Abstract
Overexpression of the immunosuppressive cytokine TGF-β is one strategy that tumors have evolved to evade effective immunesurveillance. Using transplantable models of breast and colon cancer, we made the unexpected finding that CD8+ cells in tumor-bearing animals can directly promote tumorigenesis, by a mechanism that is dependent on TGF-β. We showed that CD8+ splenocytes from tumor-bearing mice expressed elevated IL-17 when compared to naive mice, and that CD8+ T-cells could be induced to make IL-17 on addition of TGF-β and IL-6 in vitro. Treatment of mice with anti-TGF-β antibodies in vivo reduced IL-17 expression both in the tumor and the locoregional lymph nodes. Although IL-17 has not previously been shown to act as a survival factor for epithelial cells, we found that IL-17 suppressed apoptosis of several tumor cell lines in vitro, suggesting that this altered T-cell polarization has the potential to promote tumorigenesis directly, rather than indirectly through inflammatory sequelae. Consistent with this hypothesis, knockdown of the IL-17 receptor in 4T1 mouse mammary cancer cells enhanced apoptosis and decreased tumor growth in vivo. Thus, in addition to suppressing immune surveillance, tumor-induced TGF-β may actively subvert the CD8+ arm of the immune system into directly promoting tumor growth by an IL-17 dependent mechanism.
Keywords: IL-17, TGF-β, immune surveillance, cancer, mouse models
Introduction
The immune surveillance system represents an important barrier to tumor development, as mice that are immunocompromised by genetic or functional ablation frequently show enhanced spontaneous tumorigenesis (1;2). However, if the incipient tumor is not totally eliminated, the ongoing selection pressure exerted on the tumor by the immune system drives the outgrowth of tumor variants that have evolved strategies for immune evasion (1;3). Such strategies include down-regulation of tumor cell surface molecules involved in immune recognition, and secretion of immunosuppressive cytokines, such as IL-10 and TGF-β (4).
TGF-βs are evolutionarily ancient and highly pleiotropic cytokines that play key regulatory roles in many aspects of immune system function (5). Not only can TGF-βs directly inhibit cytolytic activity of NK cells, macrophages and CD8+ cytotoxic T-cells (6-8), but they also inhibit instruction, activation and expansion of tumor-specific helper and cytotoxic T-cell populations (9;10), and they enhance the generation of immunosuppressive regulatory T-cells (Tregs) (11;12). Thus the presence of TGF-β in the microenvironment of the developing tumor is predicted to disable effective immune surveillance by multiple mechanisms, most of which converge on the impairment of tumor cell killing by immune effector cells. Indeed, many human tumors overexpress TGF-β, and elevated expression frequently correlates with tumor progression and poor prognosis (13). In addition, immunoregulatory cells, such as Tregs and NKT cells may either make or induce production of TGF-β (14;15). Furthermore, there is a growing body of data suggesting that strategies to antagonize TGF-β can suppress tumorigenesis by enhancing or restoring effective anti-tumor immune surveillance (15-21), thus underscoring the potential relevance of this mechanism to carcinogenic progression.
Recently TGF-β was shown to be critical for the development of a newly discovered lineage of CD4+ T cells, the pro-inflammatory Th17 cells, which have been implicated in defence against extracellular bacteria, and in the pathogenesis of several autoimmune diseases (22). Th17 cells secrete high levels of IL-17, which interacts with the ubiquitously expressed IL-17 receptor to induce the secretion of chemokines and cytokines that mobilize neutrophils for pathogen clearance (22). There are conflicting data on a possible role for IL-17 in carcinogenesis. Forced overexpression of IL-17 ectopically in tumor cells can either suppress tumor progression through enhanced anti-tumor immunity (23;24), or promote it through an increase in inflammatory angiogenesis (25;26). However, the role of endogenous IL-17 in tumorigenesis has not been assessed.
While probing the mechanism of action of a TGF-β monoclonal antibody in suppressing experimental tumorigenesis, we have uncovered evidence to suggest that TGF-β in the tumor environment can actively subvert the CD8+ arm of the immune system into making IL-17, which then promotes tumor growth through direct prosurvival effects on the tumor cell. This novel mechanism contributes to the ability of the tumor to sculpt a microenvironment that actively promotes tumor progression, and provides further rationale for the development of strategies to antagonize TGF-β therapeutically.
Materials and Methods
Cell culture and reagents
The 4T1 mouse mammary carcinoma cell was provided by Dr. Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, MI). The CT26 mouse colon carcinoma cell line was provided by Dr. N. Restifo (NCI, NIH, Bethesda, MD, USA). The EMT6 mouse mammary carcinoma cell line was provided by Dr. Sara Rockwell (Yale University, New Haven, CT) and the Hs578t, MDA MB435 and MDA MB231 human breast carcinoma cell lines were obtained from American Type Culture Collection (Manassas, VA). TGF-β1, mouse IL-17, human IL-17, mouse IL-23, mIL-17 blocking antibody and isotype-matched IgG antibody were purchased from R&D Systems (Minneapolis, MN). Staurosporine was purchased from Sigma-Aldrich. Anti-CD8 monoclonal antibody (clone 2.43) for in vivo depletion was obtained from Harlan Bioproducts for Science Inc., Indianaopolis, IN. The anti-TGF-β murine monoclonal antibody, 1D11, which neutralizes all three isoforms of TGF-β (27), and an isotype-matched IgG1 monoclonal antibody 13C4, which was raised against Shigella toxin and serves as a control, were provided by Genzyme Corp., Framingham, MA.
In vivo tumorigenicity and metastasis assay
All animals were maintained according to the National Cancer Institute's Animal Care and Use Committee guidelines, under approved animal study protocols. For 4T1 tumors, 4 x 104 4T1 cells were inoculated into the sugically exposed #2 mammary fat pad of 7-week-old female BALB/cANCr mice. Mice were then randomized into two treatment groups, with 10-15 animals/group. Anti-TGF-β antibody (1D11; 5 mg/kg body weight) or isotype control (13C4) were administered three times per week i.p., starting one day after cell inoculation. Primary tumors were excised on day 10 for analysis, except for time-course experiments where the short (s) and long (l) dimensions of the tumor in situ were measured 3x each week using calipers, and the tumor volume was calculated using the formula v=0.52 x s2 x l. For CT26 tumorigenesis, 5 x 105 cells were injected subcutaneously on the flank of BALB/cANCr mice, and tumors were removed at day 21. For metastasis studies, mice were injected with 4,500 4T1 cells/mouse into the tail vein, and on day 21 lungs were removed, inflated and fixed for analysis. The diameter of lung surface nodules was measured using the Digimatic Caliper (Mitutoyo Corp., Japan), and metastases were subsequently confirmed histologically. For CD8+ depletion, mice were injected i.p. with 0.25 mg of anti-CD8 antibody (clone 2.43) at -4, -3, -2, 3, 10, 17 and 24 days relative to the tumor implantation.
