Abstract
TDP-43 is found in ubiquitinated inclusions (UBIs) in some frontotemporal dementias (FTD-U). One form of FTD-U, due to mutations in VCP, occurs with an inclusion body myopathy (IBMPFD). Since IBMPFD brain has TDP-43 in UBIs, we looked for TDP-43 inclusions in IBMPFD muscle. In normal muscle TDP-43 is present in nuclei. In IBMPFD muscle TDP-43 is additionally present as large inclusions within UBIs in muscle cytoplasm. TDP-43 inclusions were also found in 78% of sIBM muscles. In IBMPFD and sIBM muscle TDP-43 migrated with an additional band on immunoblot similar to that reported in FTD-U brains. This study adds sIBM and hereditary inclusion body myopathies to the growing list of TDP-43 positive inclusion diseases.
Keywords: TDP-43, inclusion body myositis, ubiquitin, frontotemporal dementia
Introduction
TAR DNA binding protein-43 (TDP-43) is one component of the ubiquitinated inclusions in brains of patients with FTD-U and ALS 1. Moreover, a phosphorylated form of TDP-43 was identified to be more prevalent specifically in FTD-U tissue as well 1. Little is known about the function of TDP-43. It is a ubiquitously expressed, highly conserved nuclear protein that may be a transcription repressor or activator of exon skipping as well as a scaffold for nuclear bodies through interactions with survival motor neuron protein 1. The significance of TDP-43 accumulation and phosphorylation is currently unclear but may relate to ubiquitin-proteasome system (UPS) dysfunction. One rare form of FTD-U, inclusion body myopathy, Paget’s disease of the bone and frontotemporal dementia (IBMPFD) has ubiquitinated and TDP-43 positive inclusions in affected neurons 2, 3. While IBMPFD muscle contains rimmed vacuoles and UBIs consistent with an inclusion body myopathy (IBM) 4.
The pathogenesis of IBM and the more common sporadic inclusion body myositis (sIBM) is unknown but may also be due to UPS dysfunction 5. Affected muscle has UBIs that contain proteins, such as β-amyloid and phosphorylated tau, known to aggregate in CNS degenerative disorders 6, 7. This has led to the suggestion that sIBM is related pathophysiologically to neurodegenerative diseases. It is not known whether TDP-43 is a component of the inclusions in IBMPFD and sIBM muscle tissue. We evaluated the localization of TDP-43 in normal, IBMPFD and sIBM skeletal muscle tissue.
Methods
Patients with sIBM had typical patterns of muscle weakness on physical examination, an abnormal EMG with myopathic motor units and spontaneous activity, and a muscle biopsy with myopathic changes, rimmed vacuoles within muscle fibers and endomysial inflammation with focal invasion of muscle fibers 8. Five patients with IBMPFD and missense mutations in the p97/VCP gene (four with R155H and one with N387H) 9 were participants in the IRB approved study. All muscle biopsies were processed and evaluated in the Washington University Neuromuscular Laboratory. Cryostat sections of rapidly frozen muscle were processed for muscle histochemistry and immunocytochemistry in our standard fashion 4, 9. The presence of vacuoles was evaluated via routine histochemical methods such as hematoxylin and eosin or modified gomoritrichrome stains. Immunocytochemistry for each antibody was performed on tissue from patients and compared to normal tissue controls processed simultaneously. Primary antibodies used in this study were directed against CD8 (clone M7103), phosphorylated neurofilaments or other phosphorylated epitopes (SMI-31 Covance, Berkley, CA), FK2 antibody to ubiquitinated proteins (PW8810-0500 BioMol, Plymouth Meeting, PA), TDP-43 antibodies: rabbit polyclonal antibody (ProteinTech Antibody Group, Chicago, IL) and mouse monoclonal antibody 2E2-D3 (Abnova, Taipei, Taiwan). Double-labeling immunofluorescence was performed as previously described using Alexa Fluor 488 and 594 conjugated secondary antibodies (Molecular Probes, Eugene, OR). Immunoperoxidase was performed as previously described using peroxidase conjugated secondary antibodies (Sigma; St. Louis, MO, USA). The specificity of TDP-43 immunostaining was confirmed with two different commercial antibodies and incubation with secondary antibody alone (either fluorescent or peroxidase conjugated). Immunoblots were performed as previously described 10.
