Abstract
Recent analyses of collagen, elastin and fibronectin matrix assembly, organization and remodeling have been facilitated by the use of tagged proteins that can be visualized without the need for antibody labeling. Here, we report the generation of C-terminal tagged, full-length and “processed” (α3ΔLG4-5) human α3 as well as C-terminal tagged, full-length human β3 laminin subunits in adenoviral vectors. Human epidermal keratinocytes (HEKs) and human bronchial epithelial (BEP2D) cells, which assemble laminin-332-rich matrices, as well as primary rat lung alveolar type II (ATII) cells, which elaborate a fibrous network rich in laminin-311, were infected with adenovirus encoding the tagged human laminin subunits. In HEKs and BEP2D cells, tagged, full-length α3, α3ΔLG4-5 and β3 laminin subunits incorporate into arrays of matrix organized into patterns that are comparable to those observed when such cells are stained using laminin-332 subunit antibody probes. Moreover, HEKs and BEP2Ds move over these tagged, laminin-332-rich matrix arrays. We have also used the tagged β3 laminin subunit-containing matrices to demonstrate that assembled laminin-332 arrays influence laminin matrix secretion and/or assembly. In the case of rat ATII cells, although tagged α3 laminin subunits are not detected in the matrix of rat ATII cells infected with virus encoding full-length human α3 laminin protein, processed human α3 laminin subunits are incorporated into an extracellular fibrous array. We discuss how these novel laminin reagents can be used to study the organization, processing and assembly of laminin matrices and how they provide new insights into the potential functional importance of laminin fragments.
1. Introduction
Cell-extracellular matrix (ECM) interactions play crucial roles in cell motility, adhesion, and differentiation. There has been much interest in the mechanisms by which the cells deposit and organize their ECM and how the architecture of the matrix impacts cell behavior. Though initial assembly of most matrix proteins begins in the cytoplasm, directed by the innate properties of the structure of the monomers themselves, the secretion, deposition, and ultimate formation of the ECM is mediated in large part by the cell itself. Once secreted, integrins and other cell surface receptors have been shown to be necessary for the proper assembly and organization of various ECM components [see, for example, (Wierzbicka-Patynowski and Schwarzbauer, 2003)].
One approach to studying ECM assembly has been through the use of tagged matrix molecules (Krahn et al., 2006; Sehgal et al., 2006). Studies using fluorophore-tagged proteins or proteins fused to green fluorescent protein (GFP) or GFP derivatives have facilitated studies examining assembly/incorporation of fibronectin, elastin, and collagen into the ECM [see, for example, (Johnson and Galis, 2003; Kozel et al., 2006; Krahn et al., 2006; Larsen et al., 2006; Sivakumar et al., 2005)]. Specifically, Erickson and co-workers have followed the formation of a fibronectin matrix in live 3T3 cells by tracking fibronectin/YFP (yellow fluorescent protein) and moesin/GFP, a marker for the actin cytoskeleton (Ohashi et al., 2002). Moreover, the study of the dynamics of elastic fiber formation has been greatly advanced through the use of a tropoelastin/Timer fusion protein (Kozel et al., 2006). The Timer protein changes from red to green over time and has allowed investigators to follow the time course of the assembly of elastic fibers in the ECM.
As is the case with other ECM proteins, laminin matrix assembly is a highly regulated process, involving the cytoskeleton and integrin receptors (Colognato et al., 1999; Li et al., 2002; Sehgal et al., 2006). Laminins are heterotrimeric molecules composed of three different subunits, termed α, β, and γ {Aumailley, 2005 #7}. Our work has focused on a number of laminin isoforms including laminin 332 (α3, β3, γ2), a major laminin found in the basement membrane of the epidermis and other epithelial tissues, and laminin-311 (α3, β1, γ1), which is deposited by alveolar type II (ATII) lung epithelial cells (Aumailley et al., 2005; Borradori and Sonnenberg, 1996; DeBiase et al., 2006; Goldfinger et al., 1998; Jones et al., 1998; Jones et al., 2005). To study the organization of the laminin ECM and its impact on cell behavior, we have generated adenoviral vectors encoding full-length laminin α3, a C-terminal truncation of laminin α3 (α3ΔLG4-5), which is equivalent to the proteolytically processed version of the α3 laminin subunit found in the matrix of many cultured epithelial cells, and full-length β3 subunit fused to fluorescent tags (Goldfinger et al., 1998). We describe several examples where these tagged laminin subunits are allowing us to visualize laminin matrix assembly in live and fixed cells. We also present data relating to how their use has the potential to provide new insights into the structure and processing of laminin proteins.