CD4+ and CD8+ T cell Isolation and FACS analysis
CD4+ and CD8+ cells were purified from BALB/c splenocytes using magnetic beads coated with anti-CD4 or anti-CD8 (Miltenyi Biotech). For the generation of T-cell conditioned medium, 2 x 105 cells were cultured in vitro in a 96 well plate in 200μl of X Vivo 20 medium (BioWhittaker, Inc), with or without stimulation with anti-CD3 and anti-CD28. Conditioned medium was harvested after 3 days. For FACS analysis, purified CD4+ or CD8+ splenocytes were stimulated for three days using plated anti-CD3 and anti-CD28 (5μg/mL) in media alone (Th0 conditions), or with TGF-β (5ng/mL), IL-6 (5-10ng/mL), anti-IFNγ (10μg/mL), and anti IL-4 (10μg/mL) (Th17 conditions). After three days cells were stimulated for 3-4 hours using PMA (10ng/ml) and ionomycin (250ng/ml) in the presence of GolgiPlug (BD Pharmingen). Cells were fixed and stained with antibodies overnight. Alexa-488 or APC-labeled anti-CD4+, APC-labeled anti-CD8+, PE-labeled anti-IL-17, and APC-labeled anti-IFN-g were obtained from BD Pharmingen. After washing, the cells were analyzed by FACScan TM or FACSCAliberTM, using CELLQuestTM (BD Biosciences) or FlowJo (Treestar) software. For determination of gene expression in cell populations of the tumor-draining lymph nodes, brachial and axillary lymph nodes from ≥5 mice/treatment group were pooled. CD8+ and CD4+ cells were sequentially purified by positive selection using first anti-CD8, and then anti-CD4-coated magnetic beads (Miltenyi Biotech). Following FACS confirmation of purity, cell populations were immediately used for RNA purification.
Stable transfection
Myc-tagged dominant negative type II TGF-β receptor (DNR) (28) was ligated into the pβ vector (29) and stably transfected into 4T1 cells. DNR activity was confirmed using the Smad3-responsive pCAGA12LUC construct and by immunoblotting for phospho-Smad2 were performed as described previously (30). Short-hairpin RNAs (shRNA) to mouse IL-17R ( #1, cat no: RMM1766-97044109 ; #2, RMM1766-96879844 ; #3, RMM1766-96879284) or the non-silencing control shRNA (RHS1703) were purchased from Open Biosystems (Huntsville, AL). To minimize the effect of clonal variation, we pooled populations of transfected clones.
Apoptosis assays
Apoptosis was assessed using the Cell Death Detection ELISA assay kit (Roche). Apoptosis was induced by serum deprivation for 48 hours (4T1, CT26, EMT6 cells), or by treatment with 0.1μM staurosporine (MDA-MB231, Hs578t, MDA MB435 cells) for 24 hours prior to assay. T-cell conditioned medium (1:4 final dilution), IL-17 (0.1 - 25ng/ml) or TGF-β (5ng/ml) were added 48 hours prior to assessment of apoptosis. Immunostaining of formalin-fixed samples for cleaved caspase-3 was performed using the SignalStain Cleaved Caspase-3 IHC detection kit (Cell Signaling Technology). Cleaved Caspase-3 staining in primary tumors was individually evaluated by quantitating the number of stained cells/high power field (200x magnification) for a cumulative total of 10 randomly selected fields/tumor.
Quantitative reverse-transcription polymerase chain reaction (RTQ-PCR)
Real-time quantitative PCR was performed using the iCycler iQ Real-time PCR Detection System (Bio-Rad) using SYBR green dye (Stratagene, Cedar Creek, TX). The primer sets used were: IL-17R, 5′-GCACCCAAGCAAAGTGGAAA-3′ (forward primer) and 5′-AAACAACGTAGGTGCCGAAGC-3′ (reverse primer); and 28S rRNA, 5′-GGGTGGTAAACTCCATCTAA-3′ (forward primer) and 5′-AGTTCTTTTCAACTTTCCCT -3′ (reverse primer). Primer sets for mouse and human IL-17, TGF-β type I receptor (TβRI), TGF- β type II receptor (TβRII) and cyclophilin A (PPIA) were purchased from Superarray (Frederick, MD). PPIA mRNA or 28SrRNA were used as normalization controls.
Cytokine array, immunoblotting, and ELISA assays
The RayBio Mouse Cytokine Array II (RayBiotech, Inc., Norcross, GA) was used to screen for cytokines present in pooled conditioned medium (CM) of CD8+ T cells from naïve mice or 4T1 tumor-bearing mice (n = 5 mice/group). Quantification of TGF-β1, IL-17 and IL-6 was performed by ELISA (Quantikine Immunology, R&D Systems). TGF-β in conditioned media was acid-activated prior to assay. Immunoblotting was performed as described previously (31). Antibodies were as follows: anti-Smad2 (Zymed Laboratories, South San Francisco, CA), anti-Phospho-Smad2, anti-Cleaved Caspase-3 and anti-Cleaved PARP (Cell Signaling Technology, Beverly, MA), anti-IL-17R (R&D Systems Inc) and anti-β-actin (Clone AC-15, Sigma-Aldrich).
Clinical Samples
RNA from matched human breast cancer and normal breast tissue were obtained from Oncomatrix Inc., (San Marcos, CA). Details of the individual specimens are given in Supplementary Table 1.
Statistical analysis
Unpaired parametric Student t test and non-parametric Mann-Whitney U tests were used to analyze the data, unless otherwise indicated in the text. All tests were two-tailed.
