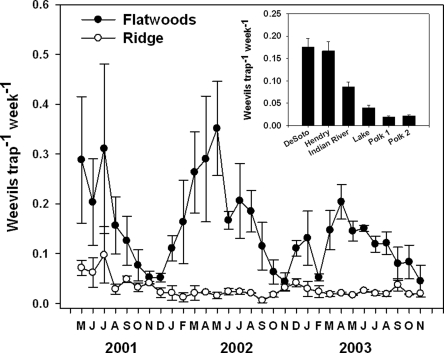
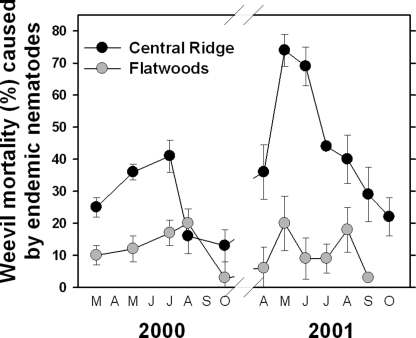
Observations by growers and researchers indicate that D. abbreviatus is much more abundant and more frequently a problem for citrus in the Flatwoods than on the Central Ridge (McCoy et al., 2004; Futch et al., 2005). A three-year study in which adult weevils were monitored with Tedders traps in a series of groves indicated that the abundance of weevils was more than five-fold greater in Flatwoods groves than in those on the Central Ridge (Futch et al., 2005; Fig. 4). Interestingly, studies involving the mortality of caged sentinel weevil larvae also indicate that there is a greater diversity and prevalence of endemic EPN in groves on the Central Ridge than in those in the Flatwoods (Duncan et al., 2003b; Fig. 5). Indeed, in certain groves at certain times of the year, endemic communities of EPN are responsible for 70 to 80% mortality among caged sentinel weevil larvae. This high level of natural weevil control by endemic EPN could be the primary reason that root weevils are seldom a major problem for citrus growers on the Central Ridge, whereas depauperate EPN communities might explain why root weevils can be devastating in Flatwoods citrus groves. Differing soil characteristics might also explain why EPN augmentations are often so successful on the Central Ridge but sometimes fail in the Flatwoods. Whatever the cause, there is a current need to either improve the success of augmentations in Flatwoods groves or find a way to bolster the natural EPN community to provide greater levels of natural control.
Fig. 4.
Numbers of Diaprepes abbreviatus root weevils captured in Tedders traps during a three-year study in citrus groves on the Central Ridge (Lake and Polk counties) and in the Flatwoods (DeSoto, Hendry and Indian River counties) (from Futch et al., 2005).
Fig. 5.
Percentage mortality among caged sentinel weevil larvae of Diaprepes abbreviatus exposed for one-week intervals during various months in citrus groves on the Central Ridge and in the Flatwoods (from Duncan et al., 2003).
Augmentation vs. conservation of entomopathogenic nematodes
As indicated above, augmentation of entomopathogenic nematodes has been an effective strategy for controlling root weevils in many Florida citrus groves, but soil type, especially the occurrence of finer-textured soils in the Flatwoods, apparently limits the usefulness of this tactic to particular groves in particular areas (Duncan et al., 2001; McCoy et al., 2002). Although the use of different species of EPN or higher rates of application in the Flatwoods might alleviate this problem to some extent (McCoy et al., 2007), convincing evidence for the effectiveness of such strategies is lacking, and higher application rates apparently do not work in certain sites (McCoy et al., 2002). Moreover, we now know that endemic EPN communities are more diverse and prevalent in citrus groves on the Central Ridge than in the Flatwoods and that endemic nematodes on the Central Ridge are capable of suppressing root weevils to a remarkable extent (Duncan et al., 2003b). This raises questions regarding the impact that EPN augmentations might have on endemic EPN communities on the Central Ridge, when, where, and under what conditions EPN augmentations might be most effectively used, what biotic and abiotic factors might be limiting EPN populations in Central Ridge versus Flatwoods groves, and what strategies might be useful to conserve EPN communities and enhance natural weevil control across the range of soil conditions that exist in Florida citrus groves.
Limiting factors for entomopathogenic nematode communities
The population biology of entomopathogenic nematodes has received little study, and there is relatively little information on the various biotic and abiotic factors that might effectively limit entomopathogenic nematode communities in various soil ecosystems (Barbercheck, 1992; Neher and Barbercheck, 1999; Glazer, 2002; Strong, 2002; Stuart et al., 2006). Indeed, EPN applications generally have been made with little knowledge or concern regarding the possible ramifications that this infusion of nematodes might have for soil community dynamics except to determine whether the particular target pest has been suppressed, at least temporarily, and whether the particular crop or commodity of interest shows improvement. Consequently, the factors that regulate EPN communities remain largely a matter of speculation. Nonetheless, evidence indicates that abiotic factors such as soil moisture, porosity, texture and bulk density can limit EPN movement, persistence and effectiveness (Barbercheck, 1992; Glazer, 2002; Stuart et al., 2006) and that biotic factors such as host abundance, mortality by various trapping and endoparasitic nematophagous fungi, predation by microarthropods, etc., competition from certain free-living bactivorous nematodes that invade host cadavers, and encumberance by Paenibacillus species can also be involved under certain circumstances (Neher and Barbercheck, 1999; Duncan et al., 2003a; El-Borai et al., 2005; Stuart et al., 2006; Duncan et al., 2007). The extent to which these various possible limiting factors might influence EPN communities in Florida citrus groves on the Central Ridge and in the Flatwoods is the subject of current research. Specifically, a food web study by Duncan et al. (2007) illustrates some important points and will be discussed below.