2. Results and discussion
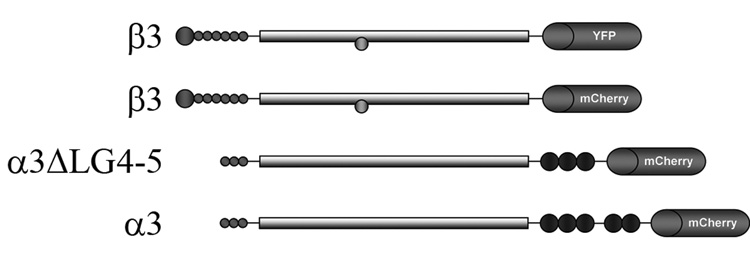
To facilitate analyses of the structure and function of laminin matrices we have developed adenoviruses encoding tagged α3 and β3 laminin subunits. Since the β3 laminin subunit is only proteolyzed in certain cell types we have placed either an mCherry or yellow fluorescent protein (YFP) tag at its C-terminal end (Udayakumar et al., 2003)(Fig. 1). We already described the generation of virus encoding β3 laminin tagged with YFP at its C-terminus in a previous report (Sehgal et al., 2006). The placement of the tag on the α3 laminin subunit is more challenging. Addition of the tag to the N-terminus of the α3 laminin will result in loss of the label, because an N-terminal signal peptide required for targeting the protein for secretion is removed prior to the incorporation of the protein into matrix. Thus, we fused the sequence of the mCherry tag to the 3’ end of the sequence encoding full-length α3 laminin sequence for expression in an adenoviral vector (Fig. 1). However, this scheme may also be problematic since the α3 laminin subunit is subject to proteolysis at its C-terminus, globular (G) domain (Goldfinger et al., 1998; Marinkovich et al., 1992; Matsui et al., 1995). Specifically, in certain epithelial cells, the α3 laminin undergoes proteolytic processing upon secretion, reducing its molecular weight from 190kD to 165 kD (Marinkovich et al., 1992). This processing involves a cleavage within the G domain at the junction between LG3 and LG4 (Goldfinger et al., 1998; Marinkovich et al., 1992; Matsui et al., 1995). Thus, we deleted sequences encoding the LG4-5 domains and fused this partial α3 laminin subunit cDNA encoding α3ΔLG4-5 to cDNA encoding the mCherry tag for expression in an adenoviral vector (Fig. 1). In all instances, we used adenoviral delivery because of its efficiency over plasmid-based delivery systems in epithelial cells.
Fig. 1.
Diagrams of the α3 and β3 laminin subunits, bearing tags that were used in these studies.
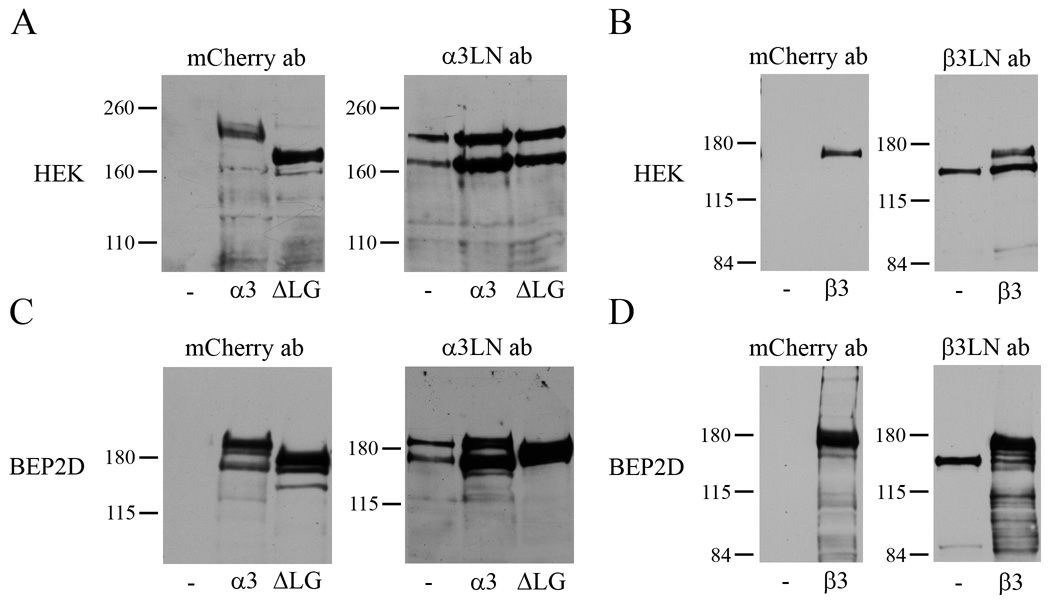
HEKs and BEP2D cells were infected with virus encoding the two different tagged α3 and the β3 laminin subunits. Western immunoblotting of cell extracts using antibody against mCherry reveals that the tagged laminin subunits are expressed by the infected cells and migrate in SDS-PAGE at approximately 20 kD greater than their non-tagged native equivalents due to the addition of the mCherry tag (Fig. 2). Specifically, tagged full-length α3, α3ΔLG4-5 and β3 laminin laminin subunit migrate at approximately 210 kD, 180 kD and 160 kD, respectively. In addition, each of these tagged subunits is recognized by their corresponding laminin-332 subunit antibodies (Fig. 2). The latter also recognize the endogenous laminin-332 proteins produced by the cells. Thus, since the tagged full-length α3 and α3ΔLG4-5 laminin subunits migrate close to the 190 kD endogenous unprocessed α3 laminin subunit expressed by both HEKs and BEP2D cells, they cannot always be resolved clearly on immunoblots probed with a3 laminin subunit antibodies (Fig. 2).
Fig. 2.