Results
CD8+ T-cells promote tumor growth by a TGF-β-dependent mechanism
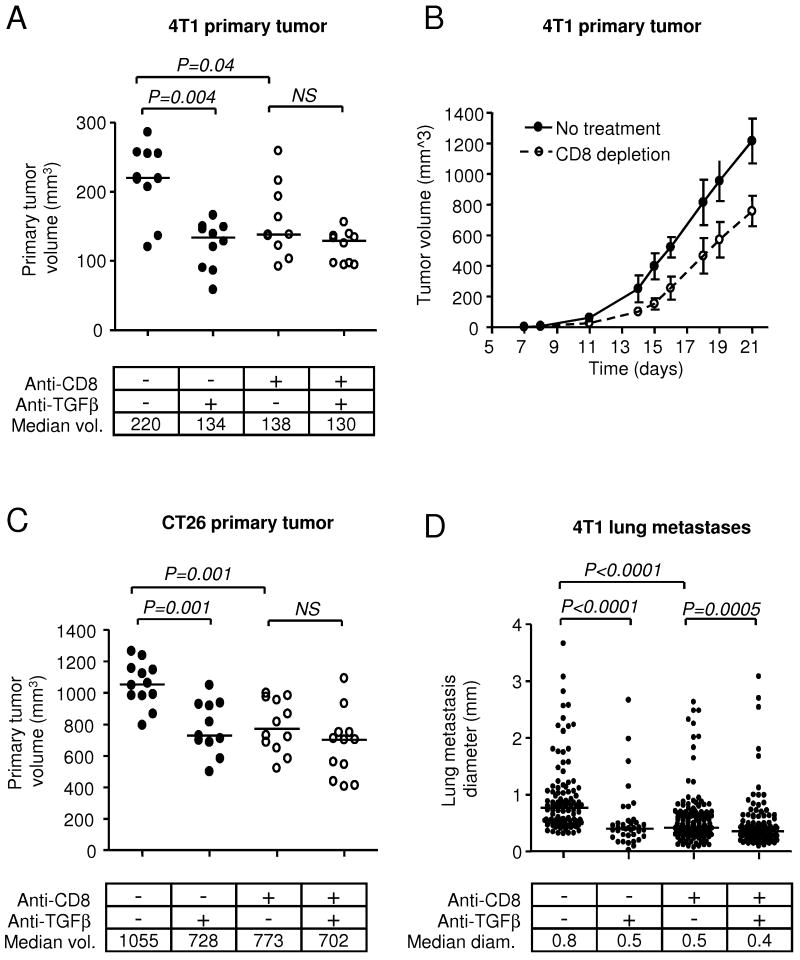
The 4T1 cell line, which is syngeneic to BALB/c mice, is a widely-used model of metastatic breast cancer (32). Treatment of mice bearing 4T1 mammary tumors with an anti-TGF-β monoclonal antibody caused a 40% reduction in tumor volume over 10 days, showing that neutralization of TGF-β can suppress tumor growth in this model (Fig. 1A, compare columns 1 and 2). Since TGF-β is known to impair functioning of the adaptive arm of the immune system (5), we tested whether the efficacy of the antibody was dependent on an intact immune system by selectively depleting the mice of CD8+ cells. Unexpectedly, we found that depletion of CD8+ T-cells alone caused a paradoxical reduction in tumor volume that was similar to that seen on treatment with anti-TGF-β antibody, and the combination of both interventions had no further effect (Fig. 1A). The inhibitory effect of depleting CD8+ T cells was proportionately greatest at earlier timepoints, (ratio of median tumor volume in CD8-depleted mice to that in intact mice: 0.42 at day 11; 0.62 at day 21), but persisted for at least 21 days (Fig. 1B). This effect of CD8-depletion or TGF-β antagonism on tumor growth was not limited to the 4T1 model, as we saw the same response in the CT26 colon carcinoma model (Fig. 1C). A similar effect was also seen on the size of 4T1 metastases that arise in the lung following injection of 4T1 cells into the tail vein (Fig. 1D), suggesting that growth of the developing tumor may be affected by the same mechanism at both the primary and metastatic sites. It is evident from Fig. 1D that TGF-β antagonism also reduces the number of lung metastases, but this effect involves a different mechanism and is addressed elsewhere (Nam et al. submitted as companion manuscript). Based on these observations, we hypothesized that CD8+ cells may actively promote growth of the 4T1 and CT26 tumors by a TGF-β-dependent mechanism, and we focused on the 4T1 model for further mechanistic analysis.
Figure 1. Effect of CD8+ cell depletion and TGF-β antagonism on primary tumorigenesis and metastasis.
A. Effect of CD8+ cell depletion and TGF-β antagonism on the size of 4T1 primary mammary tumors following orthotopic implantation of tumor cells into the mammary fat pad of syngeneic mice. Mice were treated with either anti-TGF-β antibody (1D11) or control 13C4 antibody (CON) starting one day after implantation. Select cohorts of mice were depleted of CD8+ cells by treatment with anti-CD8 antibody prior to injection of tumor cells. Lines indicate median values for the volume of excised tumors at the time of harvest (day 10 after implantation). B. Timecourse of effect of CD8+ cell depletion on the growth of 4T1 tumors. Tumor volume in situ was determined by caliper measurement. Results are means +/- S.D. for 18 tumors/treatment group. C. Effect of CD8+ cell depletion and TGF-β antagonism on the growth of CT26 colon carcinoma tumors implanted subcutaneously and then treated as in (A). D. The size of lung metastases following injection of 4T1 cells into the tail vein of syngeneic mice treated with therapeutic antibody and/or CD8+ depletion as in (A) was determined by assessing the diameter of lung surface nodules at day 21. The median diameter is indicated (n = 10 mice for each group; all metastases are represented).
Activated CD8+ T-cells from tumor-bearing animals secrete IL-17 which synergizes with TGF-β to promote survival of 4T1 cells
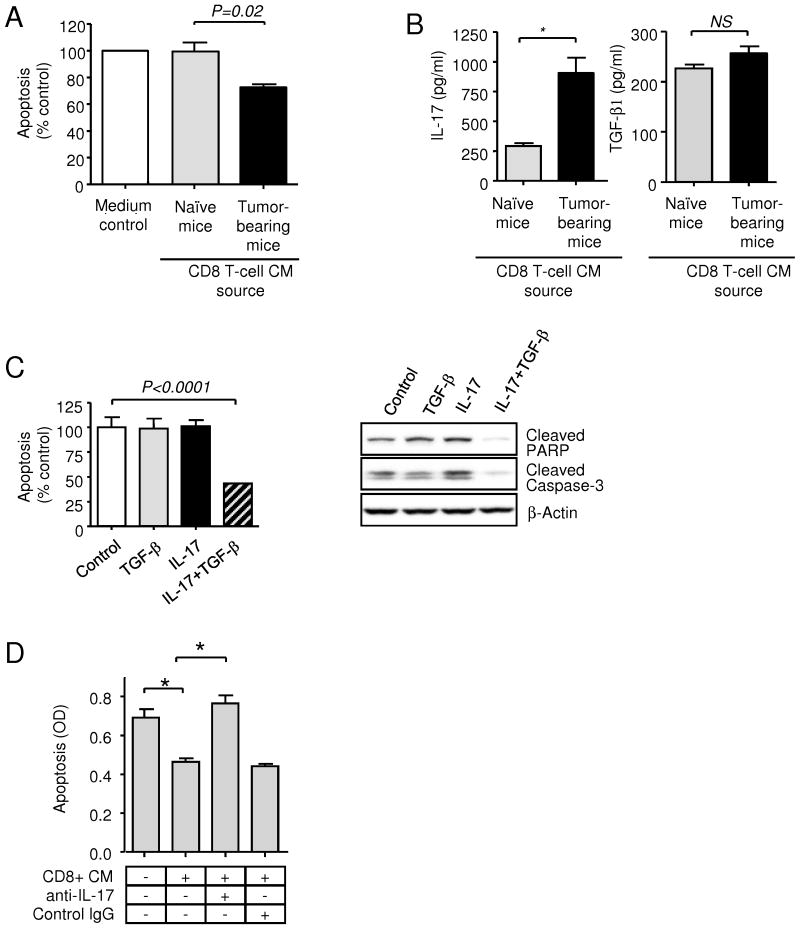
Since TGF-β alone does not affect proliferation or survival of 4T1 tumor cells (data not shown, and see Fig. 2C), we hypothesized that CD8+ T-cells were secreting a factor that synergized with TGF-β to promote growth or survival. To test this hypothesis, we prepared CD8+ T cells from the spleens of naive or tumor-bearing mice, and activated the cells ex vivo with anti-CD3 antibodies. While no effect was seen on cell proliferation (data not shown), the medium conditioned by activated CD8+ T-cells derived from tumor-bearing, but not naive mice, suppressed apoptosis of the 4T1 cells (Fig. 2A). Cytokine array analysis showed IL-17 to be the cytokine that was most differentially expressed between activated CD8+ cells from naive compared with tumor-bearing mice (Suppl. Fig 1). Activated CD8+ T-cells from tumor-bearing mice made >3x more IL-17 than CD8+ T cells from naive mice (Fig. 2B), while IL-17 was barely detectable in medium conditioned by unstimulated T cells from either source (<0.01pg/ml; data not shown). In contrast, TGF-β secretion by stimulated CD8+ T cells was unaffected by the presence of tumor (Fig. 2B).