Food web study: Augmenting with EPN and manure mulch
Duncan et al. (2007) conducted a two-year study to investigate the effects of EPN augmentation and the use of composted animal manure mulches on the mortality of D. abbreviatus larvae and the prevalence of various organisms in the soil food web associated with EPN in a Florida citrus grove. The organisms assessed included nematophagous fungi (trappers and endoparasites), Paenibacillus spp., other soil nematodes (bacterivores, fungivores, omnivores and phytoparasitic), microarthropods (mites and collembola) and enchytraeid worms. Manure mulches were studied because they previously have been shown to increase the numbers of free-living bactivorous nematodes (FLBN) that sometimes compete with EPN for resource-rich insect cadavers (Peters, 1996; Duncan et al., 2003a). Duncan et al. (2007) measured temporal responses to the treatments to detect patterns that might indicate either direct or indirect interactions between organisms in the food web. The initial hypotheses were that endemic EPN were regulated at least in part by density-dependent natural enemies and that EPN augmentation would initiate a trophic cascade that would lead to a temporary increase in EPN natural enemies and a subsequent decrease in EPN, which would be detrimental to season-long weevil biological control.
The food web study involved 40 plots, each encompassing three adjacent trees, and was conducted in a commercial citrus orchard near Bartow on the typical sandy soil of Florida's Central Ridge (96% sand, 2% silt, 2% clay). The four treatments consisted of augmentations of (1) EPN, (2) animal manure mulch, (3) both EPN and animal manure mulch, and (4) an untreated control. In the mulched plots, composted horse manure was applied in March 2004, and composted chicken manure in June 2005. For EPN augmentation, Steinernema riobrave (BioVector) were applied on 10 August 2004, 17 June 2005 and 8 September 2005, at rates of 30 IJ/cm2 on the initial and final treatment date and at 300 IJ/cm2 on the second treatment date. Periodic monitoring assessed the mortality of caged sentinel D. abbreviatus larvae, the prevalence of entomopathogenic and other soil nematodes, nematophagous fungi, Paenibacillus spp., certain microarthropods (mites and collembola), enchytraeid worms and adult D. abbreviatus, as well as fruit yield.
Food web study: Effects of EPN augmentation
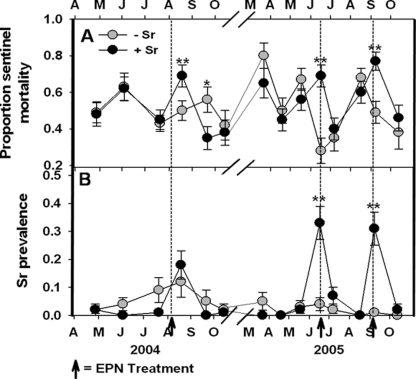
In Duncan et al. (2007), each of the three applications of S. riobrave (Sr) increased the rate of sentinel weevil mortality as assessed by placing caged larvae in the soil (Fig. 6). The average mortality rate of sentinel larvae that were buried on the day of treatment (2005) or 10 days after treatment (2004) ranged from 38% to 146% higher in EPN-augmented compared to non-augmented plots throughout the experiment. Thus, with Abbott's correction (Abbott, 1925), for the three successive augmentation dates, Sr was apparently responsible for killing 44%, 81% and 66% of weevils in the bare plots and 32%, 42% and 37% in the mulched plots that would not have been killed otherwise. However, the beneficial effects of augmentation for weevil mortality were apparently brief. Five weeks following EPN augmentation in 2004, significantly fewer sentinel weevils died in the EPN-treated plots (Fig. 6), and EPN (both endemic species and the exotic Sr) recovered from sentinel weevil cadavers were more prevalent in augmented plots only in September 2005, whereas they were less prevalent on several other sampling dates. Steinernema riobrave was detected in EPN-augmented and non-augmented plots, but Sr prevalence did not differ among treatments except during the two treatment months in 2005 (Fig. 6).
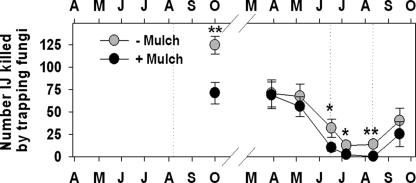
Fig. 6.
Effects (mean + SE) of augmenting the entomopathogenic nematode community beneath citrus trees with Steinernema riobrave (Sr) on the mortality of Diaprepes abbreviatus sentinel larvae (top) and the prevalence of insect cadavers containing Sr (bottom). Treatment differences based on two-way ANOVA of arcsin transformed data: @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Arrows indicate Sr application dates (from Duncan et al., 2007).
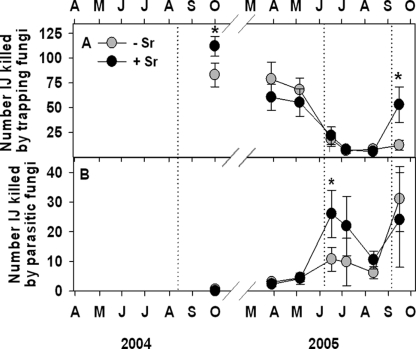
Trapping nematophagous fungi (NF) identified as Arthrobotrys oligospora Fresenius, A. musiformis Drechsler, and Gamsylella gephyropaga (Drechsler) M. Scholler, Hagedorn and A. Rubner ( = Monacrosporium cionopagum (Drechsler)) killed only 74% as many EPN in bioassaysof samples from untreated compared to EPN-augmentedplots, seven weeks after EPN treatment in 2004 (Fig. 7A). EPN augmentation did not again affect the prevalence of NF until the week following the final EPN treatment, when trapping NF killed 4.4 times as many EPN in bioassays of treated compared to untreated plots. More than twice as many EPN were killed by Catenaria spp. in bioassays of augmented compared to non-augmented plots during the two months following EPN treatment in June 2005 (Fig. 7B).
Fig. 7.
Effects (mean + SE) of augmenting the entomopathogenic nematode community beneath citrus trees with Steinernema riobrave (Sr) on bioassay measurements of the numbers of entomopathogenic nematodes killed by trapping NF (A), or endoparasitic NF (B). Treatment differences based on two-way ANOVA of arcsin transformed data: @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Dotted lines indicate Sr application dates (from Duncan et al., 2007).