Extracts of HEKs (A,B) or BEP2D cells (C,D) were processed for immunoblotting. In A and C immunoblots of extracts of uninfected HEKs or BEP2D cells (-) or cells infected with virus encoding full-length, tagged α3 laminin (α3) or tagged α3ΔLG4-5 laminin (ΔLG) were probed with mCherry or α3 laminin subunit antibodies. In B and D immunoblots of uninfected HEKs and BEP2D cells (-) or cells infected with virus encoding tagged β3 laminin (β3) were probed with mCherry or β3 laminin subunit antibodies. Since there is differential expression of each of the tagged proteins in extracts of HEKs and BEP2D cells, the amount of protein loaded into the lanes of each gel in A and C was adjusted to optimize the reactivity with the mCherry probe. Molecular weight standards are marked at the left.
Incorporation of tagged laminin subunits into laminin-332-rich matrix of fixed and live HEKs and BEP2D cells
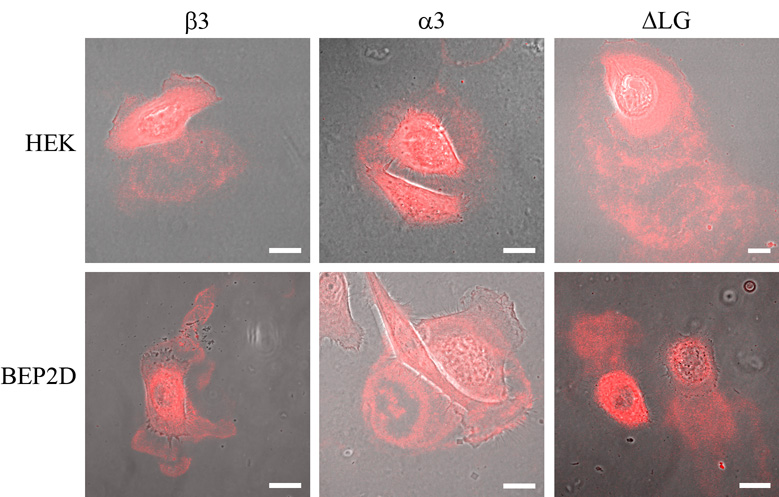
We first investigated whether the tagged laminin subunit molecules incorporate into the matrices of HEKs and BEP2D cells using confocal fluorescence microscopy. We observe a robust fluorescent signal in preparations of HEKs and BEP2D cells, following infection with virus encoding the tagged full-length α3, α3ΔLG4-5 and β3 laminin subunits (Fig. 3). This signal appears as arcs and circles along the substratum-attached surface of the infected cells. Tagged protein is also detected in areas that are not directly covered by cells, consistent with evidence that cells deposit trails of laminin-332 matrix as they move over their substrate (Fig. 3)(Sehgal et al., 2006). Taken together, these results indicate that tagged laminin subunits are incorporated into the matrix of the cells (Fig. 3). Moreover, this localization is identical to that observed when skin cells are processed for indirect immunofluorescence using laminin-332 antibodies, as shown by a number of different workers (see, for example, (Goldfinger et al., 1998; Sehgal et al., 2006; Sigle et al., 2004)).
Fig. 3.
HEKs (upper panels) and BEP2D cells (lower panels) infected with virus encoding full-length, tagged α3 laminin (α3), tagged α3ΔLG4-5 laminin (ΔLG) and tagged β3 laminin (β3), as indicated, were visualized by confocal fluorescence and phase contrast microscopy. Phase/fluorescence overlays are shown. The focal plane is close to the substratum-attached surface of the cells. Bars, 20µm.
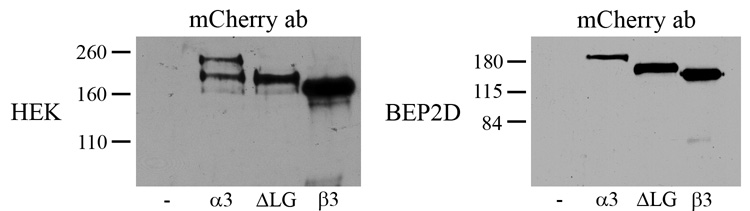
To assay the nature of the tagged proteins in the ECM of the cells, we analyzed isolated matrix by immunoblotting (Fig. 4). Immunoblotting analyses using mCherry antibody reveal that the appropriately sized tagged proteins (210, 190 and 160 kD) are detectable in the matrix HEKs and BEP2D cells expressing full-length α3, α3ΔLG4-5 or full-length β3 laminin subunits, respectively (Fig. 4). In the matrix of HEKs expressing full-length α3, we also detect a 190 kD mCherry antibody reactive polypeptide, indicating that there is N-terminal processing of the full length molecule following its secretion (Fig. 4). It also should be noted that we have been unable to detect a tagged laminin fragment representing the LG4-5 subunits in the matrix of cells induced to express a full-length tagged α3 laminin subunit (not shown). This implies that the fluorescent protein that is observed in the cells expressing tagged full-length α3 laminin in Figure 3 represents the full length protein and not a proteolyzed fragment.
Fig. 4.
The matrices of uninfected HEKs and BEP2D cells (-) or matrices of HEKs and BEP2D cells infected with virus encoding full-length, tagged α3 laminin (α3), tagged α3ΔLG4-5 laminin (ΔLG) and tagged β3 laminin (β3) were processed for immunoblotting with antibodies against mCherry as indicated. Molecular weight standards are marked at the left of each blot.