Results
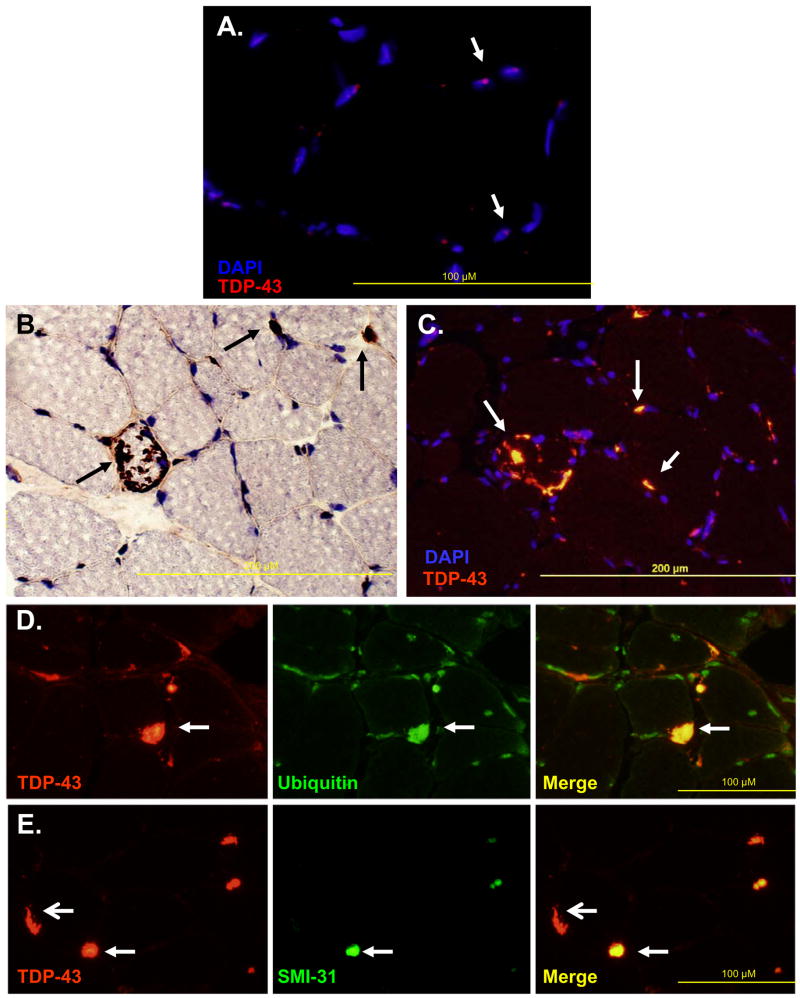
In 4 normal muscle biopsies TDP-43 was localized within scattered myonuclei with no evidence of sarcoplasmic staining (Figure 1A). In contrast, all IBMPFD patients muscle tissue had large peripherally based TDP-43 positive sarcoplasmic inclusions that did not localize to myonuclei (Figure 1B–C). These inclusions consistently co-localized with FK2, an antibody that recognizes ubiquitinated proteins (Figure 1D) and in some cases with other proteins known to aggregate. SMI-31 binding was less prominent than TDP-43 in IBMPFD patient muscle tissue (Figure 1E).
Figure 1.
