Abstract
CB1 cannabinoid receptors are distinctly expressed at high density within several regions of zebra finch telencephalon including those known to be involved in song learning (lMAN and Area X) and production (HVC and RA). Because: (1) exposure to cannabinoid agonists during developmental periods of auditory and sensory-motor song learning alters song patterns produced later in adulthood and; (2) densities of song region expression of CB1 waxes-and-wanes during song learning, it is becoming clear that CB1 receptor-mediated signaling is important to normal processes of vocal development. To better understand mechanisms involved in cannabinoid modulation of vocal behavior we have investigated the dose-response relationship between systemic cannabinoid exposure and changes in neuronal activity (as indicated by expression of the transcription factor, c-Fos) within telencephalic brain regions with established involvement in song learning and/or control. In adults we have found that low doses (0.1 mg/kg) of the cannabinoid agonist WIN-55212-2 decrease neuronal activity (as indicated by densities of c-fos-expressing nuclei) within vocal motor regions of caudal telencephalon (HVC and RA) while higher doses (3 mg/kg) stimulate activity. Both effects were reversed by pretreatment with the CB1-selective antagonist rimonabant. Interestingly, no effects of cannabinoid treatment were observed within the rostral song regions lMAN and Area X, despite distinct and dense CB1 receptor expression within these areas. Overall, our results demonstrate that, depending on dosage, CB1 agonism can both inhibit and stimulate neuronal activity within brain regions controlling adult vocal motor output, implicating involvement of multiple CB1-sensitive neuronal circuits.
Keywords: Drug Abuse, Birdsong, c-Fos, cannabinoids
Introduction
Songbirds like the zebra finch have been essential to investigations of the neurobiology underlying vocal development (reviewed by Troyer and Bottjer, 2001). Because zebra finch song is a form of vocal communication learned during distinct late-postnatal periods (Doupe and Kuhl, 1999), we have employed these animals as a pharmacological model to study drug effects on learning during “periadolescent” development (Spear 2000). We have found that single daily treatments with a modest dosage (1 mg/kg) of the cannabinoid agonist WIN55212-2 (WIN) from 50 – 100 days of age (the time-course of zebra finch post-natal development is similar to that of the rat) alters vocal learning by reducing: (1) the number of note-types produced and; (2) song stereotypy (a measure of song quality developed by Scharff and Nottebohm, 1991). Because these changes did not occur in adults administered the same treatment, the effect is restricted to periods of vocal development (Soderstrom and Johnson, 2003). Further experiments have revealed that these effects on note number and stereotypy are produced independently: stereotypy is reduced by WIN exposure from 50 – 75 days; while note numbers are altered by exposure from 75–100 days (Soderstrom and Tian, 2004).
Coordinated control of song learning, perception and production involves a discrete set of interconnected midbrain, thalamic and telencephalic brain regions (see Bottjer and Johnson, 1997 for review). CB1 cannabinoid receptors are densely and distinctly expressed in several of these song regions through adulthood (e.g. all of the areas indicated in Fig 1, Soderstrom et al., 2004). The density and pattern of distinct CB1 expression in several of these song regions notably waxes and wanes over the course of song learning, implicating cannabinoid signaling as important to the normal course of vocal development. Song regions notable for particularly distinct changes in the density and pattern of CB1 expression during vocal learning include the rostral telencephalic regions lMAN and Area X, and caudal regions HVC and RA (Soderstrom and Tian 2006). The goal of the current project was to characterize effects of cannabinoid agonism on neuronal activity within these distinctly receptor-expressing telencephalic song regions. Activity was studied as a function of expression of the immediate early gene, c-Fos. This knowledge will be important to understanding how the acute effects of cannabinoids result in reduced song output and locomotor activity in adult animals (Soderstrom and Johnson 2001) and will determine normal patterns of cannabinoid-induced changes in neuronal activity to which patterns associated with altered vocal development can be compared.
Figure 1.
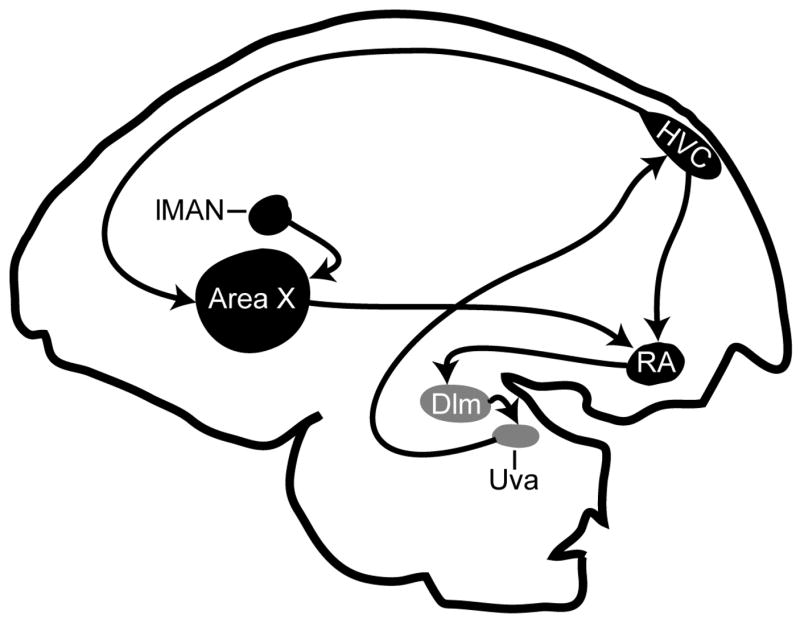
Parasagittal diagram of song regions with established distinct expression of CB1 receptors (adapted from (Soderstrom and Tian 2006). Rostral is left, dorsal top. Telencephalic regions containingdense CB1 expression are indicated in black and include telencephalic song regions lateral magnocellular nucleus of the anterior nidopallium (lMAN), Area X within songbird medial striatum (Area X), HVC and the robust nucleus of the arcopallium (RA). Thalamic regions nucleus uvaformis (Uva) and dorsal lateral nucleus of the medial thalamus (DLM) that also distinctly express CB1 receptors are shown in grey and included to illustrate known interconnections between song regions (indicated by arrows, (Bottjer and Johnson 1997).