The above results are intriguing for a number of reasons. First, it has been suggested that the LG4-5 domains of the α3 laminin subunit mediate deposition of laminin-332 into matrix (Sigle et al., 2004). This is not the case in HEKs and BEP2D cells since α3ΔLG4-5 is incorporated into their extracellular matrix. Second, the existence of full-length α3 laminin subunits in the matrix of epithelial cells has been controversial (Goldfinger et al., 1999; Goldfinger et al., 1998; Tsubota et al., 2005). However, our immunochemical and fluorescence data clearly reveal that tagged, full-length α3 laminin subunits occur in the matrix of both HEKs and BEP2D cells. Since laminin-332 containing a full-length α3 laminin subunit has been reported to support cell migration while the LG4-5 domain is known to contain a syndecan binding site and enhances integrin signaling, full-length α3 laminin subunits in the matrix of HEKs and BEP2D cells likely modulates their motile behavior and integrin-mediated signaling (Goldfinger et al., 1999; Goldfinger et al., 1998; Mizushima et al., 1997; Nguyen et al., 2000; Tsubota et al., 2005; Utani et al., 2001).
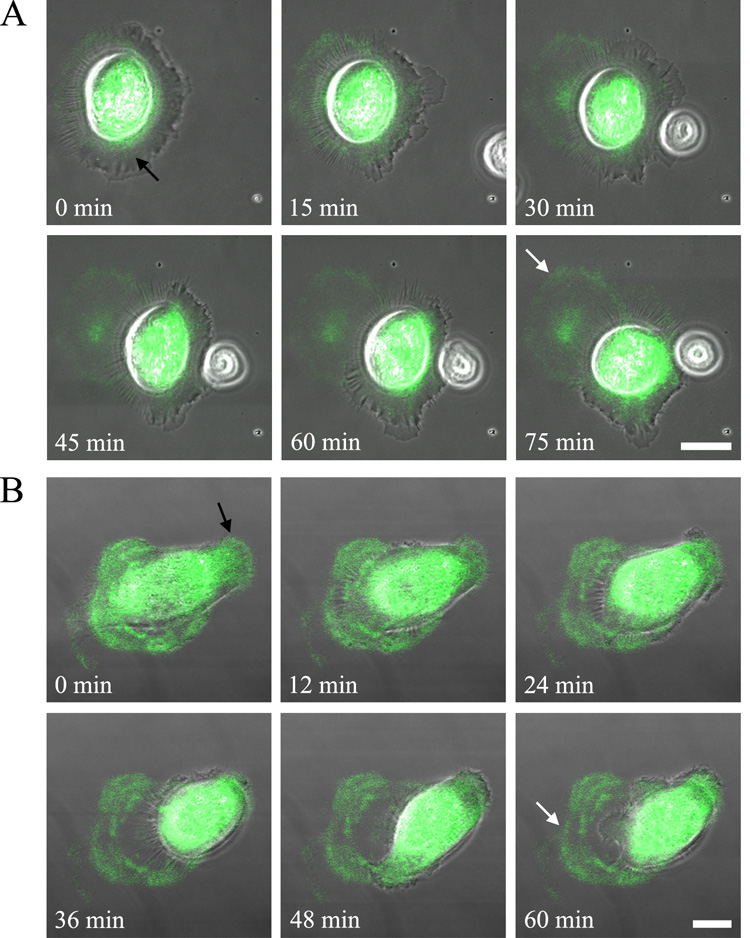
We have also analyzed live HEKs and BEP2D cells infected with virus encoding tagged β3 laminins. In both instances, the infected cells secrete labeled matrix towards the leading from of the cells and leave labeled matrix behind as they move over their substrate (Fig. 5A,B). This is consistent with recent reports from our group that laminin-332 matrices are key to the regulation of epidermal motile behavior (Kligys et al., 2007; Sehgal et al., 2006).
Fig. 5.
Live HEKs (A) or BEP2D cells (B) infected with virus encoding tagged β3 laminin were cells were plated onto glass coverslips and visualized 8 h later. Phase contrast and fluorescent images of the cells were then taken at either 5 min intervals for 75 min (A) or every 2 min for 60 min (B). Phase/fluorescence overlays at the indicated time points are shown. The black arrow in the first panel of each series of images indicates labeled protein towards the leading front of the moving cell while the white arrow in the last image of the series marks matrix left behind. The focal plane is close to the substratum-attached surface of the cells. Bars, 20µm.