The application of EPN had few measurable effects on the numbers of other nematodes at any trophic level. Between June (pre-treatment) and August (post-treatment) 2004, the average number of nematodes in plots treated with Sr declined by 35% compared to just 6% in untreated plots. Plant-parasitic nematodes accounted for much of the difference, and declined by 23% vs. 61% in untreated and Sr-treated plots, respectively. A similar trend was observed for omnivorous nematodes that declined by 10% in control plots compared to 35% in Sr-treated plots. Bactivorous nematodes increased by 44%, and mycophagous nematodes declined by 29% during this time, but without evidence of effects by Sr augmentation.
Overall, EPN augmentation directly or indirectly affected the prevalence of organisms at several trophic levels, but the effects were sometimes ephemeral and inconsistent. EPN augmentation always increased the mortality of sentinel weevil larvae, the prevalence of free-living nematodes in sentinel cadavers and the prevalence of trapping NF. Following the insecticidal effects of EPN augmentation in 2004, but not in 2005, EPN became temporarily less prevalent, and fewer sentinel weevil larvae died in EPN-augmented compared to non-augmented plots. The temporal and spatial relationships in the data indicate that EPN augmentation can initiate trophic cascades with successive bottom up (population growth by natural enemies of nematodes) and top down (greater predation of EPN) non-target effects that could result in reduced season-long biological control of root weevils. However, these data also show that population levels of most organisms affected by treatment with Sr in 2004 returned to background (i.e., untreated) levels quite quickly, and it is noteworthy that significant non-target effects on EPN did not occur following either of two Sr applications in 2005.
Food web study: Effects of manure mulch
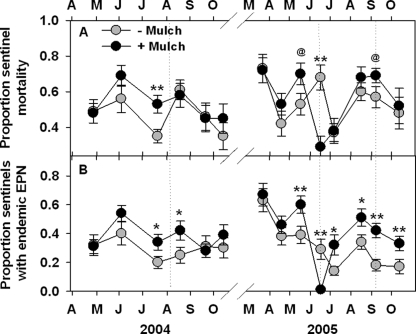
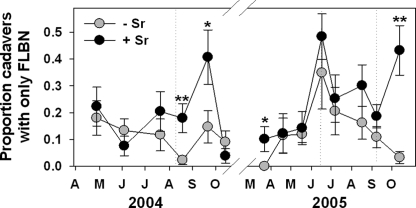
Following the application of chicken manure in June 2005, the prevalence of EPN in mulched plots decreased dramatically, and the mortality of sentinel weevils was reduced to less than half that observed in bare plots (Fig. 8). However, this effect was brief, and the mortality of sentinel larvae generally tended to be higher in mulched plots than in bare plots. Percent sentinel mortality, excluding June 2005, was 16% greater in mulched than in non-mulched plots. The prevalence of endemic EPN (Sr excluded) was consistently greater in mulched plots (Fig. 8B), as was the prevalence of all EPN, even though mulch apparently impeded infection of sentinels by exotic Sr. Excluding June 2005, sentinel weevil mortality for total EPN and endemic EPN was 28% and 43% greater, respectively, in mulched than in non-mulched plots. The proportion of cadavers containing FLBN increased immediately following the application of chicken manure, but otherwise was lower in mulched plots on several occasions across both years. The mean proportion of cadavers containing FLBN in plots during year 2 was higher in bare soil treated with Sr than in the other three treatments (e.g., Fig. 9), which underlines previous indications that the exotic Sr is less competitive with endemic FLBN than are some endemic EPN (Duncan et al., 2003a).
Fig. 8.
Effects (mean + SE) of composted animal manure applied as a mulch beneath citrus trees on the mortality of Diaprepes abbreviatus sentinel larvae (A) and the prevalence of endemic entomopathogenic nematodes in dead sentinel larvae (EPN) (B). Treatment differences based on two-way ANOVA of arcsin transformed data: @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Composted horse manure was applied in March 2004, and composted chicken manure was applied in the same plots on 10 June 2005. Dotted lines indicate Sr application dates (from Duncan et al., 2007).
Fig. 9.
Effects (mean + SE) of augmenting the entomopathogenic nematode community beneath citrus trees with Steinernema riobrave (Sr) on the prevalence of insect cadavers containing free-living, bactivorous nematodes (FLBN). A composted animal manure mulch was applied to half of the plots in each treatment, and the remaining plots remained bare. Treatment differences based on two-way ANOVA of arcsin transformed data: @(P ≤ 0.10), *(P ≤ 0.05) or **P ≤ 0.01). Dotted lines indicate Sr application dates (from Duncan et al., 2007).
The composted manure mulches consistently suppressed trapping NF (Fig. 10) but effects on endoparasitic NF varied over time. The prevalence of endoparasitic NF increased from 2004 to 2005 and tended to be lower in mulched plots until late in the summer of 2005, when endoparasitic NF from mulched plots infected greater numbers of EPN in the bioassays. A similar pattern occurred for enchytraeid worms, which were consistently suppressed in mulched plots until the trend was reversed after mid-summer 2005. Fewer microarthropods (both mites and collembola) were recovered from mulched plots in April and June 2005.
Fig. 10.
Effects (mean + SE) of composted animal manure applied as a mulch beneath citrus trees on bioassay measurements of the numbers of entomopathogenic nematodes killed by trapping NF in assay plates. Treatment differences based on two-way ANOVA of arcsin transformed data: @(P ≤ 0.10), *(P ≤ 0.05) or **(P ≤ 0.01). Composted horse manure was applied in March 2004, and composted chicken manure was applied to the same plots on 10 June 2005. Dotted lines indicate Sr application dates (from Duncan et al., 2007).