Methods
Except where noted, all materials and reagents were purchased from Sigma or Fisher Scientific. Immunochemicals were purchased from Vector laboratories (Burlingame, CA) and Santa Cruz Biotechnology (Santa Cruz, CA). We have employed the recently revised system of nomenclature in descriptions of zebra finch neuroanatomy (Reiner et al, 2004).
Animals
Adult male zebra finches bred in our aviary and sexed at ~ 25 days via PCR (Soderstrom et al. 2007) were used in these experiments. Prior to the start of experiments, birds were housed in flight aviaries with mixed seeds (SunSeed VitaFinch), grit, water, and cuttlebone freely available. Each flight aviary contained several perches. The light–dark cycle was controlled at LD 14:10 h and ambient temperature was maintained at 78° F.
To eliminate possible variance associated with staining conditions during immunohistochemistry, tissue from animals from each treatment group were processed simultaneously.
Animals were cared for and experiments conducted according to protocols approved by East Carolina University’s Animal Care and Use Committee.
Treatments
Drug treatments were given by IM injection of 50 mcl into pectoralis. Drug dilutions for injection were made from 10 mM DMSO stocks to produce a final vehicle of 1:1:18 DMSO:Alkamuls (Rhodia, Cranberry, NJ):PBS (pH = 7.4). WIN55212-2 was purchased from Sigma, rimonabant (SR141716A) was a gift from Sanofi Recherche. Because (1) song production and perception is known to alter expression of c-Fos in zebra finch song regions (Whitney et al. 2003) and; (2) zebra finches are inactive and don’t sing in the dark, treatments were given immediately prior to the beginning of light cycles to prevent potential song- and activity-related c-Fos expression. Preliminary experiments indicated peak c-Fos expression occurred 90 min following treatments, and therefore this period was used for all studies. For antagonist experiments, rimonabant was given ten minutes prior to the agonist WIN55212-2, which was given immediately preceding the beginning of light phases, and 90 min prior to perfusion for immunohistochemistry. To reverse effects of the low, 0.3 mg/kg WIN55212-2 dosage, we employed a half-log higher (1 mg/kg) dosage of rimonabant in order to minimize the fraction of agonist-bound receptors in our system. In the case of the higher 3 mg/kg WIN55212-2 dosage, we were unable to prepare an even suspension of a half-log higher rimonabant dosage to deliver in a reasonable volume (50 mcl). Therefore we employed a rimonabant dosage of only twice that of the agonist dosage (6 mg/kg rimonabant).
Anti-c-Fos immunohistochemistry
Ninety minutes following treatments, birds were killed by Equithesin overdose and transcardially perfused with phosphate-buffered saline (PBS, pH = 7.4) followed by phosphate-buffered 4 % paraformaldehyde, pH = 7.0. After brains were removed and immersed overnight in buffered 4 % paraformaldehyde, they were blocked down the midline and left hemispheres were sectioned parasagittally (lateral to medial) on a vibrating microtome. Immunohistochemistry was performed using a standard protocol reported in (Whitney et al., 2000) except that anti-c-Fos primary antibody was employed. For immunohistochemistry experiments, 30 mcm sections of zebra finch brain were reacted with a 1:3000 dilution of polyclonal anti-c-Fos antibody raised in rabbit (Santa Cruz Biotechnology, cat# sc-253). Note that this antibody has previously been successfully used with zebra finch tissue (Bolhuis et al. 2001). Tissue sections were rinsed in 0.1 % H2O2 for 30 min, blocked with 5 % goat serum for 30 min, and incubated overnight in blocking solution containing anti-c-Fos antibody (1:3000). After antibody exposure, sections were rinsed in PBS (pH = 7.4), incubated in blocking solution containing biotinylated anti-rabbit antiserum (1:500) for 1 hour, rinsed with PBS again, and then submerged in avidin-biotin-peroxidase complex solution (purchased as a kit from Vector Laboratories) for 1 hour. Antibody labeling was visualized with DAB solution. Control sections that were not incubated in primary antibody were not immunoreactive.