Use of differentially colored laminin subunits to study laminin secretion by HEKs plated onto preformed matrix
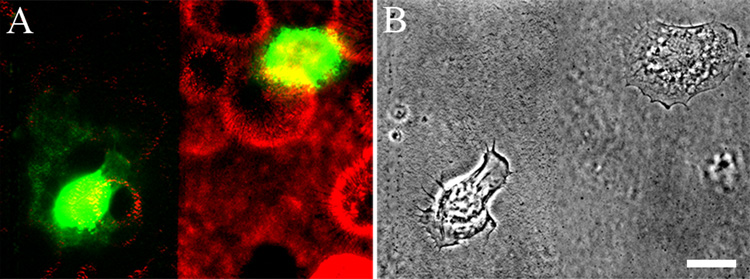
We and others have undertaken studies where we analyzed the motile and adhesive behavior of epithelial cells plated onto preformed laminin-332 matrices (see, for example, (Baker et al., 1997; Goldfinger et al., 1998; Sehgal et al., 2006; Sigle et al., 2004; Xia et al., 1996)). In such studies, there is an inherent assumption that the matrix secreted by the cell under analysis does not influence either the functions or structure of the matrix upon which the cell has been plated. However, there is no direct evidence to support this. We wanted to test this assumption by using cells expressing differentially tagged matrix molecules to analyze the secretion of laminin subunits when cells are plated onto preformed, tagged laminin matrix. For these studies, we allowed HEKs infected with virus encoding mCherry tagged β3 laminin to secrete a matrix onto their substrate for about 48 h. The cells were then removed leaving their matrix behind on the substrate, following a procedure originally detailed by Gospodarowicz (Gospodarowicz, 1984). We then scraped some of the labeled matrix using a pipetteman tip, leaving stripes of uncoated substrate surrounded by labeled matrix. Subsequently, we plated HEKs infected with virus encoding YFP-tagged β3 laminin onto the mCherry labeled matrix. We visualized the latter cells at 8 h after plating. Those HEKs that had adhered to labeled matrix appear blocked in their ability to deposit a YFP tagged laminin subunit into the preexisting matrix (Fig. 6; cell on the right). In sharp contrast, HEKs that adhere to areas of the substrate denuded of matrix assemble arcs and circles of YFP tagged laminins (Fig. 6; cell on the left). This indicates that there is a feed back mechanism in which laminin heterotrimer secretion and/or deposition is inhibited by preexisting laminin matrix.
Fig. 6.
HEKs expressing mCherry-tagged β3 laminin were plated onto glass coverslips. 24 h later the confluent monolayer of cells was removed, leaving the matrix deposited by the cells behind (Gospodarowicz, 1984). Part of this matrix was scraped off as indicated. Subsequently, HEKs expressing YFP-tagged β3 laminin were plated sparsely onto the coverslips and the cells visualized 8 h later. In A the dual color image of the cells and their matrix is shown while in B, the phase contrast image is presented. The focal plane is close to the substratum-attached surface of the cells. Note that the cell on the left of the image in A has adhered to the region of the coverslip from which matrix was scraped off. This cell has elaborated a YFP-matrix. In contrast, the cell on the right of the image, which has adhered to the mCherry tagged matrix, has not assembled a YFP matrix, despite expressing YFP-tagged protein in the cytoplasm. This is representative of three different trials. Bar in B, 20µm .
Assembly of laminin-311 matrix by ATII cells
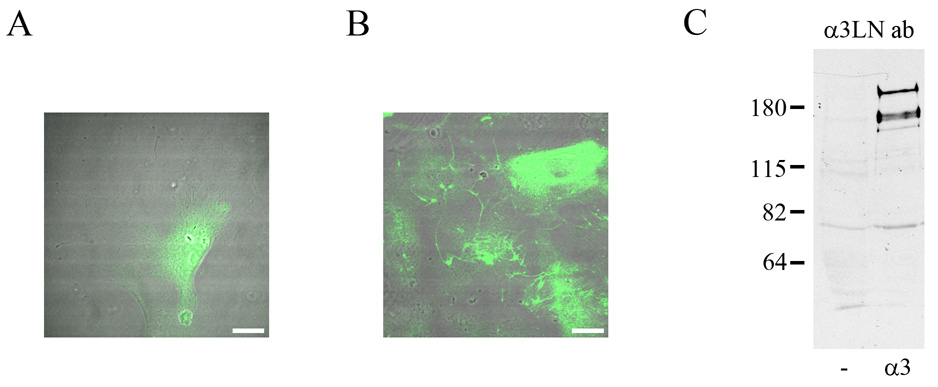
In certain circumstances a loss of the tag on a laminin subunit can be informative. This is the case for rat ATII cells. There is little, if any, laminin-332 in the matrix of ATII cells maintained in vitro (Jones et al., 2005). Rather, rat ATII cells assemble a matrix rich in laminin-311 fibers (Jones et al., 2005). Our previous published data suggest that the α3 subunit of laminin-311 in the matrix of rat ATII cells is processed (Jones et al., 2005). We confirmed this by observing cells infected with virus encoding tagged full-length α3 laminin subunit. We observe only background signal in the matrix of the infected, fluorescent ATII cells (Fig. 7A). However, when we stained these cells with an antibody (RG13) specific to the human α3 laminin subunit, we have detected numerous fluorescent fibers in the matrix of the cells (Fig. 7B). Moreover, a 210 kD polypeptide (i.e. unprocessed human α3 laminin, lacking its tag) is also recognized in Western immunoblots of extracts off the infected rat ATII cells probed with the human specific α3 laminin antibodies (Fig. 7C). The latter also recognize an additional 190 kD species which likely represents the processed α3 laminin subunit (Fig. 7C). Together, these results indicate that the tagged human α3 laminin subunit is rapidly and very efficiently processed following secretion by the rat ATII cells, unlike the case of HEKs and BEP2D cells detailed above. This suggests the possibility that processing of the α3 laminin is enhanced or facilitated when the α3 laminin subunit is a component of laminin-311 versus laminin-332. Intriguingly, another obvious difference between HEKs, BEP2D and ATII cells, is that ATII cells infected with adenovirus encoding α3ΔLG4-5 subunit are induced to detach from their substrate. Thus, in ATII cells, the truncated α3 laminin subunit appears to act in a dominant-negative fashion and induces dysadhesion of cells (not shown). This is not because of a problem in expressing laminin subunits in a rat cell line since tagged unprocessed and processed human α3 laminin subunits are expressed by 804G cells, a rat bladder epithelial cell type, without any impact on cell adhesion (not shown)(Langhofer et al., 1993). Rather, we speculate that the ability of the α3ΔLG4-5 laminin subunit to induce dysadhesion of ATII cells may result from an inhibition in laminin-311 protein secretion and/or matrix assembly.