Figure 2. Pro-survival effects of cytokines secreted by CD8 T-cells derived from tumor-bearing mice.
A. Quantitation of apoptosis in 4T1 cells in vitro after 48h exposure to conditioned medium (CM) from polyclonally activated CD8+T cells derived from the spleens of naïve and 4T1 tumor bearing mice. Apoptosis was measured using the Cell Death Detection ELISA assay and normalized to the apoptosis level seen on treatment with X-Vivo medium alone (medium control). CM was assayed at a final dilution of 1:4. Values represent mean ± SEM (n = 5). B. Quantitation of IL-17 and TGF-β1 protein in stimulated CD8+ T-cell conditioned medium using specific ELISA assays. Values represent mean ± SEM (n = 5). C. Effect of TGF-β1 and IL-17 on apoptosis of 4T1 cells in response to serum deprivation. Values represent mean ± SEM (n = 3). Apoptosis was assessed by ELISA, or by Western blot analysis of cleaved PARP, and cleaved caspase-3 in 4T1 cells 48 hours following treatment with either TGF-β1 or IL-17. β-actin was used as a normalization control. D. The pro-survival effect of CD8+ cell CM on 4T1 cells is neutralized by anti-IL-17 antibodies. Values represent mean ± SEM (n = 3).
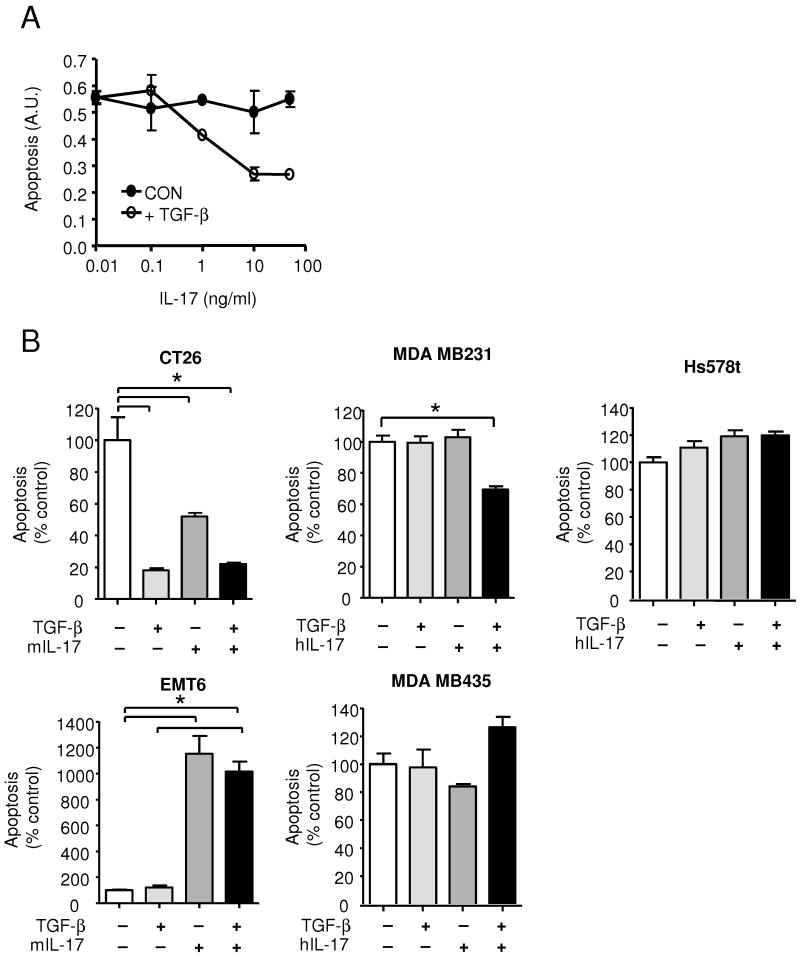
Although IL-17 has not previously been shown to function as a survival factor for tumor cells, we found that IL-17 and TGF-β synergistically protected 4T1 cells from apoptosis induced by serum deprivation in vitro, though neither factor alone was effective (Fig. 2C). IL-17 was confirmed as the critical pro-survival factor for 4T1 cells in the CD8+ T-cell conditioned medium by neutralization with anti-IL-17 antibodies (Fig. 2D). The pro-survival response to IL-17 in 4T1 cells was dose-dependent, and maximal at 10 ng/ml (Fig. 3A). In assessing a panel of tumor cells lines, we found that the pro-survival response to IL-17 was not confined to the 4T1 cells, and that it did not always show an obligatory dependence on the simultaneous presence of TGF-β (Fig 3B). Thus, TGF-β and IL-17 synergized to protect MDA MB231 human breast cancer cells against apoptosis, while either TGF-β or IL-17 were individually capable of suppressing apoptosis in the mouse colon carcinoma line CT26. However, in some cell lines IL-17 had little effect (MDA MB435, Hs578t), or actually enhanced apoptosis (EMT6), suggesting that, as for TGF-β (33), the cellular response to IL-17 is contextual. There was no obvious correlation between expression levels of IL-17Rα, TβRI or TβRII and the cellular response to IL-17 and TGF-β (Suppl. Fig. 2), so the underlying molecular mechanisms are likely to be complex.
Figure 3. The effect of IL-17 on tumor cell survival is dose- and cell type-dependent.
A. Dose dependence of the pro-survival effect of IL-17 on 4T1 cells. Where indicated, TGF-β was used at 5ng/ml. B. Effect of IL-17 and TGF-β on survival of various mouse and human cell lines in culture. Apoptosis was induced by serum deprivation (CT26, EMT6), or by treatment with staurosporine (MDA MB231, Hs578t and MDA MB435). TGF-β was used at 5ng/ml and IL-17 at 10ng/ml. Results are expressed as a percent of the no cytokine control, and are the mean +/- SEM for 3 determinations. * indicates P<0.05.
The presence of the tumor enhances differentiation of CD8+ T-cells to an IL-17 secreting phenotype
The development of helper T-cell lineages is very dependent on the specific cytokine milieu. In the presence of IL-6, TGF-β preferentially promotes differentiation of CD4+ T-cells to into pro-inflammatory Th17 cells, characterized by secretion of high levels of IL-17 (34). Since CD8+ T cells have also been shown to be capable of IL-17 secretion (35), we hypothesized that the 4T1 tumor might be creating a cytokine milieu that promotes differentiation of CD8 T-cells into an IL-17 secreting phenotype. We showed that 4T1 cells in culture make both TGF-β1 (1-4 ng/106 cells/48h) and IL-6 (9.0 +/- 2.5 pg/106 cells/48h), and furthermore that IL-6 production by 4T1 cells is strongly stimulated by TGF-β treatment (7.4-fold stimulation to 66.6 +/- 5.1 pg/106 cells/48h) following treatment with 5ng/ml TGF-β, P<0.0001). 4T1 tumors in vivo also made both TGF-β (221 +/- 24 ng/g wet weight; n=5) and IL-6 (739 +/- 233 ng/g total protein; n=5), and intratumoral IL-6 levels were reduced nearly two-fold by treatment of mice with anti-TGF-β antibody (to 443 +/- 159 ng/g; n=5; p=0.04).