For double-labeling experiments our anti-zebra finch CB1 was employed at a dilution of 1:5000 as described above for c-Fos staining and reacted with DAB to produce a rust-brown stain. After anti-CB1 staining, sections were washed three times in buffered saline solution (pH = 7.4) and blocked in 5 % goat serum for 30 min. Following the blocking step sections were exposed to anti-c-Fos primary antibody diluted 1:3000 in 5 % goat serum for 18 hours. After the second primary antibody exposure, sections were rinsed in PBS (pH = 7.4), incubated in blocking solution containing biotinylated anti-rabbit antiserum (1:500) for 1 hour, rinsed with PBS again, and then submerged in avidin-biotin-peroxidase complex solution (purchased as a kit from Vector Laboratories) for 1 hour. Antibody labeling was visualized with DAB solution with addition of a nickel chloride reagent to produce blue-grey staining.
Staining was examined in various brain regions at 12.5, 100 and 600 X using an Olympus BX51 microscope with Nomarski DIC optics. Images were captured using a Spot Insight QE digital camera and Image-Pro Plus software (MediaCybernetics, Silver Spring, MD) under identical, calibrated exposure conditions. These images were background-corrected, converted to grey scale and borders of brain regions traced manually. Two dimensional counts of labeled nuclei from images, and areas enclosed within traced areas were determined without knowledge of treatment condition for each brain region of interest from five separate sections per animal using Image-Pro Plus software. Counts were made independently by two investigators and pooled for analysis. Mean densities (within region counts of stained nuclei/area of the region) were compared across treatment group and brain region using two-way ANOVA as described below.
Statistical Analyses
Relationships between drug treatments and anti-c-Fos-reactive cell densities were determined through 2-way ANOVA with treatment (WIN dosage, or vehicle vs. WIN vs. WIN + rimonabant vs. rimonabant), and brain region (HVC vs. RA vs. lMAN vs. Area X) as factors. To best illustrate basal c-fos expression differences across rostral and caudal regions of telencephalon, raw densities of immunoreactive cells were analyzed for initial experiments investigating agonist-induced changes. For antagonist reversal experiments of effects within caudal regions only, because low- and high-dose experiments were done independently, to minimize cross-experiment variance, raw immunoreactive cell densities were transformed to fractions of respective vehicle control values. Following ANOVA determination that mean cell densities or transformed values differed across treatment and brain regions (p ≤ 0.05), Student-Neuman-Keuls post-tests were done.
Results
Effects of Various WIN55212-2 Dosages on Song Region c-Fos Expression
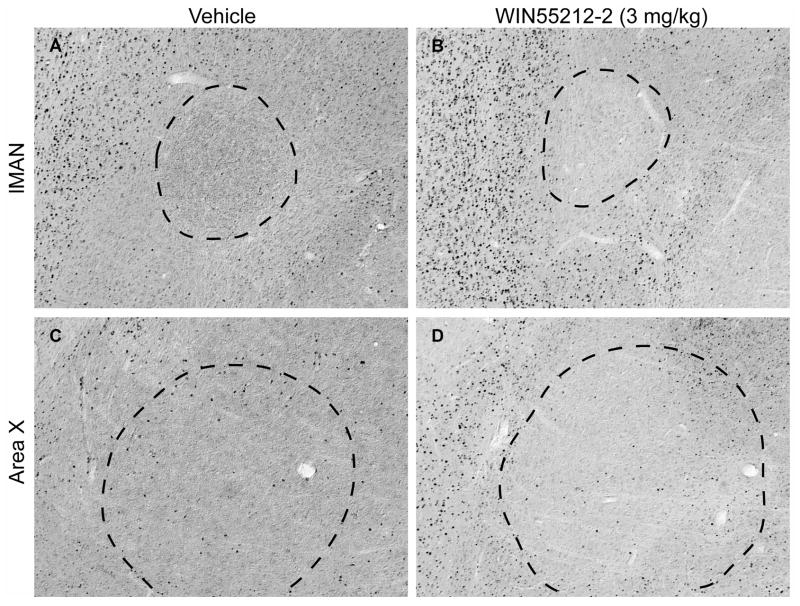
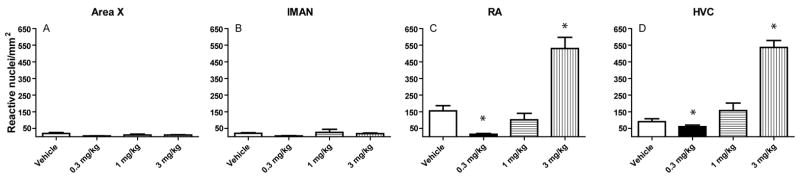
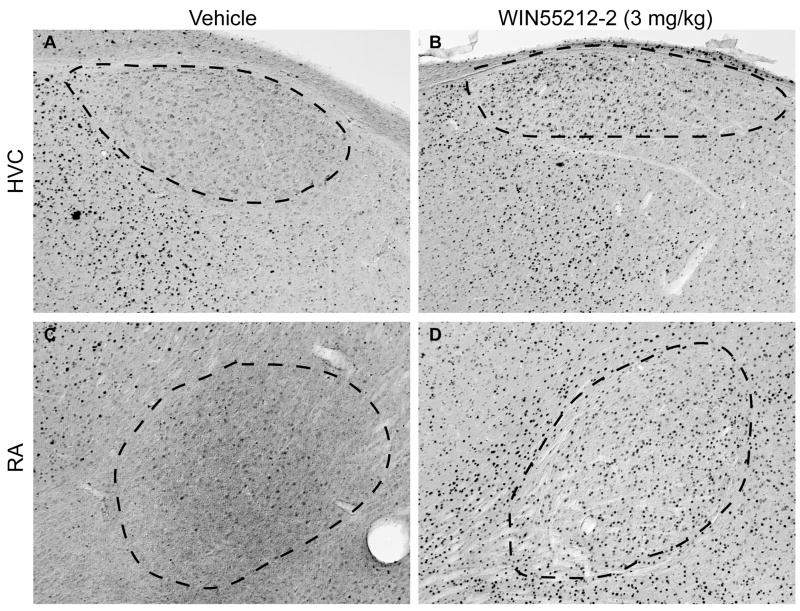