A) Normal muscle immunostained with anti-TDP-43 antibody and counterstained with DAPI to allow visualization of nuclei. Figure is overlay of anti-TDP-43 and DAPI images. Arrows denote blue nuclei with TDP-43 (red dots) in scattered myonuclei. B) IBMPFD patient tissue immunostained with anti-TDP-43 (brown) and counterstained with Congo red to allow visualization of nuclei and myofibers. Arrows denote large inclusions, some of which are peripherally based. C) Overlay of anti-TDP-43 (orange) and DAPI (blue) of IBMPFD patient tissue. Note that large peripheral inclusions do not localize within nuclei (arrows). D) IBMPFD patient tissue co-immunostained with anti-TDP-43 (red) and FK2 (green). Note that TDP-43 inclusions co-localize with FK2 (ubiquitinated proteins) (arrows). E) IBMPFD patient tissue co-immunostained with anti-TDP-43 (red) and SMI-31, an antibody against phosphorylated tau epitopes (green). Note that some TDP-43 inclusions co-localize with SMI31 (closed arrows) and others do not (open arrows).
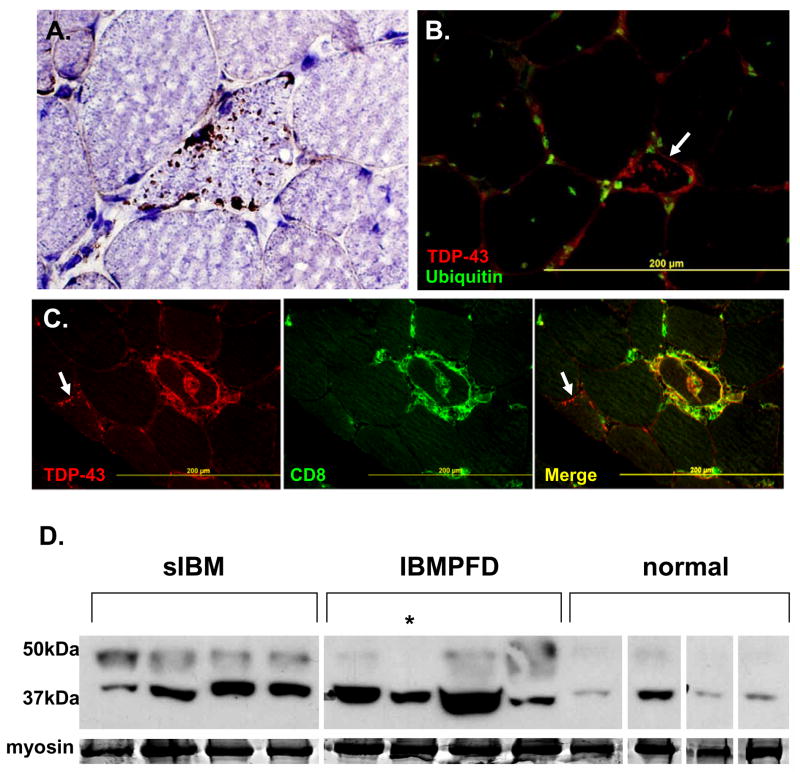
A distinctively different pattern of TDP-43 immunostaining was seen in 21 of 27 sIBM patients muscle. TDP-43 immunostained multiple small sarcoplasmic aggregates, most commonly in small angular muscle fibers (Figure 2A). These inclusions did not co-localize with myonuclei. TDP-43 was also present in debris surrounding some rimmed vacuoles (Figure 2A). The TDP-43 inclusions in sIBM were usually ubiquitin negative (Figure 2B), but occasionally co-localized with FK2. TDP-43 also co-localized with T-cells at sites of inflammatory infiltrates (Figure 2C). In contrast to sIBM, TDP-43 positive inclusions were found in only 1 of 12 steroid responsive polymyositis patient biopsies.
Figure 2.