Fig. 7.
Uninfected ATII cells (A) of ATII cells infected with virus encoding expressing human full-length, tagged α3 laminin (B) were prepared for indirect immunofluorescence microscopy using an antibody specific for the human α3 laminin subunit. The focal plane is close to the substratum-attached surface of the cells. The fluorescent images have been overlaid on phase contrast views of the fixed and stained cells. The diffuse staining in the cells in A represents background. While there is no fibrous staining in A, the human α3 laminin subunit antibody stains a network of fibers in B. In C, extracts of uninfected ATII cells and ATII cells infected with virus encoding expressing human full-length, tagged α3 laminin were processed for immunoblotting using the human α3 laminin subunit. Equal amounts of protein were loaded in the two lanes. Molecular weight standards are marked at the left. Bars in A and B, 20µm .
In summary, our data indicate the utility of an adenoviral delivery system to induce expression of tagged laminin subunits in a variety of cell types derived from diverse epithelial tissues. These tagged molecules have enabled us to visualize laminin matrix in live and fixed cells without the need for antibody localization and assay the ability of laminin matrix to support migration in live cells. Based on our preliminary studies, we predict that tagged laminin subunits will provide novel insights into the structure and functions of laminin matrices. For this reason, we will make these reagents freely available to the scientific community. To date, we have focused our efforts on generating tagged versions of the “smaller” laminin subunits but we are now in the process of preparing adenovirus encoding tagged γ1 and β1 laminin subunits. Moreover, although we have only used our virus to induce expression of the tagged molecules in cultured cells, we also suggest that they may be used in vivo in the oral cavity and lung, for example, since viral delivery of proteins to these tissues has been demonstrated by others (Auricchio et al., 2002 ; Factor et al., 2000; Factor et al., 1998; Mastrangeli et al., 1994).
3. Experimental procedures
3.1 Reagents, Cell Culture and Antibodies
HEKs, immortalized with HPV genes E6 and E7, were maintained in defined keratinocyte serum free media supplemented with a 1% penicillin/streptomycin/neomycin mixture (Invitrogen Corp., Carlsbad, CA) and grown at 37°C as described previously. BEP2D cells were generously provided by Curtis C. Harris of the NIH (Willey et al., 1991). They were cultured in serum-free LHC-8 medium supplemented with growth factors as described by others (Lechner and LaVeck, 1985). ATII cells were isolated from pathogen-free male Sprague Dawley rats and maintained in culture as previously detailed (DeBiase et al., 2006; Jones et al., 2005). A rabbit polyclonal antibody against mCherry was purchased from Clontech (Palo Alto, CA), respectively. Mouse monoclonal antibody RG13 against the human α3 laminin subunit was described previously (Gonzales et al., 1999). Clone 17, a mouse monoclonal antibody against the β3 laminin subunit, was purchased from BD Transduction Laboratories (San Jose, CA).
3.2 Adenoviral constructs
Adenovirus encoding YFP-tagged β3 laminin was described previously (Sehgal et al., 2006). In addition, full-length cDNAs encoding the α3 and β3 laminin subunits were cloned separately into the pENTR4 plasmid which contains the mCherry-N1 coding region and polylinker (Invitrogen Corp., Carlsbad, CA). To generate a truncated α3 laminin subunit, missing LG4-5 (α3ΔLG4-5), an AgeI restriction site was created at base pair 4002 in the full-length α3 laminin/pENTR4 plasmid by site-directed mutagenesis following the protocol of the manufacturer (Stratagene, La Jolla, CA). This base pair is close to the sequences encoding the major cleavage site within the G domain of the α3 laminin subunit (Hirosaki et al., 2000; Tsubota et al., 2000). An AgeI digest was performed, removing sequences between this AgeI site in the α3 laminin and the AgeI site in the polylinker. Upon religating the plasmid, sequences encoding the LG4-5 domain were removed while maintaining the reading frame through the mCherry tag. All constructs were sequenced to confirm the correct reading frame of the fusion proteins. Each pENTR4 plasmid was then used in an LR recombination reaction to transfer the cassette into the pAd/CMV/V5-DEST adenoviral vector. Following amplification, each of the adenoviral expression clones was introduced into 293A cells by lipofectamine-mediated transfection. After 10–12 days, the crude viral lysate was harvested and used to amplify the adenovirus as previously described (Sehgal et al., 2006). The amplified viral stock was titred and epithelial cells were infected at a multiplicity of infection (MOI) of 1:10 to 1:50 in cell medium.