Basal densities of c-Fos-reactive cells within the rostral telencephalic song regions lMAN and Area X were remarkably low (Fig 2 panels A and C), and did not vary as a function of acute exposure to the cannabinoid agonist WIN55212-2 (Fig 2 B and D, and Fig 3 A and B). Densities of c-Fos-reactive nuclei following agonist exposure within each brain region are summarized in Figure 3. In contrast to rostral regions, the caudal song regions HVC and RA showed appreciable levels of basal c-Fos expression (Fig 4 panels A and C, Fig 3 C and D). WIN55212-2 elicited a biphasic effect on c-Fos-reactive nuclei: 0.3 mg/kg produced a significant reduction in the density of immunoreactive nuclei in both HVC (from 90.4 ± 17.5 to 59.6 ± 11.0/mm2) and RA (from 155.5 ± 31.2 to 15 ± 4.8/mm2), 1 mg/kg produced no overall change, while 3 mg/kg produced significantly increased densities in both HVC (to 536.1 ± 41.5/mm2) and RA (to 530.2 ± 67.6/mm2, *p < 0.05 in each case).
Figure 2.
Immunohistochemical staining of lMAN and Area X regions of rostral telencephalon with anti-c-Fos antibody as a function of vehicle (A and C) or WIN55212-2 (3 mg/kg, B and D) treatment. Medial parasaggital sections represent planes about 1.5 mm lateral from the midline. Rostral is left, dorsal is top, magnification is 100 X. Dark puncta represent stained nuclei. Note relatively low-level expression in lMAN (indicated by dashed outline in panels A and B) and Area X (outlined in panels C and D) relative to that within the caudal song regions HVC and RA (shown in Fig 3).
Figure 3.
Immunohistochemical staining of HVC (indicated by dashed outline in panels A and B) and RA (outlined in panels C and D) regions of caudal telencephalon with anti-c-Fos antibody as a function of vehicle (A and C) or WIN55212-2 (3 mg/kg, B and D) treatment. Medial parasaggital sections represent planes about 1.5 mm lateral from the midline. Rostral is left, dorsal is top, magnification is 100 X. Dark puncta represent stained nuclei. Note relatively high-level expression in HVC and RA relative to that within caudal song regions (shown in Fig 2).
Figure 4.
The cannabinoid agonist WIN55212-2 increases c-Fos expression within a subset of telencephalic brain regions known to control song learning and control. Birds were killed 90 min following treatment and perfused for immunohistochemistry. Densities of immunoreactive nuclei within each region (n = 6 animals within each treatment group) are summarized. Mean densities were generated from counts within at least five separate tissue sections from each animal. Two-way ANOVA followed by post-tests revealed no significant density changes within rostral regions lMAN (panel A) and Area X (panel B), while increased densities of c-Fos immunoreactive nuclei were noted within the caudal regions HVC (panel C) and RA (panel D) 90 min following treatment with the cannabinoid agonist WIN55212-2 (3 mg/kg, *p < 0.05).
Antagonist Reversal of Cannabinoid-Altered c-Fos Expression within Caudal Song Regions of Telencephalon
Effects of the CB1 receptor antagonist rimonabant were evaluated within caudal telencephalic song regions (HVC and RA) that were initially found sensitive to effects of the agonist WIN55212-2. Within both regions, antagonist pretreatment effectively reversed cannabinoid-altered c-fos expression. These reversals included effects of both the higher stimulatory- (3 mg/kg, Fig 5 A and B, †p < 0.05) and lower (0.3 mg/kg, Fig 5 C and D, †p < 0.05) inhibitory-dosages of the agonist.
Figure 5.