Horse manure mulch did not influence total numbers of nematodes in soil samples in 2004, but the application of chicken manure in 2005 increased the numbers of bactivorous nematodes by more than 16-fold and total nematodes by nine-fold in June 2005. Total nematode numbers and numbers of bactivorous nematodes remained 70% and 338% higher, respectively, in mulched than in bare plots in September 2005. Omnivorous nematodes in mulched plots were 72% and 63% as numerous as in bare plots in June and September 2005, respectively. Mulches did not affect total numbers of plant-parasitic nematodes or mycophagous nematodes on any measurement date.
In this study, manure mulch had variable effects on endoparasitic NF, but consistently decreased the prevalence of trapping NF and increased the prevalence of EPN and sentinel weevil larvae mortality. Shapiro et al. (1999) found that fresh manure from cattle and chickens had deleterious effects on the virulence of S. carpocapsae to black cutworm and greater wax moth larvae in the field, whereas composting those materials rendered them non-detrimental. The results of Duncan et al. (2007) were more similar to those of Bednarek and Gaugler (1997), who reported three-fold higher numbers of endemic S. feltiae (Filipjev) in field plots amended in alternate years for 20 years with 20 to 30 tons of animal manure/ha compared to unamended plots. Bednarek and Gaugler (1997) attributed the positive effect of manure on EPN to a presumed increase in numbers of soil-inhabiting insects in amended plots. Their hypothesis is an equally plausible explanation for the corresponding increase of EPN in the study by Duncan et al. (2007). Additionally, the suppression of trapping NF in the mulched plots may have reduced predation on EPN sufficiently to result in higher EPN prevalence. Jaffee et al. (1994) found that the NF Hirsutella rhossiliensis Minter and Brady parasitized fewer plant-parasitic nematodes if soil was amended with composted chicken manure in field plots and laboratory assays. However, endoparasitic and trapping NF responded to chicken manure mulch differently from one another in Duncan et al. (2007), and the methods could not reveal the relative predation rates by various NF. Some NF respond to environmental factors with strong saprophytic growth, but exhibit little trapping activity (Jaffee, 2004). The NF assay by Duncan et al. (2007) was designed to favor the detection of predation rates rather than propagule density by transferring nematodes rather than soil or soil suspensions from the field plots onto the agar plates and by quantifying EPN cadavers with identifiable fungi rather than NF colonies. Nevertheless, it is an indirect, qualitative assay of predation that occurs under artificial conditions. If EPN in the Duncan et al. (2007) study were partly regulated by NF, then the fact that EPN were favored by mulch suggests that an apparent inhibitory effect of mulch on trapping NF may have been more important than an apparent stimulatory effect of mulch on endoparasitic NF. Whatever the mechanism, EPN in mulched plots in the study by Duncan et al. (2007) appear to have increased sufficiently to provide greater biological control of D. abbreviatus than occurred in plots with bare soil. These results support the suggestion by Bednarek and Gaugler (1997) that the use of animal manures might represent a useful tactic for conservation biological control.
Food web study: Spatial and temporal patterns
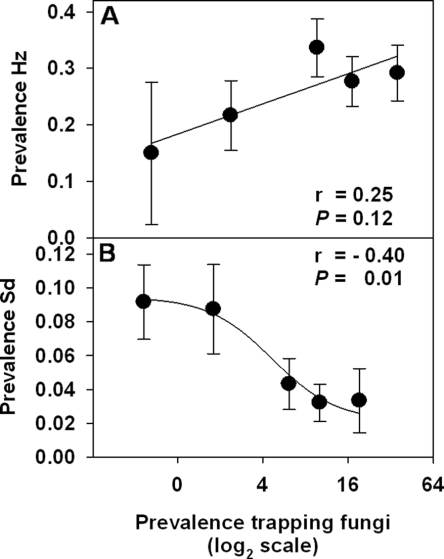
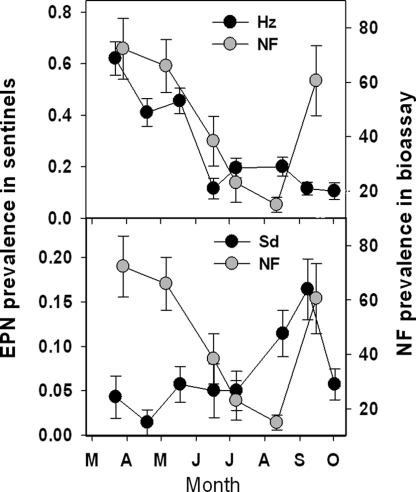
In the Duncan et al. (2007) study, the endemic EPN community was dominated by Heterorhabditis zealandica Poinar (Hz), which infected approx. 25–27% of sentinel weevil larvae each year compared to 5% by Steinernema diaprepesi Nguyen and Duncan (Sd) and 4% by H. indica Poinar, Karunaker and David (Hi). Based on the sentinel data, Hz, Sd and Hi were detected in 37, 28 and 18 plots, respectively, during the experiment. These endemic EPN species tended to predominate in mutually exclusive areas within the boundaries of the experiment, and these enclaves were relatively stable over time. The prevalence of Hz measured on six sampling dates in 2005 was directly related to the prevalence of trapping NF on the preceding sampling date, whereas Sd prevalence was inversely related to prior NF prevalence (Fig. 11). The average prevalence per plot of NF in 2005 was also spatially related to that of Sd but not to that of Hz (Fig. 12).
Fig. 11.
Spatial relationships (mean + SE) between the average prevalence during 2005 of unidentified nematophagous fungi and Heterorhabditis zealandica (Hz) (top) or Steinernema diaprepesi (bottom) beneath 40 citrus trees in a 1.6 ha area of a Florida citrus orchard (from Duncan et al., 2007).
Fig. 12.
Prevalence (mean + SE) in bioassays of NF and of sentinel weevils infected by Heterorhabditis zealandica (Hz) (top), or Steinernema diaprepesi (Sd) (bottom) during March through October 2005 in a Florida citrus orchard (from Duncan et al., 2007).