3.3 Live Cell Assays and Fluorescence Microscopy
For live cell imaging, cells were plated onto uncoated 35 mm glass-bottomed culture dishes (MatTek Corp., Ashland, MA) 18 h prior to the assay. Cells were maintained at 37°C in a cell culture, stage top incubator (Tokai Hit Co., Ltd, Shizuokaken, Japan) and then viewed on a Zeiss laser-scanning microscope (LSM) 510 confocal microscope (Zeiss Inc., Thornwood, NY). Images were taken at 2–5 min intervals over 2 h. In the case of fixed cell preparations, cells plated onto glass coverslips were processed for microscopical analyses as detailed elsewhere (Kligys et al., 2007; Sehgal et al., 2006). All preparations were viewed with a Zeiss laser-scanning microscope (LSM) 510 confocal microscope. For some assays, cells were allowed to adhere to coverslips overnight. The cells were rinsed with PBS and treated with 20mM NH4OH for 5 min to remove cells, leaving matrix behind (Gospodarowicz, 1984). The coverslips were washed extensively with water and rinsed with PBS. Cells infected with adenovirus were plated directly onto the matrix, allowed to adhere for 8 h, fixed and then imaged in the confocal microscope, as above. Images were exported as TIFF files, and figures were generated using Adobe Photoshop software.
3.4 SDS-PAGE, Immunoblotting and Immunoprecipitation
Whole cell extracts were prepared by solubilization in 1% SDS, 8M urea, 10% glycerol, 5% β-mercaptoethanol, 25mM Tris-HCl, pH 6.5 (sample buffer). Cell matrix was prepared as described above (Gospodarowicz, 1984). Matrix proteins were collected from the culture dish by solubilization in sample buffer as above. The proteins were separated by SDS-PAGE, transferred to nitrocellulose, and processed for immunoblotting as previously detailed (Harlow and Lane, 1988; Klatte et al., 1989; Laemmli, 1970).
Acknowledgments
This work was made possible by grants from the National Institutes of Health (RO1 AR054184 and PO1 HL071643). KK was supported by a training grant from the National Institutes of Health (T32 HL076139).
List of abbreviations
- HEKs
human epidermal keratinocytes
- BEP2D cells
immortalized human bronchial epithelial cells
- ATII
alveolar type II epithelial cells
- GFP
green fluorescent protein
- YFP
yellow fluorescent protein
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Auricchio A, O’Connor E, Weiner D, Gao G-P, Hildinger M, Wang L, Calcedo R, Wilson JM. Noninvasive gene transfer to the lung for systemic delivery of therapeutic proteins. J. Clin. Invest. 2002;110:499–504. doi: 10.1172/JCI15780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SE, Skalli O, Goldman RD, Jones JC. Laminin-5 and modulation of keratin cytoskeleton arrangement in FG pancreatic carcinoma cells: Involvement of IFAP300 and evidence that laminin-5/cell interactions correlate with a dephosphorylation of alpha 6A integrin. Cell Motil. Cytoskel. 1997;37:271–286. doi: 10.1002/(SICI)1097-0169(1997)37:3<271::AID-CM9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Hemidesmosomes: Role in adhesion, signaling and human diseases. Curr. Op. Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J. Cell Biol. 1999;145:619–631. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBiase PJ, Lane K, Budinger S, Ridge K, Wilson M, Jones JCR. Laminin-331 (laminin-6) fiber assembly by type I-like alveolar cells. J. Histo. Cytochem. 2006;54:665–672. doi: 10.1369/jhc.5A6889.2006. [DOI] [PubMed] [Google Scholar]
- Factor P, Dumasius V, Saldias F, Brown LAS, Sznajder JI. Adenovirus-mediated transfer of an Na/K-ATPase beta1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum. Gene Ther. 2000;11:2231–2242. doi: 10.1089/104303400750035753. [DOI] [PubMed] [Google Scholar]
- Factor P, Saldias F, Ridge K, Dumasius V, Jabner Z, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder JI. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase beta1 subunit gene. J. Clin. Invest. 1998;102:1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JCR. The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci. 1999;112:2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JCR. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Haan K, Baker SE, Fitchmun MI, Todorov I, Weitzman S, Jones JCR. A cell signal pathway involving laminin-5, α3β1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol. Bio. Cell. 1999;10:259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz DIE. Preparation of extracellular matrices produced by cultured bovine corneal endothelial cells and PF-HR-9 endodermal cells: their use in cell culture. In: Alan R, editor. Methods for preparation of media, supplements, and substrata for serum-free animal cell culture. New York: Alan R. Liss. Inc.; 1984. pp. 275–293. [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. pp. 485–510. [Google Scholar]
- Hirosaki T, Mizushima H, Tsubota Y, Moriyama K, Miyazaki K. Structural requirement of carboxyl-terminal globular domains of laminin α3 chain for promotion of rapid cell adhesion and migration by laminin-5. J. Biol. Chem. 2000;275:22495–22502. doi: 10.1074/jbc.M001326200. [DOI] [PubMed] [Google Scholar]