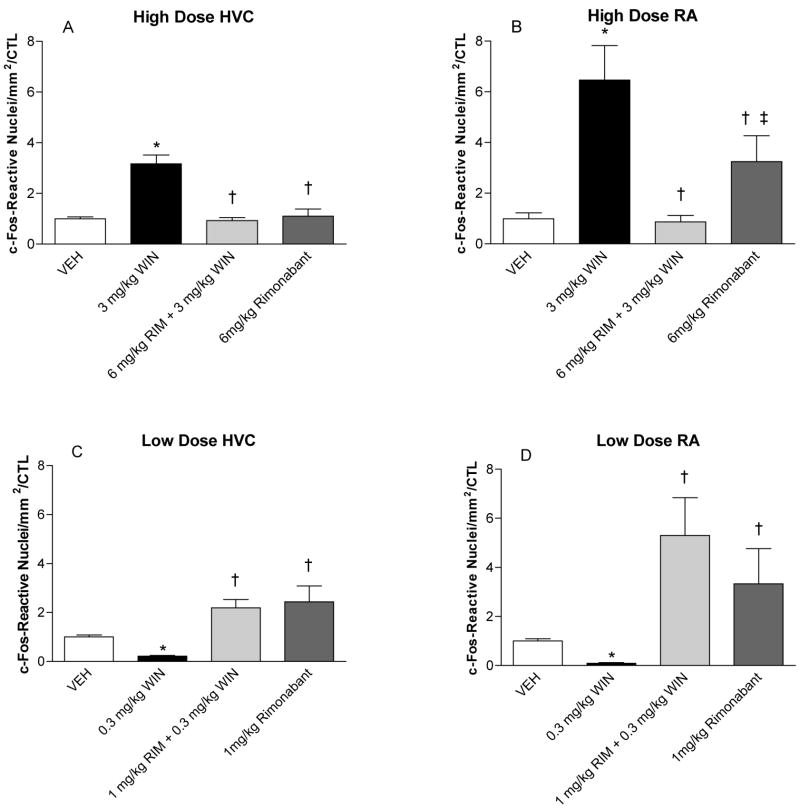
The CB1-selective antagonist rimonabant (RIM) reverses both high- (3 mg/kg) and low-dosage (0.3 mg/kg) effects of the cannabinoid agonist WIN55212-2. Densities of anti-c-Fos reactive cells within HVC (panels A and C) or RA (panels B and D) following high- (panels A and B) and low-WIN dosage treatments (panels C and D) are shown as a fraction of vehicle controls (VEH). Significant differences from VEH groups following 2-way ANOVA and Student-Neuman-Keuls post-tests are indicated by asterisks (*p < 0.05). Differences from WIN treatments (3 or 0.3 mg/kg WIN) are indicated by single daggers (†p < 0.05). The double-dagger in panel B indicates a significant difference from the combined treatment of 6 mg/kg rimonabant and 3 mg/kg WIN55212-2 (‡p < 0.05).
Following the high WIN55212-2 dosage (3 mg/kg), within HVC mean fractions of c-Fos-expressing neurons (relative to vehicle controls [VEH]) were significantly increased to 3.17 +/− 0.35 of control. This increase was significantly reversed to 0.93 +/− 0.12 of control by pretreatment with 6 mg/kg rimonabant (Fig 5A, †p < 0.05). Similarly within RA, 3 mg/kg of WIN55212-2 resulted in a significant increase in c-Fos densities to 6.46 +/− 1.36 of controls. This increase within RA was significantly reversed to 0.87 +/− 0.25 of control following pretreatment with 6 mg/kg rimonabant (Fig 5B, †p < 0.05). Interestingly, within RA, the high rimonabant dosage (6 mg/kg) significantly increased densities of c-Fos-reactive nuclei relative to effects of a combination of 6 mg/kg rimonabant with 3 mg/kg WIN55212-2 (indicated by a double dagger in Fig 5 B, ‡p < 0.05). The potential significance of this is discussed below.
Following the lower WIN55212-2 dosage (0.3 mg/kg), significant reductions of c-Fos reactive cells were observed within both HVC (to 0.21 +/− 0.04 of VEH) and RA (to 0.10 +/− 0.02 of VEH). Pretreatment with 1 mg/kg rimonabant prevented these reductions in both brain regions, resulting in 2.19 +/− 0.34 and 5.31 +/−1.54 of VEH in HVC and RA respectively (†p < 0.05 in each case, Fig 5 C and D).
Although within RA rimonabant administered alone tended to increase densities of c-fos-reactive nuclei relative to vehicle controls, differences were not significant (within either HVC or RA at low or high agonist dosages, p > 0.05 in each case). In HVC of animals treated with both 0.3 mg/kg and 3 mg/kg WIN55212-2, significant differences between combined rimonabant and WIN55212-2 and rimonabant alone were not observed.