A) sIBM patient tissue immunostained with anti-TDP-43 (brown) and counterstained with Congo red to allow visualization of nuclei and myofibers. TDP-43 inclusions are small, in angular fibers and occasionally surround rimmed vacuoles. B) Overlay of sIBM patient biopsy immunostained with TDP-43 (red) and FK2, for ubiquitinated proteins (green). TDP-43 inclusions do not co-localize with ubiquitin. C) sIBM patient tissue co-immunostained with anti-TDP-43 (red) and anti-CD8 (green at focal sites of inflammation). Note the co-localization of TDP-43 and CD8. This is in contrast to an adjacent fiber (arrow) with sarcoplasmic TDP-43 inclusions that does not co-localize with CD8. D) Muscle tissue from sIBM, IBMPFD and normal patient biopsies were homogenized and separated by SDS-PAGE. The subsequent gel was transferred to nitrocellulose and immunoblotted with an antibody to TDP-43. Normal tissue has a discrete band at 43kDa consistent with TDP-43, while sIBM and IBMPFD have a more prominent band and also a higher migrating band. Myosin is show as a loading control. All tissues are from flash frozen biopsies except the lane noted with an * which is from autopsy muscle. All bands are from the same autoradiograph and moved for presentation purposes.
Immunoblots of normal, IBMPFD and sIBM patient tissue with an antibody to TDP-43 demonstrated an increase in TDP-43 immunoreactivity present at 43kDa as well as a higher migrating band similar to the phosphorylated form seen in FTD-U patient tissue (Figure 2D).
Discussion
Disruptions in the UPS may be associated with the pathogenesis of several degenerative disorders. In particular, IBM muscle and FTD-U brain have UBIs that contain aggregated proteins. However, the principal molecular constituents of the UBIs seen in these diseases have been incompletely defined. Recent studies have identified TDP-43 as a component of the UBIs in FTD-U, including IBMPFD, and ALS brain tissue 1, 3. Since IBMPFD muscle also has UBIs, we examined the localization of TDP-43 in normal, sIBM and IBMPFD patient skeletal muscle. We found that TDP-43 localized to myonuclei in normal muscle, but, in IBMPFD and sIBM patient muscle, TDP-43 was additionally present as sarcoplasmic inclusions. This is associated with an increase in TDP-43 protein levels as well as a higher molecular weight band seen in sIBM and IBMPFD patient muscle tissue via immunoblot when compared with normal patient tissue. A similar higher molecular weight band was identified as phosphorylated TDP-43 in patients with FTD-U and ALS 1. Whether the band seen in IBMPFD and sIBM muscle tissue is the same or another post-translationally modified form (i.e. ubiquitinated) is not known.
TDP-43 inclusions were present in 100% of IBMPFD and 78% of sIBM patient muscle biopsies, while 0% of normal muscle and 8% of steroid responsive polymyositis patient muscle biopsies had similar TDP-43 inclusions. This suggests that TDP-43 immunohistochemistry may be helpful in confirming the diagnosis of sIBM. At present, the most reliable antibody marker for the diagnosis of sIBM is SMI-31 (an antibody against phosphorylated tau epitopes) which is not present in all sIBM biopsies and found in other diseased muscle tissue 11. For example in our hands SMI-31-positive aggregates are present in 66% of sIBM patients compared with 17% of steroid responsive polymyositis patients (unpublished observations).
It is notable that the TDP-43 immunostaining pattern was different when comparing sIBM and IBMPFD muscle. sIBM had small punctuate TDP-43 positive inclusions throughout the sarcoplasm that occasionally co-localized with ubiquitin. IBMPFD muscle had large peripherally based TDP-43 positive inclusions that always co-localized with ubiquitin. Both sIBM and IBMPFD patient muscle had evidence of a higher molecular weight TDP-43 species on immunoblot.
It is unknown if TDP-43 accumulation in FTD-U, ALS or IBM is pathogenic or merely a pathologic feature of the diseased tissue 12. Our findings in skeletal muscle are similar to those found in CNS disease 1. TDP-43 inclusions in FTD-U and ALS are cytoplasmic, similar to the predominantly sarcoplasmic inclusions in IBM muscle. The presence of ubiquitinated TDP-43 in disparate tissues (i.e. IBMPFD muscle and brain) in which the only commonality is mutant VCP, suggests that VCP dysfunction may play a role in TDP-43 aggregation.