- Johnson C, Galis ZS. Quantitative assessment of collagen assembly by live cells. J. Biomed. Mat. Res. 2003;67A:775–784. doi: 10.1002/jbm.a.10136. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. BioEssays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Lane K, Hopkinson SB, Lecuona E, Geiger RC, Dean DA, Correa-Meyer E, Gonzales M, Campbell K, Sznajder JI, Budinger S. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechano-signal transduction via a dystroglycan-dependent, integrin-independent, mechanism. J. Cell Sci. 2005;118:2557–2565. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte DH, Kurpakus MA, Grelling KA, Jones JCR. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J. Cell Biol. 1989;109:3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligys K, Claiborne JN, DeBiase P, Hopkinson SB, Mizuno K, Jones JCR. The Slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization and motility behavior of keratinocytes. J. Biol. Chem. 2007;282:32520–32528. doi: 10.1074/jbc.M707041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel BA, Rongsih BJ, Czirok A, Zach A, Little CD, Davis EC, Knutsen RH, Wagensil JE, Levy MA, Mecham RP. Elastic fiber fomration: A dynamic view of extracellular matrix assembly using timer reporters. J. Cell. Physiol. 2006;207:87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- Krahn KN, Bouten CVC, van Tuijl S, Zandvoort MAMJ, Merkx M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell cultures. Anal. Biochem. 2006;350:177–185. doi: 10.1016/j.ab.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JCR. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J. Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Larsen M, Wei C, Yamada KM. Cell and fibronectin dynamics during branching morphogenesis. J. Cell Sci. 2006;119:3376–3384. doi: 10.1242/jcs.03079. [DOI] [PubMed] [Google Scholar]
- Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J. Tissue Culture Methods. 1985;9:43–48. [Google Scholar]
- Li S, Harrison D, Carbonetto S, Fässler R, Smyth N, Edgar D, Yurchenco PD. Matrix assembly, regulation, and survival functions of laminin and its receptors in embryonic stem cell differentiation. J. Cell Biol. 2002;157:1279–1290. doi: 10.1083/jcb.200203073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J. Biol. Chem. 1992;267:17900–17906. [PubMed] [Google Scholar]
- Mastrangeli A, O'Connell B, Aladib W, Fox PC, Baum BJ, Crystal RG. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am. J. Physiol. 1994;266:G1146–G1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- Matsui C, Wang CK, Nelson CF, Bauer EA, Hoeffler WK. The assembly of laminin-5 subunits. J. Biol. Chem. 1995;270:23496–23503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- Mizushima H, Takamura H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K. Identification of integrin-dependent and -independent cell adhesion domains in COOH-terminal globular region of laminin-5 α3 chain. Cell Growth Differen. 1997;8:979–987. [PubMed] [Google Scholar]
- Nguyen BP, Ren X-D, Schwartz MA, Carter WG. Ligation of integrin α3β1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J. Biol. Chem. 2000;276:43860–43870. doi: 10.1074/jbc.M103404200. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Kiehart DP, Erickson HP. Dual labeling of the fibronectin matrix and actin cytoskeleton with green fluorescent protein variants. J. Cell Sci. 2002;115:1221–1229. doi: 10.1242/jcs.115.6.1221. [DOI] [PubMed] [Google Scholar]
- Sehgal BU, Debiase P, Matzno S, Chew T-L, Claiborne JN, Hopkinson SB, Russell A, Marinkovich PM, Jones JCR. Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 (laminin-5) matrix organization. J. Biol. Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigle RO, Gil SG, Bhattacharya M, Ryan MC, Yang T-M, Brown TA, Boutaud A, Miyashita Y, Olerud J, Carter WG. Globular domains 4/5 of the laminin α3 chain mediate deposition of precursor laminin 5. J. Cell Sci. 2004;11:4481–4494. doi: 10.1242/jcs.01310. [DOI] [PubMed] [Google Scholar]
- Sivakumar P, Czirok A, Rongsih BJ, Divakara VP, Wang Y, Dallas SL. New insights into extracellular matrix assembly and reorganization from dynamic imaging of extracellular matrix proteins in living osteoblasts. J. Cell. Sci. 2005;119:1350–1360. doi: 10.1242/jcs.02830. [DOI] [PubMed] [Google Scholar]
- Tsubota Y, Mizushima H, Hirosaki T, Higashi S, Yasumitsu HKM. Isolation and activity of proteolytic fragment of laminin-5 alpha3 chain. Biochem. Biophysic. Res. Commun. 2000;278:614–620. doi: 10.1006/bbrc.2000.3851. [DOI] [PubMed] [Google Scholar]
- Tsubota Y, Yasuda C, Kariya Y, Ogawa T, Hirosaki T, Mizushima H, Miyazaki K. Regulation of biological activity and matrix assembly of laminin-5 by COOH-terminal ,LG4-5 domain of α3 chain. J. Biol. Chem. 2005;280:14370–14377. doi: 10.1074/jbc.M413051200. [DOI] [PubMed] [Google Scholar]
- Udayakumar TS, Chen ML, Bair EL, Von Bredow DC, Cress AE, Nagle RB, Bowden GT. Membrane type-1-matrix metalloproteinase expressed by prostate carcinoma cells cleaves human laminin-5 beta3 chain and induces cell migration. Cancer Res. 2003;63:2292–2299. [PubMed] [Google Scholar]
- Utani A, Nomizu M, Matsuura H, Kato K, Kobayashi T, Takeda U, Aota S, Nielsen PK, Shinkai H. A unique sequence of the laminin alpha 3 G domain binds to heparin and promotes cell adhesion through syndecan-2 and -4. J. Biol. Chem. 2001;276:28779–28788. doi: 10.1074/jbc.M101420200. [DOI] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer J. The ins and outs of fibronectin matrix assembly. J. Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- Willey JC, Broussoud A, Sleemi A, Bennett WP, Cerutti P, Harris CC. Immortalization of normal human bronchial epithelial cells by human papillomaviruses 16 or 18. Cancer Res. 1991;51:5370–5377. [PubMed] [Google Scholar]
- Xia Y, Gill SG, Carter WG. Anchorage mediated by integrin α6β4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane associated 80-kD protein. J. Cell Biol. 1996;132:727–740. doi: 10.1083/jcb.132.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]