The inverse spatial and temporal relationships between the prevalence of NF and Sd indicate that NF play a role in regulating this nematode, whereas the spatial independence of Hz and NF, together with the positive correlation between Hz and NF in time, indicate that this nematode is not suppressed by NF. These relationships could have resulted if Hz benefited from greater predation by NF on steinernematid competitors of Hz than on Hz itself. The tendency of heterorhabditid IJ to retain the second-stage cuticle as a sheath affords them protection from some NF, whereas steinernematid IJ are more susceptible prey because they readily lose the sheath while migrating in soil (Timper and Kaya, 1989, 1992). Jaffee and Strong (2005) found that the population density of Arthrobotrys oligospora in the vicinity of insect cadavers infected by S. glaseri (Steiner) increased 10-fold more than in the vicinity of cadavers infected by H. marelatus Liu and Berry. Moreover, as with hetero-rhabditids in the Duncan et al. (2007) study, no spatial relationships were evident between H. marelatus and 12 species of nematophagous fungi isolated from sites at Bodega Bay in California (Jaffee et al., 1996).
Food web study: Long-term effects
In the Duncan et al. (2007) study, five months after the last application of chicken manure in February 2006, the numbers of juvenile T. semipenetrans in bare plots were 3.6-fold higher than in mulched plots. Much of the mulch effect on this plant-parasitic nematode can be attributed to the density of fibrous roots, which was 45% less in the mulched than in the bare plots. During 12 months between October 2005 and 2006, only 23 emerging adult weevils were caught in the ground traps placed on the experimental plots, but half as many adult weevils were trapped in mulched as in bare soil, and EPN augmentation reduced the recovery of weevil adults by 56%. This level of adult weevil suppression is similar to previous reports (Bullock et al., 1999; Duncan et al., 1999, 2002, 2003b) and reinforces the view that augmentation with S. riobrave is an effective strategy for reducing adult weevil emergence in Central Ridge citrus groves. Neither mulch nor augmentation of EPN significantly affected fruit yield by the end of this study.
Habitat manipulation and conservation biological control
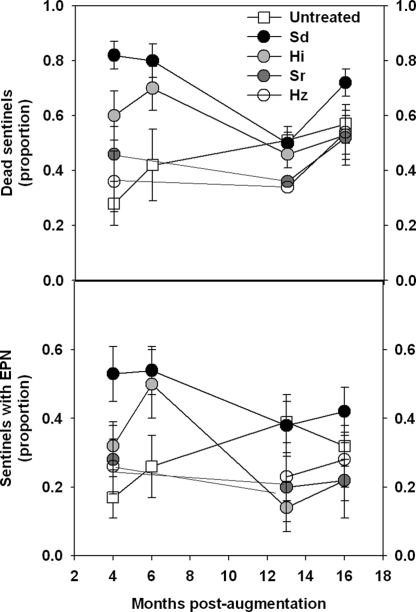
As indicated above, the addition of composted animal manure mulch might constitute a useful technique for conserving and enhancing EPN populations for biological control purposes. However, another habitat manipulation strategy, one that was initiated for other purposes by citrus growers with Flatwoods groves on Florida's east coast, might prove even more effective. These growers have begun planting new trees in oversized planting holes (approx. 1-m diam. × 30-cm deep) filled with coarse sand of the kind found on the Central Ridge to improve drainage, reduce infection by Phyto-phthora nicotianae Breda de Haan and promote tree growth. Incidentally, this management practice might also provide an ideal habitat for EPN, and we were intrigued to see how EPN from the Central Ridge might respond to being inoculated into these small, coarse sand islands in the midst of a Flatwoods grove. Consequently, in May 2006, we designated a series of single-tree experimental plots centered on four-year-old citrus trees in a commercial citrus grove that had been planted using this strategy. These plots were inoculated with nematodes of one of four species (H. indica, H. zealandica, S. riobrave or S. diaprepesi) or left as untreated controls. We monitored EPN prevalence by burying 5 caged sentinel weevil larvae/plot at periodic intervals. The initial results of this experiment indicate that the nematodes are persisting well in the coarse sand plots and providing levels of mortality of caged sentinel weevil larvae that are much higher than normally seen in Flatwoods groves and comparable to that seen on the Central Ridge (Fig. 13). The rate of weevil mortality in plots inoculated with S. diaprepesi averaged 71% per week (range = 50–82%) during 2006 and 2007. Steinernema diaprepesi is a large, long-lived species that is often the dominant species in groves on the Central Ridge and has yet to be isolated from other areas in Florida or elsewhere (Duncan et al., 2003b, 2007). Our previous research indicates that this nematode competes better than other EPN with certain free-living nematodes inside the insect cadaver (Duncan et al., 2003a) and also is less susceptible to predation by some commonly encountered nematophagous fungi (El-Borai et al., 2008). Heterorhabditis indica, the only endemic EPN in this commercial grove, has shown the tendency to colonize uninoculated (control) plots and to induce levels of sentinel weevil mortality that are higher than that typically seen in Flatwoods groves. However, the level of sentinel mortality in control plots in 2006 was still significantly less than in plots inoculated with S. diaprepesi. In 2007, sentinel weevil mortality was similar in plots inoculated with various EPN species and in uninoculated plots but, unfortunately, there were no trees planted in native soil in this grove for comparison. This is the first documented case in which applied EPN have been shown to persist at high levels for longer than one or two weeks after application in a Florida citrus grove.
Fig. 13.
Proportion of dead sentinel Diaprepes abbreviatus weevil larvae (top) and sentinel larvae containing entomopathogenic nematodes observed after one-week exposures in coarse-sand plots established in a commercial Flatwoods citrus grove and inoculated with one of four species of nematodes or left untreated: Sd = Steinernema diaprepesi, Sr = S. riobrave, Hi = Heterorhabditis indica, Hz = H. zealandica (Duncan, personal communication).