Double immunohistochemical labeling with anti-c-Fos and anti-zebra finch CB1 receptor antibodies
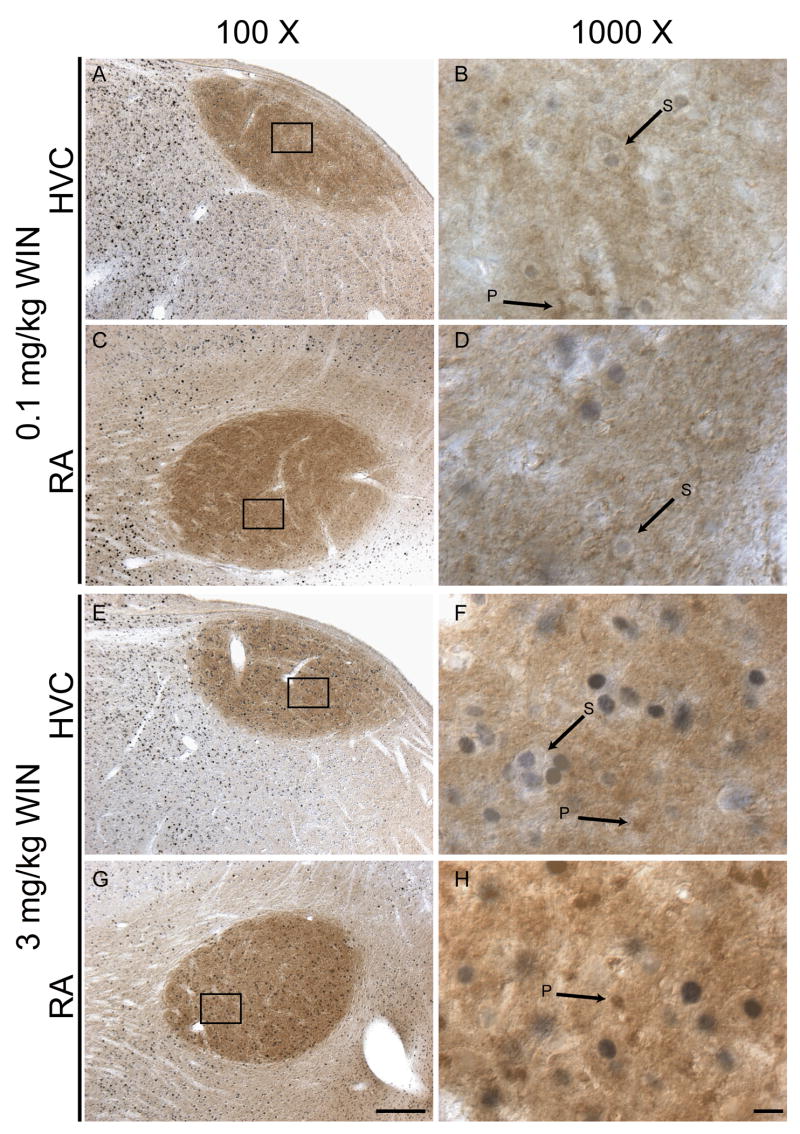
To evaluate the relative distribution of CB1 receptors and c-Fos-expressing cells, a series of double-labeling immunohistochemistry experiments were completed. As found previously (Soderstrom and Tian 2006; Soderstrom et al. 2004) CB1 receptors are expressed densely in neuropil and within distinct puncta of both HVC and RA song regions of zebra finch telencephalon. This pattern of CB1 receptor expression did not appear to change as a function of acute administration of various WIN55212-2 dosages. The distinctly-labeled puncta are irregularly shaped and smaller than c-Fos-labeled nuclei, and occasionally appear to surround the cell bodies of c-Fos expressing cells but do not otherwise appear to colocalize with nuclear c-Fos expression or within cell bodies surrounding c-Fos-labeled nuclei (see Fig 6).
Figure 6.
Double-immunohistochemcial labeling with c-Fos and CB1 cannabinoid receptor antibodies. c-Fos-labeled nuclei are stained blue-grey, CB1 receptor staining is rust-brown. The pattern of anti-CB1 staining within HVC and RA consists of diffuse neuropil staining with distinct small and irregularly shaped puncta (indicated with arrows labeled ‘P’). c-Fos-labeled nuclei surrounded by unstained cytoplasm are indicated with arrows labeled ‘S’). Dorsal is top, rostral left. 100 X bars = 200 microns, 1000 X bars = 10 microns.
Discussion
The ability of cannabinoid agonists to stimulate immediately early gene expression has been established since the mid-1990s (Mailleux et al. 1994). Prior studies of cannabinoid stimulation of c-Fos expression within a subset of rat brain regions have provided important insight into the role of cannabinoid signaling in the mammalian brain: For example, discovery of increased activity within nucleus accumbens is consistent with rewarding properties of these compounds, increased activity within caudate putamen is consistent with locomotor effects, and activity within the paraventricular nucleus of the hypothalamus is consistent with known effects on the hypothalamic-pituitary-adrenal axis and involvement in stress responses (McGregor et al. 1998; Patel et al. 1998; Patel and Hillard 2003).
Although there has been some indication of the ability of low cannabinoid agonist dosages to inhibit neuronal activity in mammalian systems (c.f. Fig 3, Patel and Hillard 2003), clear effects have not been previously reported. Prior lack of appreciation of this phenomenon may be due to differences in basal neuronal activity between our avian species and the rodents previously studied, or perhaps more likely, to differences in anatomy of the brain regions studied. Rather than the laminar arrangement of groups of neurons characteristic of mammalian forebrain, the avian telencephalon is organized in a nuclear manner (e.g. Fig 1 and Reiner 2005). The resulting discrete aggregations of functionally-related neurons are particularly well-suited to spatial analysis, effectively increasing the signal in our studies and allowing precise measurement of c-Fos expression levels.
Our goal was to characterize changes in neural activity within CB1-expressing telencephalic song regions after systemic cannabinoid agonist exposure. Our hypothesis was that changes in activity within all four cannabinoid-receptor-expressing regions studied; lMAN, Area X, HVC and RA, would be observed following cannabinoid treatments. Therefore the finding of altered activity only within the caudal regions, HVC and RA, was unexpected.
The function of the rostral regions, lMAN and Area X, are critical for successful zebra finch vocal development. Lesions of either of these areas prior to completion of song learning results in impaired vocal development, while adult ablation of these regions in does not alter already-learned song (Bottjer et al. 1984). This raises the possibility that cannabinoid signaling systems known to be present within these rostral song regions serve a learning-related function that is completed prior to maturation, and accompanied by decreased activity. This hypothesis is supported by a distinct increase in CB1-receptor densities and changes in expression patterns within these regions during vocal learning that wanes in adulthood (Soderstrom and Tian 2006). This also suggests that altered vocal learning produced by exogenous cannabinoid exposure during late-postnatal development (Soderstrom and Johnson 2003; Soderstrom and Tian 2004) may be attributable to a premature reduction in cannabinoid-sensitive activity within rostral song regions, a possibility that merits further study.