Using purified CD8+ and CD4+ splenocytes from naive mice stimulated in vitro with plate-bound anti-CD3 and soluble anti-CD28 for 3 days under various polarizing conditions, we confirmed that TGF-β in combination with IL-6 could induce a significant increase in the number of IL-17 positive CD8+ T-cells (Suppl. Fig. 3). The most robust induction of IL-17 was observed in the additional presence of neutralizing antibodies against IFN-γ and IL-4 (Suppl. Fig. 2). Thus, as has recently been shown by Liu et al in mixed lymphocyte culture (36), our data confirm that TGF-β and IL-6 can polarize CD8+ T cells towards an IL-17 secreting “Tc17” fate, as they do for CD4+ T cells.
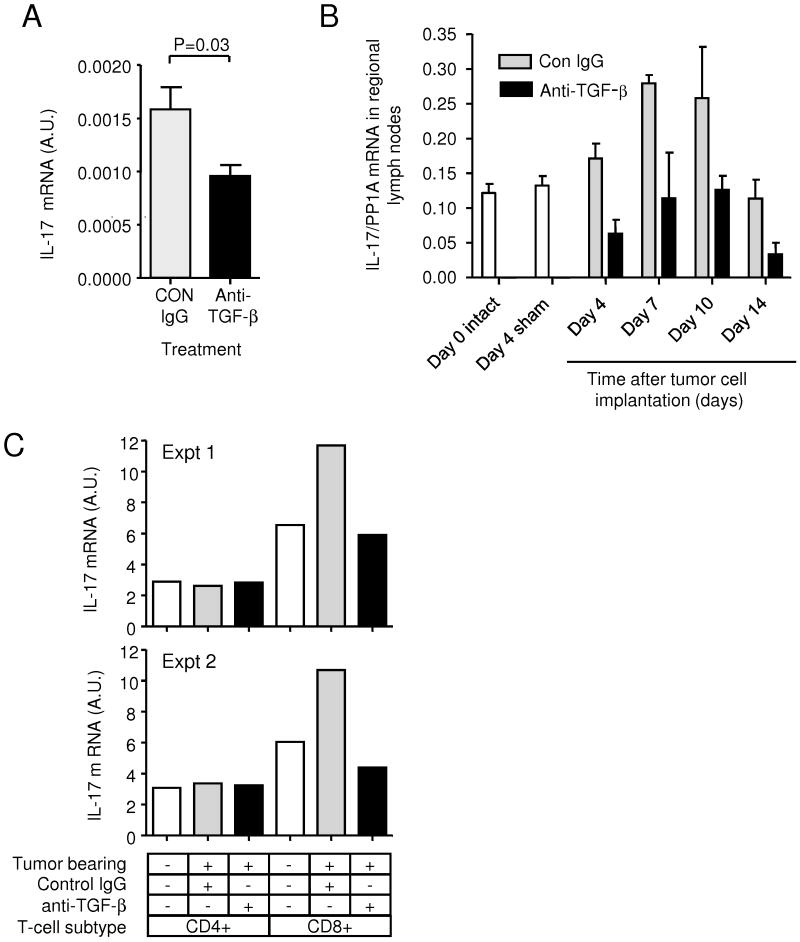
Given that the 4T1 tumor makes cytokines that could promote IL-17 production by CD8+ T cells, we assessed IL-17 expression levels in the tumor. By RTQ-PCR, we found that treatment with anti-TGF-β caused a ∼2-fold reduction in the IL-17 mRNA level in 4T1 tumors (Fig. 4A), presumably reflecting a change in IL-17 expression by infiltrating CD8+ cells. Analysis of IL-17 expression in the locoregional lymph nodes showed that the presence of the tumor caused an increase in IL-17 mRNA that peaked at days 7-10 after tumor cell implantation and was suppressed by anti-TGF-β treatment (Fig. 4B). The tumor-induced increase in IL-17 in the lymph nodes was seen specifically in CD8+ cells, but not CD4+ cells (Fig. 4C). The effects on IL-17 expression appeared to be local to the tumor and regional lymph nodes, as we did not see significant increases in circulating TGF-β, IL-6 or IL-17 in serum or plasma from tumor-bearing animals (data not shown).
Figure 4. TGF-β antagonism reduces IL-17 expression in tumors and lymph nodes of tumor-bearing mice.
A. RTQ-PCR analysis of IL-17 mRNA expression in 4T1 primary tumors derived from the mice treated with anti-TGF-β or control antibody (CON), and harvested at day 10 after tumor cell implantation. Values represent mean ± SD for 5 independent primary tumors/treatment group. B. Timecourse of IL-17 expression in locoregional lymph nodes after implantation of 4T1 tumor cells in the mammary fat pad. Lymph nodes were harvested at different times after tumor cell implantation, and IL-17 mRNA levels were determined by RTQ-PCR. Levels in lymph nodes from naive or sham-operated mice are shown for comparison. Results are mean +/- SEM for 3 mice/group. C. Cellular origin of IL-17 in locoregional lymph nodes from tumor bearing mice. Lymph nodes were harvested from naive or tumor-bearing mice treated with anti-TGF-β or control antibody (CON) at day 10 after tumor cell implantation. Lymph nodes were pooled for 5 mice for each group, and CD4+ or CD8+ cells were purified and assessed for expression of IL-17 mRNA by RTQ-PCR. The results of two independent experiments are presented.