These preliminary observations of habitat manipulation and EPN inoculation in a Flatwoods grove suggest that this kind of conservation biological control strategy might be a practical way to extend the usefulness of EPN for root weevil control to sites that have not been amenable to EPN augmentation because of adverse soil conditions. If successful, the basic paradigm of creating conducive mesocosms of coarse sand in orchards in which the native soil is less amenable for EPN has several potential advantages over currently available IPM tactics for root weevil control. It is more economical for growers because a single inoculative application of EPN in the coarse sand is likely to provide greater and more sustained control of root weevils than multiple applications to the native soil. Moreover, it should provide maximum protection to the portion of the root system that sustains the most critical root weevil damage, the crown.
We have initiated additional experiments across a range of sites that incorporate larger sand mesocosms and appropriate native soil controls to further test the feasibility of this technique and to determine the best methods for implementation of this cultural practice. For example, the establishment of relatively small individual mesocosms around each tree might not be optimal for the maintenance of EPN populations of various species, whereas planting entire rows of young trees in trenches filled with coarse sand might be an extremely practical approach for growers and could lead to a much higher probability of EPN population growth and persistence, especially for EPN species that are rare or absent in the surrounding native soil. Further questions concerning the optimal size and structure of coarse sand mesocosms, both for EPN and to optimize tree growth and yield, remain to be explored. This kind of conservation biological control program could serve as an important model for future EPN conservation programs in diverse agroecosystems worldwide.
Conclusions
In Florida, endemic communities of entomopathogenic nematodes are generally more diverse and more prevalent in citrus groves on the Central Ridge than in those on the Flatwoods, whereas the Diaprepes root weevil, a major insect pest of citrus, is typically less abundant in Central Ridge groves than in Flatwoods groves. The difference in weevil abundance appears to be directly related to EPN prevalence in groves with different soil types, and soil type appears to influence EPN prevalence either directly by restricting nematode movement and host searching ability or indirectly through soil food web interactions. Certain strategies of soil habitat manipulation are showing potential for enhancing EPN populations and could enable improved and sustained suppression of root weevils. An experiment in which composted animal manure mulch was added to experimental plots resulted in increased prevalence of EPN and could be a useful conservation strategy. Moreover, in the Flatwoods, some growers have adopted the practice of planting young trees in coarse sand from the Central Ridge to improve drainage and tree survival. Research indicates that these coarse sand islands or mesocosms around the roots of the trees also provide improved habitat for EPN and thereby will likely provide additional protection for the trees from root weevil larvae. In the future, both augmentation and conservation of EPN will likely contribute to our continuing efforts to improve root weevil management in Florida citrus groves.
Footnotes
This research was supported by the Florida Agricultural Experiment Station and a grant from the T-STAR Special Grants Program.
Symposium paper presented at the 46th Annual Meeting of the Society of Nematologists, July 28–August 1, 2007, San Diego, CA.
This manuscript was edited by David Bird.
Literature Cited
- Abbott WS. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology. 1925;18:265–267. [Google Scholar]
- Adair RC. A four-year field trial of entomopathogenic nematodes for control of Diaprepes abbreviatus in a flatwoods citrus grove. Proceedings of the Florida State Horticultural Society. 1994;107:63–68. [Google Scholar]
- Adair RC, Nigg HN, Simpson SE, Le Fevre L. Observations on the oviposition process of Diaprepes abbreviatus (Coleoptera: Curculionidae) Florida Entomologist. 1999;82(2):362–365. [Google Scholar]
- Barbercheck ME. Effect of soil physical factors on biological control agents of soil insect pests. Florida Entomologist. 1992;75:539–548. [Google Scholar]
- Bednarek A, Gaugler R. Compatibility of soil amendments with entomopathogenic nematodes. Journal of Nematology. 1997;29:220–227. [PMC free article] [PubMed] [Google Scholar]
- Boemare N. Biology, taxonomy, and systematics of Photorhabdus and Xenorhabdus . In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI Publishing; 2002. pp. 35–56. [Google Scholar]
- Bullock RC, Pelosi RR, Killer EE. Management of citrus root weevils (Coleoptera: Curculionidae) on Florida citrus with soil-applied entomopathogenic nematodes (Nematoda: Rhabditida) Florida Entomologist. 1999;82:1–7. [Google Scholar]
- Campos-Herrera R, Manuel Gómez-Ros JM, Escuer M, Cuadra L, Barrios L, Gutiérrez C. Diversity, occurrence, and life characteristics of natural entomopathogenic nematode populations from La Rioja (Northern Spain) under different agricultural management and their relationships with soil factors. Soil Biology and Biochemistry. 2008;40:1474–1484. [Google Scholar]
- Duncan LW, Dunn DC, Bague G, Nguyen K. Competition between entomopathogenic and free-living bactivorous nematodes in larvae of the weevil Diaprepes abbreviatus . Journal of Nematology. 2003a;35:187–193. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Genta JG, Zellers J. Efficacy of Steinernema riobrave against larvae of Diaprepes abbreviatus in Florida soils of different texture. Nematropica. 2001;31:130. [Google Scholar]