The caudal telencephalic song regions, HVC and RA are critical for vocal motor output of adult song (Nottebohm et al. 1976). The ability of systemic WIN55212-2 to alter activity within these motor regions is consistent with results of behavioral experiments demonstrating cannabinoid agonist inhibition of adult song production (Soderstrom and Johnson 2001) and the well-established effects of cannabinoid agonists to reduce locomotor activity in other vertebrate species (including amphibians, e.g. Soderstrom et al. 2000 and reviewed by Chaperon and Thiebot 1999). These results also suggest that low cannabinoid dosages may produce behavioral effects that oppose those of higher dosages. In the case of song production, low doses tend to increase output, while higher dosages inhibit it (see Fig 2A, Soderstrom and Johnson 2001).
Distinct dose-dependent effects of WIN55212-2 are particularly interesting, and suggest presence of multiple cannabinoid-sensitive systems within the vocal motor regions HVC and RA. In mammalian species, evidence supports a presynaptic modulatory role for CB1 signaling (Elphick and Egertova 2001) that involves a reduced probability of neurotransmitter release following inhibition of calcium- and activation of potassium-channels (Mackie and Hille 1992; Mackie et al. 1995). Through this mechanism, accumulating evidence suggests that cannabinoid signaling is an essential component of “depolarization-induced suppression of inhibition” or DSI (Wilson and Nicoll 2002). DSI is a retrograde process wherein postsynaptic activity promotes presynaptic inhibition of transmitter release. From this it seems likely that the altered neuronal activity measured in our avian system may be attributable to reduced transmitter release within the rostral vocal motor song regions, HVC and RA. From this it follows that low dosage effects, characterized by reduced neuronal activity (0.3 mg/kg WIN55212-2, see Fig 3 C and D) are likely attributable to reduced excitatory neurotransmitter release, while higher dosage effects (3 mg/kg WIN55212-2) follow reduced inhibitory input. This hypothesis suggests that: (1) vocal motor song regions contain both excitatory and inhibitory input; (2) the excitatory input is more sensitive to cannabinoid agonists than the inhibitory and; (3) an inhibitory tone predominates within vocal motor regions of zebra finch telencephalon. Pending detailed studies of the neuroanatomy of HVC and RA, similar to the one recently completed for the striatal region Area X (Reiner et al. 2004) it is difficult to predict which neurotransmitter systems are likely involved in the biphasic cannabinoid effects observed.
The studies done with the antagonist rimonabant were essential to demonstrate involvement of CB1 receptors in the agonist effects we measured. WIN55212-2 is an effective agonist of both CB1 and CB2 receptor subtypes, while rimonabant (referred to prior to clinical development as SR141716A) is CB1 selective (Pertwee 1997). In the case of WIN55212-2-altered activity within HVC and RA, both the inhibitory low-dose and stimulatory higher dose effects were reversed by rimonabant and therefore are both attributable to CB1 receptor activation (Fig 5). Although 6 mg/kg rimonabant was effective in reversing effects of the agonist administered alone (Fig 5 A and B), within RA delivery of 6 mg/kg rimonabant alone resulted in significantly higher levels of c-Fos-reactive cells than did a combination of 6 mg/kg rimonabant with 3 mg/kg WIN55212-2. This effect may be attributable to incomplete displacement of agonist-receptor complexes within RA, resulting in an effectively reduced agonist dosage to levels associated with inhibitory effects on activity (similar to those produced by 0.3 mg/kg WIN55212-2 alone, see Fig 5 D). Rimonabant is a problematic antagonist. In some systems (and in most behavioral systems) it appears to function as a true CB1-selective antagonist (reviewed by Fowler 2007). In other systems, particularly those in vitro, it clearly has the ability to function as an inverse agonist, possibly through promoting functional coupling to Gs (Glass and Felder 1997). Therefore we cannot be sure if effects measured following administration of rimonabant alone (those presented in Fig 5B) are attributable to inverse agonism, or to antagonism of endocannabinoid tone within RA.
Double labeling experiments allowed us to assess the relative patterns of expression of CB1 receptors and c-Fos-expressing nuclei within the caudal telencephalic song regions HVC and RA (see Fig 6). Within these regions, the distinct expression of CB1 within neuropil suggests a generalized expression throughout neuronal processes that surround the cell bodies of c-Fos-expressing neurons. Densely stained puncta, some of which also surround c-Fos-expressing cell bodies, are consistent in size and appearance with pre-synaptic densities, such as those previously described to express aromatase in zebra finch telencephalon (Peterson et al. 2005). This dual pattern of CB1 receptor expression may provide additional insight into the distinct efficacies of low- and high- cannabinoid agonist dosages described above. For example, signaling coupled to the dense CB1-expressing puncta is likely to be more sensitive to receptor activation than that within the more diffusely-expressing neuropil. Because CB1 signaling is most clearly associated with presynaptic inhibition of synaptic release, (Elphick and Egertova 2001) our hypothesis suggests that at low agonist dosages, CB1 expression within puncta likely functions to reduce excitatory input to c-Fos expressing neurons, decreasing their activity from basal levels. As agonist dosages increase, effective activation of the lower density, but more wide-spread population of neuropil receptors may become more significant. Because higher agonist dosages are associated with increased neural activity (as indicated by increased c-Fos expression) this suggests that neuropil expression may function to mitigate inhibitory neural input. These interesting possibilities will be the subject of further experimentation.