Knockdown of IL-17 response reduces tumor growth and enhances apoptosis in vivo
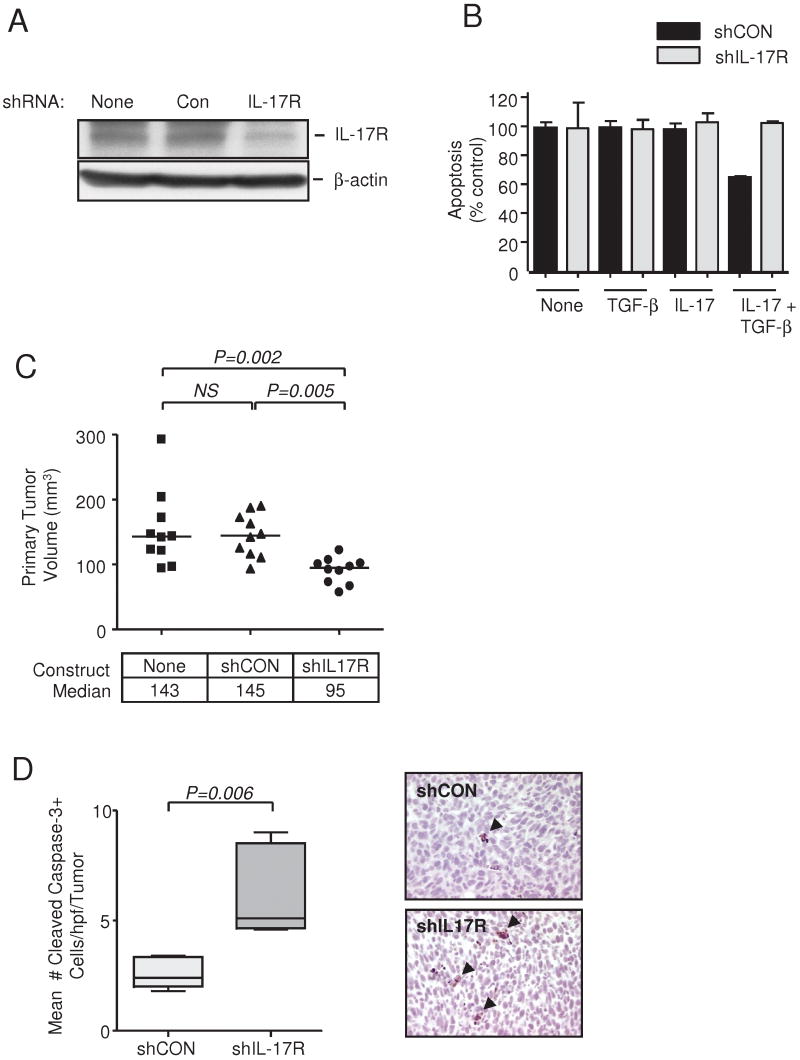
Having established that the presence of a tumor enhances local IL-17 production in a TGF-β-dependent manner, we then wished to test whether this phenomenon was biologically significant. To test whether IL-17 acts directly on the tumor cell to promote tumorigenesis in vivo, we knocked down IL-17 response in 4T1 cells using shRNA targeted to the IL-17 receptor-α. Three independent shRNA constructs suppressed IL-17Rα mRNA and protein by up to 60% (Suppl Fig 4 and Fig 5A). The shRNA construct #2 completely blocked the anti-apoptotic effect of IL-17 and TGF-β in vitro and was used for further experiments (Fig. 5B). IL-17R knockdown suppressed tumorigenesis in vivo, to a similar extent to that seen with TGF-β neutralization or CD8 depletion (Fig. 5C; compare Fig 1A). Apoptosis was significantly increased in tumors expressing the shIL17R (Fig. 5D). Since IL-17 has previously been shown to upregulate the production of angiogenic factors by tumor cells (26), we determined whether the smaller tumor size and enhanced apoptosis following IL-17R knockdown might be secondary to changes in tumor vascularization. However, we saw no difference in blood vessel density between control and IL-17R knockdown tumors (Suppl. Fig. 5). Thus the data support the hypothesis that IL-17 serves as a direct pro-survival factor for the 4T1 tumor cells in vivo.
Figure 5. IL-17 directly promotes the growth of 4T1 tumors in vivo.
A. IL-17R protein levels in 4T1 cells following transfection with IL-17R shRNA or a non-silencing shRNA (Con) as determined by Western blot. β-actin is the loading control. B. Knockdown of IL-17R blocks the prosurvival effect of IL-17 + TGF-β on 4T1 cells in vitro. Apoptosis was quantitated using the Cell Death Detection ELISA. Values represent mean ± SEM (n = 3). C. Effect of IL-17R knockdown on growth of 4T1 tumors in the mammary fat pad. 4T1 cells were stably transfected with the IL-17R shRNA or Con shRNA. Primary tumors were excised and measured at day 10. The median value is indicated for each group (n = 10). NS, not significant. D. Immunohistochemical staining for Cleaved Caspase-3 in primary tumors of 4T1 cells stably transfected with the IL-17R shRNA or Con shRNA. Left panel, boxes show median values with upper and lower quartiles, and whiskers indicate range (n = 10 for each group). Right panel, the magnification is X 400. Arrows indicate apoptotic cells.
IL-17 expression is upregulated in some human breast cancers
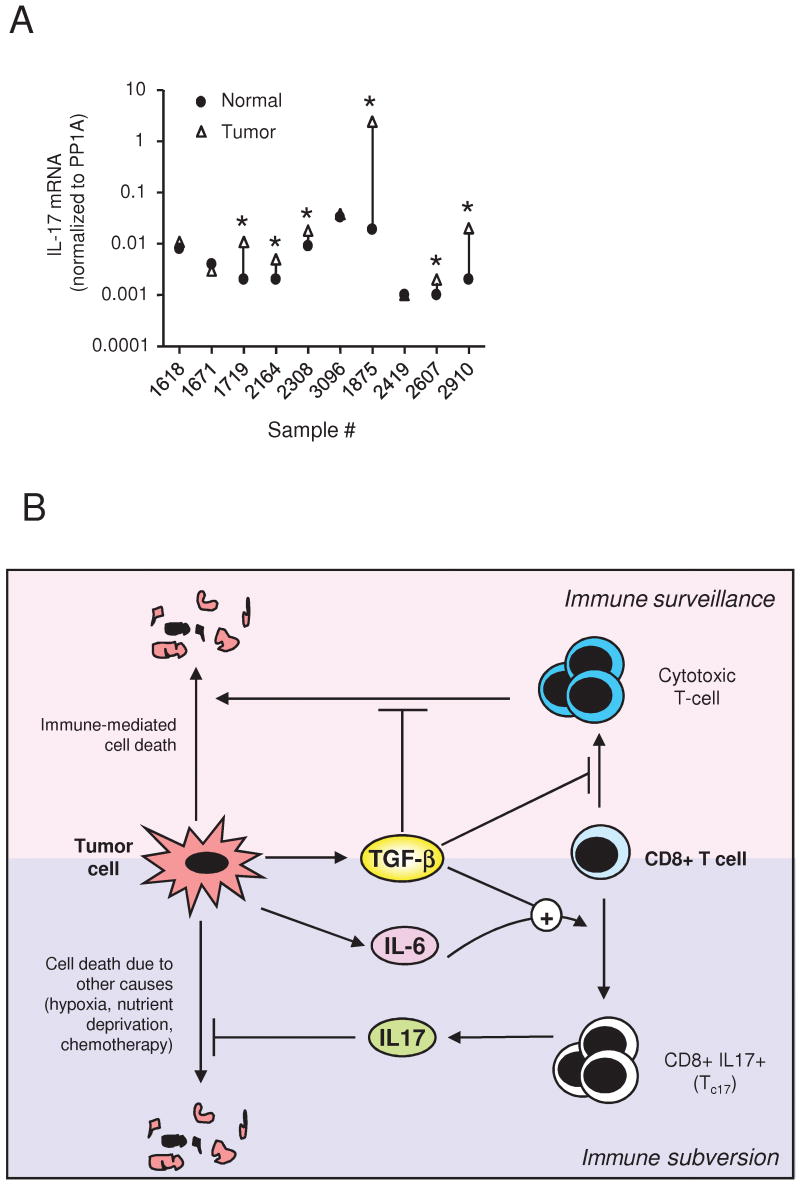
To determine whether this mechanism has any relevance for human breast cancer, we analyzed expression of IL-17 mRNA expression in a small cohort of matched normal human breast and breast cancer samples. IL-17 mRNA was elevated ≥2-fold in the tumor compared with normal breast in 6/10 cases (P=0.004, paired Wilcoxon signed rank test; Fig. 6A). IL17Rα mRNA was detectable in all samples (data not shown).