TDP-43 contains RNA binding motifs that may be associated with its function. Interestingly, another hereditary IBM, oculopharyngeal muscular dystrophy, is due to mutations in another RNA binding protein PABPN1 13. Mutant PABPN1 forms intranuclear inclusions that contain ubiquitin and polyadenylated mRNA 14.It is not known whether a similar process occurs with TDP-43 in sIBM and IBMPFD.
Diseases that develop TDP-43 inclusions include FTD-U, ALS, IBMPFD and sIBM 1, 3 suggesting that similar pathogenic mechanisms may be present. Further studies will be needed to further define the role of these proteins and their dysregulation in central nervous system and skeletal muscle tissue.
Footnotes
Statement: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article to be published in Journal of Neurology, Neurosurgery & Psychiatry editions and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence http://jnnp.bmjjournals.com/ifora/licence.pdf.
References
- 1.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 2.Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, et al. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65(6):571–81. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, et al. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66(2):152–7. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- 4.Hubbers CU, Clemen CS, Kesper K, Boddrich A, Hofmann A, Kamarainen O, et al. Pathological consequences of VCP mutations on human striated muscle. Brain. 2006 doi: 10.1093/brain/awl238. [DOI] [PubMed] [Google Scholar]
- 5.Askanas V, Engel WK. Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition. Neurology. 2006;66(2 Suppl 1):S39–48. doi: 10.1212/01.wnl.0000192128.13875.1e. [DOI] [PubMed] [Google Scholar]
- 6.Askanas V, Alvarez RB, Engel WK. beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol. 1993;34(4):551–60. doi: 10.1002/ana.410340408. [DOI] [PubMed] [Google Scholar]
- 7.Askanas V, Engel WK, Bilak M, Alvarez RB, Selkoe DJ. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol. 1994;144(1):177–87. [PMC free article] [PubMed] [Google Scholar]
- 8.Dalakas MC. Sporadic inclusion body myositis--diagnosis, pathogenesis and therapeutic strategies. Nat Clin Pract Neurol. 2006;2(8):437–47. doi: 10.1038/ncpneuro0261. [DOI] [PubMed] [Google Scholar]
- 9.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36(4):377–81. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 10.Weihl CC, Dalal S, Pestronk A, Hanson PI. Inclusion body myopathy-associated mutations in p97/VCP impair endoplasmic reticulum-associated degradation. Hum Mol Genet. 2006;15(2):189–99. doi: 10.1093/hmg/ddi426. [DOI] [PubMed] [Google Scholar]
- 11.van der Meulen MF, Hoogendijk JE, Moons KG, Veldman H, Badrising UA, Wokke JH. Rimmed vacuoles and the added value of SMI-31 staining in diagnosing sporadic inclusion body myositis. Neuromuscul Disord. 2001;11(5):447–51. doi: 10.1016/s0960-8966(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 12.Rothstein JD. TDP-43 in amyotrophic lateral sclerosis: pathophysiology or patho-babel? Ann Neurol. 2007;61(5):382–4. doi: 10.1002/ana.21155. [DOI] [PubMed] [Google Scholar]
- 13.Brais B, Bouchard JP, Xie YG, Rochefort DL, Chretien N, Tome FM, et al. Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet. 1998;18(2):164–7. doi: 10.1038/ng0298-164. [DOI] [PubMed] [Google Scholar]
- 14.Calado A, Tome FM, Brais B, Rouleau GA, Kuhn U, Wahle E, et al. Nuclear inclusions in oculopharyngeal muscular dystrophy consist of poly(A) binding protein 2 aggregates which sequester poly(A) RNA. Hum Mol Genet. 2000;9(15):2321–8. doi: 10.1093/oxfordjournals.hmg.a018924. [DOI] [PubMed] [Google Scholar]