- Duncan LW, Graham JH, Dunn DC, Zellers J, McCoy CW, Nguyen K. Incidence of endemic entomopathogenic nematodes following application of Steinernema riobrave for control of Diaprepes abbreviatus . Journal of Nematology. 2003b;35:178–186. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Zellers J. Profitability of applications of Steinernema riobrave, metalaxyl and supplemental fertilization for management of Diaprepes abbreviatus and Phytophthora nicotianae in a Florida citrus orchard. Fourth International Congress of Nematology. 2002;4:192. (Abstr.) [Google Scholar]
- Duncan LW, Graham JH, Zellers J, Bright D, Dunn DC, El-Borai FE, Porazinska DL. Food web responses to augmenting the entomopathogenic nematodes in bare and animal manure-mulched soil. Journal of Nematology. 2007;39(2):176–189. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, McCoy CW. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Curculionidae) of two species of entomogenous nematodes (Rhabditida: Steinernematidae; Heterorhabditidae) Environmental Entomology. 1996;25:174–178. [Google Scholar]
- Duncan LW, McCoy CW, Terranova AC. Estimating sample size and persistence of entomogenous nematodes in sandy soils and their efficacy against the larvae of Diaprepes abbreviatus in Florida. Journal of Nematology. 1996;28:56–67. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Shapiro DI, McCoy CW, Graham JH. Entomopathogenic nematodes as a component of citrus root weevil IPM. . In: Polavarapu S, editor. Proceedings of workshop on optimal use of insecticidal nematodes in pest management; August 28–30; New Brunswick, NJ: Rutgers University; 1999. pp. 69–78. [Google Scholar]
- El-Borai, F.E., Bright, D.B., Graham, J.H., Stuart, R.J., and Duncan, L.W. 2008. Differential susceptibility of entomopathogenic nematodes to nematophagous fungi from Florida citrus orchards. Nematology (in press)
- El-Borai FE, Duncan LW, Preston JF. Bionomics of a phoretic association between Paenibacillus sp. and the entomopathogenic nematode Steinernema diaprepesi . Journal of Nematology. 2005;37:18–25. [PMC free article] [PubMed] [Google Scholar]
- Forst S, Clarke D. Bacteria-nematode symbiosis. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI Publishing; 2002. pp. 57–77. [Google Scholar]
- French JV, Skaria M. Citrus root weevil identified. Texas A & M Kingsville Newsletter. 2000;18:1–4. [Google Scholar]
- Futch SH, Duncan LW, Zekri M. Validation of an area-wide extension program to estimate the seasonal abundance of adult citrus root weevils with un-baited pyramidal traps. Proceedings of the Florida State Horticultural Society. 2005;117:143–147. [Google Scholar]
- Georgis R, Koppenhöfer AM, Lacey LA, Bélair G, Duncan LW, Grewal PS, Samish M, Tan L, Torr P, van Tol RWHM. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. [Google Scholar]
- Glazer I. Survival biology. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI Publishing; 2002. pp. 169–187. [Google Scholar]
- Grafton-Cardwell, E. E., Godfrey, K. E., Peña, J. E., McCoy, C. W., and Luck, R. F. 2004. Diaprepes Root Weevil. 8 pp. Regents of the University of California, Division of Agriculture and Natural Resources. Publication 8131. http://anrcatalog.ucdavis.edu/pdf/8131.pdf
- Graham JH, Bright DB, McCoy CW. Phytophthora-Diaprepes weevil complex: Phytophthora spp. relationship with citrus rootstocks. Plant Disease. 2003;87:85–90. doi: 10.1094/PDIS.2003.87.1.85. [DOI] [PubMed] [Google Scholar]
- Jaffee BA. Do organic amendments enhance the nematode-trapping fungi Dactylellina haptotyla and Arthrobotrys oligospora? Journal of Nematology. 2004;36:267–275. [PMC free article] [PubMed] [Google Scholar]
- Jaffee BA, Ferris H, Stapleton JJ, Norton MVK, Muldoon AE. Parasitism of nematodes by the fungus Hirsutella rhossiliensis as affected by certain organic amendments. Journal of Nematology. 1994;26:152–161. [PMC free article] [PubMed] [Google Scholar]
- Jaffee BA, Strong DR. Strong bottom-up and weak top-down effects in soil: Nematode-parasitized insects and nematode-trapping fungi. Soil Biology and Biochemistry. 2005;37:1011–1021. [Google Scholar]
- Jaffee BA, Strong DR, Muldoon AE. Nematode-trapping fungi of a natural shrubland: Tests for food chain involvement. Mycologia. 1996;88:554–564. [Google Scholar]
- Kaya HK, Bedding RA, Akhurst RJ. An overview of insect-parasitic and entomopathogenic nematodes. In: Bedding R, Akhurst R, Kaya H, editors. Nematodes and the biological control of insect pests. East Melbourne, Australia: CSIRO Publications; 1993. pp. 1–10. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Lapointe SL, Borchert DM, Hall DG. Effect of low temperatures on mortality and oviposition in conjunction with climate mapping to predict spread of the root weevil, Diaprepes abbreviatus, and introduced natural enemies. Environmental Entomology. 2007;36(1):73–82. doi: 10.1603/0046-225x(2007)36[73:eoltom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lewis EE, Campbell JF, Gaugler R. A conservation approach to using entomopathogenic nematodes in turf and landscapes. In: Barbosa P, editor. Conservation biological control. New York: Academic Press; 1998. pp. 235–254. [Google Scholar]
- Lewis EE, Campbell J, Griffin C, Kaya H, Peters A. Behavioral ecology of entomopathogenic nematodes. Biological Control. 2006;38:66–79. [Google Scholar]
- McCoy CW. Arthropod pests of citrus roots. In: Timmer LW, Duncan LW, editors. Citrus health management. St. Paul, Minn: APS Press; 1999. pp. 149–156. [Google Scholar]
- McCoy CW, Castle WS, Graham JH, Syvertsen JP, Schu-mann AW, Stuart RJ. Pesticide suppression of Diaprepes abbreviatus (L.) (Coleoptera: Curculionidae) promoted differential growth and survival of “Hamlin” orange trees budded to five rootstocks in a Phytophthora infested grove. Proceedings of the Florida State Horticultural Society. 2004;117:167–173. [Google Scholar]