Figure 6. Expression of IL-17 in human breast tumors, and model for the role of TGF-β in subverting the CD8+ T-cell response.
A. Expression of IL-17 in human breast tumors. IL-17 mRNA levels were determined by RTQ-PCR on a panel of 10 human breast cancer samples compared with patient-matched normal breast, and expression levels were normalized to cyclophilin A (PP1A). * indicates samples where expression in the tumor was ≥2-fold higher than in the matched normal sample. B. Model for the role of TGF-β in subverting the CD8+ T-cell response. TGF-β secreted by tumor cells is known to suppress the immune surveillance response by preventing the generation and effector function of cytotoxic T-cells. However, if the tumor also makes IL-6, the simultaneous presence of TGF-β and IL-6 in the vicinity of the tumor promotes expansion and/or differentiation of local CD8+ T-cells into an IL-17-secreting form (Tc17). The IL-17 derived from the subverted T-cells can then promote tumor survival by directly blocking apoptosis of the tumor cells. In some tumors, TGF-β can further promote the subversion process by enhancing IL-6 expression by the tumor, as well as synergizing with IL-17 to enhance tumor cell survival (not shown)
Discussion
The immune system plays a complex role in tumorigenesis. While full activation of an effective adaptive immune response can lead to tumor eradication, it is clear that unresolved inflammatory responses can promote tumorigenesis through multiple mechanisms, including mutagenic or pro-proliferative effects on the tumor, enhanced angiogenesis, and suppression of cytotoxic T-cell responses (2;37). Thus deficiency of innate immune cells frequently attenuates spontaneous or experimental carcinogenesis, while deficiency of adaptive immune cells generally enhances tumorigenesis in mouse models (2). Interestingly however, although immune suppression in humans is associated with a dramatically enhanced cancer risk for many tumors (38;39), it is associated with a somewhat decreased relative risk of breast, prostate, and bladder cancer (38;40), suggesting that the adaptive immune response may be weakly tumor promoting for these tumor types.
Here we have shown for the first time that a tumor can subvert the CD8+ arm of the immune surveillance system into promoting tumorigenesis through direct effects on tumor cell survival. Using the 4T1 model of metastatic breast cancer, we showed that the presence of a tumor secreting both IL-6 and TGF-β causes local polarization or expansion of CD8+ T-cells into an IL-17-secreting state (Tc17). We further showed that IL-17 could directly promote survival of 4T1 tumor cells, both in vitro and in vivo, thus providing a plausible explanation for the tumor promoting effect of CD8+ T-cells in this model. Many tumors are already known to evade immune surveillance by compromising the development or activity of tumor-specific cytotoxic T-cells, often in a TGF-β-dependent manner (3;15;16;19;37;41;42). However, our data suggest that some tumors may go further, creating a local cytokine environment that actively skews local differentiation or expansion of CD8+ T cells to a state where they directly promote tumor growth. This effect is dependent on the presence of TGF-β at one or more steps (see model in Fig. 6B), and turns a key anti-tumor defense mechanism against the host organism.
To our knowledge, ours is the first report of a direct pro-survival effect of IL-17 on tumor cells, though overexpression of an IL-17 receptor-like gene (IL17RL), activated by an unknown ligand, was recently shown to suppress apoptosis in prostate cancer cell lines (43). The underlying mechanism is currently not known, but we have found that IL-17 and TGF-β synergize to suppress expression of the death receptor ligand TRAIL in 4T1 cells, which may contribute to this effect (data not shown). The anti-apoptotic effect is not just confined to the 4T1 model, as in a preliminary screen of a small panel of cancer cell lines, we found that IL-17 could promote the in vitro survival of 3/6 cell lines tested, with a variable requirement for the simultaneous presence of TGF-β. However, in one case (EMT6 mammary carcinoma), IL-17 actually promoted apoptosis, suggesting that as for TGF-β (33), the nature of the apoptotic response to IL-17 depends on the cellular context, and the specific determinant of the response is not yet known.
IL-17 production is defining for a new lineage of pro-inflammatory T-cells whose role in tumorigenesis has not been extensively explored (22). In a recent study, it was demonstrated that both IL-17+ CD4+ and IL-17+CD8+ T-cells are present in the tumor microenvironment of several different human and mouse tumor types (44), suggesting that local production of IL-17 by tumor-infiltrating T-cells may be a relatively widespread phenomenon in tumorigenesis. Indeed we have preliminary evidence, in a small cohort of breast cancer patients, that IL-17 mRNA is significantly upregulated in the tumor when compared with normal breast from the same patient. However, in contrast to Kryczek et al., in our preclinical model we saw a tumor-induced increase in IL-17 production in the locoregional lymph nodes as well as in the tumor, and within the lymph nodes the increase in IL-17 was specific to CD8+ T-cells and not CD4+ T cells. Thus the dominant site of IL-17 production and the originating cell type may vary among tumors.
The production of IL-17 is amplified and sustained by IL-23 in mouse CD4+ T-cells (22). Recently IL-23 was shown to be overexpressed in several different human cancer types, and to promote tumorigenesis in a preclinical model of chemically-induced skin carcinogenesis, and in transplantable tumors (37). The tumor-promoting effect of IL-23 in this study was attributed to the upregulation of inflammatory responses, including enhanced matrix metalloproteinase expression and angiogenesis, and to a reduced infiltration of cytotoxic T-cells into the transformed tissue. While IL-17 clearly has the potential to exert similar effects (25;26), in our current work we believe that the direct effect of IL-17 on tumor cell survival predominates over alternative mechanisms, since blockade of IL-17 response in the 4T1 tumor cell was as effective as CD8+ cell depletion in suppressing tumorigenesis. However, the relative contributions of direct and indirect effects of IL-17 on tumorigenesis are likely to vary between tumor types, depending at least in part on whether the tumor cell can use IL-17 as a direct pro-survival factor.
Both preclinical and clinical studies support the concept that the adaptive arm of the immune system can be tumor promoting under certain circumstances (reviewed in (2)). Although this effect is often due to the dominance of immunosuppressive CD4+ regulatory T-cells (Tregs), a number of CD8+ T cell subclasses with the potential to promote tumorigenesis have now been described. Similar to our findings with the 4T1 breast and CT26 colon carcinoma models, Girardi et al have recently shown that CD8+ T-cells can promote skin tumorigenesis induced by a two-step chemical initiation/promotion protocol using DMBA and TPA (41). They identified a putative tumor promoting CD8+ population of tumor-infiltrating lymphocytes (“T-pro”), that had a phenotype consistent with activated effector memory cells (αβTCR+CD8+CD44+CD62L-) and expressed inflammatory mediators (IFN-γ, TNF-α and COX2), but were deficient in perforin (41). Since the DMBA/TPA carcinogenic regimen upregulates both TGF-β and IL-6 (45;46), and would be predicted to cause Tc17 skewing, it will be interesting to determine whether the T-pros also make IL-17, or whether an additional tumor promoting IL-17+CD8+ subset may also exist in this model. A number of other CD8+ subsets have also been shown to have immunosuppressive activity, including CD8+CD28- cells, CD8+CD45R+ cells, CD8+CD122+, and IL-10-secreting CCR7+CD45RO+CD8+ T-cells (47-50). With the exception of the CCR7+CD45RO+CD8+ T cells (50), these suppressor CD8+ T cells have mostly been identified in the context of autoimmunity and self-tolerance, and roles in promoting tumorigenesis have yet to be demonstrated. In all cases however, the predicted mode of action of these cells is the suppression of primary T-cell responses. The CD8+ tumor promoting mechanism that we have described here is novel in that it involves direct effects on the tumor cell itself.