Overall, results reported herein demonstrate for the first time dose-dependent effects of cannabinoid receptor activation to both inhibit and stimulate neuronal activity within a subset of CB1 receptor-expressing brain regions. This knowledge will be helpful for interpretation of the complex neuromodulary effects of cannabinoids in other systems, and improves our understanding of the nature and function of cannabinoid signaling within the vertebrate brain.
Acknowledgments
We are grateful to Bin Luo who managed the breeding aviary and assisted in these experiments.
This work was supported by NIDA grants R01DA020109 and R21DA14693
List of non-standard abbreviations
- lMAN
lateral magnocellular nucleus of the anterior nidopallium
- Area X
Area X within songbird medial striatum
- RA
robust nucleus of the arcopallium
- Uva
nucleus uvaformis
- DLM
dorsal lateral nucleus of the medial thalamus
- HVC
is used as a proper name (per Reiner et al, 2004) to indicate a prominent vocal motor nucleus of zebra finch telencephalon
References
- Reiner A. Organization and evolution of the avian forebrain. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2005;287A:1080–1102. doi: 10.1002/ar.a.20253. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Hetebrij E, Den Boer-Visser AM, De Groot JH, Zijlstra GGO. Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. European Journal of Neuroscience. 2001;13:2165–2170. doi: 10.1046/j.0953-816x.2001.01588.x. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Thiebot MH. Behavioral effects of cannabinoid agents in animals. Critical Reviews In Neurobiology. 1999;13:243–281. doi: 10.1615/critrevneurobiol.v13.i3.20. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408\. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. The pharmacology of the cannabinoid system--a question of efficacy and selectivity. Mol Neurobiol. 2007;36:15–25. doi: 10.1007/s12035-007-0001-6. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Verslype M, Preud’homme X, Vanderhaeghen JJ. Activation of multiple transcription factor genes by tetrahydrocannabinol in rat forebrain. Neuroreport. 1994;5:1265–1268. doi: 10.1097/00001756-199406020-00028. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Arnold JC, Weber MF, Topple AN, Hunt GE. A comparison of delta 9-THC and anandamide induced c-fos expression in the rat forebrain. Brain Res. 1998;802:19–26. doi: 10.1016/s0006-8993(98)00549-6. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Patel NA, Moldow RL, Patel JA, Wu G, Chang SL. Arachidonylethanolamide (AEA) activation of FOS proto-oncogene protein immunoreactivity in the rat brain. Brain Res. 1998;797:225–233. doi: 10.1016/s0006-8993(98)00364-3. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Cannabinoid-induced Fos expression within A10 dopaminergic neurons. Brain Research. 2003;963:15. doi: 10.1016/s0006-8993(02)03797-6. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272:2089–96. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Laverghetta AV, Meade CA, Cuthbertson SL, Bottjer SW. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J Comp Neurol. 2004;469:239–61. doi: 10.1002/cne.11012. [DOI] [PubMed] [Google Scholar]
- Reiner APDJ, Bruce L, Butler AB, Csillag A, Kunzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised Nomenclature for Avian Telencephalon and Some Related Brainstem Nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. The Zebra Finch CB1 Cannabinoid Receptor: Pharmacology and In Vivo and In Vitro Effects of Activation. J Pharmacol Exp Ther. 2001;297:189–197. [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. Cannabinoid exposure alters learning of zebra finch vocal patterns. Brain Res Dev Brain Res. 2003;142:215–7. doi: 10.1016/s0165-3806(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Leid M, Moore FL, Murray TF. Behavioral, Pharmacological and Molecular Characterization of an Amphibian Cannabinoid Receptor. Journal of Neurochemistry. 2000;75:413–423. doi: 10.1046/j.1471-4159.2000.0750413.x. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Qin W, Leggett MH. A minimally invasive procedure for sexing young zebra finches. J Neurosci Methods. 2007 doi: 10.1016/j.jneumeth.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Distinct Periods of Cannabinoid Sensitivity During Zebra Finch Vocal Development. Developmental Brain Research. 2004;153:225–232. doi: 10.1016/j.devbrainres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Developmental Pattern of CB1 Cannabinoid Receptor Immunoreactivity in Brain Regions Important to Zebra Finch (Taeniopygia guttata) Song Learning and Control. J Comp Neurol. 2006;496:739–758. doi: 10.1002/cne.20963. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q, Valenti M, Di Marzo V. Endocannabinoids link feeding state and auditory perception-related gene expression. J Neurosci. 2004;24:10013–21. doi: 10.1523/JNEUROSCI.3298-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000;24:115–23. [PMC free article] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. CB1 cannabinoid receptor activation inhibits a neural correlate of song recognition in an auditory/perceptual region of the zebra finch telencephalon. J Neurobiol. 2003;56:266–74. doi: 10.1002/neu.10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid Signaling in the Brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]