- McCoy CW, Rogers ME, Futch SH, Graham JH, Duncan LW, Nigg HN. Citrus root weevils. In: Rogers ME, Timmer LW, editors. 2007 Florida citrus pest management guide, SP-43. Gainesville, FL: Coop. Extension Service, University of Florida, Institute of Food and Agricultural Sciences; 2007. pp. 61–65. [Google Scholar]
- McCoy CW, Shapiro DI, Duncan LW, Nguyen K. Entomopathogenic nematodes and other natural enemies as mortality factors for larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) Biological Control. 2000;19:182–190. [Google Scholar]
- McCoy CW, Stuart RJ, Duncan LW, Nguyen K. Field efficacy of two commercial preparations of entomopathogenic nematodes against larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in alfisol type soil. Florida Entomologist. 2002;85:537–544. [Google Scholar]
- McCoy CW, Stuart RJ, Nigg HN. Seasonal life stage abundance of Diaprepes abbreviatus in irrigated and non-irrigated citrus plantings in central Florida. Florida Entomologist. 2003;86:34–42. [Google Scholar]
- McCoy CW, Stuart RJ, Shapiro-Ilan DI, Duncan LW. Application and evaluation of entomopathogens for citrus pest control. In: Lacey LA, Kaya HK, editors. Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests. 2nd edition. Dordrecht, Netherlands: Kluwer Academic Publishers; 2006. pp. 567–581. [Google Scholar]
- Neher D, Barbercheck ME. Diversity and function of soil mesofauna. In: Collins, Qualset CO, editors. The Biodiversity of agroecosystems. Boca Raton, FL: CRC Press LLC; 1999. pp. 27–47. in. W. [Google Scholar]
- Nigg HN, Simpson SE, Stuart RJ, Duncan LW, McCoy CW, Gmitter FG., Jr Abundance of Diaprepes abbreviatus (L.) (Coleoptera: Curculionidae) neonates falling to the soil under tree canopies in Florida citrus groves. Journal of Economic Entomology. 2003;96:835–843. doi: 10.1093/jee/96.3.835. [DOI] [PubMed] [Google Scholar]
- Nigg HN, Simpson SE, Stuart RJ, Yang LK, Adair RC, Bas B, Ur-Rehman S, Cuyler NW, Barnes JI. Reproductive potential of Florida populations of Diaprepes abbreviatus (Coleoptera: Curculionidae) Journal of Entomological Science. 2004;39:251–266. [Google Scholar]
- Obreza, T. A., and Collins, M. E. 2002. Common soils used for citrus production in Florida. http://edis.ifas.ufl.edu/SS403
- Peña JE, Hall DG, McCoy CW. Natural enemies of the weevil Diaprepes abbreviatus (Coleoptera: Curculionidae), a serious pest of citrus in Florida. Proceedings of the International Society of Citriculture. 2000;2:785–788. [Google Scholar]
- Peters A. The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Science and Technology. 1996;6:389–402. [Google Scholar]
- Quintela ED, Fan J, McCoy CW. Development of Diaprepes abbreviatus (Coleoptera: Curculionidae) on artificial and citrus root substrates. Journal of Economic Entomology. 1998;91:1173–1179. [Google Scholar]
- Schroeder WJ. Comparison of two steinernematid species for control of the root weevil Diaprepes abbreviatus . Journal of Nematology. 1994;26:360–362. [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Lewis LC, Obrycki JJ, Abbas M. Effects of fertilizers on suppression of black cutworm (Agrostis ipsilon) damage with Steinernema carpocapsae . Supplement to the Journal of Nematology. 1999;31:690–693. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Duncan LW, Lacey LA, Han R. Orchard crops. In: Grewal P, Ehlers R-U, Shapiro-Ilan D, editors. Nematodes as biological control agents. St. Albans, UK: CABI; 2005. pp. 215–230. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhöfer AM. Factors affecting commercial success: Case studies in cotton, turf and citrus. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI; 2002. pp. 333–356. [Google Scholar]
- Simpson SE, Nigg HN, Coile NC, Adair RC. Diaprepes abbreviatus (Coleoptera: Curculionidae): Host plant associations. Environmental Entomology. 1996;25:333–349. [Google Scholar]
- Stansly PA, Mizell RF, McCoy CW. Monitoring Diaprepes abbreviatus with Tedders traps in southwest Florida citrus. Proceedings of the Florida State Horticultural Society. 1997;110:22–26. [Google Scholar]
- Strong DR. Populations of entomopathogenic nematodes in foodwebs. In: Gaugler R, editor. Entomopathogenic nematology. Wallingford, UK: CABI; 2002. pp. 225–240. [Google Scholar]
- Stuart RJ, Barbercheck ME, Grewal PS, Taylor RAJ, Hoy CW. Population biology of entomopathogenic nematodes: Concepts, issues and models. Biological Control. 2006;38:80–102. [Google Scholar]
- Stuart RJ, Jackson IW, McCoy CW. Predation on neonate larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in Florida citrus: Testing for daily patterns of neonate drop, ant predators, and chemical repellency. Florida Entomologist. 2003;86(1):61–72. [Google Scholar]
- Stuart RJ, McCoy CW, Castle WS, Graham JH, Rogers ME. Diaprepes, Phytophthora and hurricanes: Rootstock selection and pesticide use contribute to differential growth and survival of ‘Hamlin’ orange trees in a central Florida citrus grove. Proceedings of the Florida State Horticultural Society. 2008;119:128–135. [Google Scholar]
- Stuart RJ, Rogers ME. Battling the evil weevil: Recent advances in the war on Diaprepes abbreviatus . Citrus Industry. 2006;87:7–11. [Google Scholar]
- Timper P, Kaya HK. Role of the 2nd-stage cuticle of entomogenous nematodes in preventing infection by nematophagous fungi. Journal of Invertebrate Pathology. 1989;54:314–321. [Google Scholar]
- Timper P, Kaya HK. Impact of a nematode-parasitic fungus on the effectiveness of entomopathogenic nematodes. Journal of Nematology. 1992;24:1–8. [PMC free article] [PubMed] [Google Scholar]
- Wolcott GN. The life history of Diaprepes abbreviatus, L., at Rio Piedras, Puerto Rico. Journal of Agriculture of the University of Puerto Rico. 1936;20:883–914. [Google Scholar]
- Woodruff RE. A Puerto Rican weevil new to the United States (Coleoptera: Curculionidae). Florida Department of Agriculture. Plant Industry Entomology Circular. 1964;77:1–4. [Google Scholar]