In summary, here we have shown that presence of TGF-β in a tumor bed contributes to a local cytokine milieu that can direct inappropriate CD8+ T-cell differentiation or expansion down a pro-inflammatory, IL-17-secreting path. In tumor cells that have evolved to use IL-17 as a survival factor, this subversion of the CD8+ T-cell response can promote tumorigenesis directly, converting the adaptive arm of the immune system from a form in which it attacks the tumor into one in which it provides direct trophic support for the tumor, and thus promotes tumor progression.
Supplementary Material
Acknowledgments
We thank Dr. Mario Anzano and Anthony Vieira for expert technical assistance with the mice.
Reference List
- 1.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 3.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 4.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 5.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–71. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Rook AH, Kehrl JH, Wakefield LM, et al. Effects of transforming growth factor β on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986;136:3916–20. [PubMed] [Google Scholar]
- 7.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334:260–2. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 8.Thomas DA, Massague J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Gorelik L, Flavell RA. Transforming growth factor-β in T-cell biology. Nat Rev Immunol. 2002;2:46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 10.Ranges GE, Figari IS, Espevik T, Palladino MA., Jr Inhibition of cytotoxic T cell development by transforming growth factor β and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987;166:991–8. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor FoxP3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-β signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold LI. The role for transforming growth factor-β (TGF-β) in human cancer. Crit Rev Oncog. 1999;10:303–60. [PubMed] [Google Scholar]
- 14.Chen W, Wahl SM. TGF-β: the missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003;14:85–9. doi: 10.1016/s1359-6101(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 15.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-β signaling in T cells. Nat Med. 2001;7:1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 17.Ge R, Rajeev V, Ray P, et al. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-β type I receptor kinase in vivo. Clin Cancer Res. 2006;12:4315–30. doi: 10.1158/1078-0432.CCR-06-0162. [DOI] [PubMed] [Google Scholar]
- 18.Uhl M, Aulwurm S, Wischhusen J, et al. SD-208, a novel transforming growth factor β receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 2004;64:7954–61. doi: 10.1158/0008-5472.CAN-04-1013. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki E, Kapoor V, Cheung HK, et al. Soluble type II transforming growth factor-β receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clin Cancer Res. 2004;10:5907–18. doi: 10.1158/1078-0432.CCR-03-0611. [DOI] [PubMed] [Google Scholar]
- 20.Schlingensiepen KH, Schlingensiepen R, Steinbrecher A, et al. Targeted tumor therapy with the TGF-β2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–39. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki E, Kim S, Cheung HK, et al. A novel small-molecule inhibitor of transforming growth factor β type I receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res. 2007;67:2351–9. doi: 10.1158/0008-5472.CAN-06-2389. [DOI] [PubMed] [Google Scholar]
- 22.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Benchetrit F, Ciree A, Vives V, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 24.Hirahara N, Nio Y, Sasaki S, et al. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 25.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–89. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 26.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 27.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-β. Bioactivity neutralization and transforming growth factor β2 affinity purification. J Immunol. 1989;142:1536–41. [PubMed] [Google Scholar]
- 28.Tang B, Vu M, Booker T, et al. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Invest. 2003;112:1116–24. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinkas J, Leder P. MEK1 signaling mediates transformation and metastasis of EpH4 mammary epithelial cells independent of an epithelial to mesenchymal transition. Cancer Res. 2002;62:4781–90. [PubMed] [Google Scholar]
- 30.Nam JS, Suchar AM, Kang MJ, et al. Bone sialoprotein mediates the tumor cell-targeted prometastatic activity of transforming growth factor β in a mouse model of breast cancer. Cancer Res. 2006;66:6327–35. doi: 10.1158/0008-5472.CAN-06-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–6. [PubMed] [Google Scholar]
- 32.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 33.Sanchez-Capelo A. Dual role for TGF-β1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 35.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-Producing T Cells Are Important in Effector Functions for the Elicitation of Contact Hypersensitivity Responses. J Immunol. 2006;177:6852–8. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu SJ, Tsai JP, Shen CR, Sher YP, Hsieh CL, Yeh YC, et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-{β} and interleukin-6. J Leukoc Biol. 2007;82:354–360. doi: 10.1189/jlb.0207111. [DOI] [PubMed] [Google Scholar]
- 37.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher B, Wang Z, Schymura MJ, Kahn A, Fordyce EJ. Cancer incidence in New York State acquired immunodeficiency syndrome patients. Am J Epidemiol. 2001;154:544–56. doi: 10.1093/aje/154.6.544. [DOI] [PubMed] [Google Scholar]
- 39.Haagsma EB, Hagens VE, Schaapveld M, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84–91. doi: 10.1016/s0168-8278(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 40.Stewart T, Tsai SC, Grayson H, Henderson R, Opelz G. Incidence of de-novo breast cancer in women chronically immunosuppressed after organ transplantation. Lancet. 1995;346:796–8. doi: 10.1016/s0140-6736(95)91618-0. [DOI] [PubMed] [Google Scholar]
- 41.Roberts SJ, Ng BY, Filler RB, et al. Characterizing tumor-promoting T cells in chemically induced cutaneous carcinogenesis. Proc Natl Acad Sci U S A. 2007;104:6770–5. doi: 10.1073/pnas.0604982104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Q, Yang X, Pins M, et al. Adoptive transfer of tumor-reactive transforming growth factor-β-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer. Cancer Res. 2005;65:1761–9. doi: 10.1158/0008-5472.CAN-04-3169. [DOI] [PubMed] [Google Scholar]
- 43.You Z, Shi XB, DuRaine G, et al. Interleukin-17 receptor-like gene is a novel antiapoptotic gene highly expressed in androgen-independent prostate cancer. Cancer Res. 2006;66:175–83. doi: 10.1158/0008-5472.CAN-05-1130. [DOI] [PubMed] [Google Scholar]
- 44.Kryczek I, Wei S, Zou L, et al. Cutting Edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 45.Akhurst RJ, Fee F, Balmain A. Localized production of TGF-β mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988;331:363–5. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- 46.Vasunia KB, Miller ML, Andringa S, Baxter CS. Induction of interleukin-6 in the epidermis of mice in response to tumor-promoting agents. Carcinogenesis. 1994;15:1723–7. doi: 10.1093/carcin/15.8.1723. [DOI] [PubMed] [Google Scholar]
- 47.Najafian N, Chitnis T, Salama AD, et al. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–48. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xystrakis E, Dejean AS, Bernard I, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294–301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 49.Rifa'I M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–34. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–6. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.